Abstract
The effects of supernatant from the bacterial strain Serratia marcescens 2170 (CS-2170) on the viability of different haematopoietic cancer cell lines (Jurkat, NSO, HL-60 and Ramos) and nonmalignant cells (NIH-3T3 and MDCK) was studied. We examined whether this cytotoxic effect was due to apoptosis, and we purified the molecule responsible for this effect and determined its chemical structure.
Using an MTT assay we showed a rapid (4 h) decrease in the number of viable cells. This cytotoxic effect was due to apoptosis, according to the fragmentation pattern of DNA, Hoechst 33342 staining and FACS analysis of the phosphatidylserine externalization. This apoptosis was blocked by using the caspase inhibitor Z-VAD.fmk, indicating the involvement of caspases.
Prodigiosin is a red pigment produced by various bacteria including S. marcescens. Using mutants of S. marcescens (OF, WF and 933) that do not synthesize prodigiosin, we further showed that prodigiosin is involved in this apoptosis. This evidence was corroborated by spectroscopic analysis of prodigiosin isolated from S. marcescens.
These results indicate that prodigiosin, an immunosuppressor, induces apoptosis in haematopoietic cancer cells with no marked toxicity in nonmalignant cells, raising the possibility of its therapeutic use as an antineoplastic drug.
Keywords: Apoptosis, cancer cell lines, chemotherapy, immunosuppressor, prodigiosin
Introduction
Apoptosis is a form of cell death in which cells actively participate in their own destructive processes. This process is characterized by morphological (Kerr et al., 1994) and biochemical/molecular (Rowan & Fisher, 1997; Reed, 1999; Wickremasinghe & Hoffbrand, 1999) criteria. Cells undergoing apoptosis shrink and lose their normal intercellular contacts and subsequently exhibit cytoplasmic and chromatin condensation and internucleosomal cleavage of DNA. In the final stages, cells become fragmented into small apoptotic bodies, which are then eliminated by phagocytosis.
Several bacterial pathogens have been identified as mediators of apoptosis in vitro and during pathogenesis (Zychlinsky & Santonetti, 1997). These pathogens have developed different strategies to survive inside the host, overcome natural defences and thus cause disease. Induction of host immunosuppression by triggering apoptosis in phagocytes, like polymorphonuclear neutrophils (PMN) and macrophages, might represent an advantage in bacterial invasion, since these are the most dangerous cells for bacteria. Several pathogens like Shigella spp (Chen & Zychlinsky, 1994) and Salmonella spp (Chen et al., 1996; Lindgren et al., 1996; Monack et al., 1996) induce apoptosis in macrophages. Furthermore, the induction of PMN apoptosis by Actinobacillus actinomycetemcomitans has been suggested (Kato et al., 1995).
Bacterial toxins like leukotoxin, α-toxin and hemolysin form pores in the eukaryotic cell membrane and disrupt the cell via osmotic swelling (Mangan et al., 1991; Hildebrand et al., 1991; Jonas et al., 1993). Other toxins like diphtheria toxin and exotoxin A inhibit protein synthesis, causing apoptosis in eukaryotic cells (Morimoto & Bonavida, 1992; Kochi & Collier, 1993). Yoshida et al. (1998) identified an acidic glycoprotein purified from the crude extract of Streptococcus pyogenes Su, which showed cell growth inhibition in vitro and antitumour activity in vivo. Verotoxin 1, the active component of the bacteriocin preparation from Escherichia coli, induces apoptosis in human cancer cell lines (Arab et al., 1998) and eliminates human astrocytoma xenografts (Arab et al., 1999). The prevention of neoplasia by agents from bacteria that inhibit cancer cell proliferation but are not toxic to healthy cells is an exciting prospect. In our laboratory, during screening of potential anticancer drugs, we observed that the supernatant from cultures in stationary phase (growth) of the bacterial strain S. marcescens 2170 induced death in several cancer cell lines. On the basis of this reproducible observation we pursued three objectives. First, we examined the effects of supernatant on the viability of several haematopoietic cancer cell lines and nonmalignant cells and assessed whether this cytotoxic effect was due to apoptosis. Second, we identified the molecule contained in the supernatant of S. marcescens that was responsible for the induction of apoptosis. Third, we purified this molecule and determined its chemical structure. Our results confirm the molecule as an apoptotic factor with interesting anti-cancer properties.
Methods
Chemical and reagents
Meat peptone was purchased from Difco (Detroit, MI, U.S.A.). Glycerol was bought from Merck (Darmstadt, Germany). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and propidium iodide (PI) were purchased from Sigma Chemicals Co (St Louis, MO, U.S.A.). N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (Z-VAD.fmk) was obtained from Enzyme Systems Products (Dublin, CA, U.S.A.). Deionized water further purified with a Millipore Milli-Q system (Bedford, MA, U.S.A.). was used.
Bacterial strains
Serratia marcescens 2170 environmental isolated is a wild type strain that produces prodigiosin. Mutants strains of S. marcescens-OF, -WF and -933 were deficient in prodigiosin biosynthesis (Mody et al., 1990). NR1 is a lipopolysaccharide (LPS)-deficient mutant strain of S. marcescens which does not have the O-antigen (Palomar et al., 1993).
Bacterial cell cultures
Each bacterial strain (S. marcescens-2170, -OF, -WF, -933 and -NR1) was inoculated into 25 ml of peptone glycerol (PG) medium, containing 1% meat peptone and 10% glycerol in distilled water, and cultivated for 8 h at 30°C with vigorous shaking, and then transferred to 250 ml of PG medium and cultivated for 48 h at 30°C with vigorous shaking. Bacteria were then harvested by centrifugation at 6800×g for 15 min at 4°C.
