Abstract
We previously reported activation of an inhibitory adrenergic and a non-adrenergic non-cholinergic (NANC) pathway during abdominal surgery relaxing the rat gastric fundus. In the present study, we investigated the possible role of nitric oxide (NO) and vasoactive intestinal polypeptide (VIP) in the NANC part of the surgery-induced fundic relaxation.
The effect of the NO biosynthesis inhibitor NG-nitro-L-arginine (L-NOARG), the non-selective VIP receptor antagonist [D-p-Cl-Phe6,Leu17]-VIP and the selective VIP1 receptor antagonist [Acetyl-His1,D-Phe2,Lys15,Arg16,Leu17]-VIP was investigated on the non-adrenergic fundic relaxation induced by manipulation of the small intestine followed by resection of the caecum.
Guanethidine partly reduced the manipulation-induced fundic relaxation. Addition of L-NOARG reduced this non-adrenergic component, whereas the non-selective VIP receptor antagonist had no significant effect. Combination of L-NOARG and the non-selective VIP antagonist however further reduced the relaxation to manipulation.
The selective VIP1 receptor antagonist reduced the mean and maximal relaxation induced by abdominal surgery in the presence of guanethidine. When combined with L-NOARG, the relaxation of the gastric fundus was almost completely abolished. The VIP1 receptor antagonist alone had no significant effect on the mean and maximal relaxation, but enhanced recovery of fundic tone.
In conclusion, as VIP1 receptors are not present in the rat gastric fundus, these results suggest that the NANC inhibitory pathway activated during abdominal surgery involves VIP1 receptors, most likely in the afferent limb. The inhibitory neurotransmitters released at the level of the gastric fundus smooth muscle are NO and a substance different from VIP.
Keywords: Gastric fundus, gastrointestinal motility, ileus, nitric oxide, non-adrenergic non-cholinergic, vasoactive intestinal polypeptide
Introduction
Postoperative ileus is an iatrogenic condition, which complicates every abdominal surgical procedure and causes significant morbidity and prolonged hospitalization. Animal studies have clearly shown that laparotomy and handling of the intestines relaxes the stomach (Abrahamsson et al., 1979; Glise & Abrahamsson, 1980; Holzer et al., 1992; Zittel et al., 1994) and delays gastric emptying (Barquist et al., 1996; Junghardrquist et al., 1992; Plourde et al., 1993; Tache et al., 1991) mainly by presynaptic inhibition of cholinergic neurotransmission mediated by spinal adrenergic reflex pathways (Glise et al., 1980). Blockade of adrenergic neurotransmission with guanethidine, 6-hydroxydopamine or adrenergic receptor antagonists indeed partly prevent or reduce surgery-induced gastric retention of dye or gastric relaxation (De Winter et al., 1997; Dubois et al., 1973; Holzer et al., 1986; Sagrada et al., 1987). However this blockade is not complete, suggesting that other non-adrenergic pathways should be involved.
At present, it is becoming increasingly clear that more intense and nociceptive stimuli will activate an additional non-adrenergic non-cholinergic (NANC) inhibitory pathway. For example, in the cat, mechanical handling of the intestine and surgical intervention resulted in gastric relaxation mediated by a vagal inhibitory pathway (Glise & Abrahamsson, 1980). In the rat, gastric reflex relaxation resistant to adrenergic and cholinergic blockade was also reported during colonic distension or pain stimulation (Bojo et al., 1993; Bojo & Cassuto, 1992). Recently, we showed similar findings illustrating that manipulation of the rat intestine followed by resection of the caecum triggered a gastric relaxation that was only partly reduced by adrenergic and cholinergic blockade (Boeckxstaens et al., 1999). This NANC component was blocked by vagotomy illustrating that this reflex relaxation resulted from vagally mediated activation of inhibitory NANC nerves.
Nitric oxide (NO) and vasoactive intestinal polypeptide (VIP) are considered as the main inhibitory NANC neurotransmitters in the stomach. Abundant evidence has been reported illustrating that NO mediates NANC relaxation of the stomach. In vitro, inhibitors of NO synthase reduce nerve-induced relaxation (Boeckxstaens et al., 1992; D'Amato et al., 1992a; Li & Rand, 1990) and in addition, NO is released during NANC nerve stimulation (Boeckxstaens et al., 1991). Also in vivo, gastric relaxation to distension or to duodenal lipid infusion is blocked by NO inhibitors (Desai et al., 1991; Meulemans & Schuurkes, 1995). Similarly, VIP plays an important role in relaxing the stomach. VIP receptor antagonists or antiserum to VIP reduce NANC nerve-mediated relaxation (D'Amato et al., 1988a; De Beurme & Lefebvre, 1988). Furthermore, VIP is released during nerve stimulation (D'Amato et al., 1992b). VIP receptors can be subdivided into VIP1/PACAP (VIP1) receptors and VIP2/PACAP (VIP2) receptors.
