Abstract
Correolide (1–10 μM), a nortriterpene purified from Spachea correae and a selective blocker of Kv1 potassium channels, elicits repetitive twitching in guinea-pig ileum. This effect is not seen in guinea-pig duodenum, portal vein, urinary bladder or uterine strips, nor in rat or mouse ileum.
The time course and amplitude of the correolide-induced twitches in guinea-pig ileum are similar to those elicited by electrical stimulation of the enteric nervous system.
The correolide-induced twitching is not affected by pre-treatment with capsaicin (1 μM), but is facilitated by the NO synthase inhibitor, NG-nitro-L-arginine methyl esther (L-NAME, 200 μM).
The correolide-induced twitching is abolished by tetrodotoxin (1 μM) or hexamethonium (100 μM), and is markedly inhibited by nifedipine (0.3 μM) or atropine (0.2 μM). The atropine-resistant component is inhibited by selective antagonists of NK1 and NK2 tachykinin receptors, namely GR 82334 and GR 94800 (1 μM each). The former compound is more effective in inhibiting the correolide-induced, atropine-resistant activity.
Correolide intensified the twitching of ileum segments exposed to saturating concentrations of margatoxin (MgTX), which suggests that Kv1 sub-types other than Kv1.1 (Kv1.4 or Kv1.5) are involved in the relatively greater degree of stimulation of the enteric nervous system by correolide, as compared to MgTX.
We propose that blockade of Kv1 channels by correolide increases the excitability of intramural nerve plexuses promoting release of acetylcholine and tachykinins from excitatory motor neurons. This, in turn, leads to Ca2+-dependent action potentials and twitching of the muscle fibres.
Keywords: Potassium channels, smooth muscle motility, enteric nervous system, tachykinins, nitric oxide, scorpion toxins
Introduction
The role of the enteric nervous system in the control of intestinal smooth muscle contractility and peristalsis is now well established, and a number of populations of enteric neurons have been identified and characterized. The availability of potent and selective receptor ligands has greatly facilitated the assessment of the individual roles of various neurotransmitters in the complex interplay of both excitatory and inhibitory inputs to the smooth muscle fibres in the longitudinal and circular muscle layers of guinea-pig ileum (for reviews, see Costa & Brookes, 1994; Surprenant, 1994; Goyal & Hirano, 1996). The accumulated evidence indicates that acetylcholine and tachykinins, such as substance P and neurokinins (NKA, NKB), are co-transmitters released by excitatory motor neurons, whereas vasoactive intestinal peptide (VIP), adenosine-triphosphate (ATP) and nitric oxide (NO) are major inhibitory neurotransmitters.
Isolated segments of guinea-pig ileum bathed in physiological saline solutions display spontaneous myogenic activity in the form of contractions of small amplitude and irregular frequency and time course. In some segments, relatively large twitches, sensitive to blockade by TTX, are superposed on the myogenic contractions. We have recently shown that nanomolar concentrations of peptidyl K+ channel inhibitors, such as margatoxin (MgTX; Garcia-Calvo et al., 1993), kaliotoxin (KTX, Crest et al., 1992), the agitoxins AgTX1 and AgTX2 (Garcia et al., 1994) and alpha-dendrotoxin (α-DaTX, Dufton & Harvey, 1998) induce TTX-sensitive twitches in guinea-pig ileum strips (Suarez-Kurtz et al., 1999). Given the specificity of these different peptides as inhibitors of sub-types of Shaker-type voltage-gated K+ channels (Kv1), it was proposed that high-affinity blockade of Kv1.1 underlies these peptide's ability to stimulate excitatory motor pathways in the enteric nervous system and elicit twitches in guinea-pig ileum (Suarez-Kurtz et al., 1999).
Kv1 is one of nine described families of voltage-gated K channels identified through molecular cloning techniques (Robertson, 1997). High-affinity peptidyl inhibitors, many obtained from scorpion venoms, have provided significant information about these channels and served as selective ligands for the development of high-throughput screening assays to detect channel modulators (Garcia et al., 1997). Screening for small molecule natural product inhibitors of Kv1.3 has recently yielded the purification and structural elucidation of the nortriterpene, correolide (Figure 1), isolated from the Costa Rican tree Spachea correae (Goetz et al., 1998). Correolide is a highly-selective inhibitor of the Kv1 family, being devoid of activity against many other receptor and ion channel targets (Felix et al., 1999). Thus, correolide represents an important new tool for investigating the function of Kv1 in tissues of interest and the potential role of these channels as pharmacological targets for therapeutic use. The present study shows that correolide, but not its weakly active analogue, C-4 desacetyl correolide THF C-3 acid (Felix et al., 1999), elicits TTX-sensitive twitches in guinea-pig ileum strips. The mechanism of this effect was investigated using selective pharmacological tools to block nicotinic and muscarinic cholinergic receptors, neurokinin receptors, NO synthesis and L-type Ca2+ channels. In addition, capsaicin was used to examine the role of afferent C-fibres in the correolide-induced twitching in guinea-pig ileum.
Figure 1.