Supernatants from the bacterial strains were passed through a 0.22 μm filter (Nalge Nunc Int. Rochester, NY, U.S.A.) and concentrated by a centricon® Plus-20 (Millipore Iberica, Barcelona, Spain). The resulting concentrated samples (named CS-2170, CS-OF, CS-WF, CS-933 and CS-NR1) were divided into aliquots and stored at −20°C. Protein concentration of the CS were estimated by the Bradford method (Bradford, 1976), and used as a measure of the CS quantity added to the growth medium of the cell lines.
Cell lines and culture conditions
Acute human T cell leukaemia cells (Jurkat clone E6-1), myeloma cells (NSO), human promyelocytic leukaemia cells (HL-60), human Burkitt lymphoma cells (Ramos), Swiss mouse embryo cells (NIH-3T3) and MDCK (NBL-2) nonmalignant canine epithelial cells were obtained from the American Type Culture Collection (Rockville, MD, U.S.A.). Cells were cultured in RPMI 1640 medium (Biological Industries, Beit Haemek, Israel) except NIH-3T3, which was cultured in DMEM medium (Biological Industries, Beit Haemek, Israel) and MDCK, which was cultured in MEM (Sigma). The three media were supplemented with 10% heat-inactivated FBS, 100 u ml−1 penicillin, 100 μg ml−1 streptomycin (all from GIBCO–BRL, Paisley, U.K.), and 2 mM L-glutamine (Sigma).
Cell viability assay
Cell viability was determined by the MTT assay (Mosmann, 1983). Briefly, 20×103 cells were incubated in 96-well microtiter cell culture plates, in the absence (control cells) or in the presence of increasing amounts of CS-2170, CS-OF, CS-WF, CS-933 and CS-NR1, in a final volume of 100 μl. After 4 h incubation, 10 μM of MTT (diluted in PBS) was added to each well for an additional 4 h. The blue MTT formazan precipitate was dissolved in 100 μl of isopropanol:1N HCl (24 : 1) and the absorbance at 550 nm was measured on a multiwell plate reader. Cell viability was expressed as a percentage of control. Data are shown as the mean±standard deviation of triplicate cultures.
Analysis of DNA fragmentation
Analysis of DNA fragmentation by agarose gel electrophoresis was performed as described previously (Bellosillo et al., 1997). Briefly, 1×106 cells per ml were exposured to 10 μg ml−1 of CS-2170, CS-OF, CS-WF, CS-933 and CS-NR1 and incubated overnight (16 h). Cells were washed in PBS and resuspended in ice-cold lysis buffer (10 mM Tris-HCl pH 7.4, 1 mM EDTA, 0.2% Triton X-100). After incubating for 15 min at 4°C, cell lysates were centrifuged at 14,000×g for 15 min to separate low molecular weight DNA from intact chromatin. The supernatant was treated with 0.2 mg ml−1 of proteinase K in a buffer containing (mM) NaCl 150, Tris-HCl 10 pH 8.0, EDTA 40 and 1% SDS for 4 h at 37°C. The DNA preparations were phenol/chloroform extracted twice to remove proteins. DNA was precipitated with 140 mM NaCl and two volumes of ethanol at −20°C overnight. DNA precipitates were recovered by centrifugation at 14,000×g for 15 min at 4°C, washed twice in cool 70% ethanol and air dried. DNA pellets were resupended in 15 μl of TE (10 mM Tris-HCl pH 8.0, 1 mM EDTA) and treated with RNase-DNase free (Boehringer Mannheim, Mannheim, Germany) for 1 h at 37°C. 3.2 μl of loading buffer was added to each tube and the DNA preparations were electrophoresed in 1% agarose gels which contained ethidium bromide. Gels were placed on a UV light box to visualize the DNA ladder pattern.
Hoechst staining
Cell morphology was evaluated by fluorescence microscopy following Hoechst 33342 DNA staining (Sigma Chemicals Co.). Cells (4×105 per ml) from each cell line were incubated in the absence (control cells) or in the presence of CS-2170 (5 μg ml−1) and 300 nM of prodigiosin for 6 h. Cells were then washed in PBS and resuspended in PBS plus Hoechst 33342 to a final concentration of 2 μg ml−1 and incubated for 30 min at 37°C in the dark. After incubation, cells were washed in PBS and the sections were examined with a Leitz Diaplan microscope and photographed with a Wild MPS 45 Photoautomat system. Apoptotic cells were identified by features characteristic of apoptosis (e.g. nuclear condensation, formation of membrane blebs and apoptotic bodies).
Analysis of apoptosis by annexin V binding
Exposure of phosphatidylserine was quantified by surface annexin V-FITC (Bender MedSystems, Boehringer Mannheim) staining as described previously (Koopman et al., 1994; Bellosillo et al., 1998). 4×105 cells per ml were incubated for 4 h with 5 and 10 μg ml−1 of CS-2170. After this, cells were washed in PBS and resuspended in 200 μl binding buffer (mM: HEPES/NaOH 10 pH 7.4, NaCl 140, CaCl2 2.5) plus 0.6 μl of annexin V-FITC Kit and incubated for 30 min at room temperature in the dark. After incubation, we added 200 μl of binding buffer and propidium iodide to a final concentration of 5 μg ml−1. Cells were analysed using a Becton Dickinson FACS Calibur flow cytometer (Mountain View, CA, U.S.A.). Samples were acquired and analysed using Cell Quest software and data were analysed with the Paint-a-gate Pro software (Becton Dickinson).
Annexin V binds to those cells that express phosphotidylserine on the outer layer of the membrane, and propidium iodide stains the cellular DNA of those cells with a compromised cell membrane. This allows the discrimination of live cells (unstained with either fluorochrome) from early apoptotic cells (stained only with annexin V) and advanced apoptotic and necrotic cells (stained with both annexin V and propidium iodide).