Studies on the inhibitory NANC neurotransmitters released during surgery are rather scarce. Recently, improvement of postoperative small bowel transit in the rat was reported after treatment with a non-selective VIP receptor antagonist (Espat et al., 1995). In addition, we reported enhancement of intestinal transit by NO synthase inhibition (De Winter et al., 1997) and by a specific VIP1 receptor antagonist suggesting the involvement of NO and VIP1 receptors in surgery-induced ileus (De Winter et al., 1998). Measurement of intestinal transit however does not allow determining whether gastric and/or small intestinal motility is affected, therefore the role of NO and VIP in reducing gastric motility following surgery remains to be determined. Furthermore, VIP receptors are abundantly present both on afferent and efferent pathways, and thus the exact site of action of the VIP receptor antagonists in vivo is unclear. Previous studies in the rat have shown that VIP2 receptor mRNA is abundantly present in the stomach whereas VIP1 receptor mRNA is mostly present in the small intestine (Usdin et al., 1994). Based on this knowledge, we anticipated that if VIP receptor antagonism would enhance gastric motility, the site of action might be clarified using the selective VIP1-receptor antagonist [Acetyl-His1,D-Phe2,Lys15,Arg16, Leu17]-VIP. Therefore, we studied the effect of the non-selective VIP receptor antagonist [D-p-Cl-Phe6,Leu17]-VIP and the selective VIP1-receptor antagonist [Acetyl-His1,D-Phe2, Lys15,Arg16,Leu17]-VIP on manipulation-induced NANC relaxation of the rat proximal stomach. In addition, the effect of NO biosynthesis inhibition was investigated.
Methods
Animals
Male Sprague Dawley rats (250–350 g) were fasted overnight but had free access to water. They were initially anaesthetized with i.p. urethane (0.55 g) to place the i.v. lines.
Experimental protocol
Adequate air passage was ensured by a tracheal cannula. Thereafter, anaesthesia was continued by i.v. urethane (0.3–0.7 g per rat). Cannulas were placed in the tail vein, jugular vein and the carotid artery. The tail cannula was used for continuous infusions of saline and administration of drugs. The jugular vein was used for intravenous administration of sodium nitroprusside (SNP; 50 μg kg−1 body weight). Blood pressure was continuously monitored via the carotid cannula. A non-compliant balloon was orally introduced into the stomach, positioned in the gastric fundus and inflated with 3 ml of air to obtain an intra-balloon pressure of 5 mmHg (Boeckxstaens et al., 1999). Blood pressure and intra-balloon pressure were measured by a pressure transducer. Signals were digitized and stored on a computer for further analysis using commercially available software (Poly Physiological Analysis Package, Inspector Research Systems BV Amsterdam, The Netherlands). Temperature was kept constant between 36.0 and 37.0°C using a heating pad.
After placement of the cannulas and inflation of the balloon, the animals were allowed to recover during 45 min. Infusion of saline (1 ml h−1) was started to avoid dehydration. Before the protocol, basal fundic pressure was assessed by an i.v. bolus injection of SNP (50 μg kg−1). This dose results in a maximal relaxation abolishing the basal fundic pressure to post-mortem level. Thereafter, the effect of a median skin incision (SI) of 5 cm on fundic pressure was evaluated, followed after 10 min by a median laparotomy (LT). The opened abdomen was covered with moisturized gauze and 20 min later, the small intestine and the caecum were exteriorized and manipulated during 5 min. Immediately thereafter, a silk threat was tightened around the neck of the caecum and the caecum was excised without interruption of gut continuity. This procedure lasted approximately 1 min. The intestines were then returned in the abdominal cavity and the linea alba and the skin were closed separately. Fundic pressure was recorded during and after the above described surgical procedures until 90 min after the start of the manipulation. Between the different surgical procedures, a bolus of sodium nitroprusside was given to determine the level of 100% relaxation. The effect of guanethidine, NG-nitro-L-arginine (L-NOARG) and the specific and non-specific VIP receptor antagonists was studied on the manipulation-induced relaxation. Guanethidine was added as a bolus of 1 ml (6 mg kg−1 body weight). L-NOARG was injected as a bolus (5 mg kg−1 body weight in 1 ml) followed by a maintenance infusion (5 mg kg−1 h−1). The non-selective VIP antagonist (350 μg) and the specific VIPI receptor antagonist (5 μg) were administered i.v. over a 15 min period starting 5 min before manipulation of the small intestine.
After the experiment, animals were killed by an overdose of nembutal after which the position of the balloon was checked. In addition, the post-mortem fundic pressure was checked confirming that complete relaxation was obtained with SNP. Study protocols were approved by the Animal Ethical Committee of the Academic Medical Center.
Drugs
The effect of the following drugs was evaluated: guanethidine monosulphate, L-NOARG, sodium nitroprusside, the non-selective VIP receptor antagonist [D-p-Cl-Phe6,Leu17]-VIP (Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands) and the selective VIP1-receptor antagonist [Acetyl-His1,D-Phe2, Lys15,Arg16,Leu17]-VIP (gift from Prof Dr P. Robberecht, Universite Libre de Bruxelles, Brussels, Belgium).
Data analysis and statistics
The degree of relaxation is referred to the lowest level (end-expiratory) of intra-balloon pressure following an i.v. bolus injection of 50 μg kg−1 SNP. This dosage of SNP induces maximal relaxation of the gastric fundus similar to the post-mortem intragastric level. As such, SNP-induced relaxation was used throughout the experiment to estimate that part of the intra-balloon pressure resulting from active tonic contraction of the fundus. Fundic relaxation induced by manipulation and caecum resection was measured at several time points (0, 0.5, 1, 2, 4, 6, 8, 10, 15, 20, 25, 30, 45, 60, 75 and 90 min after the start of the manipulation) and is expressed as percentage of the relaxation to bolus injection of SNP prior to the experiment (=basal fundic pressure). In addition, the maximal relaxation, irrespective of its timing relative to the onset of the manipulation, was determined.
