Abstract
In rat pulmonary artery pre-contracted with phenylephrine, the mechanisms of relaxation to the nitric oxide (NO) donor, spermine NONOate, were investigated.
Responses to spermine NONOate were only partially blocked by the soluble guanylate cyclase inhibitor, ODQ (1H-[1,2,4]Oxadiazolo-[4,3,-a]quinoxalin-1-one) at concentrations up to 30 μM. Ten μM ODQ gave maximal inhibition. Endothelium removal had no effect on the potency of spermine NONOate or its inhibition by ODQ.
The protein kinase G inhibitor, Rp-8-Br-cGMPS (100 μM), caused minimal inhibition of spermine NONOate despite causing marked inhibition of glyceryl trinitrate and isosorbide dinitrate.
Spermine NONOate (100 μM) caused a 35 fold increase in guanosine 3′5′ cyclic monophosphate (cyclic GMP) above basal levels in pulmonary artery rings. ODQ (3 μM) abolished this cyclic GMP production but did not inhibit corresponding relaxant responses. Similar results were seen with another NONOate (MAHMA NONOate; 10 μM).
ODQ-resistant relaxation to spermine NONOate (i.e. relaxation seen in the presence of 10 μM ODQ) was inhibited by potassium (80 mM), charybdotoxin (300 nM), iberiotoxin (300 nM), apamin (100 nM), ouabain (1 mM) or thapsigargin (100 nM) but not by 4-aminopyridine (3 mM), glybenclamide (10 μM) or diltiazem (10 μM).
Potassium, charybdotoxin, ouabain and thapsigargin also inhibited ODQ-resistant relaxation to FK409 ((±)-E-4-ethyl-2-[E-hydroxyimino]-5-nitro-3-hexenamide).
We conclude that, on rat pulmonary artery, spermine NONOate can produce cyclic GMP-independent relaxation that involves, at least in part, activation of Na+/K+-ATPase, sarco-endoplasmic reticulum Ca2+-ATPase and calcium-activated potassium channels.
Keywords: Calcium-activated potassium channels, cyclic GMP-independent relaxation, FK409, Na+/K+-ATPase, nitric oxide donors, rat pulmonary artery, sarco-endoplasmic reticulum Ca2+-ATPase, spermine NONOate
Introduction
Nitric oxide (NO) is conventionally thought to cause relaxation of vascular smooth muscle by activation of soluble guanylate cyclase and elevation of intracellular guanosine 3′ 5′ cyclic monophosphate (cyclic GMP; Schmidt et al., 1993). Hence vasorelaxant responses to NO and to NO donor drugs are inhibited by the guanylate cyclase inhibitor, ODQ (1H-[1,2,4]Oxadiazolo-[4,3,-a]quinoxalin-1-one; Brunner et al., 1996; van der Zypp & Majewski, 1998; Homer et al., 1999; Feelisch et al., 1999). However there is now evidence that NO can also act by mechanisms that do not involve the guanylate cyclase/cyclic GMP pathway. These alternative mechanisms, that are not blocked by guanylate cyclase inhibitors, include activation of (i) potassium channels in the cell membrane (Yuan et al., 1996; Mistry & Garland, 1998), (ii) Na+/K+-ATPase (Gupta et al., 1994) and/or (iii) sarco-endoplasmic reticulum Ca2+-ATPase (SERCA; Trepakova et al., 1999).
In a previous study on rat pulmonary artery relaxant responses to glyceryl trinitrate, isosorbide dinitrate and sodium nitroprusside were virtually abolished by ODQ (3 μM), whereas responses to four other NO donors, viz. spermine NONOate, MAHMA NONOate, SIN-1 (3-morpholinosydnonimine) and FK409 ((±)-E-4-ethyl-2-[E-hydroxyimino]-5-nitro-3-hexenamide), were only partially inhibited by this guanylate cyclase inhibitor (Homer et al., 1999). The four NO donors that were only partially inhibited all generate NO ‘spontaneously' at physiological pH, without requiring activation in the tissue (Feelisch & Stamler, 1996). The data indicated that responses to these particular NO donors probably had an ODQ-resistant component.
In this study we further examine the effects of spermine NONOate on rat pulmonary artery. Substituted diazeniumdiolates, the group of compounds to which spermine NONOate belongs, are useful in pharmacological studies because of their reliable generation of NO (Keefer et al., 1996). Spermine NONOate is one of the most widely used compounds in this group, largely because of its convenient half-life (39–73 mins; Keefer et al., 1996; Homer & Wanstall, 1998). The first aim of the study was to establish whether or not ODQ-resistant relaxation by spermine NONOate was cyclic GMP-independent; this cannot be assumed automatically because there may be pools of guanylate cyclase that are not inhibited by ODQ. To address this aim (i) the effects of an inhibitor of protein kinase G, the target for cyclic GMP, have been examined and (ii) cyclic GMP production has been compared with relaxation, in the presence and absence of ODQ. The second aim was to investigate possible mechanisms that could account for any cyclic GMP-independent relaxation. To address this aim we have investigated the effects of inhibitors of potassium channels, Na+/K+-ATPase and SERCA on relaxant responses in the presence of ODQ.
Preliminary accounts of these data were presented at Pulmonary Circulation VII (International Symposium), Prague, Czech Republic, June 1999 (Wanstall & Homer, 1999) and to a meeting of the Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists, Sydney, December 1999 (Homer & Wanstall, 1999).
Methods
Rat pulmonary artery preparations
Male Wistar rats (body weight 293±3.4 g, n=140) were anaesthetized with pentobarbitone (90 mg kg−1, i.p.), the thorax was opened and heparin (2500 IU kg−1) injected into the left ventricle to prevent blood clotting in the pulmonary artery. The main pulmonary artery was removed, cleared of adhering connective tissue and from it a single ring preparation, 3 mm in length, was obtained.