Purification of prodigiosin
Prodigiosin was extracted by shaking the S. marcescens 2170 cells with a mixture of methanol/1N HCl (24 : 1). After centrifugation (6800×g for 15 min), the solvent of the supernatant was evaporated under vacuum. Atmospheric pressure liquid chromatography of the extract was performed on silica gel with chloroform and methanol as solvents. The eluted pigmented fractions were pooled and the chloroform/methanol extract was vacuum evaporated, redissolved in H2O and lyophilized. The isolated pigment was redissolved in methanol and analysed by electrospray ionisation mass spectrometry (ESI-MS) using a VG-Quattro triple quadrupol mass spectrometer (Micromass, VG-Biotech, U.K.). The isolated pigment was repurified by subsequent semipreparative HPLC carried out on a Shimadzu instrument (Kyoto, Japan). A Nucleosil C18 reversed-phase column (250×4 mm, 10 μm) was used with a 0–100% linear gradient in 30 min (A: 0.01 M ammonium acetate, pH 7, B: 100% acetonitrile). The elution was monitored both using diode-array UV detector (SPD-M10AVP Shimadzu) and by ESI-MS. After repeated injections the pooled fractions containing the major peak were vacuum evaporated, redissolved in H2O, lyophilized and characterized by ESI-MS and 1H-NMR. ESI, m/z 324.4 (M+H)+, (C20H25N3O requires 323.4381 (MW average)). 1H-NMR (CD3OD, 500 MHz, p.p.m.); 10.71 (m, NH), 8.54 (m, NH), 7.08 (s, 1H), 6.95 (s, 1H), 6.88 (m, 1H), 6.83 (m, 1H), 6.30 (m, 1H), 6.25 (s, 1H), 3.96 (s, 3H), 2.43 (t, 2H), 1.58 (s, 3H), 1.2–1.4 (m, 6H), 0.91 (t, 3H). The concentration of prodigiosin was determined as described (Goldschmidt & Williams, 1968).
Results
CS-2170 decreased the viability of haematopoietic cancer cells
The effect of the concentrated supernatant from S. marcescens 2170 (CS-2170) on the viability of different haematopoietic cancer cell lines (Jurkat, NSO, HL-60 and Ramos) and nonmalignant cell lines (NIH-3T3 and MDCK) was studied. Cell lines were incubated for 4 h with several doses of CS-2170, ranging from 1 to 10 μg ml−1, and then cell viability was determined by the MTT assay. A dose-dependent decrease in the number of viable cells was observed in all the cell lines studied except NIH-3T3 and MDCK (Figure 1A). The IC50 was 1.45 μg ml−1 for Jurkat, 3.03 μg ml−1 for Ramos, 3.14 μg ml−1 for HL-60 and between 5 and 10 μg ml−1 for NSO. We did not observe a significant decrease in the viability of the NIH-3T3 and MDCK at the doses used in the experiment or in other nonmalignant cell lines like Swiss-3T3. The viability study of these cell lines treated with prodigiosin at different times (4–24 h) did not show differences (data not shown).
Figure 1.
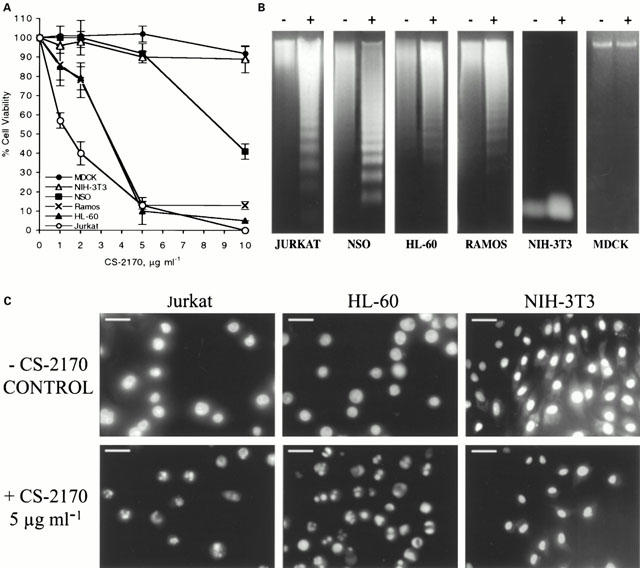
CS-2170 induces apoptosis in haematopoietic cancer cell lines. (A) Dose-response of the effect of CS-2170 on cell viability. Haematopoietic cancer cells (Jurkat, NSO, HL-60 and Ramos) and nonmalignant cell lines (MDCK and NIH-3T3) were incubated for 4 h with 1–10 μg ml−1 of CS-2170. Cell viability was determined by the MTT assay as described in Methods and it is expressed as a percentage with respect to control cells. (B) DNA fragmentation induced by CS-2170 was observed in the agarose gel electrophoresis as described in Methods. Jurkat, NSO, HL-60, Ramos, MDCK and NIH-3T3 cells were untreated (−) or incubated for 16 h with 10 μg ml−1 of CS-2170 (+). (C) Fluorescence microscopic analysis of Jurkat, HL-60 and NIH-3T3 nuclei with Hoechst 33342 staining. Cells were untreated (-CS-2170) or treated with 5 μg ml−1 of CS-2170 for 6 h (bar=10 μm).
CS-2170 induced apoptosis in haematopoietic cancer cells
In order to determine whether this cytotoxic effect was due to apoptosis, we analysed whether CS-2170 induces DNA fragmentation. Agarose gel electrophoresis of DNA showed the characteristic ladder pattern of apoptosis in all haematopoietic cancer cell lines incubated overnight in the presence of 10 μg ml−1 of CS-2170. However, DNA laddering was not observed in the NIH-3T3 and MDCK at this dose of CS-2170 (Figure 1B).
We corroborated these results at microscopic level using the Hoechst 33342 staining. Fluorescence microscope allowed the visualization of apoptotic cells with condensed or fragmented nuclei in Jurkat and HL-60 cells but not in NIH-3T3 cells (Figure 1C).