Statistical analysis was performed with a repeated measurement analysis of variance. Specifically a linear mixed model was used with time and treatment as factors allowing for random variation between animals and an auto-regressive within animal error structure. Discrimination between models was done using BIC (Bayesian information criterion) following testing between treatments using the Student t-test statistic. We tested a model without treatment, a model with treatment added and a model with time and treatment interaction. Using BIC, the model with added treatment appeared to be the best. This means that all effects can be described independent of time. Using this model, the mean relaxation over the entire 90 min post-manipulation period was calculated for each experiment (Figures 2 and 5). This parameter, comparable to calculation of the total area under the curve, thus represents the averaged degree of relaxation, not at a certain time point, but for the entire 90 min period. In contrast, the data shown in Figures 1 and 4 are not calculated by the model but represent the mean at each individual time point calculated from the percentage relaxation measured at that particular point in time.
Figure 2.
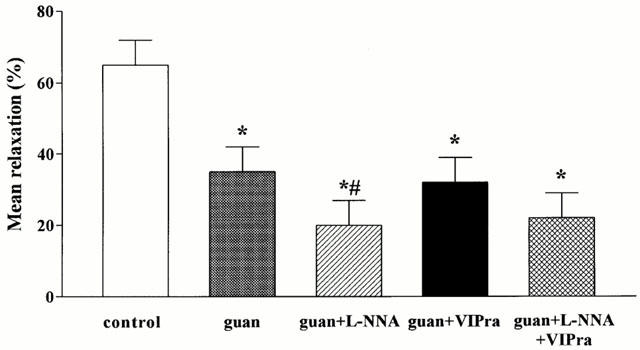
Effect of guanethidine (guan), guanethidine+L-NNA, guanethidine+the non-selective VIP receptor antagonist (VIPra) and guanethidine+L-NNA+the non-selective VIP receptor antagonist on the mean relaxation induced by manipulation of the small intestine and caecum resection. Results are expressed as per cent of the SNP-induced relaxation and are shown as mean±s.e.mean for n=3–7 animals. *P<0.05, different from control, Student's t-test; #P<0.05, different from guanethidine, Student's t-test.
Figure 5.
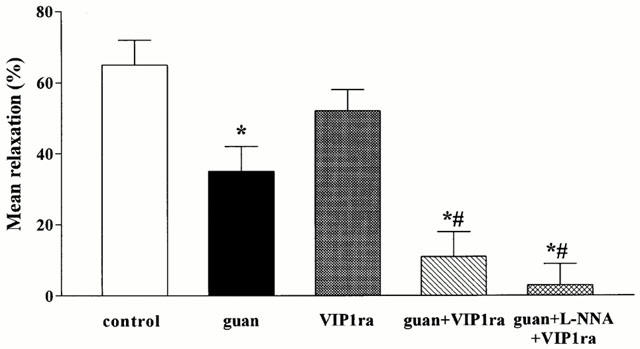
Effect of the selective VIP1 receptor antagonist (VIP1ra), guanethidine (guan), guanethidine+the selective VIP1 receptor antagonist and the combination of guanethidine+L-NNA+the selective VIP1 receptor antagonist on the mean relaxation induced by manipulation of the small intestine and caecum resection. Results are expressed as per cent of the SNP-induced relaxation and are shown as mean±s.e.mean for n=3–7 animals. *P<0.05, different from control, Student's t-test; #P<0.05, different from guanethidine, Student's t-test.
Figure 1.
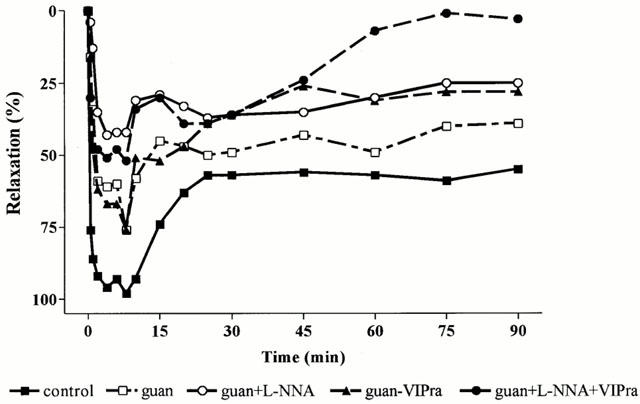
Effect of guanethidine (guan), guanethidine+L-NNA, guanethidine+the non-selective VIP receptor antagonist (VIPra) and guanethidine+L-NNA+the non-selective VIP receptor antagonist on the relaxation of the rat gastric fundus induced by manipulation of the small intestine followed by caecum resection. Results are expressed as per cent of the SNP-induced relaxation (n=5–7).
Figure 4.