Measurement of vasorelaxant responses
Artery preparations and general experimental protocol
Rings of main pulmonary artery were mounted around two stainless steel wires in a vertical organ bath containing physiological salt solution (PSS) at 37°C and gassed with 95% O2 /5% CO2. The composition of the PSS was (mM): NaCl 118, KCl 5.9, CaCl2 1.5, MgSO4 0.72, NaHCO3 25, glucose 11.7, NaEDTA 0.025. In some experiments the endothelium was removed mechanically by gently rubbing the lumen of the vessel with the tip of a pair of fine forceps. Resting force was 10 mN and changes in force in the circular muscle were recorded isometrically with a Statham Universal Transducer (UC3+UL5) attached to a micrometer (Mitutoyo, Tokyo, Japan). All preparations were allowed to equilibrate for 1 h (PSS replaced at 15 min intervals). They were then contracted submaximally with phenylephrine (0.1 μM) and, once the contraction reached a plateau, acetylcholine (1 μM) was added. A relaxant response confirmed the endothelial integrity of the preparation (mean acetylcholine response (n=96): 65±1.4% reversal of the phenylephrine contraction; minimum value 23% reversal). The preparations from which the endothelium was removed did not relax in response to acetylcholine (i.e. there was zero reversal of the phenylephrine contraction). A reference contraction to potassium-depolarizing PSS (in which 80 mM NaCl was replaced with 80 mM KCl) was then obtained. The tissues were washed, pre-contracted with phenylephrine (0.1 μM) and relaxant responses to an NO donor were determined as described below. In the absence of ODQ, concentration-response curves to the NO donors were reproducible.
Specific experimental protocols
In the first series of experiments, cumulative concentration-response curves were obtained to spermine NONOate in the absence (control curve) and then in the presence of 0.1, 3, 10 or 30 μM ODQ (guanylate cyclase inhibitor; 30 min incubation).
In the second series of experiments cumulative concentration-response curves were obtained to spermine NONOate, glyceryl trinitrate or isosorbide dinitrate in the absence and then in the presence of the protein kinase G inhibitor, Rp-8-Br-cGMPS (100 μM; 45 min incubation).
In the third series of experiments cumulative concentration-response curves to spermine NONOate or FK409 were obtained (i) in the absence of any inhibitor (control), then (ii) in the presence of 10 μM ODQ alone and finally (iii) in the presence of 10 μM ODQ together with one of the following: potassium (80 mM), charybdotoxin (300 nM), iberiotoxin (300 nM), apamin (100 nM), 4-aminopyridine (3 mM), glybenclamide (10 μM), diltiazem (10 μM), ouabain (1 mM) or thapsigargin (100 nM). In the experiments with potassium, 80 mM KCl was substituted for 80 mM NaCl in the PSS. In these experiments, 10 μM diltiazem was present before and during the third curve, to prevent any contraction to K+; it was also present during the second curve to provide an appropriate control. Preliminary experiments showed that this concentration of diltiazem had no effect on the concentration-response curves to either spermine NONOate or FK409 in the absence of ODQ. Phenylephrine contractions in the absence and presence of ODQ (10 μM) were (as a per cent of the reference contraction to potassium) 43±1.3, n=92 and 74±1.7, n=62, respectively. Retrospective analysis of the data showed that, in the absence of ODQ, slopes of plots of negative log IC25 values for spermine NONOate (n=60) or FK409 (n=37) vs. phenylephrine contraction (with contractions ranging from 11 to 79% of the potassium contraction) were 0.001 and 0.003, respectively, and were not significantly different from zero (P>0.2). Thus the negative log IC25 values of spermine NONOate and FK409 (used to determine the shifts in the curves by ODQ; see below) were shown to be independent of the size of the submaximal contraction to phenylephrine. Therefore the increase in the size of this contraction in the presence of ODQ would not have influenced our findings on the effects of ODQ on the NO donor concentration-response curves.
In a fourth series of experiments, the effects of ODQ on responses to a single concentration of either spermine NONOate or MAHMA NONOate were determined, using the same protocol and NONOate concentrations as those used in the preparation of samples for the cyclic GMP assays (see below). Thus in these experiments there was no preliminary phenylephrine/acetylcholine response and no contraction to potassium-depolarizing PSS. Preparations were contracted with 0.1 μM phenylephrine and responses to the NO donors were obtained in the presence of zaprinast±ODQ (10 and 3 μM respectively; 30 min incubation). The concentration of each NO donor that gave a just-maximal response (spermine NONOate 100 μM; MAHMA NONOate, 10 μM) was selected from previously obtained concentration-response curve data. The different concentrations reflect the 10 fold difference in potency between these two NONOates (Homer & Wanstall, 1998).
Data analysis
Contractions to phenylephrine were measured in mN and expressed as a percentage of the contraction to potassium depolarizing PSS. Relaxant responses to the NO donors were measured from the plateau of the phenylephrine contraction and were expressed as ‘per cent reversal' of the phenylephrine contraction. The IC25 (concentration giving 25% reversal of the phenylephrine contraction) was interpolated from the plot of response versus log molar concentration of NO donor. The magnitude of the shift in the NO donor concentration-response curve by an inhibitor drug was measured as the ‘log unit shift', defined as [negative log IC25 (control)−negative log IC25 (inhibitor(s) present)]. ‘Log unit shifts' were measured at the level of the IC25 (rather than IC50) because, in the presence of some of the inhibitors, 50% relaxation was not always achieved. This was because 100 μM was the highest concentration of spermine NONOate or FK409 that could confidently be used in the analyses since, at higher concentrations, part of the relaxation to each of these NO donors was probably due to decomposition products other than NO (see below).
Decomposition products of the NONOates and FK409
Solutions of spermine NONOate and MAHMA NONOate (0.1 M; in 1 M HCl) and of FK409 (0.01 M; in 0.01 M NaOH) were allowed to decompose spontaneously for 2 weeks (MAHMA NONOate) or 3–6 weeks (spermine NONOate and FK409), i.e. for 400 to 1000 half-lives, to allow release of all NO, as advocated by Keefer et al. (1996). These solutions were then tested for relaxant effects on phenylephrine-contracted pulmonary artery rings (the NONOate solutions in 1 M HCl were first neutralized with NaOH). At concentrations greater than 100 μM, the decomposition products for spermine NONOate caused partial relaxation of the artery preparations; this is in keeping with the known biological effects of the polyamine, spermine (de Meis, 1967; Keefer et al., 1996). Similar results were obtained for decomposed FK409 and MAHMA NONOate. Therefore no data for concentrations of the NONOates or FK409 greater than 100 μM have been included in the study.
Measurement of cyclic GMP levels in pulmonary artery rings
Single ring preparations of main pulmonary artery were weighed, placed in a test tube containing 10 ml of physiological salt solution (PSS) at 37°C and gassed with 95% O2 - 5% CO2, and allowed to equilibrate for 1 h. The cyclic GMP phosphodiesterase inhibitor, zaprinast (10 μM), with or without ODQ (3 μM), was then added, followed 30 min later by phenylephrine (0.1 μM). Ten minutes after the addition of phenylephrine, preparations were exposed to a single dose of either spermine NONOate (100 μM) or MAHMA NONOate (10 μM) for time periods ranging from 1 to 8 min, before being rapidly frozen in liquid nitrogen. For measurements of basal cyclic GMP, the NO donor was excluded from the protocol. Cyclic GMP was extracted from the tissues by immersion in 6% w v−1 trichloroacetic acid (TCA) followed by sonification for 15 min and centrifugation (1500×g) for 10 min. The precipitate was discarded and TCA was removed from the supernatant by extraction with ether (5 ml; three times). Residual ether was removed by heating the samples to 70°C for 7 min. The samples were acetylated and cyclic GMP concentration was determined using a competitive enzyme immunoassay kit (Cayman Chemicals, Ann Arbor, MI, U.S.A.). The cyclic GMP content of each sample was divided by tissue wet weight and expressed as pmol mg−1.