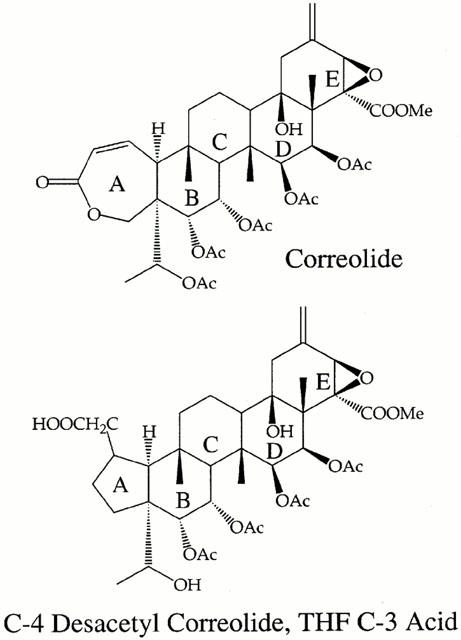
Chemical structures of correolide and C-4 desacetyl correolide, THF C-3 acid.
Methods
Preparations
Experiments were performed at 37°C on the portal vein, segments of duodenum or ileum, and strips of urinary bladder or uterus muscle obtained from adult guinea-pigs, and on isolated ileum segments from adult rats (Wistar) or mice (Swiss-55). Animals were kept following the precepts of humane care, in rooms with temperature control and light/ dark cycle, and were asphyxiated by CO2 inhalation. For recording muscle tension, the preparations were mounted vertically between two metal stirrups, of which the lower was fixed and the upper was attached to a rigid wire connected to a force-displacement transducer (Grass FT-03; Grass Instruments Co., Quincy, MA, U.S.A.). The tension recordings from ileum strips, which was used in most experiments, reflect predominantly the activity of the longitudinal muscle. A 1-g load was initially applied to the guinea-pig and rat preparations and a 0.5-g load to mouse ileum segments, as previous studies from our laboratories have shown that this allows stable tension recordings from these tissues for several hours (Suarez-Kurtz et al., 1991; 1999). The transducer signals were amplified and recorded on a Grass polygraph (Model 7). Integration of the isometric tension recordings was used to quantify the effects of atropine or the neurokinin antagonists on correolide-induced twitching of guinea-pig ileum segments (Suarez-Kurtz et al., 1991). Briefly, the zero level for integration was set at 5% of the average amplitude of the ‘basal' spontaneous tension oscillations, recorded between 40 and 60 min after mounting the preparations in the muscle chamber and immediately before addition of correolide to the bath. Integrated activity during exposure to correolide is expressed relative to the basal activity. The inhibitory effect of atropine and/or the neurokinin antagonists is expressed relative to the integrated activity recorded after 10–20 min exposure to correolide. In some experiments, electrical stimulation was applied to the guinea-pig ileum strips, via two platinum ring electrodes, placed around the segments, at 6 mm distance from each other. Stimulation consisted of single pulses or trains (10 s, 10 Hz) of square wave pulses of 0.2 ms duration and supramaximum intensity. The electrically-elicited contractions were completely blocked by 1 μM TTX, and therefore result from stimulation of excitatory motor pathways of the enteric nervous system.
Solutions and chemicals
The physiological saline solution, a modified Krebs-Henseleit solution, had the following composition (in millimolar): NaCl 120, KCl 5.9, CaCl2 2.5, MgCl2 1.1, NaHCO3 15, NaH2PO4 1.2, glucose 11, and HEPES 10. The pH of this solution after equilibration with 95% O2 and 5% CO2 was 7.3 at 37°C. Correolide (Figure 1) was isolated from extracts of the Costa Rican tree Spachea correae Cuatrec. & Croat (Malpighiaceae), as described by Goetz et al. (1998). An analogue of correolide with weak Kv1 blocking activity, C-4 desacetyl correolide, THF C-3 acid (Figure 1), was prepared as described by Baker et al. (1998). Stock solutions (20 mg ml−1) of correolide or its analogue were made in DMSO, and all subsequent dilutions were made in the physiological saline. Thus, the highest concentration of DMSO in the bath, when applying 10 μM correolide or C-4 desacetyl correolide, THF C-3 acid, was 0.05%, which has no effect in the spontaneous motility of the tissues studied. TTX, atropine sulfate, hexamethonium hydrobromide and NG-nitro-L-arginine methyl esther (L-NAME) were from Sigma Chemical Co. (St. Louis, MO, U.S.A.), whereas the tachykinin antagonists GR 82334 (pGlu-Ala- Asp-Pro- Asn- Lys- Phe-Tyr-Pro(spiro-γ-lactam) Leu-Trp-NH2; Hall et al., 1992) and GR 94800 (Phenyl-CO-Ala-Ala-D-Trp-Phe-D-Pro-Nle-NH2; McElroy et al., 1992), were purchased from Research Biochemicals International (Natick, MA, U.S.A.).
Pooled data from identical experiments are presented as means±s.e.mean. Student's t-test was used for statistical analysis of the data. The significance level was set at P<0.05.