Furthermore, we confirmed these results by measuring apoptosis in the Jurkat cell line by FACS analysis with annexin V-FITC and propidium iodide staining (Figure 2). We incubated Jurkat cells with 0, 5 and 10 μg ml−1 of CS-2170. The mean values of the early apoptotic populations (IP−/Annexin V+ cells) were 4±2, 34±8 and 45±3%, respectively.
Figure 2.
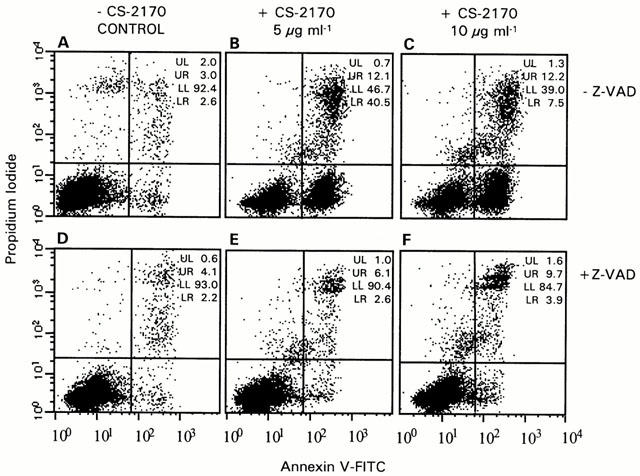
Involvement of caspases in induction of phosphatidylserine externalization by CS-2170. A representative annexin-V/IP assay with Jurkat is shown. This assay reveals the early phosphatidylserine translocation to the surface of apoptotic cells by incubating the cells with FITC-labelled annexin V and the latest stages of apoptotic or necrotic processes were detected with the vital dye propidium iodide (PI). Cells staining were analysed using the Becton Dickinson FACS Calibur flow cytometer as described in Methods. (A) Control cells. (B, C) Cells were incubated for 4 h with 5 and 10 μg ml−1 of CS-2170 respectively. (D) Cells were incubated with 50 μM Z-VAD.fmk. (E, F). Cells were incubated with 5 and 10 μg ml−1 of CS-2170 respectively in the presence of 50 μM Z-VAD.fmk.
To demonstrate the involvement of caspase activation in the apoptotic effect, we analysed whether the caspase inhibitor Z-VAD.fmk prevented apoptosis (García-Calvo et al., 1998). When cells were incubated with 0, 5 and 10 μg ml−1 of CS-2170 in the presence of 50 μM Z-VAD.fmk, the mean values of the early apoptotic population were 2±0.2, 3±0.4 and 5±2% respectively. These results show that Z-VAD.fmk inhibits the apoptotic action of CS-2170. Furthermore, Z-VAD.fmk blocked DNA fragmentation (data not shown).
Identification from CS-2170 of the molecule responsible of apoptosis in haematopoietic cancer cells
Samples of CS-2170 were heated to 100°C for 15 min or subjected to different protease treatments with trypsin, proteinase K, and protease V8 in order to eliminate active proteins from the sample. In all cases, the samples continued inducing apoptosis (data not shown).
S. marcescens is a Gram-negative bacillus containing LPS, which is responsible for the biological activity of endotoxin and is located in the outer membrane of the bacteria. LPS can be discharged from the outer membrane to the culture medium. In order to rule out LPS as the molecule involved in the apoptosis induced by CS-2170, we used the S. marcescens NR1 mutant which is LPS deficient. Both CS-2170 and CS-NR1 induced similar DNA laddering pattern (Figure 3), indicating that LPS is not responsible for the apoptotic action.
Figure 3.
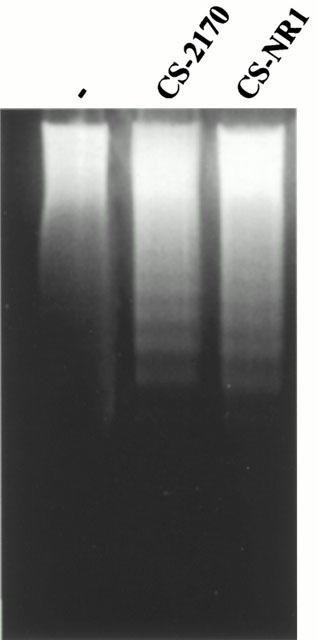
DNA fragmentation induced by CS-2170 and CS-NR1. Jurkat cells were untreated (−) or incubated overnight (16 h) with 10 μg ml−1 of CS-2170 and CS-NR1. DNA was extracted and subjected to agarose gel electrophoresis as described in Methods.
Samples from mutants defective in prodigiosin biosynthesis (Figure 4) were examined to clarify the involvement of this pigment in the apoptosis activity observed. Prodigiosin (2-methyl-3-pentyl-6-methoxyprodigiosene) is synthesized in S. marcescens by the coupling of 2-methyl-3-n-amylpyrrole (MAP) with 4-methoxy-2,2′-bipyrrole-5-carboxaldehyde (MBC). Mutant OF does not form MBC, but forms 4-hydroxy-2,2′-bipyrrole-5-carboxaldehyde (HBC) instead, which couples with MAP to form norprodigiosin (2-methyl-3-pentyl-6-hydroxyprodigiosene). Mutant WF does not form HBC or MBC but forms the volatile pyrrole MAP. Mutant 933 does not form MAP but forms MBC (Mody et al., 1990) Figure 4. Only CS-2170 and CS-OF induced a significant decrease in the viability of Jurkat cells (Figure 5A), and the characteristic DNA ladder pattern of apoptosis (Figure 5B). CS-WF and CS-933 decreased cell viability but with less efficiency than CS-2170 and CS-OF and with no evidence of DNA laddering (Figure 5B). Furthermore, the addition of purified prodigiosin to CS-WF and CS-933 allowed the induction of apoptosis in Jurkat cells (data not shown). These evidences indicate that prodigiosin and norprodigiosin (produced by OF mutant) are involved in this apoptosis.
Figure 4.