Drugs and solutions
Sources of drugs were as follows: FK409 (gift from Fujisawa, Japan); glyceryl trinitrate (GTN-POHL; ampoules; Rhone-Poulenc Rorer); MAHMA NONOate and spermine NONOate (Cayman); ODQ (Tocris Cookson); Rp-8-Br-cGMPS and thapsigargin (Sapphire Bioscience); all other drugs (Sigma). Solutions of drugs were prepared as follows: acetylcholine (10 mM), 4-aminopyridine (1 M), apamin (1 mM), charybdotoxin (50 μM), diltiazem (10 mM), iberiotoxin (50 μM), ouabain (10 mM), Rp-8-Br-cGMPS (100 mM) and thapsigargin (100 μM) in deionized water, MAHMA NONOate and spermine NONOate (10 mM) in 10 mM NaOH; zaprinast (10 mM) in 50 mM NaOH; FK409 (10 mM) in 0.1 mM HCl; phenylephrine (10 mM) in 10 mM HCl; glybenclamide (10 mM) and ODQ (10 mM) in dimethylsulphoxide; isosorbide dinitrite (40 mM) in absolute ethanol. Ampoules of glyceryl trinitrate contained 22 mM in ethanol. Dilutions, when required, were made in PSS except for MAHMA NONOate and spermine NONOate which were diluted in 10 mM NaOH and FK409 which was diluted in 0.1 mM HCl. During the experiments all drug dilutions were kept on ice and dilutions of NO donors were protected from light. None of the vehicles, at the concentrations used, caused any response in the artery preparations.
Statistics
Mean values were calculated from data obtained in preparations from a number (n) of different animals and are quoted with their s.e.mean. Differences between mean values have been assessed either by one-way analysis of variance (ANOVA) followed by a Tukey Kramer post hoc test or by paired or unpaired t-test. Differences between complete concentration-response curves in the presence of ODQ with and without other inhibitors have been analysed by two-way ANOVA.
Results
Effects of ODQ on relaxant responses to spermine NONOate
Removal of the endothelium had no effect on the potency of spermine NONOate (negative log IC25, n=4: endothelium present 7.03±0.04, n=4; endothelium absent 7.10±0.12, n=4; P>0.05; unpaired t-test) or on the effect of ODQ on the concentration-response curves to spermine NONOate (log unit shifts, 3 μM ODQ, n=4: endothelium present 1.79±0.10, n=4; endothelium absent 2.04±0.08, n=4; P>0.05; unpaired t-test). In these experiments, relaxant responses to acetylcholine ranged from 47–72% reversal in the endothelium-intact preparations but were absent (zero reversal) in the endothelium-denuded preparations. The remainder of the data in the study has been obtained on endothelium-intact preparations.
The effects of various concentrations of ODQ on concentration-response (relaxation) curves to spermine NONOate are illustrated in Figure 1. All concentrations tested caused parallel shifts in the curves and even in the presence of the highest concentration of ODQ (30 μM) pronounced relaxant responses to spermine NONOate remained (Figure 1). The effects of 30 μM (log unit shift 2.49±0.13, n=4) and 10 μM (log unit shift 2.18±0.04, n=4) were not significantly different (P>0.05) but both concentrations had a greater effect than 3 μM (log unit shift 1.79±0.10, n=4, P<0.05; one-way ANOVA). This indicated that the effect of 10 μM ODQ was probably maximal and that there is an ODQ-resistant component to the responses to spermine NONOate.
Figure 1.
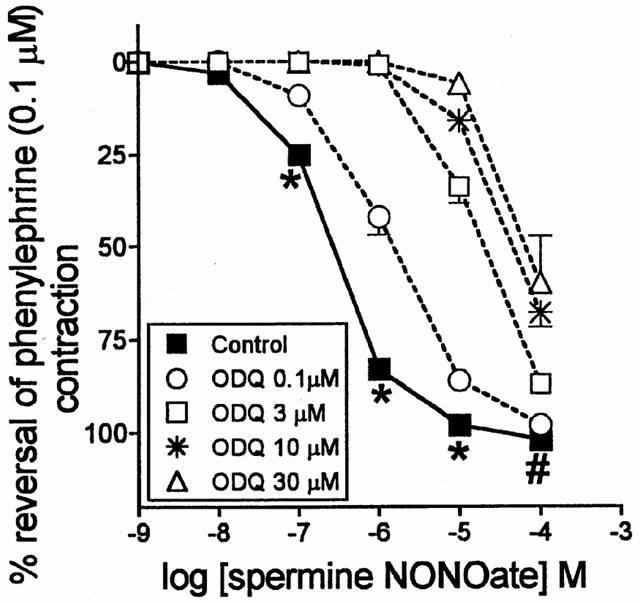
Mean concentration-response curves to spermine NONOate on rat main pulmonary arteries pre-contracted with phenylephrine (0.1 μM) in the absence (control; n=16) and in the presence of ODQ (preincubation 30 min; n=4), 0.1, 3, 10 or 30 μM. Relaxation responses are expressed as % reversal of the phenylephrine-induced contraction. Points are mean values with s.e.mean shown by vertical bars except when smaller than the size of the symbols. *P<0.001 when compared with corresponding responses in the presence of ODQ (0.1, 3, 10 or 30 μM). #P<0.001 when compared with corresponding responses in the presence of ODQ (3, 10 or 30 μM) (one way ANOVA, Tukey-Kramer post hoc test).