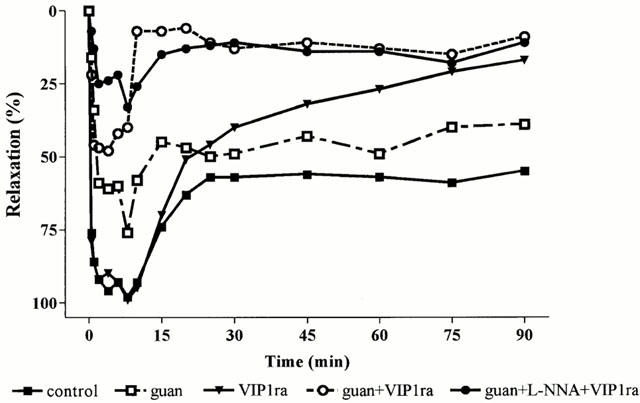
Effect of the selective VIP1 receptor antagonist (VIP1ra), guanethidine (guan), guanethidine+the selective VIP1 receptor antagonist and the combination of guanethidine+L-NNA+the selective VIP1 receptor antagonist on the fundic relaxation induced by manipulation of the small intestine and caecum resection. Results are expressed as per cent of the SNP-induced relaxation and are shown as mean for n=3–7 animals.
Results
Effect of guanethidine on the fundic relaxation induced by skin incision, laparotomy and manipulation
As previously described (Boeckxstaens et al., 1999), skin incision and laparotomy induced short lasting relaxations that were completely abolished by guanethidine. In contrast, manipulation of the small intestine followed by caecum resection resulted in a long lasting relaxation (Figure 1). Ninety minutes after the start of the manipulation the fundic pressure was not recovered and the fundus was still relaxed to 63±6% of the SNP-induced maximal relaxation (Figure 1). Guanethidine partly reduced this relaxation (Figure 1) resulting in a significant reduction of the mean relaxation, as determined by the curve fitting, from 65±7 to 35±7% (Figure 2). The maximal relaxation was reduced from 100±1 to 84±5%, not significantly different from control (Figure 3).
Figure 3.
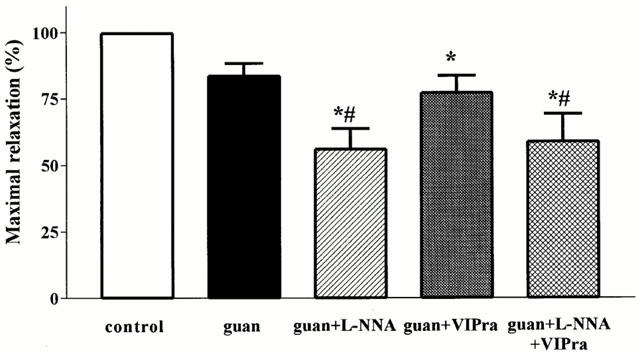
Effect of guanethidine (guan), guanethidine+L-NNA, guanethidine+the non-selective VIP receptor antagonist (VIPra) and guanethidine+L-NNA+the non-selective VIP receptor antagonist on the maximal relaxation induced by manipulation of the small intestine and caecum resection. Results are expressed as per cent of the SNP-induced relaxation and are shown as mean±s.e.mean for n=3–7 animals. *P<0.05, different from control, Student's t-test; #P<0.05, different from guanethidine, Student's t-test.
Effect of L-NOARG and the non-selective VIP receptor antagonist [D-p-Cl-Phe6,Leu17]-VIP on the guanethidine-resistant relaxation induced by manipulation
When guanethidine was combined with L-NOARG, the fundic relaxation induced by manipulation and caecum resection recovered faster, especially during the first 15 min (Figure 1). This resulted in a further reduction of the mean relaxation to 20±7% and of the maximum relaxation to 56±8%, significantly different from control and guanethidine (Figures 2 and 3).
When the non-selective VIP receptor antagonist was combined with guanethidine, no additional effect on the mean relaxation, the initial relaxation or the maximal relaxation was observed compared to guanethidine (Figures 1, 2 and 3).
Finally, when both L-NOARG and the non-selective VIP receptor antagonist were combined with guanethidine, the relaxation to manipulation was further reduced (Figure 1), especially the later part of the relaxation. Although not statistically significant, there was a tendency to better recovery of fundic tone after 45 min compared to guanethidine and the combination of guanethidine with L-NOARG. The mean relaxation and the maximal relaxation were however not significantly different from guanethidine (Figures 2 and 3).
Effect of the selective VIP1-receptor antagonist [Acetyl-His1,D-Phe2,Lys15,Arg16,Leu17]-VIP on the guanethidine-resistant relaxation induced by manipulation
In order to determine the type of VIP/PACAP receptor subtype involved, the effect of the selective VIP1 receptor antagonist was evaluated in the presence of guanethidine (Figure 4). Addition of the VIP1 receptor antagonist further reduced the mean fundic relaxation from 35±7 to 11±7% (Figure 5). The maximal relaxation was significantly reduced to 53±9% which is significantly different from control and guanethidine (Figure 6). Addition of L-NOARG further reduced the first part of the manipulation-induced relaxation (Figure 4) with reduction of the maximal relaxation to 36±9% (Figure 6). The mean relaxation was reduced to 3±6%, which is significantly different from guanethidine (Figure 5). Already after 15 min, fundic tone was almost completely recovered when the animals were treated with the VIP1 antagonist in combination with guanethidine or guanethidine and L-NOARG (Figure 4). When administered alone, the selective VIP1 receptor antagonist did not affect the mean relaxation, the first part of the manipulation-induced fundic relaxation or the maximal relaxation (Figures 4, 5 and 6). However, the fundic tone recovered better compared to control with only 16±11% relaxation remaining after 90 min (Figure 4).