Results
Effects of correolide on smooth muscle contractility
We have recently reported (Suarez-Kurtz et al., 1999) that peptidyl blockers of Shaker-type Kv1 channels elicit twitches in guinea-pig ileum, and that this effect is not seen in several other guinea-pig smooth muscle preparations. Figure 2 shows that correolide reproduces this pattern: within 4 min of exposure to 3 μM correolide, spike-like twitches were recorded from the ileum segment (Figure 2A), whereas no such effect was observed in detrusor muscle (Figure 2B), portal vein (Figure 2C), uterus (Figure 2D), or duodenum strips (n=4, not shown) during 20-min exposures to either 3 or 10 μM correolide. A small (<10%) increase of the integrated activity of portal vein and detrusor muscles was observed in some preparations, exposed to 10 μM correolide (Figure 2). This effect, which differs markedly, both in time course and magnitude, from the twitching elicited in ileum segments, was not further investigated in the present study.
Figure 2.
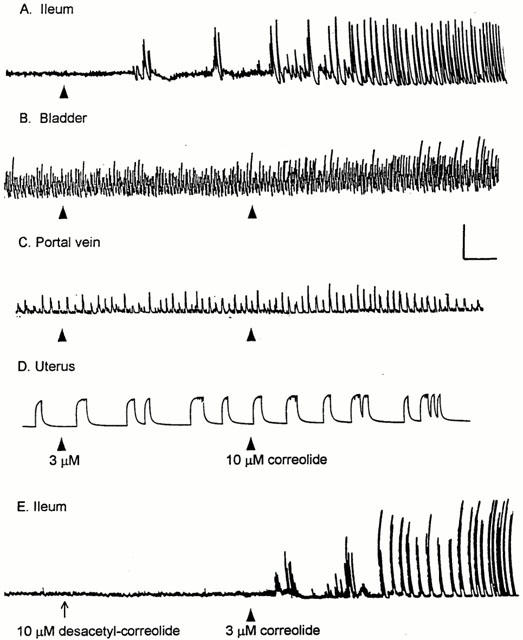
The effect of correolide and C-4 desacetyl correolide, THF C-3 acid on the contractility of guinea-pig smooth muscles. Isometric tension recordings were made from ileum (A, E), bladder (B) portal vein (C) and uterus (D) Preparations A–D were initially exposed to 3 μM correolide (arrowheads); after 20 min the correolide concentration was increased to 10 μM in B–D (arrowheads). Preparation E was initially exposed to 10 μM C-4 desacetyl correolide, THF C-3 acid (desacetyl-correolide, arrow). After 20 min, this compound was removed from, and 3 μM correolide was added to the bathing medium (arrowhead). Correolide, but not its analogue, induced twitching in the ileum segments. Calibration bars: Horizontal, 4 min; vertical, 1 g.
The ability of correolide to induce twitching in guinea-pig ileum segments is not reproduced by its analogue, C4 desacetyl correolide, THF C3 acid (Figure 1E), which possesses 100 fold reduced Kv1 channel blocking activity than the parent compound (Felix et al., 1999). The pattern of twitching elicited by correolide in guinea-pig ileum was not seen in rat or mouse ileum segments, studied under similar experimental conditions (n=3–5, not shown).
Correolide-induced twitching in guinea-pig ileum segments
Figure 3 shows data on the concentration-response curve for the correolide-induced twitching in guinea-pig ileum. Two patterns of twitching activity were observed during exposure to increasing correolide concentrations (1–10 μM). Bursts of twitches, occurring randomly at variable intervals, were observed in some segments exposed to 1 and/or 3 μM correolide (Figure 3B,C), whereas sustained twitching was consistently observed in 67 ileum segments exposed to 10 μM correolide (Figure 3D) and in five out of 16 segments challenged with 3 μM compound (Figure 2A). The time-course of the correolide-induced twitches is similar to that of twitches evoked by electrical field stimulation (Figure 3A,D). The occurrence of sustained twitching following exposure to correolide was accompanied by a decrease in the basal myogenic activity (Figures 2A and 3D). This slight decrease in tone is probably caused by release of inhibitory neurotransmitters, as a transient relaxation can be observed after either correolide-induced twitches or electrically-evoked trains of pulses (Figure 3A,D). Removal of correolide from the bathing medium after sustained twitching had been recorded for 20–30 min does not reverse the drug effect (up to 1 h observation). The occurrence of either pattern of twitching described above, during 20 min exposures to increasing concentrations of correolide (0.1–10 μM), was used to construct a quantal concentration-effect curve (frequency plot) for the compound (Ponte et al., 2000). The plot in Figure 3E shows that 1 μM correolide is the minimal concentration required for inducing twitches, whereas all segments respond to 10 μM compound. Prolonging to 90 min the exposure time to 0.1–0.3 μM correolide still failed to elicit twitching in guinea-pig ileum (n=6, not shown).
Figure 3.