Effects of the G-kinase inhibitor, Rp-8-Br-cGMPS, on responses to spermine NONOate, glyceryl trinitrate and isosorbide dinitrate
Rp-8-Br-cGMPS (100 μM) caused only a very small parallel shift in the concentration-response curve to spermine NONOate without any depression in maximum response (Figure 2a). This was in contrast to the effects of Rp-8-Br-cGMPS on the curves for glyceryl trinitrate and isosorbide dinitrate where marked shifts in the curves occurred together with significant depressions in maximum response (Figure 2b, c). The shift in the spermine NONOate curve (0.58±0.06 log units, n=4) was significantly smaller (P<0.05; one-way ANOVA) than the shifts for glyceryl trinitrate and isosorbide dinitrate (0.99±0.08 and 1.37±0.09 log units (n=4), respectively). These data indicated that the involvement of cyclic GMP-dependent protein kinase is less important for relaxation to spermine NONOate than for relaxation to either of the organic nitrates, and suggested that part of the response to spermine NONOate might be cyclic GMP-independent.
Figure 2.
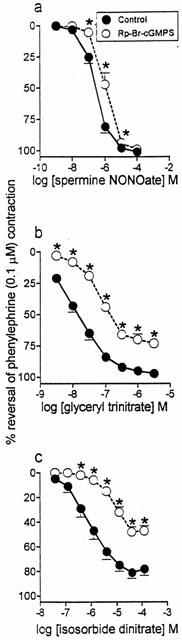
Mean concentration-response curves to (a) spermine NONOate (n=4), (b) glyceryl trinitrate (n=4) and (c) isosorbide dinitrate (n=4) on rat main pulmonary arteries pre-contracted with phenylephrine (0.1 μM) in the absence (control) and then in the presence of Rp-8-Br-cGMPS (100 μM; preincubation 45 min). Relaxation responses are expressed as % reversal of the phenylephrine-induced contraction. Points are mean values with s.e. mean shown by vertical bars except when smaller than the size of the symbols. *Response significantly less than the corresponding response in the absence of Rp-8-Br-cGMPS (P<0.05, paired t-test).
Comparison of cyclic GMP production and vasorelaxation in the absence and presence of ODQ
In this series of experiments we examined not only spermine NONOate but also another diazeniumdiolate, MAHMA NONOate (see Discussion for reason). The basal level of cyclic GMP in pulmonary artery rings was 0.66±0.07 pmol mg−1 (n=4). Spermine NONOate (100 μM) and MAHMA NONOate (10 μM) each caused increases in cyclic GMP and relaxation of the tissues (Figure 3). Cyclic GMP production was greatest during the first minute of incubation with each of the NONOates. Therefore an incubation time of 1 min was used for the experiments in which data with and without ODQ were compared.
Figure 3.
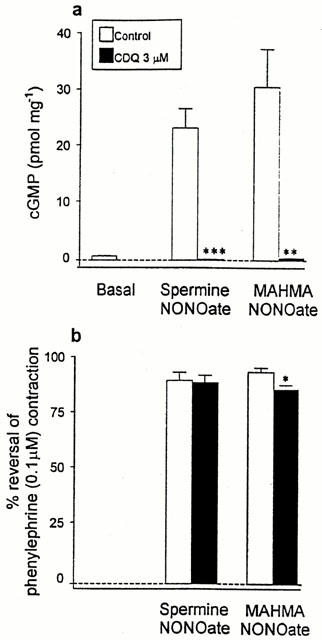
(a) Mean cyclic GMP content in rat main pulmonary arteries (zaprinast, 10 μM, and phenylephrine, 0.1 μM, present) in the absence (control) or presence of ODQ (3 μM). Basal levels (no NO donor) and levels after exposure to either spermine NONOate (100 μM) or MAHMA NONOate (10 μM) are shown. Cyclic GMP content is expressed as pmol mg−1 wet weight of tissue. (b) Mean relaxation responses to either spermine NONOate (100 μM) or MAHMA NONOate (10 μM) on rat main pulmonary arteries obtained under identical conditions to those used for the cyclic GMP determinations shown in (a). Relaxation responses are expressed as % reversal of phenylephrine-induced contraction. Data in (a) and (b) are mean values with s.e.mean shown by the vertical lines (n=4–7 preparations). * 0.05>P>0.01, ** 0.01>P>0.001, ***P<0.001 when compared with corresponding data in the absence of ODQ 3 μM (unpaired t-test).
In the absence of ODQ, the amounts of cyclic GMP produced by spermine and MAHMA NONOates, respectively, were 35 and 46 fold greater than basal levels (Figure 3a). Corresponding relaxation responses (obtained under the same experimental conditions as were used for determining cyclic GMP) were 92 and 96% reversal of the phenylephrine contraction (Figure 3b). In the presence of ODQ (3 μM), cyclic GMP production by both NONOates was abolished, i.e. cyclic GMP levels were reduced below basal levels (spermine NONOate 0.12±0.03 pmol mg−1; MAHMA NONOate 0.41±0.04 pmol mg−1; n=4; P<0.05 when compared with basal levels; one-way ANOVA); however the two drugs still caused ⩾90% relaxation (Figure 3a, b).
These data suggested that spermine NONOate (and also MAHMA NONOate) can cause relaxation by a mechanism(s) that does not involve cyclic GMP.
The effects of inhibitor drugs on relaxant responses in the presence of ODQ
The effects of (i) ODQ (10 μM) alone and (ii) ODQ (10 μM) plus various inhibitor drugs on concentration-response curves to spermine NONOate are summarized in Table 1. Consecutive concentration-response curves in the presence of ODQ (10 μM) without any other inhibitor drug were reproducible (Table 1).
Table 1.
Effects of inhibitors on concentration-response curves to spermine NONOate in the presence of 10 μM ODQ
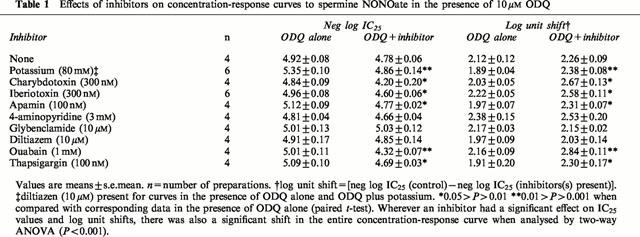
ODQ (10 μM) alone produced a consistent shift in the spermine NONOate curves of the order of 2 log units (Table 1). In the presence of ODQ (10 μM), potassium (80 mM; 10 μM diltiazem present to prevent calcium influx through L-type calcium channels; see Methods for details) caused a significant additional shift in the curves to spermine NONOate. This suggested that opening of potassium channels might contribute to the ODQ-resistant component of the responses to spermine NONOate (Figure 4; Table 1). Various selective potassium channel antagonists were then tested. The calcium-activated potassium channel (KCa) antagonists, charybdotoxin (300 nM; Figure 4), iberiotoxin (300 nM; Figure 4) and apamin (100 nM), like potassium, caused significant additional shifts in the curves, but 4-aminopyridine (3 mM; voltage-dependent potassium channel (Kv) antagonist) and glybenclamide (10 μM; ATP-dependent potassium channel (KATP) antagonist; Figure 4) did not (Table 1). Because the effects of potassium, charybdotoxin, iberiotoxin and apamin were only small, alternative mechanisms were investigated.
