Abstract
The effect of acetyl–tyrosyl-valyl-alanyl-aspartyl–chloromethylketone (ac-YVAD-cmk), an irreversible caspase-1 (IL-1β converting enzyme, ICE) inhibitor on mortality, leukocyte and platelet counts and cytokine levels was investigated in a double-blind rat model of endotoxaemia. Intravenous (i.v.) bolus administration of lipopolysaccharide (LPS) (25–75 mg kg−1, n=12 per group) to anaesthetized rats induced a dose dependent increase in mortality over 8 h (LD50=48 mg kg−1). During this period, animals became leukopenic and thrombocytopenic. Serum levels of IL-β, IL-6, and TNF-α were highly elevated. Pretreatment of rats with ac-YVAD-cmk at a dose of 12.5 μmol kg−1 significantly reduced mortality from 83 to 33% using Log Rank analysis. However, ac-YVAD-cmk did not modify blood cell counts or cytokine profiles as compared with the LPS-drug vehicle group. These data lay credence to the potential importance of caspase-1-inhibition in modifying the inflammatory response to endotoxin. Further investigations are warranted in understanding the relationship between caspase-1 inhibition, cytokine production and animal survival in different experimental paradigms of sepsis.
Keywords: Caspase-1, ICE-1, endotoxaemia, ac-YVAD-cmk, cytokines
Introduction
Deciphering the sequelae of inflammatory events and understanding mediator interactions in experimental models of sepsis and in the clinic still represents a major medical challenge in the hope to discover effective pharmacotherapies (Cohen, 1999). Inflammatory mediator production (e.g. cytokines, chemokines, adhesion molecules) in various animal models of sepsis is controlled at the levels of transcription, translation and enzyme-mediated processing. Evidence has accumulated to suggest that cleavage of pre-formed, inactive mediators to their biologically active molecules may represent an important pathway which ultimately leads to the inflammatory response (Fantuzzi & Dinarello, 1999). Therefore, inhibition of this pathway could provide a feasible target site for drug intervention.
Caspase-1 (Interleukin-1-Converting Enzyme, ICE-1), is a member of a growing family of more than 10 different intracellular cysteine proteases and exerts an important role in the processing inflammatory molecules such as Interleukin-1-β and IL-18 (Fantuzzi & Dinarello, 1999). These pro-inflammatory mediators share many biological properties and play central roles in the development of inflammation in different animal models (Fantuzzi & Dinarello, 1999). Although some deleterious effects of endotoxin are prevented in IL-1-β (Fantuzzi et al., 1996) and IL-18 knockout mice (Sakao et al., 1998), both types of mice succumb to endotoxin-induced lethality. In contrast, caspase-1-deficient mice, who are defective in the production of IL-1α, IL-1β and IL-18, are resistant to endotoxic shock (Li et al., 1995).
In the present study, we aimed to investigate the effects of a specific and permeable caspase-1 inhibitor, ac-YVAD-cmk, on mortality, blood parameters and cytokine production in a rat model of endotoxaemia. Our data shows for the first time that ac-YVAD-cmk afforded protection in this model despite the fact that animals still displayed acute properties of endotoxaemia based on leukopenic and thrombocytopenic responses and exaggerated levels of blood cytokines.
Methods
Chemicals and reagents
The caspase-1 inhibitor acetyl–tyrosyl-valyl-alanyl-aspartyl–chloromethylketone (ac-YVAD-cmk) was purchased from Bachem Fine Biochemicals (Heidelberg, Germany). Lipopolysaccharide (Salmonella typhimurium) was obtained from Sigma Aldrich (Deisenhofen, Germany). Ketamine (Ketanest® 50 mg) was supplied by Parke Davis (Berlin, Germany) and Xylazine (Rompun® 20 mg ml−1) was supplied by Bayer Leverkusen (Germany). Intramedic® PE 50 catheter (inner diameter 0.58 mm, outer diameter 0.965 mm) was purchased from Becton Dickinson (Heidelberg, Germany).
Animals
All experiments were performed using male Sprague-Dawley rats of 350±25 g body weight (Harlan Winkelmann, Borchen, Germany). Animals were housed under standardized conditions in single cages with free access to diet and water. All experiments conformed to the Deutsches Tierschutzgesetz and NIH guidelines for animal research. Animal protocol approval was authorized by the animal committee (Municipality of Cologne).