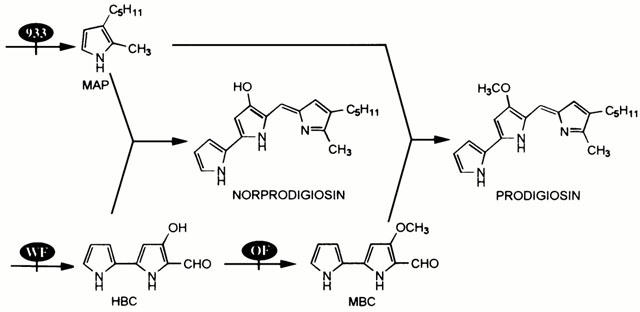
Scheme of prodigiosin biosynthesis by S. marcescens. OF, WF and 933 mutants of the prodigiosin biosynthesis are indicated.
Figure 5.
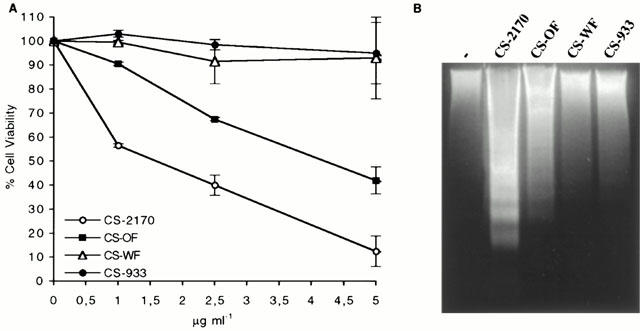
Effect of CS from different mutants in prodigiosin biosynthesis. (A) Dose-response of the effect of CS-2170, CS-OF, CS-WF and CS-933 on cell viability. Jurkat cells were incubated for 4 h with 1–5 μg ml−1 of CS-2170, CS-OF, CS-WF and CS-933. Cell viability was determined by the MTT assay as described in Methods. (B) DNA fragmentation induced by CS-2170, CS-OF, CS-WF and CS-933 was analysed by agarose gel electrophoresis as described in Methods. Jurkat cells were untreated (−) or incubated overnight with 10 μg ml−1 of CS-2170, CS-OF, CS-WF and CS-933.
Purification and characterization of prodigiosin from CS-2170 as the apoptotic factor synthesized from S. marcescens 2170
Furthermore, the apoptotic factor was purified by methanol/HCl extraction followed by silica gel chromatography and semipreparative reverse-phase HPLC. Electrospray ionization mass spectrometry gave a molecular weight of 323.4, consistent with the expected value for prodigiosin (C20H25N3O). The structure of prodigiosin was further confirmed by high-field 1H-NMR spectroscopy. Jurkat and NIH-3T3 cell lines were incubated with 60 to 1200 nM of prodigiosin for 4 h, and cell viability was determined by MTT assay. Prodigiosin decreased viability of Jurkat cells. The IC50 was higher for the NIH-3T3 cells (Figure 6A). The DNA fragmentation pattern and was observed in Jurkat cells but not in NIH-3T3 cells (Figure 6B). The nuclei shown a strong blue fluorescence and were condensed and fragmented in Jurkat cells (Figure 6C). Furthermore, we confirmed these results by measuring apoptosis in Jurkat cells by FACS analysis with annexin V-FITC and propidium iodide staining (Figure 6D). We incubated the cells with 0, and 300 nM of prodigiosin. The mean values of the early apoptotic populations (IP−/Annexin V+ cells) were 3±0.7 and 38±2%, respectively.
Figure 6.
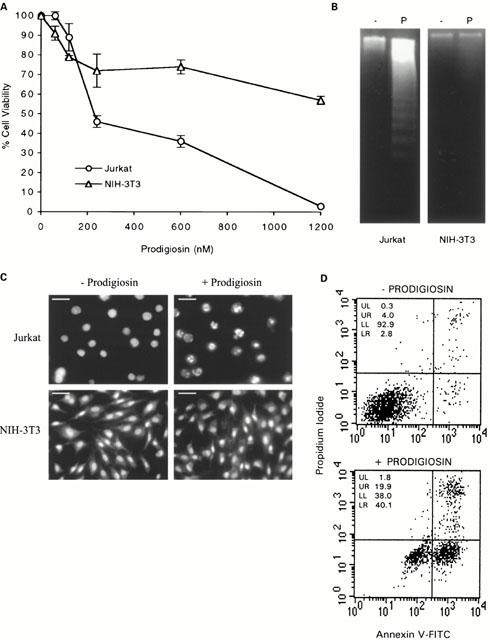
Purified prodigiosin induces apoptosis in Jurkat cells. (A) Dose-response of the effect of prodigiosin on cell viability. Jurkat and NIH-3T3 cell lines were incubated with 60 to 1200 nM of prodigiosin for 4 h. Cell viability was determined by the MTT assay as described in Methods and it is expressed as a percentage with respect to control cells. (B) DNA fragmentation induced by prodigiosin. 1×106 Jurkat and NIH-3T3 cells in 1 ml were untreated (−) or incubated (P) with 300 nM of prodigiosin for 16 h. DNA was extracted and subjected to agarose gel electrophoresis as described in Methods. (C) Fluorescence microscopic analysis of Jurkat and NIH-3T3 nuclei with Hoechst 33342 staining. Jurkat and NIH-3T3 cells were untreated (−Prodigiosin) or treated (+Prodigiosin) with 300 nM of prodigiosin for 6 h (bar=10 μm). (D) Detection of apoptosis in Jurkat cells detected by annexin-V/PI assay after 4 h exposure to 300 nM of prodigiosin.