Figure 6.
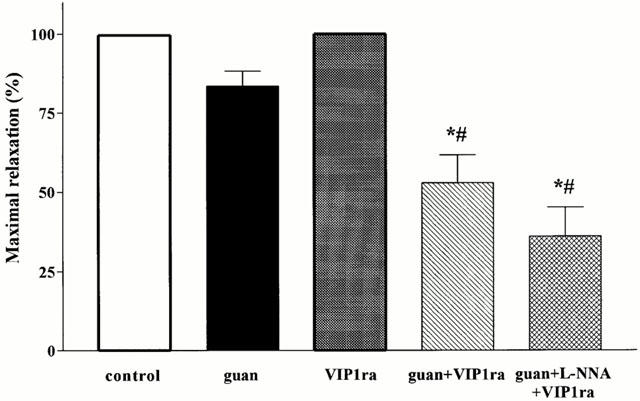
Effect of the selective VIP1 receptor antagonist (VIP1ra), guanethidine (guan), guanethidine+the selective VIP1 receptor antagonist and the combination of guanethidine+L-NNA+the selective VIP1 receptor antagonist on the maximum relaxation induced by manipulation of the small intestine and caecum resection. Results are expressed as per cent of the SNP-induced relaxation and are shown as mean+s.e.mean for n=3–7 animals *P<0.05, different from control, Student's t-test; #P<0.05, different from guanethidine, Student's t-test.
Discussion
We previously demonstrated that more intense nociceptive stimulation of the gut during manipulation relaxes the proximal stomach, mainly by a vagally-mediated NANC mechanism (Boeckxstaens et al., 1999). Here we confirm the importance of this mechanism and illustrate that NO is mainly involved in the initial part of the manipulation-induced relaxation. We further illustrate that VIP1 receptor antagonism potently enhances recovery of fundic tone, suggesting the involvement of VIP in the underlying neural pathway. As no VIP1 receptors have been demonstrated in the rat proximal stomach (Robberecht et al., 1998; Usdin et al., 1994), we hypothesize that most likely VIP1 receptors in the afferent pathway or in the central nervous system are activated during manipulation of the small intestine.
It is becoming increasingly clear that NANC nerves play an important role in the inhibition of gastrointestinal motility following nociceptive intestinal stimulation (Abrahamsson et al., 1979; Bojo & Cassuto, 1992; Glise et al., 1980; Glise & Abrahamsson, 1980). Previously, De Winter et al. (1997) showed that mechanical stimulation of the small intestine and caecum triggers a non-adrenergic pathway contributing to the delay in postoperative small intestinal transit. In addition, we recently demonstrated that manipulation of the small intestine during abdominal surgery activated a NANC neural pathway relaxing the proximal stomach (Boeckxstaens et al., 1999). This relaxation lasted at least 30 min after the end of the mechanical stimulation. In the present study, we confirmed the presence of a guanethidine resistant component that lasted up to 90 min. These findings support the concept that NANC nerves are involved in the pathogenesis of postoperative ileus.
Although NO is one of the main neurotransmitters released by NANC nerves, evidence supporting a role in postoperative ileus is rather scarce. Recently, De Winter et al. (1997) demonstrated that NO biosynthesis blockade completely reversed the non-adrenergic component of the delay in small intestinal transit following abdominal surgery. In the present study, L-NOARG only had a minor effect on the guanethidine-resistant relaxation induced by small intestinal manipulation. Mainly the relaxation occurring during the manipulation of the small intestine and closure of the abdomen was further reduced. In contrast, blockade of NO biosynthesis had no additional effect on the component that persisted after closure of the abdomen. These findings may suggest that NO is mainly released during activation of nociceptive/mechanoreceptors inducing a short lasting relaxation whereas a second non-nitrergic neurotransmitter is mainly involved in the more prolonged component. Similar findings have been reported in vitro (Boeckxstaens et al., 1992; D'Amato et al., 1992a; Takahashi & Owyang, 1995). NANC nerve stimulation at high frequency and vagal nerve stimulation also results in a fast and a prolonged relaxation of the rat gastric fundus. Blockade of NO biosynthesis reduces the fast initial part of the relaxation but has no effect on the prolonged component.
In vitro studies provide strong evidence that the second neurotransmitter involved is VIP. VIP antiserum or receptor antagonists and trypsin indeed block the prolonged relaxation of the stomach evoked by NANC nerve or vagal nerve stimulation (D'Amato et al., 1988b; De Beurme & Lefebvre, 1988; Takahashi & Owyang, 1995). In our study, VIP receptor blockade also further reduced the guanethidine resistant and prolonged relaxation of the rat gastric fundus induced by intestinal manipulation. Interestingly, the effect of the VIP1 receptor antagonist was clearly more pronounced than that of the non-selective VIP receptor antagonist which may be explained by the higher affinity (Ki in the nM range) of the VIP1 receptor antagonist compared to the non-selective VIP antagonist (Gourlet et al., 1997). These findings suggest that VIP may be the second neurotransmitter and thus corroborate earlier findings suggesting involvement of VIP in postoperative ileus (De Winter et al., 1998; Espat et al., 1995). In these studies, delay in gastrointestinal transit following intestinal stimulation was indeed partly reversed by non-selective VIP receptor blockade. Using a selective VIP1 receptor antagonist, De Winter et al. (1998) hypothesized that manipulation of the small intestine increased VIP release by inhibitory neurons acting on VIP1 receptors. In our hands, the same VIP1 receptor antagonist indeed almost completely abolished the guanethidine-resistant part of the manipulation-induced relaxation. However, the rat stomach rather contains VIP2 receptor mRNA (Usdin et al., 1994) and lacks functional VIP1 receptors (Robberecht et al., 1998). Therefore, it is unlikely that the effect of VIP1 receptor blockade results from an effect at the smooth muscle. The exact nature of the non-nitrergic neurotransmitter released by the inhibitory NANC nerves in the stomach in response to abdominal surgery therefore still remains to be determined.