Experimental protocol
Anaesthesia was initiated by intraperitoneal injection of 0.6 ml ketamine/xylazine solution containing 38 mg ketamin and 4.8 mg xylozin per ml. Following implantation of femoral venous and arterial lines, anaesthesia was maintained by i.v. injection of 0.1 ml ketamine/xylazine. The venous line was used for administration of fluids and drugs whilst blood was withdrawn from the arterial line. Blood samples (550 μl), replaced with saline, were collected using EDTA as an anticoagulant.
Animal groups
Animals were randomized into four groups, each animal receiving either PBS containing 2.8% DMSO (group 1, control group, n=12), or the caspase-1 inhibitor ac-YVAD-cmk at a dosage of 1.25 μmol kg−1 (group 2, n=12), 6.25 μmol kg−1 (group 3, n=12) or 12.5 μmol kg−1 (group 4, n=12), respectively. Thirty minutes after administration of ac-YVAD-cmk, endotoxaemia was induced by bolus i.v. of 65 mg kg−1 Lipopolysaccharide (LPS) (=LD80). Surviving animals were sacrificed after the last blood sample had been collected.
Laboratory procedures
Blood samples were centrifuged for 10 min at 1000×g at 4°C and sera aliquots (110 μl) stored at −80°C. IL-1β, IL-6 and TNFα were measured using commercially available rat ELISA kits (BioSource, Germany and Diaclone, France). White blood cell count (leukocytes, haematocrit, haemoglobin, erythrocytes and platelets) were counted automatically (Blood Center, University of Cologne).
Statistical Analyses
Statistical calculations were performed using SPSS 8.0 for Windows. All values are given as mean±s.d. Significance between control and treated groups was determined using Chi square test for analysis of survival rate. Wilcoxon U-test was used to compare the means of cytokine levels and blood count between groups. ANOVA followed by post hoc LSD testing was performed to compare the means of cytokine levels and blood counts at different time points. P values of <0.05 were regarded as significant.
Results
Following LPS administration mortality was monitored for 8 h. Administration of 65 mg kg−1 LPS (LD80) with drug vehicle resulted in 83% mortality (10/12) after 8 h. Pretreatment of two groups with (1.25 and 6.25 μmol kg−1 YVAD) 30 min prior to LPS administration did not improve survival as compared with the control group at the 8 h time point (1.25 YVAD: 92% mortality, 11/12 and 6.25 YVAD 83% mortality, 10/12). In contrast, a dose of 12.5 μmol kg−1 significantly attenuated LPS induced mortality (33% mortality, 4/12, P=0.0130 vs Group 1, 0.0032 vs Group 2 and 0.0130 vs Group 3). (Figure 1)
Figure 1.
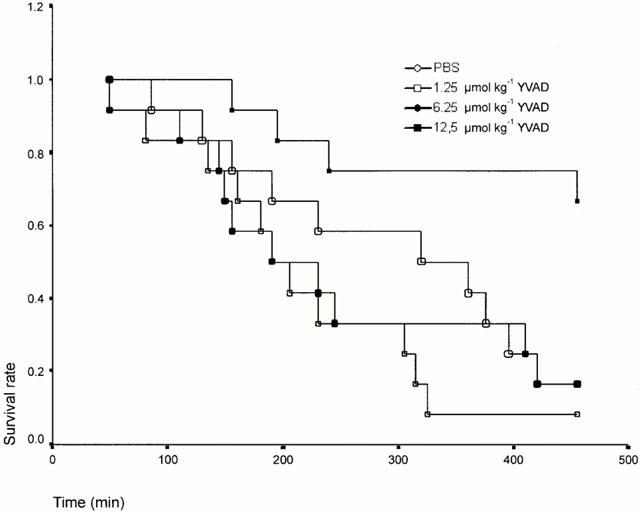
Mortality profile of ac-YVAD-cmk treated rats in a model of endotoxaemia. Rats were pretreated with the various doses of ac-YVAD-cmk 30 min prior to an i.v. administration of LPS (65 mg kg−1).
LPS caused a rapid leukopenia 1 h post administration (13.95*103–5.16*103 μl−1 average of all groups) which was unaffected by the various doses of ac-YVAD-cmk. Thereafter, there was a further slight decrease in leukocyte numbers in all groups (Figure 2). In parallel with leukopenia, platelet counts decreased during the 8 h period in all experimental groups (830*103 to 276*103 μl−1 average of all groups, Figure 3). Levels of haemoglobin, erythrocytes, and haematocrit remained unaltered for all experimental groups (data not shown).