Figure 4.
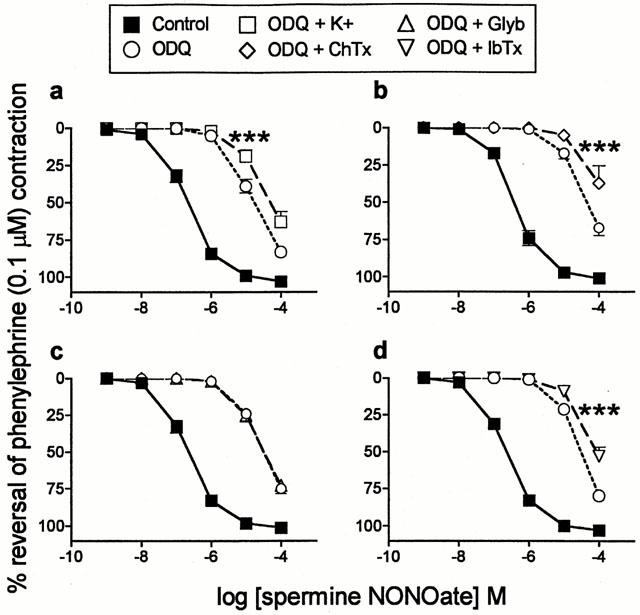
Mean concentration-response curves to spermine NONOate (n=4–6) on rat main pulmonary arteries pre-contracted with phenylephrine (0.1 μM) in the absence of any inhibitor (control), in the presence of 10 μM ODQ alone and in the presence of 10 μM ODQ plus (a) potassium (K+, 80 mM), (b) charybdotoxin (ChTx, 300 nM), (c) glybenclamide (glyb., 10 μM) or (d) iberiotoxin (IbTx, 300 nM). In (a) diltiazem (10 μM) was present for curves in the presence of ODQ with and without potassium. Relaxation responses are expressed as % reversal of the phenylephrine-induced contraction. Points are mean values with s.e.mean shown by vertical bars except when smaller than the size of the symbols. ***Concentration-response curve significantly different from the corresponding curve obtained in the presence of ODQ (10 μM) alone (P<0.001; two-way ANOVA).
Ouabain (1 mM; Na+/K+-ATPase inhibitor; Figure 5a; Table 1) and thapsigargin (100 nM; SERCA inhibitor; Figure 6a; Table 1) significantly inhibited responses to spermine NONOate in the presence of ODQ. The L-type calcium channel blocker, diltiazem (10 μM), had no effect (Table 1).
Figure 5.
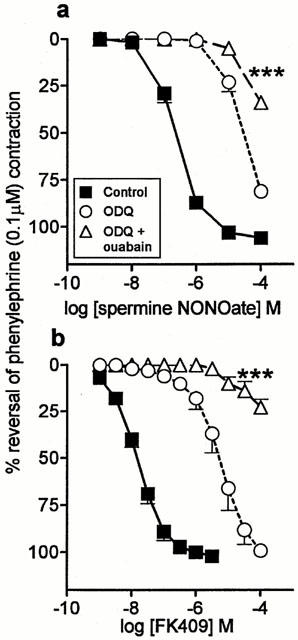
Mean concentration-response curves to (a) spermine NONOate (n=4) and (b) FK409 (n=4) on rat main pulmonary arteries pre-contracted with phenylephrine (0.1 μM) in the absence of any inhibitor (control), in the presence of 10 μM ODQ alone and in the presence of 10 μM ODQ plus 1 mM ouabain. Relaxation responses are expressed as % reversal of the phenylephrine-induced contraction. Points are mean values with s.e.mean shown by vertical bars except when smaller than the size of the symbols. ***Concentration-response curve significantly different from the corresponding curve in the presence of ODQ alone (P<0.001; two-way ANOVA).
Figure 6.
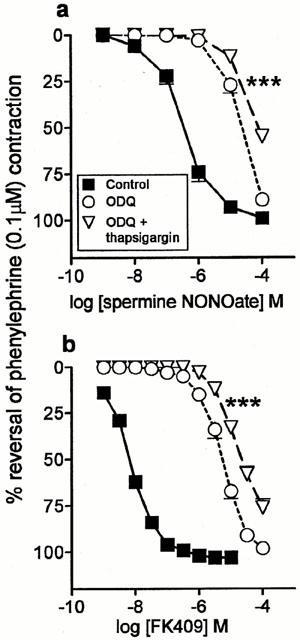
Mean concentration-response curves to (a) spermine NONOate (n=4) and (b) FK409 (n=4) on rat main pulmonary arteries pre-contracted with phenylephrine (0.1 μM) in the absence of any inhibitor (control), in the presence of 10 μM ODQ alone and in the presence of 10 μM ODQ plus 100 nM thapsigargin. Relaxation responses are expressed as % reversal of the phenylephrine-induced contraction. Points are mean values with s.e.mean shown by vertical bars except when smaller than the size of the symbols. ***Concentration-response curve significantly different from the corresponding curve in the presence of ODQ alone (P<0.001; two-way ANOVA).
In the presence of ODQ (10 μM), both charybdotoxin and iberiotoxin (but not potassium, ouabain or thapsigargin) consistently increased the size of the phenylephrine contraction to >79% of the reference contraction to 80 mM potassium. However this could not account for the observed inhibitory effects of charybdotoxin or iberiotoxin described above. This was ascertained in a series of experiments with spermine NONOate in which the concentration of phenylephrine, in the presence of ODQ (10 μM), was raised to 0.3 μM. The higher concentration of phenylephrine caused an increase in contraction size comparable to that caused by charybdotoxin or iberiotoxin but did not inhibit responses to the NO donor. Data (with 10 μM ODQ present) in preparations contracted first with 0.1 μM and then with 0.3 μM phenylephrine are, respectively (n=4): spermine NONOate neg log IC25 values: 4.76±0.11 and 4.65±0.06 (P>0.05; paired t-test): log unit shifts by ODQ: 2.31±0.12 and 2.42±0.07 (P>0.05; paired t-test).