Discussion
Apoptosis is involved in the action of several cancer-chemotherapeutic agents. In the last few years, the selection of new drugs associated with apoptosis that would be expected to be effective against tumours with high proliferation like leukemias and lymphomas has been introduced into screening for new anticancer drugs (Cameron & Feuer, 2000). Our findings demonstrate that prodigiosin released from S. marcescens 2170 to the culture medium induced apoptosis in four haematopoietic cancer cell lines (Jurkat, NSO, HL-60 and Ramos) but not in nonmalignant cells (NIH-3T3 and MDCK). Furthermore, prodigiosin is equally active in other cancer cell lines like SW-620, DLD-1 and HGT-1 (all of gastrointestinal origin) (Montaner et al., manuscript in preparation), and indicates that prodigiosin may have potential as new antineoplastic candidate.
S. marcescens is a ubiquitous bacterium inhabiting water, soil, plants, insects and vertebrates, and it has various characteristics including the pigment prodigiosin (Hejazi & Falkiner, 1997). We decided to study the possible involvement of prodigiosin in this apoptosis for different reasons: (a) in our culture conditions the supernatant from S. marcescens 2170 was always red, the characteristic colour of prodigiosin; (b) prodigiosin should be stable at 100°C and protease-resistant (both properties are in accordance with our previous experiments); (c) immunosuppressive activity has been described for prodigiosin family members (Tsuji et al., 1992; Kawauchi et al., 1997; Songia et al., 1997; Han et al., 1998); and (d) the immunosuppressive effects of prodigiosin family members may be due to their apoptotic effects as Kawauchi et al., (1997) and, recently, Azuma et al. (2000) suggested.
Prodigiosin is produced by S. marcescens,, Pseudomonas magnesiorubra, Vibrio psychroerythrus and other bacteria (Gerber, 1975; Paruchuri & Harshey, 1987). Microscopic observations of S. marcescens colonies showed that prodigiosin pigment was localized in vesicles (extracellular and cell associated) or intracellular granules (Matsuyama et al., 1986; Kobayashi & Ichikawa, 1991). This pigment is synthesized in a bifurcated pathway, in which mono- and bipyrrole precursors are synthesized separately and then coupled to form prodigiosin (Boger & Patel, 1988). Although, we can not rule out the possibility that CS-2170 has other minor proapoptotic components, our results using different mutant strains of S. marcescens indicate that prodigiosin and/or norprodigiosin, with very similar chemical structure, are involved in the apoptotic activity (Figure 4). Identification by spectroscopic analysis of prodigiosin released from S. marcescens to the culture medium, allow us to demonstrate that it is responsible for the induction of apoptosis in haematopoietic cancer cells. Recently, Han et al. (1998) described a T-cell specific immunosuppression associated to prodigiosin; however, they showed that prodigiosin did not cause significant decrease in the splenic lymphocyte viability for 24 h of incubation at concentrations from 1 to 1000 nM, similar to our results in nonmalignant NIH-3T3 cells (Figure 6A).
Prodigiosin 25-C (UP) and cycloprodigiosin hydrochloride (cPrG·HCl), two members of the prodigiosin family, synthesized by Streptomyces spp and Pseudoalteromonas denitrificans respectively have been identified to have immunosuppressive activity (Tsuji et al., 1992; Kawauchi et al., 1997; Songia et al., 1997). UP inhibits equally well both T and B human lymphocyte proliferation, but not transformed leukaemic cell lines (Songia et al., 1997); Songia et al. (1997) suggested that cell-cycle related proteins such as retinoblastoma (Rb) and cyclin-dependent Kinase-2 and -4 (Cdk-2 and Cdk-4) are the target molecules of UP to induce growth arrest in G1 phase in human T and B lymphocytes. Recently, Mortellaro et al. (1999) reported that a synthetic analogue of UP, PNU156804, has a biological effect indistinguishable from UP and efficiently inhibits the activation of NF-κB and AP-1 transcription factors. Inhibition of NF-κB substantially enhances the apoptotic potential of cancer therapies (Wang et al., 1999). However, Kawauchi et al. (1997) and Azuma et al. (2000) suggested that apoptosis is the mechanism of action of cPrG·HCl to induce suppression of T cell proliferation. The molecule cPrG·HCl inhibits proliferation and induces apoptosis in hepatocellular carcinoma cell lines, showing IC50 values from 276 to 592 nM, compared with 8395 nM in isolated normal rat hepatocytes, at 72 h (Yamamoto et al., 1999). Our results show an IC50 for prodigiosin of 225 nM in Jurkat in a shorter assay (4 h). However, the mechanisms underlying the apoptotic effect of prodigiosin are unknown. cPrG·HCl inhibits vacuolar ATPase (Tsuji et al., 1992), and, like other vacuolar ATPase inhibitors, it acidifies the cytoplasm and apoptosis (Gottlieb et al., 1996). The results presented in this report show that prodigiosin-induced apoptosis is blocked by Z-VAD.fmk, indicating that these caspases are involved in prodigiosin-induced apoptosis in haematopoietic cancer cell lines.
Interestingly, prodigiosin induces apoptosis in Jurkat and HL-60 cells, both of which are p53 deficient (Chen & Haas, 1990). This evidence indicates that prodigiosin-induce apoptosis by a p53-independent mechanism. Oncogenesis is often associated with defects in p53. As prodigiosin-induced apoptosis is p53-independent, this could mean an advantage over other chemotherapeutic drugs (Brown & Wouters, 1999; Bunz et al., 1999).
The elucidation of the mechanisms involved in the apoptotic action of prodigiosin and its evaluation as a possible anticancer drug warrants further investigation.
Acknowledgments
We thank Dr P. Orús, Dr M. Berlanga and Dr M. Viñas (Microbiology Dept. University of Barcelona) for the generous gift of bacterial strains and helpful discussions and Dr J. Llenas (Almirall-Prodesfarma) for reviewing the manuscript and Dr M. Dalmau and Jordi Capella for the technical assistance.