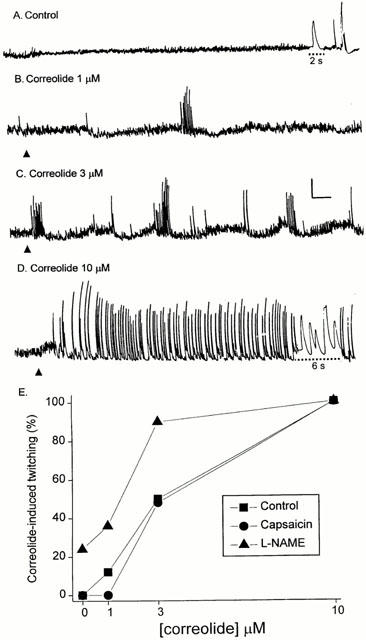
The effects of increasing concentrations of correolide on the spontaneous motility of guinea-pig ileum segments. (A–D) Tension recordings from a segment exposed successively to 1, 3 and 10 μM correolide (arrowheads). (A) Control; the phasic tensions shown at the end are two electrically-evoked twitches and one contraction elicited by tetanic stimulation (Methods). The speed of the recording device was increased during the times indicated by the dotted lines to allow observation of the time course of the twitches evoked by single pulses (A), or induced by 10 μM correolide (D). Calibration bars: horizontal, 1 min; vertical, 1 g. (E) Quantal concentration-effect curve for the correolide-induced twitching in guinea-pig ileum. Ileum segments were exposed, at 20 min intervals, to increasing concentrations of correolide (1–10 μM). Data were obtained from control preparations (no pretreatment; n=15) or from preparations pretreated with either L-NAME (200 μM; n=9) or capsaicin (1 μM; n=9), applied 30 min before the exposure to the lowest concentration of correolide (1 μM). The plot shows the frequency, expressed as a percentage of the number of experiments in which a given concentration elicited twitching in the ileum segments.
Pharmacological interaction of L-NAME or capsaicin with correolide in guinea-pig ileum
Figure 3E summarizes the effects of the NO synthase inhibitor, L-NAME, and of capsaicin, a selective activator of afferent sensory neurones, on the sensitivity of guinea-pig ileum segments to correolide. Pretreatment with L-NAME (200 μM, for 30 min) evoked twitching in two out of 10 ileum segments and facilitated the correolide-induced twitching, as indicated by a displacement to the left of the quantal concentration-response curve for this triterpene (Figure 3E). Pretreatment with capsaicin (1 μM), caused an initial, transient contraction of the ileum segments (Bartho et al., 1987), but had no effect on the quantal concentration-response curve for the correolide-induced twitching (Figure 3E).
Effects of tetrodotoxin, hexamethonium and of nifedipine on the correolide-induced twitches
TTX (1 μM, n=5) abolished the correolide-induced twitching of guinea-pig ileum (Figure 4A). This indicates that the targets for correolide, namely Kv1 channels, are located in the enteric nervous system and not in the smooth muscle cells. Accordingly, in segments pretreated with TTX, correolide (up to 10 μM) did not affect sub-maximal tensions elicited either by 0.1 μM acetylcholine or 10 nM substance P (n=3, not shown). The correolide-induced twitches are markedly inhibited by the ganglionic blocking agent, hexamethonium (100 μM, n=5, Figure 4B), which suggests that Kv1 channels are expressed by myenteric interneurons and that functional integrity of nicotinic receptors in ganglion cells of the enteric nervous system is required for twitch elicitation. Nifedipine (0.3 μM, n=5, Figure 4C), an L-type Ca2+ channel blocker, also had a distinct inhibitory effect on the correolide-induced twitching, which we ascribe to blockade of Ca2+-dependent action potentials in the smooth muscle fibres.
Figure 4.
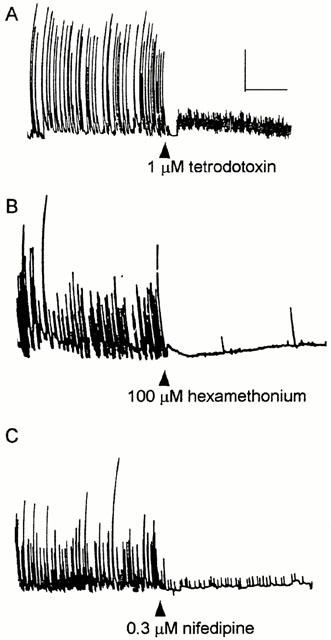
The effects of TTX, hexamethonium and nifedipine on the correolide-induced twitching in three guinea-pig ileum segments. After recording the twitches induced by 10 μM correolide for 10 min, TTX (A), hexamethonium (B) or nifedipine (C) were added to the bath media. Calibration bars: horizontal, 2 min; vertical, 1 g.