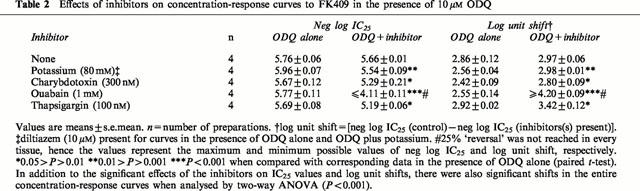
Experiments with some of the inhibitors were repeated using another NO donor, FK409 (Table 2). Each inhibitor tested (potassium, charybdotoxin, ouabain and thapsigargin) caused a significant inhibition of the FK409 curves in the presence of 10 μM ODQ, as found with spermine NONOate (Figures 5b and 6b; Table 2). However two differences between the FK409 and spermine NONOate data were observed. Firstly, the shift of the FK409 curve by 10 μM ODQ was approximately half a log unit greater than the corresponding shift for spermine NONOate (Tables 1 and 2). Secondly, the effect of ouabain was markedly greater for FK409 than for spermine NONOate (Figure 5; Tables 1 and 2).
Table 2.
Effects of inhibitors on concentration-response curves to FK409 in the presence of 10 μM ODQ
Discussion
The objective of this study was to elucidate the mechanisms responsible for relaxant responses in rat pulmonary artery to spermine NONOate, a NO donor drug that generates NO spontaneously. The study was prompted by previous data suggesting that responses to spermine NONOate, like the other drugs studied that generate NO spontaneously, might have a component that was resistant to inhibition by the soluble guanylate cyclase inhibitor, ODQ (Homer et al., 1999). In the present study this observation has been confirmed by examining additional concentrations of ODQ. Even in the presence of concentrations of ODQ as high as 10 and 30 μM, significant relaxation to spermine NONOate remained; furthermore 10 μM ODQ was shown to give maximal inhibition. Hence the present findings support the view that part of the relaxation response to spermine NONOate is ODQ-resistant.
In other blood vessel types (i.e. bovine pulmonary and coronary arteries, rat aorta, rabbit aorta and carotid arteries, and canine cerebral arteries) responses to a variety of NO donors have likewise been shown to induce responses that cannot be blocked by ODQ. The particular NO donors include S-nitrosoglutathione (Brunner et al., 1996; Moro et al., 1996; Feelisch et al., 1999), S-nitroso-N-acetyl-D,L-penicillamine (van der Zypp & Majewski, 1998; Feelisch et al., 1999), Angeli's salt, peroxinitrite (Feelisch et al., 1999), SIN-1 (Plane et al., 1996; Feelisch et al., 1999), DETA NONOate (Li et al., 1998) and DEA NONOate (Onoue & Katusic, 1998). All of these drugs, like spermine NONOate, can generate NO ‘spontaneously' (i.e. they do not require tissue activation). An ODQ-resistant component of relaxation was also identified in the response induced by ‘authentic NO' (Hussain et al., 1997; Weisbrod et al., 1998, Feelisch et al., 1999). In one of the above studies the effects of ODQ varied depending on whether or not the endothelium was present (Plane et al., 1996). This was not seen in the present study with spermine NONOate.
Two practical issues in relation to the experiments with ODQ need to be mentioned. Firstly, it has recently been suggested that at high concentrations (>10 μM) ODQ may inhibit the enzymatic processes responsible for the generation of NO from glyceryl trinitrate and sodium nitroprusside and hence cause excessively high inhibition of responses to these NO donors (Feelisch et al., 1999). However the possibility of non-selective inhibition was not an issue in the present study since neither spermine NONOate, nor the other two drugs included in experiments involving ODQ (MAHMA NONOate and FK409), undergo enzymatic activation in the tissue. Furthermore we were not seeking an explanation for excessive inhibition of responses by ODQ, but rather an explanation for responses that were resistant to blockade by ODQ. The second practical issue was the possibility that ODQ-resistant relaxation to spermine NONOate might have been due to decomposition products other than NO, i.e. spermine or nitrite. To avoid this possibility we limited our study to concentrations of spermine NONOate that gave responses that could not be attributed to either of these products, i.e. ⩽100 μM. The evidence for this was obtained from (i) the decomposition experiments described in the Methods, and (ii) data previously obtained for spermine and sodium nitrite (Homer & Wanstall, 1998).
The most probable explanation for the failure of ODQ to completely block the relaxation to spermine NONOate is that NO generated by the drug can act by a mechanism that does not involve cyclic GMP. An alternative explanation could be that ODQ-resistant relaxation to spermine NONOate was due to NO from the drug acting on a pool of guanylate cyclase that is not inhibited by ODQ, e.g. particulate membrane-bound guanylate cyclase, which is not inhibited by ODQ (Moro et al., 1996; Homer et al., 1999), or alternatively a pool of soluble guanylate cyclase that is inaccessible to ODQ. However data (i) with the G kinase inhibitor, Rp-8-Br-cGMPS and (ii) from experiments in which cyclic GMP production was directly compared with relaxation argue against this possibility.
The protein kinase G inhibitor caused remarkably little inhibition of relaxation to spermine NONOate, especially when compared with the inhibition of responses to glyceryl trinitrate or isosorbide dinitrate, suggesting a lesser involvement of cyclic GMP in responses to the NONOate. More importantly, when cyclic GMP production and relaxation were determined under identical conditions, direct evidence was obtained that vasorelaxation to spermine NONOate could occur independently of cyclic GMP production. This was also seen with MAHMA NONOate. The inclusion of MAHMA NONOate was prompted by the suggestion in the literature that the relative importance of cyclic GMP in the response to NO might be influenced by the rate of NO production (Plane et al., 1998). MAHMA NONOate has a much higher rate of decomposition than spermine NONOate (half-life in PSS at 37°C is 1.3 min compared with 73 min for spermine NONOate; Homer & Wanstall, 1998) and hence generates NO much more rapidly. The finding that, in the presence of ODQ, both spermine NONOate and MAHMA NONOate could cause almost maximal relaxation of pulmonary artery in the absence of cyclic GMP production agrees with reports in other blood vessels, not only for NO donors that can generate NO spontaneously but also for authentic NO (S-nitrosoglutathione, Brunner et al., 1996; SIN-1, Plane et al., 1996, DEA NONOate, Onoue & Katusic, 1998; NO, Weisbrod et al., 1998). The possibility has been raised that inhibition of cyclic GMP production without concomitant inhibition of relaxation may not necessarily indicate that relaxation is independent of cyclic GMP but, alternatively, that extremely small increases in cyclic GMP levels may be enough to trigger smooth muscle relaxation (Onoue & Katusic, 1998; Garcia-Pascual et al., 1999). However this explanation cannot apply to the present study because cyclic GMP levels in the presence of either spermine NONOate or MAHMA NONOate were reduced to below basal levels when ODQ was present. Thus we are confident that part of the vasorelaxation to both of these NONOates occurs in the absence of cyclic GMP production.