Using the current experimental set-up, it is impossible to exactly localize the VIP1 receptors in the reflex arch underlying the NANC relaxation, especially because VIP or its receptors are involved at several levels of neurotransmission. As VIP1 receptor mRNA is abundantly present in the small intestine (Usdin et al., 1994), VIP released during manipulation could first of all locally activate afferent fibres. On the other hand, VIP is involved in primary afferent pathways sending sensory information to the spinal cord (Kawatani & de Groat, 1991; Morgan et al., 1999) whereas VIP receptors have also been demonstrated in the vagal nerve (Lundberg et al., 1979). Finally, when VIP is administered in the brain stem, it affects rat gastric motor function via the vagal nerve (Krowicki et al., 1997). Although it is uncertain whether the VIP antagonists can pass the blood brain barrier, theoretically, the VIP receptor antagonists used in our study could thus interact with neurotransmission at each level. Additional in vitro studies are therefore necessary to further unravel the exact level of action of the VIP1 receptor antagonist.
In the absence of guanethidine, the VIP1 receptor antagonist failed to affect the initial part of the manipulation-induced relaxation but induced a faster recovery of fundic tone. As the addition of guanethidine resulted in an almost complete reversal of fundic tone, it seems that this non-VIP pathway is mainly if not completely adrenergic in nature. Therefore, we suggest that nociceptive stimulation of the small intestine triggers: (1) An adrenergic inhibitory pathway; and (2) A NANC inhibitory pathway. The NANC pathway involves VIP1 receptors, most likely in the afferent limb. The inhibitory neurotransmitters released at the level of the gastric fundus smooth muscle are NO and a substance different from VIP.
Based on our results, one might hypothesize that gastrointestinal hypomotility following abdominal surgery may be largely prevented by pharmacological blockade of these two pathways. It should be emphasized however that we only focused on the acute effect of abdominal surgery. Clearly, postoperative ileus lasts much longer than the 90 min period we studied and other mechanisms may be involved in this later phase. Recent evidence indeed clearly shows a pivotal role of inflammation induced by manipulation (Kalff et al., 1999a,1999b). Graded intestinal manipulation resulted in influx of inflammatory cells starting at 3 h and persisting beyond 24 h. Adhesion-blocking antibodies significantly prevented influx of these cells as well as concomitant dysmotility (Kalff et al., 1999b). To what extent the ‘early phase' or the inhibitory reflexes triggered by the manipulation of the gut are involved in the initiation of this inflammatory process still needs to be studied. It seems however to become clear that postoperative ileus consists of an early neural phase and a later inflammatory phase responsible for the observed dysmotility after abdominal surgery.
In conclusion, we confirmed that abdominal surgery activates both an adrenergic and a non-adrenergic inhibitory pathway acutely relaxing the gastric fundus. We provided evidence that the NANC pathway involves VIP1 receptors, most likely in the afferent limb. The inhibitory neurotransmitters released at the level of the gastric fundus smooth muscle are NO and a substance different from VIP.
Acknowledgments
The authors greatly acknowledge Prof Dr P. Robberecht (Univerite Libre de Bruxelles, Brussels, Belgium) for the generous supply of the selective VIP1 receptor antagonist [Acetyl-His1,D-Phe2,Lys15,Arg16, Leu17]-VIP. R.M.J.G.J. van den Wijngaard is supported by the Dutch Foundation for Scientific Research (NWO) (Grant no. 0803/903-50-242).