Figure 2.
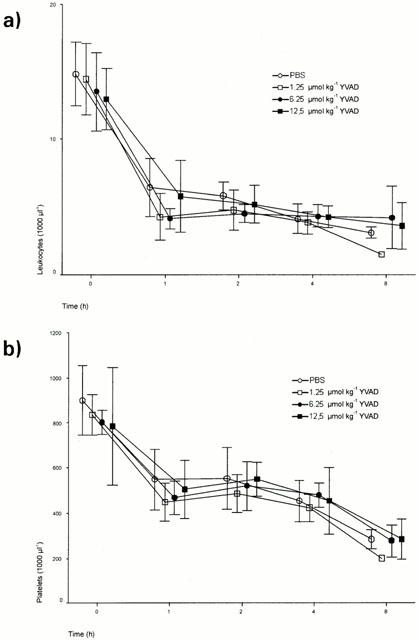
Leukocyte (a) and Platelet (b) counts in ac-YVAD-cmk-treated and non-treated rats. Thirty minutes prior to an i.v. bolus administration of LPS (65 mg kg−1). Data are means±s.d.
Figure 3.
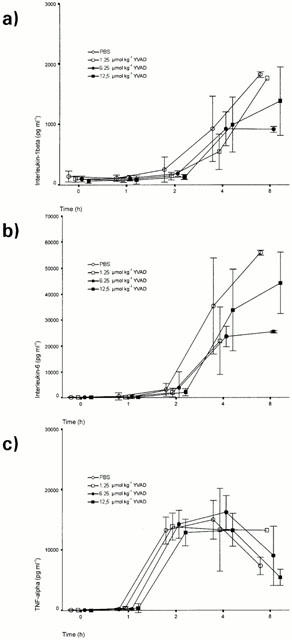
Effect of various doses of ac-YVAD-cmk on serum cytokine levels in a rat model of endotoxaemia (a) IL-1β, (b) IL-6, (c) TNF-α. Data are means±s.d.
Cytokines (TNFα, IL-β and IL-6) were measured in all four groups at time points 0, 1, 2, 4 and 8 h after administration of LPS. TNFα-levels peaked at 4 h (14,300 pg ml−1 average of all groups) and decreased at the 8 h time point (6860 pg ml−1 average of all groups). IL-1β and IL-6 production lagged behind TNFα with increasing levels observed between the 2 and 8 h time points (IL-1β: 1408 pg ml−1, IL-6 : 43,006 pg ml−1; at 8 h time point, average of all groups). In all experimental groups, ac-YVAD-cmk did not affect cytokine production.
Discussion
The present study aimed to investigate if administration of the specific caspase-1 inhibitor, ac-YVAD-cmk, could ameliorate endotoxin-induced mortality and if this effect might be attributable to a concomitant reduction in IL-1β levels. At the highest dose examined, ac-YVAD-cmk significantly attenuated mortality in the acute model of endotoxaemia without modifying circulating IL-1β levels. Furthermore, improvement of survival by ac-YVAD-cmk was not related to modifications in haematological parameters or production of TNFα or IL-6.
Previous studies investigating the effects of caspase-1 inhibition have shown its ability to suppress in part the release of IL-1β in-vitro (Schumann et al., 1998) and in-vivo (Fletcher et al., 1995 Mignon et al., 1999). However, the transient decrease in IL-1β production did not affect mortality (Fletcher et al., 1995). The lack of effect of ac-YVAD-cmk on circulating IL-1β in the present study might be attributable to: (1) suboptimal dose of ac-YVAD-cmk; (2) inability of the drug to access target sites, e.g. monocytes, macrophages; (3) short half life of ac-YVAD-cmk; and (4) existence of ICE-independent, IL-1β synthesis pathways. Evidence indicates that pro IL-1-β can be cleaved to its biologically active form by a panel of proteases (e.g. cathepsin G, elastase, metalloproteinases) present at high concentrations in inflammatory fluids (Irmler et al., 1995, Hazuda et al., 1988), at sites of cellular infiltrations as well as extracellular loci (Hazuda et al., 1988). Furthermore, in ICE deficient mice challenged with terpentine, inflammation is still driven by high levels of biologically active IL-1β (Fantuzzi et al., 1997), an effect not observed in IL-1β deficient mice (Zheng et al., 1995).