The final series of experiments was designed to identify possible mechanisms responsible for the ODQ-resistant, cyclic GMP-independent relaxation. In this series FK409 was studied in addition to spermine NONOate. FK409 also generates NO spontaneously but is an oxime, i.e. has a chemical structure that is quite different from that of spermine NONOate (Feelisch & Stamler, 1996). For both drugs, evidence was obtained that activation of Na+/K+-ATPase, SERCA and potassium channels could each account for part of the cyclic GMP-independent relaxation inasmuch as responses in the presence of ODQ were inhibited in the presence of ouabain, thapsigargin or an elevated potassium concentration. Data obtained with selective potassium channel inhibitors indicated that the particular types of potassium channel involved were KCa (large, intermediate and small conductance) but not KV or KATP potassium channels. This was despite the fact that all three types of potassium channel have been identified in pulmonary artery smooth muscle cells (Archer et al., 1994, 1996; Yuan et al., 1996; Clapp & Gurney, 1992). In other vessels activation of KCa channels has been shown to be involved in relaxation responses to NO donors, in the presence of ODQ, i.e. rat mesenteric arteries (Plane et al., 1996), rabbit carotid arteries (Plane et al., 1998) and canine cerebral arteries (Onoue & Katusic, 1998). In contrast to the present study, Yuan et al. (1996) found that NO could directly activate KV channels in rat pulmonary artery smooth muscle cells. However the data of Yuan et al. (1996) were obtained in cells from much smaller arteries than were used in the present study. Hence the discrepancy may reflect the predominance of KV channels in the majority of cells in small, but not main, pulmonary arteries (Archer et al., 1996). Compared with some of these studies, the effects of the potassium channel inhibitors in the present study were comparatively small. This is unlikely to be due to inadequate concentrations of inhibitors because the concentrations of charybdotoxin and iberiotoxin were at the higher end of the commonly used range. Hence potassium channel activation may make only a minor contribution to cyclic GMP-independent relaxation in rat main pulmonary artery.
There are precedents for each of the other mechanisms identified, viz. activation of SERCA and of Na+/K+-ATPase. NO-induced activation of SERCA, independent of cyclic GMP, has been described in human platelets (Trepakova et al., 1999) and cyclic GMP-independent activation of Na+/K+-ATPase has been observed in rabbit aorta (Gupta et al., 1994). It is possible that the cyclic GMP-independent mechanisms identified in the present study are not the only ones that have a role in rat pulmonary artery, since a combination of ODQ, ouabain, thapsigargin and elevated potassium concentration failed to abolish responses to spermine NONOate or FK409 completely (Homer & Wanstall, unpublished). However we are hesitant to draw firm conclusions from these data because of the potential interactions of such a large number of inhibitors in a single experiment.
Close inspection of the data revealed some subtle differences between spermine NONOate and FK409, even though these drugs both generate NO spontaneously. Relaxation to FK409 was blocked by ODQ (10 μM) to a greater extent than spermine NONOate (approximately 2.5 and 2 log units, respectively). Moreover, the combination of ouabain and ODQ blocked relaxations to FK409 to a much greater extent than spermine NONOate. Thus activation of soluble guanylate cyclase and Na+/K+-ATPase may be rather more important for relaxation to FK409 than to spermine NONOate. One known difference between these two drugs is the redox state of the nitrogen monoxide that they generate, i.e. predominantly NO. for spermine NONOate and NO. and NO− for FK409 (Feelisch & Stamler, 1996). The nature of the response can be influenced by the redox state of NO (Feelisch, 1998), but whether this can account for the slightly different pharmacological profiles described above is currently unknown.
In summary, the results of this study have shown that pulmonary vasorelaxant responses to spermine NONOate (a NO donor drug that does not require tissue activation to generate NO) are only partially blocked by the soluble guanylate cyclase inhibitor, ODQ. The ODQ-resistant relaxation to spermine NONOate, and also to MAHMA NONOate, can occur in the absence of cyclic GMP production. Mechanisms found to contribute to the cyclic GMP-independent component of the responses to spermine NONOate include activation of (i) Na+/K+-ATPase, (ii) SERCA and (iii) KCa channels. Each of these mechanisms was also found to occur with FK409, a structurally unrelated NO donor that, like spermine NONOate, generates NO ‘spontaneously', i.e. without tissue activation. If the action of other NO donors, especially those that require activation in the tissue, should involve a different range of mechanisms, this could have implications in relation to the choice of an appropriate NO donor drug for experimental or therapeutic purposes. This would be particularly relevant if one or more of the mechanisms of relaxation should be up- or down-regulated in a particular disease state.
Acknowledgments
The National Health and Medical Research Council of Australia supported this study and this financial support is gratefully acknowledged. J.C. Wanstall is an NH & MRC Senior Research Fellow. We would like to thank the Fujisawa Pharmaceutical Company, Japan, for kindly providing us with a sample of FK409 for use in this study.