Effects of atropine and of tachykinin antagonists on the correolide-induced twitches
Figures 5A and 6 illustrate the experimental protocols used for investigating the pharmacological interaction between correolide, atropine and/or selective tachykinin antagonists. The integration of the contractile activity (Methods) was used to quantify the correolide-induced twitching and the inhibitory effects of atropine (0.2 μM) and of the selective tachykinin antagonists (1 μM). In 21 out of 39 experiments, atropine reduced the integrated activity by >80% (as illustrated in Figure 5A), whereas variable degrees of reduction were observed in the other 18 segments. The histogram in Figure 5B shows the distribution of the atropine effect. Regardless of the degree of inhibition obtained with atropine, increasing the concentration to 1 μM caused no further reduction of the correolide-induced twitching (n=8, not shown). The 15 experiments in which the degree of atropine inhibition was in the range of 40–80% were used to evaluate the additional effect of either tachykinin antagonist on the atropine-resistant activity (Figure 6). The inhibitory effect of the NK1 antagonist, GR 82334, on the atropine-resistant activity (69%±5, n=8) was significantly greater (P<0.05, Student's t-test) than that of the NK2 antagonist, GR 94800 (41%±12, n=7).
Figure 5.
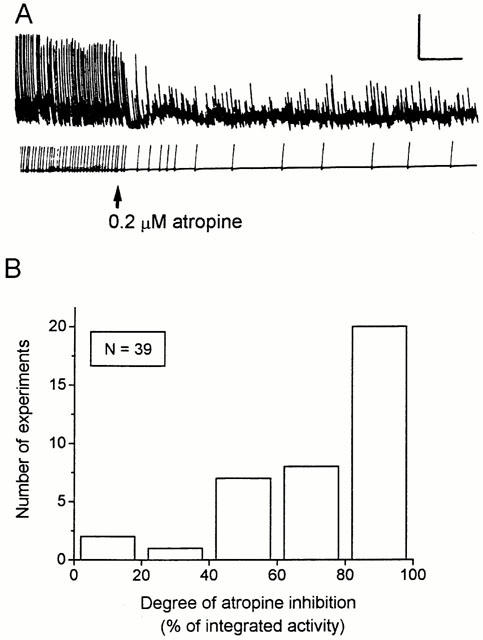
The effects of atropine on the correolide-induced twitching in guinea-pig ileum. (A) Correolide (10 μM) was applied to the bath 15 min before the beginning of the recording and atropine (0.2 μM) was added where indicated. Integrated mechanical activity, used to quantify the data is shown below the tension recording. Calibration bars: horizontal, 2 min; vertical, 1 g. (B) Histogram showing the distribution of the inhibitory effect of atropine on the correolide-induced activity (n=39 segments). The integrated activity recorded after 20–30 min exposure to 10 μM correolide was taken as 100%, and the effect of atropine is expressed as the per cent reduction of the correolide-induced activity.
Figure 6.
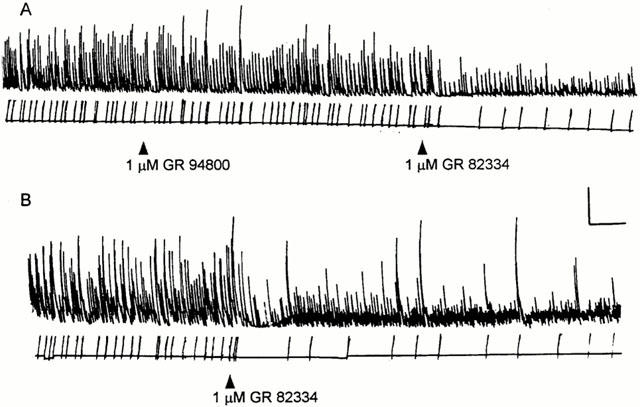
The effects of the tachykinin antagonists GR 82334 and GR 94800 on the correolide-induced, atropine-resistant activity in guinea-pig ileum. (A,B) The ileum segments were exposed to 10 μM correolide for 15 min, and then atropine (0.2 μM) was added to the bath. The tension recordings shown in (A) and (B) start 10 min after the addition of atropine. The NK antagonists (1 μM) were applied where indicated. Note the relatively greater effect of NK1 antagonist, GR 82334. Calibration bars: horizontal, 5 min; vertical, 1 g.
Comparison of the twitching induced by correolide or by MgTX
The correolide- and the MgTX-induced twitches of guinea-pig ileum share a number of common features (see Discussion), but differ in their sensitivity to blockade by atropine. This muscarinic antagonist abolishes the twitching elicited by MgTX or other peptidyl blockers of Kv1.1 channels (Suarez-Kurtz et al., 1999), but causes incomplete blockade of the correolide-induced twitching (Figure 5). This discrepancy prompted us to investigate the interaction of atropine with both correolide and MgTX, under identical conditions, in the same ileum segments. In Figure 7A, atropine abolished the bursts of twitches elicited by MgTX, but did not prevent correolide from triggering repetitive twitching; the latter was markedly inhibited by the NK1 receptor blocker, GR 82334 (Figure 7A). Figure 7B discloses another aspect of the pharmacodynamic interaction between MgTX and correolide, namely the ability of the latter to induce additional increases in twitching amplitude and frequency in the presence of saturating concentrations of MgTX (Suarez-Kurtz et al., 1999). Thus, it appears that the discrepancies between MgTX and correolide in their stimulatory effects in ileum motility cannot be entirely attributed to greater blockade of MgTX-sensitive Kv1 channels by correolide under the prevalent experimental conditions (see Discussion).
Figure 7.