The improvement in survival with 12.5 μmol kg−1 ac-YVAD-cmk in the present study is reminiscent of survival to lethal endotoxaemia in ICE deficient mice (Li et al., 1995, Kuida et al., 1995), an effect not observed in IL-1β (Fantuzzi et al., 1996) or IL-18 knockout mice (Sakao et al., 1998). IL-18 serves a pivotal role in inflammation being able to stimulate the production of chemokines and cytokines in vitro (Puren et al., 1998). However, in IL-18 knockout mice, levels of cytokines, such as IL-1β, IL-6 and TNF-α were overproduced as compared to wildtype mice indicating a negative feedback role of IL-18 on cytokine production (Sakao et al., 1998). Putative inhibition of caspase-1 activity and IL-18 production in the present study may serve as an autocrine mechanism for the production of biologically active IL-1β via an IL-18 independent pathway. This hypothesis should be addressed following the development of specific reagents, e.g. antibodies to rat IL-18.
Caspases are known to be involved in apoptosis in vitro (Fahy et al., 1999) and in vivo (Mignon et al., 1999). In the latter study, Mignon and co-workers showed that ac-YVAD-cmk prevented lethality in a TNFα-dependent mouse model of endotoxaemia primed with D-Galactosamine. Based on normal liver histology and selective inhibition of caspase-3 activity, the authors concluded that survival was due to inhibition of apoptosis in hepatocytes. In contrast, ac-YVAD-cmk did not prevent LPS-induced endotoxic shock in unsensitized mice. The protective effect of ac-YVAD-cmk in our study might be due to prevention of inflammation-induced apoptosis as opposed to multi organ failure since mortalities in our study and that of Mignon et al, occurred over a similar time course. Such an effect might be due to FAS-mediated apoptosis through a caspase-1-dependent pathway since ICE knockout mice are resistant to FAS-mediated apoptosis yet remain susceptible to other stimuli of apoptosis (Kuida et al., 1995). Alternatively, ac-YVAD-cmk may be a broad spectrum caspase inhibitor including caspase-3 as observed in FAS (Enari et al., 1995) and TGF-β mediated liver apoptosis (Inayat-Hassain et al., 1997).
The present data point to the potential importance of caspase-1 inhibition in modifying the pathophysiological outcome of experimental sepsis. Additional studies should be performed with more selective caspase inhibitors in conjuncture with improved animal models of human sepsis which are non-hypodynamic in nature (Mathiak et al., 2000, Wichterman et al., 1980).
Acknowledgments
This work was supported by Cologne Fortune program (grant no. 33/99), Faculty of Medicine, University of Cologne, Germany.
Abbreviations
- ac-YVAD-cmk
acetyl–tyrosyl-valyl-alanyl-aspartyl–chloromethylketone
- ICE-1
interleukin converting enzyme-1, caspase-1
- IL
interleukin
- LPS
lipopolysaccharide
References
- COHEN J. Adjunctive therapy in sepsis: a critical analysis of the clinical trial programme. Br. Med. Bull. 1999;55:212–225. doi: 10.1258/0007142991902222. [DOI] [PubMed] [Google Scholar]
- ENARI M., HUG H., NAGATA S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- FAHY R.J., DOSEFF A.I., WEWERS M.D. Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J. Immunol. 1999;163:1755–1762. [PubMed] [Google Scholar]
- FANTUZZI G., DINARELLO C.A. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1) J. Clin. Immunol. 1999;19 1:1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- FANTUZZI G., KU G., HARDING M.W., LIVINGSTON D.J., SIPE J.D., KUIDA K., FLAVELL R.A., DINARELLO C.A. Response to local inflammation of IL-1β-converting enzyme-deficient mice. J. Immunol. 1997;158:1818–1824. [PubMed] [Google Scholar]