Abbreviations
- cPrG·HCl
cycloprodigiosin hydrochloride
- ESI-MS
electrospray ionization mass spectrometry
- HBC
4-hydroxy-2,2′-bipyrrole-5-carboxaldehyde
- LPS
lipopolysaccharide
- MAP
2-methyl-3-n-amylpyrrole
- MBC
4-methoxy-2,2′-bipyrrole-5-carboxaldehyde
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PG
peptone glycerol
- PI
propidium iodide
- UP
uncedylprodigiosin
- Z-VAD.fmk
N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone
References
- ARAB S., MURAKAMI M., DIRKS P., BOYD B., HUBBARD S., LINGWOOD D., RUTKA J. Verotoxins inhibit the growth of and induce apoptosis in human astrocytoma cells. J. Neurol. Oncol. 1998;40:137–150. doi: 10.1023/a:1006010019064. [DOI] [PubMed] [Google Scholar]
- ARAB S., RUTKA J., LINGWOOD C. Verotoxin induces apoptosis and the complete, rapid, long-term elimination of human astrocytoma xenografts in nude mice. Oncol. Res. 1999;11:33–39. [PubMed] [Google Scholar]
- AZUMA T., WATANABE N., YAGISAWA H., HIRATA H., IWAMURA M., KOBAYASHI Y. Induction of apoptosis of activated murine splenic T cells by cycloprodigiosin hydrochloride, a novel immunosuppressant. Immunopharmacology. 2000;46:29–37. doi: 10.1016/s0162-3109(99)00153-8. [DOI] [PubMed] [Google Scholar]
- BELLOSILLO B., DALMAU M., COLOMER D., GIL J. Involvement of CED-3/ICE proteases in the apoptosis of B-chronic lymphocytic leukemia cells. Blood. 1997;89:3378–3384. [PubMed] [Google Scholar]
- BELLOSILLO B., PIQUÉ M., BARRAGÁN M., CASTAÑO E., VILLAMOR N., COLOMER D., MONTSERRAT E., PONS G., GIL J. Aspirin and Salicylate induce apoptosis and activation of caspases in B-cell chronic lymphocytic leukemia cells. Blood. 1998;92:1406–1414. [PubMed] [Google Scholar]
- BOGER D.L., PATEL M. Total synthesis of prodigiosin, prodigiosene, and desmethoxyprodigiosin: Diels-Alder reactions of heterocyclic azadienes and development of an effective palladium (II)-promoted 2,2′-bipyrrole coupling procedure. J. Org. Chem. 1988;53:1405–1415. [Google Scholar]
- BRADFORD M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BROWN J.M., WOUTERS B.G. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res. 1999;59:1391–1399. [PubMed] [Google Scholar]
- BUNZ F., HWANG P.M., TORRANCE C., WALDMAN T., ZHANG Y., DILLEHAY L., WILLIAMS J., LENGAUER C., KINZLER K.W., VOGELSTEIN B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J. Clin. Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMERON R, FEUER G.Mollecular cellular and tissue reactions of apoptosis and their modulation by drugs Handbook of experimental pharmacology 2000Vol. 142Germany, Springer; ed. Cameron, R.G. & Feuer, G [Google Scholar]
- CHEN L.M., KANIGA K., GALAN J.E. Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- CHEN E., HAAS M. Frequent mutations in the p53 tumor suppressor gene in human leukemia T-cell lines. Mol. Cell Biol. 1990;10:5502–5509. doi: 10.1128/mcb.10.10.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Y., ZYCHLINSKY A. Apoptosis induced by bacterial pathogens. Microb. Pathogenesis. 1994;17:203–212. doi: 10.1006/mpat.1994.1066. [DOI] [PubMed] [Google Scholar]
- GARCÍA-CALVO M., PETERSON E.P., LEITING B., RUEL R., NICHOLSON D.W., THORNBERRY N.A. Inhibition of human caspase by peptide-based and macromolecular inhibitors. J. Biol. Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- GERBER N.N. Prodigiosin-like pigments. Crc. Crit. Rev. Microbiol. 1975;3:469–485. doi: 10.3109/10408417509108758. [DOI] [PubMed] [Google Scholar]
- GOLDSCHMIDT M.C., WILLIAMS R.P. Thiamine-induced formation of the monopyrrole moiety of prodigiosin. J. Bacteriol. 1968;96:609–616. doi: 10.1128/jb.96.3.609-616.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTLIEB R.A., NORDBERG J., SKOWRONSKI E., BABIOR B.M. Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification. Proc. Natl. Acad. Sci. U.S.A. 1996;93:654–658. doi: 10.1073/pnas.93.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN S.B., KIM H.M., KIM Y.H., LEE C.W., JANG E.S., SON K.H., KIM S.U., KIM Y.K. T-cell specific immunosuppression by prodigiosin isolated from Serratia marcescens. Int. J. Immunopharmacol. 1998;20:1–13. doi: 10.1016/s0192-0561(97)00062-3. [DOI] [PubMed] [Google Scholar]
- HEJAZI A., FALKINER F.R. Serratia marcescens. J. Med. Microbiol. 1997;46:903–912. doi: 10.1099/00222615-46-11-903. [DOI] [PubMed] [Google Scholar]
- HILDEBRAND A., POHL M., BHAKDI S. Staphylococcus aureus α-toxin: dual mechanisms of binding to target cells. J. Biol. Chem. 1991;266:17195–17200. [PubMed] [Google Scholar]
- JONAS D., SCHULTHEIS B., KLAS C., KRAMMER P.H., BHAKDI S. Cytocidal effects of Escherichia coli hemolysin on human T lymphocytes. Infect. Immun. 1993;61:1715–1721. doi: 10.1128/iai.61.5.1715-1721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATO S., MURO M., AKIFUSA S., HANADA N., SEMBA I., FUJII T., KOWASHI Y., NISHIHARA T. Evidence for apoptosis of murine macrophages by Actinobacillus actinomycetemcomitans infection. Infect. Immun. 1995;63:3914–3919. doi: 10.1128/iai.63.10.3914-3919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAUCHI K., SHIBUTANI K., YAGISAWA H., KAMATA H., NAKATSUJI S., ANZAI H., YOKOYAMA Y., IKEGAMI Y., MORIYAMA Y., HIRATA H. A possible immunosuppressant, cycloprodigiosin hydrochloride, obtained from Pseudoalteromonas denitrificans. Biochem. Biophys. Res. Commun. 1997;237:543–547. doi: 10.1006/bbrc.1997.7186. [DOI] [PubMed] [Google Scholar]