Abbreviations
- Cyclic GMP
guanosine 3′ 5′ cyclic monophosphate
- FK409
(±)-E-4-ethyl-2-[E-hydroxyimino]-5-nitro-3-hexenamide
- KATP channel
ATP-dependent potassium channel
- KCa channel
calcium-activated potassium channel
- KV channel
voltage-dependent potassium channel
- NO
nitric oxide
- NONOates
diazeniumdiolates
- ODQ
1H-[1,2,4]Oxadiazolo-[4,3,-a]quinoxalin-1-one
- PSS
physiological salt solution
- SERCA
sarco-endoplasmic reticulum Ca2+-ATPase
- SIN-1
3-morpholinosydnonimine
- TCA
trichloroacetic acid
References
- ARCHER S.L., HUANG J.M.C., HAMPL V., NELSON D.P., SCHULTZ P.J. , WEIR E.K. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARCHER S.L., HUANG J.M.C., REEVE H.L., HAMPL V., TOLAROVA S., MICHELAKIS E., WEIR E.K. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ. Res. 1996;78:431–442. doi: 10.1161/01.res.78.3.431. [DOI] [PubMed] [Google Scholar]
- BRUNNER F., SCHMIDT K., NIELSEN E.B., MAYER B. Novel guanylyl cyclase inhibitor potently inhibits cyclic GMP accumulation in endothelial cells and relaxation of bovine pulmonary artery. J. Pharmacol. Exp. Ther. 1996;277:48–53. [PubMed] [Google Scholar]
- CLAPP L.H., GURNEY A.M. ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am. J. Physiol. 1992;262:H916–H920. doi: 10.1152/ajpheart.1992.262.3.H916. [DOI] [PubMed] [Google Scholar]
- DE MEIS L. Relaxing effect of spermine and spermidine on intact and glycerol-treated muscle. Am. J. Physiol. 1967;212:92–96. doi: 10.1152/ajplegacy.1967.212.1.92. [DOI] [PubMed] [Google Scholar]
- FEELISCH M. The use of nitric oxide donors in pharmacological studies. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:113–122. doi: 10.1007/pl00005231. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., KOTSONIS P., SIEBE J., CLEMENT B., SCHMIDT H.H.H.W. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]Oxadiazolo-[4,3-a]quinoxaline-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome P-450 enzymes involved in nitric oxide donor bioactivation. Mol. Pharmacol. 1999;56:243–253. doi: 10.1124/mol.56.2.243. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., STAMLER J.S.Donors of nitrogen oxides Methods in nitric oxide research 1996Chichester: Wiley and Sons; 71–115.ed. Feelisch, M. & Stamler, J.S. pp [Google Scholar]
- GARCIA-PASCUAL A., COSTA G., LABADIA A., JIMENEZ E., TRIGUERO D. Differential mechanisms of urethral smooth muscle relaxation by several NO donors and nitric oxide. Naunyn-Schmiederg's Arch. Pharmacol. 1999;360:80–91. doi: 10.1007/s002109900038. [DOI] [PubMed] [Google Scholar]
- GUPTA S., MCARTHUR A., GRADY C., RUDERMANN N.B. Stimulation of vascular Na+-K+-ATPase activity by nitric oxide: a cGMP-independent effect. Am. J. Physiol. 1994;266:H2146–H2151. doi: 10.1152/ajpheart.1994.266.5.H2146. [DOI] [PubMed] [Google Scholar]
- HOMER K.L., FIORE S.A., WANSTALL J.C. Inhibition by 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) of responses to nitric oxide-donors in rat pulmonary artery: influence of the mechanism of nitric oxide generation. J. Pharm. Pharmacol. 1999;51:135–139. doi: 10.1211/0022357991772240. [DOI] [PubMed] [Google Scholar]
- HOMER K.L., WANSTALL J.C. In vitro comparison of two NONOates (novel nitric oxide donors) on rat pulmonary arteries. Eur. J. Pharmacol. 1998;356:49–57. doi: 10.1016/s0014-2999(98)00511-1. [DOI] [PubMed] [Google Scholar]
- HOMER K.L., WANSTALL J.C. Characterisation of cGMP-independent pulmonary vasorelaxation by nitric oxide donors. Proc. Aust. Soc. Clin. Exp. Pharmacol. Toxicol. 1999;6:54. [Google Scholar]
- HUSSAIN A.S., MARKS G.S., BRIEN J.F., NAKATSU K. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) inhibits relaxation of rabbit aortic rings induced by carbon monoxide, nitric oxide and glyceryl trinitrate. Can. J. Physiol. Pharmacol. 1997;75:1034–1037. [PubMed] [Google Scholar]
- KEEFER L.K., NIMS R.W., DAVIES K.M., WINK D.A. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- LI P., JIN M., CAMPBELL W.B. Effect of selective inhibition of soluble guanylyl cyclase on the KCa channel activity in coronary artery smooth muscle. Hypertension. 1998;31:303–308. doi: 10.1161/01.hyp.31.1.303. [DOI] [PubMed] [Google Scholar]
- MISTRY D.K., GARLAND C.J. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BKCa) in smooth muscle cells isolated from the rat mesenteric artery. Br. J. Pharmacol. 1998;124:1131–1140. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORO M.A., RUSSELL R.J., CELLEK S., LIZASOAIN I., SU Y., DARLEY-USMAR V.M., RADOMSKI M.W., MONCADA S. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONOUE H., KATUSIC Z.S. The effect of 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and charybdotoxin (CTX) on relaxations of isolated cerebral arteries to nitric oxide. Brain Res. 1998;785:107–113. doi: 10.1016/s0006-8993(97)01393-0. [DOI] [PubMed] [Google Scholar]
- PLANE F., HURRELL A., JEREMY J.Y., GARLAND C.J. Evidence that potassium channels make a major contribution to SIN-1-evoked relaxation of rat isolated mesenteric artery. Br. J. Pharmacol. 1996;119:1557–1562. doi: 10.1111/j.1476-5381.1996.tb16072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLANE F., WILEY K.E., JEREMY J.Y., COHEN R.A., GARLAND C.J. Evidence that different mechanisms underlie smooth muscle relaxation to nitric oxide and nitric oxide donors in the rabbit isolated carotid artery. Br. J. Pharmacol. 1998;123:1351–1358. doi: 10.1038/sj.bjp.0701746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT H.H.H.W., LOHMANN S.M., WALTER U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Bioch. Bioph. Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- TREPAKOVA E.S., COHEN R.A., BOLOTINA V.M. Nitric oxide inhibits capacitative cation influx in human platelets by promoting sarcoplasmic/endoplasmic reticulum Ca2+-ATPase- dependent refilling of Ca2+ stores. Circ. Res. 1999;84:201–209. doi: 10.1161/01.res.84.2.201. [DOI] [PubMed] [Google Scholar]
- VAN DER ZYPP A., MAJEWSKI H. Effect of cGMP inhibitors on the actions of nitrodilators in rat aorta. Clin. Exp. Pharm. Physiol. 1998;25:38–43. doi: 10.1111/j.1440-1681.1998.tb02141.x. [DOI] [PubMed] [Google Scholar]
- WANSTALL J., HOMER K. Nitric oxide donors on rat pulmonary arteries: different mechanisms of action. Physiol. Res. 1999;48:47P. [Google Scholar]
- WEISBROD R.M., GRISWOLD M.C., YAGHOUBI M., KOMALAVILAS P., LINCOLN T.M., COHEN R.A. Evidence that additional mechanisms to cyclic GMP mediate the decrease in intracellular calcium and relaxation of rabbit aortic smooth muscle to nitric oxide. Br. J. Pharmocol. 1998;125:1695–1707. doi: 10.1038/sj.bjp.0702233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUAN X., TOD M.L., RUBIN L.J., BLAUSTEIN M.P. NO hyperpolarizes pulmonary artery smooth muscle cells and decreased the intracellular Ca2+ concentration by activating voltage-gated K+ channels. Proc. Natl. Acad. Sci. U.S.A. 1996;93:10489–10494. doi: 10.1073/pnas.93.19.10489. [DOI] [PMC free article] [PubMed] [Google Scholar]