Abbreviations
- L-NOARG
NG-nitro-L-arginine
- NANC
non-adrenergic non-cholinergic
- NO
nitric oxide
- VIP
vasoactive intestinal polypeptide
References
- ABRAHAMSSON H., GLISE H., GLISE K. Reflex suppression of gastric motility during laparotomy and gastroduodenal nociceptive stimulation. Scand. J. Gastroenterol. 1979;14:101–106. doi: 10.3109/00365527909179853. [DOI] [PubMed] [Google Scholar]
- BARQUIST E., BONAZ B., MARTINEZ V., RIVIER J., ZINNER M.J., TACHE Y. Neuronal pathways involved in abdominal surgery-induced gastric ileus in rats. Am. J. Physiol. 1996;270:R888–R894. doi: 10.1152/ajpregu.1996.270.4.R888. [DOI] [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., HIRSCH D.P., KODDE A., MOOJEN T.M., BLACKSHAW A., TYTGAT G.N.J., BLOMMAART P.J.E. Activation of an adrenergic and vagally-mediated NANC pathway in surgery-induced fundic relaxation in the rat. Neurogastroenterol. Motil. 1999;11:467–474. doi: 10.1046/j.1365-2982.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., PELCKMANS P.A., BOGERS J.J., BULT H., DE MAN J.G.O.L., HERMAN A.G., VAN MAERCKE Y.M. Release of nitric oxide upon stimulation of nonadrenergic noncholinergic nerves in the rat gastric fundus. J. Pharmacol. Exp. Ther. 1991;256:441–447. [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., PELCKMANS P.A., DE MAN J.G., BULT H., HERMAN A.G., VANMAERCKE Y.M. Evidence for a differential release of nitric oxide and vasoactive intestinal polypeptide by nonadrenergic noncholinergic nerves in the rat gastric fundus. Arch. Int. Pharmacodyn. Ther. 1992;318:107–115. [PubMed] [Google Scholar]
- BOJO L., CASSUTO J. Gastric reflex relaxation by colonic distension. J. Auton. Nerv. Syst. 1992;38:57–64. doi: 10.1016/0165-1838(92)90216-4. [DOI] [PubMed] [Google Scholar]
- BOJO L., LEFEBVRE R.A., NELLGARD P., CASSUTO J. Involvement of vasoactive intestinal polypeptide in gastric reflex relaxation. Eur. J. Pharmacol. 1993;236:443–448. doi: 10.1016/0014-2999(93)90483-x. [DOI] [PubMed] [Google Scholar]
- D'AMATO M., CURRO D., MONTUSCHI P. Evidence for dual components in the non-adrenergic non-cholinergic relaxation in the rat gastric fundus: role of endogenous nitric oxide and vasoactive intestinal polypeptide. J. Auton. Nerv. Syst. 1992a;37:175–186. doi: 10.1016/0165-1838(92)90039-j. [DOI] [PubMed] [Google Scholar]
- D'AMATO M., CURRO D., MONTUSCHI P., CIABATTONI G., RAGAZZONI E., LEFEBVRE R.A. Release of vasoactive intestinal polypeptide from the rat gastric fundus. Br. J. Pharmacol. 1992b;105:691–695. doi: 10.1111/j.1476-5381.1992.tb09040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'AMATO M., DE BEURME F.A., LEFEBVRE R.A. Comparison of the effect of vasoactive intestinal polypeptide and non-adrenergic non-cholinergic neurone stimulation in the cat gastric fundus. Eur. J. Pharmacol. 1988b;152:71–82. doi: 10.1016/0014-2999(88)90837-0. [DOI] [PubMed] [Google Scholar]
- D'AMATO M., DE BEURME F.A., LEFEBVRE R.A. Influence of VIP antiserum and VIP antagonists on the effect of VIP and of non-adrenergic non-cholinergic neurone stimulation in the cat gastric fundus. Pharmacol. Res. Comm. 1988a;20:421–422. doi: 10.1016/s0031-6989(88)80023-7. [DOI] [PubMed] [Google Scholar]
- DE BEURME F.A., LEFEBVRE R.A. Vasoactive intestinal polypeptide as possible mediator of relaxation in the rat gastric fundus. J. Pharm. Pharmacol. 1988;40:711–715. doi: 10.1111/j.2042-7158.1988.tb07000.x. [DOI] [PubMed] [Google Scholar]
- DESAI K.M., SESSA W.C., VANE J.R. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991;351:477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- DE WINTER B.Y., BOECKXSTAENS G.E., DE MAN J.G., MOREELS T.G., HERMAN A.G., PELCKMANS P.A. Effect of adrenergic and nitrergic blockade on experimental ileus in rats. Br. J. Pharmacol. 1997;120:464–468. doi: 10.1038/sj.bjp.0700913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE WINTER B.Y., ROBBERECHT P., BOECKXSTAENS G.E., DE MAN J.G., MOREELS T.G., HERMAN A.G., PELCKMANS P.A. Role of VIP1/PACAP receptors in postoperative ileus in rats. Br. J. Pharmacol. 1998;124:1181–1186. doi: 10.1038/sj.bjp.0701954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOIS A., WEISE V.K., KOPIN I.J. Postoperative ileus in the rat: physiopathology, etiology and treatment. Ann. Surg. 1973;178:781–786. doi: 10.1097/00000658-197312000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESPAT N.J., CHENG G., KELLEY M.C., VOGEL S.B., SNINSKY C.A., HOCKING M.P. Vasoactive intestinal peptide and substance P receptor antagonists improve postoperative ileus. J. Surg. Res. 1995;58:719–723. doi: 10.1006/jsre.1995.1113. [DOI] [PubMed] [Google Scholar]
- GLISE H., ABRAHAMSSON H. Reflex vagal inhibition of gastric motility by intestinal nociceptive stimulation in the cat. Scand. J. Gastroenterol. 1980;15:769–774. doi: 10.3109/00365528009181528. [DOI] [PubMed] [Google Scholar]