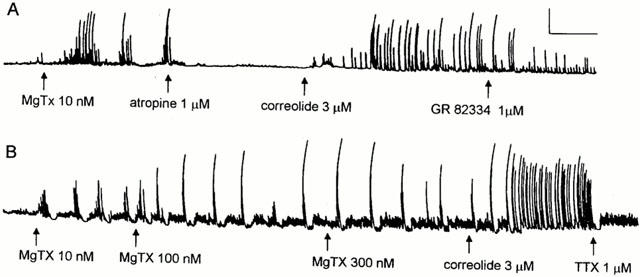
Comparison of the contractile effects of correolide and MgTX. (A) and (B) show isometric tension recordings from two guinea-pig ileum segments. (A) After complete blockade of the MgTX-induced twitching by atropine, addition of correolide to the bathing medium restored the twitches, which were inhibited by 1 μM GR 82334. (B) After recording a saturated response to increasing concentrations of MgTX (10, 100 and 300 nM), addition of correolide (10 μM) to the bath caused a marked increase in motility, which was abolished by TTX (1 μM). Calibration bars: horizontal, 4 min; vertical, 1 g.
Discussion
The present study shows that correolide elicits TTX-sensitive twitches in guinea-pig ileum segments, while the weakly active analogue C-4 desacetyl correolide THF C3 acid (Felix et al., 1999) fails to do so. Correolide, a nortriterpene extracted from Spachea correae, is the first potent (IC50<100 nM for Kv1.3; Felix et al., 1999), small molecular weight, selective inhibitor of Kv1 potassium channels to be isolated from a natural product source (Goetz et al., 1998). Correolide blocks all members of the Kv1 family, and displays marked selectivity against numerous receptors and ligand- or voltage-gated ion channels, including Kv2, Kv3 and Kv4 series of channels (Felix et al., 1999). We have recently reported that high-affinity peptidyl blockers of Kv1 channels, such as MgTX, α-DaTX, KTX, AgTX-1 and AgTX-2 induce TTX-sensitive twitching in guinea-pig ileum (Suarez-Kurtz et al., 1999). The correolide-induced twitching in guinea-pig ileum shares several characteristics previously reported for these peptidyl blockers of Kv1 channels (Suarez-Kurtz et al., 1999). First, the time course of both MgTX and correolide-induced twitches is virtually identical to that of the twitches evoked by field stimulation of the enteric nervous system, and both are inhibited by TTX. Second, the twitches induced by correolide or by peptidyl blockers of Kv1 are inhibited by hexamethonium, indicating a pre-ganglionic action in the myenteric plexus. Third, both the MgTX- and the correolide-induced twitching are reduced by nifedipine, which we ascribe to blockade of the Ca2+-action potentials in the ileum smooth muscle fibres. It is likely that these action potentials are triggered by excitatory neurotransmitters from the enteric nervous system which, in turn, activate the excitation-contraction coupling process, leading to muscle twitching. Both acetylcholine, acting on muscarinic receptors, and tachykinins, acting on NK receptors, appear to mediate the excitatory input to muscle fibres, since the correolide-induced twitching is inhibited by atropine and by selective NK receptor blockers. In view of the high selectivity of GR 82334 (Hall et al., 1992) and GR 94800 (McElroy et al., 1992) for NK1 and NK2 receptors, respectively, we propose that both receptor sub-types contribute to the correolide-induced twitches.
The correolide-induced twitching was facilitated by the NO synthase inhibitor, L-NAME. NO released from motor neurons causes gastrointestinal relaxation by both activating soluble guanylyl cyclase in smooth muscle cells (reviewed by Lefebvre, 1995) and by inhibiting the release of excitatory transmitters, such as acetylcholine and tachykinins (Hebeiss & Kilbinger, 1998). Accordingly, NO synthase inhibitors have been shown to enhance nerve-mediated cholinergic and tachykininergic contractions (Wiklund et al., 1993) and to increase depolarization-evoked release of acetylcholine from the guinea-pig myenteric plexus (Hebeiss & Kilbinger, 1996). Facilitation of the correolide-induced twitching by L-NAME suggests that Kv1 channels modulate the excitability of inhibitory motor neurons in the enteric nervous system. Accordingly, the sustained twitching induced by correolide is accompanied by a slight decrease in tone, reflecting a reduction of the basal myogenic activity. Thus, blockade of Kv1 by correolide would lead to increased release of NO (and possibly other inhibitory neurotransmitters, such as VIP), which reduces the release of the excitatory neurotransmitters, acetylcholine and tachykinins and opposes their effects on smooth muscle fibres. The release of NO is prevented by L-NAME, thus leading to facilitation of correolide-induced twitching. Alternatively, there might be a constitutive release of NO in the guinea-pig ileum, which would oppose the stimulatory action of correolide.
Capsaicin, at a concentration (1 μM) that causes stimulation of afferent C-fibres in the enteric nervous system, followed by depletion of their neurotransmitters (reviewed by Holzer, 1991), had no effect on the quantal concentration-response curve for correolide in guinea-pig ileum segments. Thus, it appears that the correolide-induced twitching does not require functional integrity of the afferent neurons in the enteric nervous system. There is also evidence that capsaicin-sensitive afferent neurons are not an integral part of the neural pathways subserving peristalsis in the guinea-pig small intestine (Bartho & Holzer, 1995).