- KERR J.F.R., WINTERFORD C.M., HARMON B.V. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI N., ICHIKAWA Y. Separation of the prodigiosin-localising crude vesicles which retain the activity of protease and nuclease in Serratia marcescens. Microbiol. Immunol. 1991;35:607–614. doi: 10.1111/j.1348-0421.1991.tb01592.x. [DOI] [PubMed] [Google Scholar]
- KOCHI S.K., COLLIER R.J. DNA fragmentation and cytolysis in U937 cells treated with diphtheria toxin or other inhibitors of protein synthesis. Exp. Cell Res. 1993;208:296–302. doi: 10.1006/excr.1993.1249. [DOI] [PubMed] [Google Scholar]
- KOOPMAN G., REUTELINGSPERGER C.P., KUIJTEN G.A., KEEHNEN R.M., PALS S.T., VAN OERS M.H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- LINDGREN S.W., STOJILKOVIC I., HEFFRON F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc. Natl. Acad. Sci. U.S.A. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANGAN D.F., TAICHMAN N.S., LALLY E.T., WAHL S.M. Lethal effects of Actinobacillus actinomycetemcomitans leukotoxin on human T lymphocytes. Infect. Immunol. 1991;56:3267–3272. doi: 10.1128/iai.59.9.3267-3272.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUYAMA T., MURAKAMI T., FUJITA M., FUJITA S., YANO I. Extracellular vesicle formation and biosurfactant production by Serratia marcescens. J. General Microbiol. 1986;132:865–875. [Google Scholar]
- MODY R.S., HEIDARYNEJARD V., PATEL A.M., DAVE P.J. Isolation and characterisation of Serratia marcescens mutants defective in prodigiosin biosynthesis. Curr. Microbiol. 1990;20:95–103. [Google Scholar]
- MONACK D.M., RAUPACH B., HROMOCKYJ A.E., FALKOW S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIMOTO H., BONAVIDA B. Diphtheria toxin and pseudomonas A toxin mediated apoptosis. J. Immunol. 1992;149:2089–2094. [PubMed] [Google Scholar]
- MORTELLARO A., SONGIA S., GNOCCHI P., FERRARI M., FORNASIERO C., D'ALESSIO R., ISETTA A., COLOTTA F., GOLAY J. New immunosuppressive drug PNU156804 blocks IL-2-dependent proliferation and NF-kappa B and AP-1 activation. J. Immunol. 1999;162:7102–7109. [PubMed] [Google Scholar]
- MOSMANN T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- PALOMAR J., MONTILLA R., FUSTÉ M.C., VIÑAS M. The role of O-antigen in susceptibility of Serratia marcescens to non-immune serum. Microbios. 1993;76:189–196. [PubMed] [Google Scholar]
- PARUCHURI D.K., HARSHEY R.M. Flagellar variation in Serratia marcescens is associated with color variation. J. Bacteriol. 1987;169:61–65. doi: 10.1128/jb.169.1.61-65.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REED J.C. Mechanisms of apoptosis avoidance in cancer. Curr. Opin. Oncol. 1999;11:68–75. doi: 10.1097/00001622-199901000-00014. [DOI] [PubMed] [Google Scholar]
- ROWAN S., FISHER D.E. Mechanisms of apoptotic cell death. Leukemia. 1997;11:457–465. doi: 10.1038/sj.leu.2400626. [DOI] [PubMed] [Google Scholar]
- SONGIA S., MORTELLARO A., TAVERNA S., FORNASIERO C., SCHEIBER E.A., ERBA E., COLOTTA F., MANTOVANI A., ISETTA A.M., GOLAY J. Characterisation of the new immunosuppressive drug undecylprodigiosin in human lymphocytes. J. Immunol. 1997;158:3987–3995. [PubMed] [Google Scholar]
- TSUJI R.F., MAGAE J., JAMASHITA M., NAGAI K., YAMASAKI M. Immunomodulating properties of prodigiosin 25-C, an antibiotic which preferentially suppresses of cytotoxic T cells. J. Antibiot. 1992;45:1295–1302. doi: 10.7164/antibiotics.45.1295. [DOI] [PubMed] [Google Scholar]
- WANG C., CUSACK J.J., LIU R., BALDWIN A.J. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-κB. Nature Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- WICKREMASINGHE R.G., HOFFBRAND A.V. Biochemical and genetic control of apoptosis: relevance to normal hematopoiesis and hematological malignancies. Blood. 1999;93:3587–3600. [PubMed] [Google Scholar]
- YAMAMOTO C., TAKEMOTO H., KUNO K., YAMAMOTO D., TSUBURA A., KAMATA K., HIRATA H., YAMAMOTO A., KANO H., SEKI T., INOUE K. Cycloprodigiosin hydrochloride, a new H+/Cl− symporter, induces apoptosis in human and rat hepatocellular cancer cell lines in vitro and inhibits the growth of hepatocellular carcinoma xenografts in nude mice. Hepatology. 1999;30:894–902. doi: 10.1002/hep.510300417. [DOI] [PubMed] [Google Scholar]
- YOSHIDA J., TAKAMURA S., NISHIO M.M. Characterization of a streptococcal antitumor glycoprotein (SAGP) Life Sci. 1998;62:1043–1053. doi: 10.1016/s0024-3205(97)01142-9. [DOI] [PubMed] [Google Scholar]
- ZYCHLINSKY A., SANTONETTI P. Apoptosis in bacterial pathogenesis. J. Clin. Invest. 1997;100:S63–S65. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]