- FANTUZZI G., ZHENG H., FAGGIONI R., BENIGNI F., GHEZZI P., SIPE J.D., SHAW A.R., DINARELLO C.A. Effect of endotoxin in IL-1β-deficient mice. J. Immunol. 1996;157:291–296. [PubMed] [Google Scholar]
- FLETCHER D.S., AGARWAL L., CHAPMAN K.T., CHIN J., EGGER L.A., LIMJUCO G., LUELL S., MACINTYRE D.E., PETERSON E.P., THORNBERRY N.A., KOSTURA M.J. A synthetic inhibitor of interleukin-1 beta converting enzyme prevents endotoxin-induced interleukin-1 beta production in vitro and in vivo. J. Interferon Cytokine Res. 1995;15:243–248. doi: 10.1089/jir.1995.15.243. [DOI] [PubMed] [Google Scholar]
- HAZUDA D.J., LEE J.C., YOUNG P.R. The kinetics of interleukin 1 secretion form activated monocytes. Differences between interleukin 1 alpha and interleukin 1 beta. J. Biol. Chem. 1988;263:8473–8479. [PubMed] [Google Scholar]
- INAYAT-HASSAIN S.H., COUET C., COHEN G.M., CAIN K. Processing/activation of CPP32-like proteases is involved in transforming growth factor β1-induced apoptosis in rat hepatocytes. Hepatology. 1997;25:1519–1526. doi: 10.1002/hep.510250634. [DOI] [PubMed] [Google Scholar]
- IRMLER M., HERIG S., MACDONALD H.R., SADOUL R., BECHERER J.D., PROUDFOOT A., SOLARI R., TSCHOPP J. Granuzmye A is an interleukin-1β-converting enzyme. J. Exp. Med. 1995;181:1917–1822. doi: 10.1084/jem.181.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUIDA K.L.J.A., KU G., HARDING M.W., LIVINGSTON D.J., SU M.S.S., FLAVELL R.A. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- LI P., ALLEN H., BANERJEE S., FRANKLIN S., HERZOG L., JOHNSTON C., MCDOWELL J., PASKIND M., RODMAN L., SALFELD J., TOWNE E., TRACEY D., WARDWELL S., WEI F.Y., WONG W., KAMEN R., SESHARDI T. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- MATHIAK G., SCZEWCZYK D., ABDULLAH F., OVADIA P., FEUERSTEIN G., RABINOVICI R. An improved clinically-relevant sepsis model in the conscious rat. Crit. Care Med. 2000;28:1947–1952. doi: 10.1097/00003246-200006000-00043. [DOI] [PubMed] [Google Scholar]
- MIGNON A., ROUQUET N., FABRE M., MARTIN S., PAGÉS J.C., DHAINAUT J.F., KAHN A., BRIAND P., JOULIN V. LPS challenge in D-galactosamine-sensitized mice accounts for caspase-dependent fulminant hepatitis, not for septic shock. Am. J. Respir. Crit. Care Med. 1999;159:1308–1315. doi: 10.1164/ajrccm.159.4.9712012. [DOI] [PubMed] [Google Scholar]
- PUREN A.J., FANTUZZI G., GU Y., SU M.S.S., DINARELLO C.A. Interleukin-18 (IFNγ-inducing factor) induces IL-8 and IL-1β via TNFα production from Non-CD14+ human blood molecular cells. J. Clin. Invest. 1988;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKAO Y., TAKEDA K., TSUTSUI H., KAISHO T., NOMURA F., OKAMURA H., NAKANISHI K., AKIRA S. IL-18-deficient mice are resistant to endotoxin-induced liver injury but highly susceptible to endotoxin shock. Int. Immunol. 1998;11:471–480. doi: 10.1093/intimm/11.3.471. [DOI] [PubMed] [Google Scholar]
- SCHUMANN R.R., BELKA C., REUTER D., LAMPING N., KIRSCHNING C.J., WEBER J.R., PFEIL D. Lipopolysaccharide activates caspase-1 (interleukin-1-converting-enzyme) in cultured monocytic and endothelial cells. Blood. 1998;91:577–584. [PubMed] [Google Scholar]
- WICHTERMAN K.A., BAUE A.E., CHAUDRY I.H. Sepsis and septic shock – a review of laboratory models and a proposal. J. Surg. Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- ZHENG H., FLETCHER D., KOZAK W., MIANG M., HOFMANN K., CONN C.C., SISZYNSKI D., GRABIEC C., TRUMBAUER M.A., SHAW A.R.K.M.J., STEVENS K., ROSEN H., NORTH R.J., CHEN H.Y., TOCCI M.J., KLUGER M.J., VAN DER PLOEG L.H.T. Resistance to fever induction and impaired acute-phase response in interleukin-1β deficient mice. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]