Based on the specificity of MgTX and other peptidyl blockers as inhibitors of sub-types of Kv1, it was suggested (Suarez-Kurtz et al., 1999) that Kv1.1, but not Kv1.2, Kv1.3, and/or Kv1.6 channels in the enteric nervous system were the relevant targets for the peptide-induced smooth muscle twitching. The present results, especially the ability of correolide to induce additional increases in twitching amplitude and frequency in the presence of saturating concentrations of MgTX, are consistent with the idea that not only Kv1.1, but also other members of the Kv1 family are involved in the correolide-induced twitching in guinea-pig ileum. Correolide blocks, with similar potency, Kv1.1 and the other five members of the Kv1 family, which might underlie the ability of correolide, not shared by MgTX, to induce twitches partially resistant to blockade by atropine. It might be speculated that blockade by correolide of other Kv1 sub-types, such as Kv1.4 or Kv1.5 (channels that are resistant to inhibition by the peptides used in the previous study) leads to a relatively greater degree of stimulation of the enteric nervous system, as compared to MgTX.
Correolide-induced twitching was not observed in other guinea-pig smooth muscle tissues, namely duodenum, portal vein, urinary bladder or uterus, nor in ileum segments from rat or mouse. Thus, Kv1 channels seem to play a relatively minor functional role in excitation-contraction coupling in the smooth muscles examined in the present study, with the notable exception of the guinea-pig ileum (Suarez-Kurtz et al., 1999). The absence of twitching in rat and mouse ileum segments is consistent with differences in functional patterns of ileum activity between guinea-pig and other rodent species, which were already recognized in the early studies of Trendelenburg (1917).
Acknowledgments
G. Suarez-Kurtz is a Senior Investigator of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and work in his laboratory is supported by grants from CNPq, Fundação Ary Frauzino, Financiadora de Estudos e Projetos (FINEP), Ministério da Ciência e Tecnologia (Pronex) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). C.F. Oliveira was supported by a student scholarship from CNPq.
Abbreviations
- AgTX1
agitoxin1
- AgTX2
agitoxin2
- ATP
adenosine triphosphate
- α-DaTX
alpha-dendrotoxin
- KTX
kaliotoxin
- Kv1 channels
Shaker-type voltage-gated potassium channels
- L-NAME
NG-nitro-L-arginine methyl esther
- MgTX
margatoxin
- NK-1
neurokinin-1
- NK-2
neurokinin-2
- NKA
neurokinin A
- NKB
neurokinin B
- NO
nitric oxide
- TTX
tetrodotoxin
- VIP
vasoactive intestinal peptide
References
- BAKER R.K., BAO J.M., KAYSER F., PARSONS W.H., RUPPRECHT K. Merck & Co, Inc.: U.S.A.; 1998. In US Patent #5,363,478. [Google Scholar]
- BARTHO L., HOLZER P. The inhibitory modulation of guinea-pig intestinal peristalsis caused by capsaicin involves calcitonin gene-related peptide and nitric oxide. Naunyn-Schmiederberg's Arch. Pharmacol. 1995;353:102–109. doi: 10.1007/BF00168922. [DOI] [PubMed] [Google Scholar]
- BARTHO L., PETHO G., ANTAL A., HOLZER P., SZOLCSANYI J. Two types of relaxation due to capsaicin in the guinea-pig isolated ileum. Neurosci. Lett. 1987;81:146–150. doi: 10.1016/0304-3940(87)90355-7. [DOI] [PubMed] [Google Scholar]
- COSTA M., BROOKES S.J. The enteric nervous system. Am. J. Gastroenterol. 1994;89 Suppl.:S129–S137. [PubMed] [Google Scholar]
- CREST M., JACQUET G., GOLA M., ZERROUK H., BENSLIMANE A., ROCHAT H., MANSUELLE P., MARTIN-EUCLAIRE M.F. Kaliotoxin, a novel peptidyl inhibitor of neuronal BK-type Ca2+-activated K+ channels characterized from Androctonus mauretanicus venom. J. Biol. Chem. 1992;267:1640–1647. [PubMed] [Google Scholar]
- DUFTON M.J., HARVEY A.L. Dendrotoxins: How does structure determine function. J. Toxicol. 1998;17:161–182. [Google Scholar]
- FELIX J.P., BUGIANESI R.M., SCHMALHOFER W.A., BORRIS R., GOETZ M.A., HENSENS O.D., BAO J.M., KAYSER F., PARSONS W.H., RUPPRECHT K., GARCIA M.L., KACZOROWSKI G.J., SLAUGHTER R.S. Identification and biochemical characterization of a novel nortriterpene inhibitor of the human lymphocyte voltage-gated potassium channel, Kv1.3. Biochemistry. 1999;38:4922–4930. doi: 10.1021/bi982954w. [DOI] [PubMed] [Google Scholar]