- GLISE H., LINDAHL B.-O., ABRAHAMSSON H. Reflex adrenergic inhibition of gastric motility by nociceptive intestinal stimulation and peritoneal irritation in the cat. Scand. J. Gastroenterol. 1980;15:673–681. doi: 10.3109/00365528009181514. [DOI] [PubMed] [Google Scholar]
- GOURLET P., DE NEEF P., CNUDDE J., WAELBROECK M., ROBBERECHT P. In vitro properties of a high affinity selective antagonist of the VIP1 receptor. Peptides. 1997;18:1555–1560. doi: 10.1016/s0196-9781(97)00230-1. [DOI] [PubMed] [Google Scholar]
- HOLZER P., LIPPE I.T.H., AMANN R. Participation of capsaicin-sensitive afferent neurons in gastric motor inhibition caused by laparotomy and intraperitoneal acid. Neuroscience. 1992;48:715–722. doi: 10.1016/0306-4522(92)90414-w. [DOI] [PubMed] [Google Scholar]
- HOLZER P., LIPPE I.T., HOLZER PETSCHE U. Inhibition of gastrointestinal transit due to surgical trauma or peritoneal irritation is reduced in capsaicin-treated rats. Gastroenterology. 1986;91:360–363. doi: 10.1016/0016-5085(86)90569-x. [DOI] [PubMed] [Google Scholar]
- JUNGHARDRQUIST E., ZINNER M., RIVIER J., TACHE Y. Abdominal surgery-induced delayed gastric emptying in rats: role of CRF and sensory neurons. Am. J. Physiol. 1992;262:G616–G620. doi: 10.1152/ajpgi.1992.262.4.G616. [DOI] [PubMed] [Google Scholar]
- KALFF J.C., BUCHHOLZ B.M., ESKANDARI M.K., HIERHOLZER C., SCHRAUT W.H., SIMMONS R.L., BAUER A.J. Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat. Surgery. 1999a;126:498–509. [PubMed] [Google Scholar]
- KALFF J.C., CARLOS T.M., SCHRAUT W.H., BILLIAR T.R., SIMMONS R.L., BAUER A.J. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology. 1999b;117:378–387. doi: 10.1053/gast.1999.0029900378. [DOI] [PubMed] [Google Scholar]
- KAWATANI M., DE GROAT W.C. A large proportion of afferent neurons innervating the uterine cervix of the cat contain VIP and other neuropeptides. Cell Tissue Res. 1991;266:191–196. doi: 10.1007/BF00678724. [DOI] [PubMed] [Google Scholar]
- KROWICKI Z.K., NATHAN N.A., HORNBY P.J. Opposing effects of vasoactive intestinal polypeptide on gastric motor function in the dorsal vagal complex and the nucleus raphe obscurus of the rat. J. Pharmacol. Exp. Ther. 1997;282:14–22. [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Nitric oxide and vasoactive intestinal polypeptide mediate non- adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur. J. Pharmacol. 1990;191:303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M., HOKFELT T., KEWENTER J., PETTERSSON G., AHLMAN H., EDIN R., DAHLSTROM A., NILSSON G., TERENIUS L., UVNAS-WALLENSTEN K., SAID S. Substance P-, VIP-, and enkephalin-like immunoreactivity in the human vagus nerve. Gastroenterology. 1979;77:468–471. [PubMed] [Google Scholar]
- MEULEMANS A., SCHUURKES J. Intralipid-induced gastric relaxation is mediated via NO. Neurogastroenterol. Mot. 1995;7:151–155. doi: 10.1111/j.1365-2982.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- MORGAN C.W., OHARA P.T., SCOTT D.E. Vasoactive intestinal polypeptide in sacral primary sensory pathways in the cat. J. Comp. Neurol. 1999;407:381–394. doi: 10.1002/(sici)1096-9861(19990510)407:3<381::aid-cne6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- PLOURDE V., WONG H.C., WALSH J.H., RAYBOULD H.E., TACHE Y. CGRP antagonists and capsaicin on celiac ganglia partly prevent postoperative gastric ileus. Peptides. 1993;14:1225–1229. doi: 10.1016/0196-9781(93)90180-o. [DOI] [PubMed] [Google Scholar]
- ROBBERECHT P., DE NEEF P., LEFEBVRE R.A. Influence of selective VIP receptor agonists in the rat gastric fundus. Eur. J. Pharmacol. 1998;359:77–80. doi: 10.1016/s0014-2999(98)00662-1. [DOI] [PubMed] [Google Scholar]
- SAGRADA A., FARGEAS M.J., BUENO L. Involvement of alpha-1 and alpha-2 adrenoceptors in the postlaparotomy intestinal motor disturbances in the rat. Gut. 1987;28:955–959. doi: 10.1136/gut.28.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TACHE Y., BARQUIST E., STEPHENS R.L., RIVIER J. Abdominal surgery-and trephination-induced delay in gastric emptying is prevented by intracisternal injection of CRF antagonist in the rat. J. Gastrointest. Mot. 1991;3:19–25. [Google Scholar]
- TAKAHASHI T., OWYANG C. Vagal control of nitric oxide and vasoactive intestinal polypeptide release in the regulation of gastric relaxation in rat. J. Physiol. 1995;484:481–492. doi: 10.1113/jphysiol.1995.sp020680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDIN T.B., BONNER T.I., MEZEY E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135:2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- ZITTEL T.T., REDDY S.N., PLOURDE V., RAYBOULD H.E. Role of spinal afferents and calcitonin gene-related peptide in the postoperative gastric ileus in anesthetized rats. Ann. Surg. 1994;219:79–87. doi: 10.1097/00000658-199401000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]