- GARCIA M.L., GARCIA-CALVO M., HIDALGO P., LEE A., MAKINNON R. Purification and characterization of three inhibitors of voltage-dependent K+ channels from Leiurus quinquestriatus var. hebraeus venom. Biochemistry. 1994;33:6834–6839. doi: 10.1021/bi00188a012. [DOI] [PubMed] [Google Scholar]
- GARCIA M.L., HANNER M., KNAUS H.-G., KOCH R., SCHMALHOFER W., SLAUGHTER R.S., KACZOROWSKI G.J. Pharmacology of potassium channels. Adv. Pharmacol. 1997;39:425–471. doi: 10.1016/s1054-3589(08)60078-2. [DOI] [PubMed] [Google Scholar]
- GARCIA-CALVO M., LEONARD R.J., NOVICK J., STEVEN S.P., SCHMALHOFER W., KACZOROWSKI G.J., GARCIA M.L. Purification, characterization, and bio-synthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibts voltage-dependent potassium channels. J. Biol. Chem. 1993;268:18866–18874. [PubMed] [Google Scholar]
- GOETZ M.A., HENSENS O.D., ZINK D.L., BORRIS R.P., MORALES F., TAMAYO-CASTILLO G., SLAUGHTER R.S., FELIX J., BALL R.G. Potent nor-triterpenoid blockers of the voltage-gated potassium channel Kv1.3 from Spachea correae. Tet. Lett. 1998;39:2895–2898. [Google Scholar]
- GOYAL R.K., HIRANO I. The enteric nervous system. N. Engl. J. Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- HALL J.M., FLOWERS J.M., MORTON I.K.M. A pharmacological study of NK1 and NK2 tachykinin receptor characteristics in the rat isolated urinary bladder. Br. J. Pharmacol. 1992;107:777–784. doi: 10.1111/j.1476-5381.1992.tb14523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBEISS K., KILBINGER H. Differential effects of nitric oxide donors on basal and electrically evoked release of acetylcholine from guinea-pig myenteric neurones. Br. J. Pharmacol. 1996;118:2073–2078. doi: 10.1111/j.1476-5381.1996.tb15646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBEISS K., KILBINGER H. Nitric oxide-sensitive guanylyl cyclase inhibits acetylcholine release and excitatory motor transmission in the guinea-pig ileum. Neuroscience. 1998;82:623–629. doi: 10.1016/s0306-4522(97)00308-4. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanisms of actions and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- LEFEBVRE R.A. Nitric oxide in the peripheral nervous system. Ann. Med. 1995;27:379–388. doi: 10.3109/07853899509002591. [DOI] [PubMed] [Google Scholar]
- MCELROY A.B., CLEGG S.P., DEAL M.J., EWAN G.B., HAGAN R.M., IRELAND S.J., JORDAN C.C., PORTER B., ROSS B.C., WARD P., WHITTINGTON A.R. Highly potent and selective heptapeptide antagonists of the neurokin NK2 receptor. J. Med. Chem. 1992;35:2582–2591. doi: 10.1021/jm00092a008. [DOI] [PubMed] [Google Scholar]
- PONTE C.G., ESTRELA R.C.E., SUAREZ-KURTZ G. Modulation of the Ca2+ release channel of sarcoplasmic reticulum by amiloride analogs. Eur. J. Pharmacol. 2000;391:11–20. doi: 10.1016/s0014-2999(00)00058-3. [DOI] [PubMed] [Google Scholar]
- ROBERTSON B. The real life of voltage-gated K+ channels: more than model behavior. Trends Pharmacol. Sci. 1997;18:474–483. doi: 10.1016/s0165-6147(97)01140-1. [DOI] [PubMed] [Google Scholar]
- SUAREZ-KURTZ G., GARCIA M.L., KACZOROWSKI G.J. Effects of charybdotoxin and iberiotoxin on the spontaneous motility and tonus of different guinea-pig smooth muscle tissues. J. Pharmacol. Exp. Ther. 1991;259:439–443. [PubMed] [Google Scholar]
- SUAREZ-KURTZ G., VIANNA-JORGE R., PEREIRA B.F., GARCIA M.L., KACZOROWSKI G.J. Peptidyl blockers of Shaker-type Kv1 channels elicit twitches in guinea-pig ileum by blocking Kv1.1 at the enteric nervous system and enhancing acetylcholine release. J. Pharmacol. Exp. Ther. 1999;289:1517–1522. [PubMed] [Google Scholar]
- SURPRENANT A. Control of the gastrointestinal track by enteric neurons. Annu. Rev. Physiol. 1994;56:117–140. doi: 10.1146/annurev.ph.56.030194.001001. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG P. Physiologische Versuche über die Dünndarmperistaltik. Arch. exp. Path. Pharmak. 1917;81:55–129. doi: 10.1007/s00210-006-0052-7. [DOI] [PubMed] [Google Scholar]
- WIKLUND C.U., OLGART C., WILKLUND N.P., GUSTAFSSON L.E. Modulation of cholinergic and substance P-like neurotransmission by nitric oxide in the guinea-pig ileum. Br. J. Pharmacol. 1993;110:833–839. doi: 10.1111/j.1476-5381.1993.tb13888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


