Abstract
Animal and epidemiological studies suggest that polyphenol constituents of red wine possess antioxidant activities that favour protection against cardiovascular disease – the so-called. ‘French paradox' – and possibly, central nervous system disorders such as Alzheimer's disease (AD) and ischaemia.
In the present study, the potential of three major red wine derived-polyphenols to protect against toxicity induced by the nitric oxide free radical donors sodium nitroprusside (SNP) and 3-morpholinosydnonimine (SIN-1) was examined in cultured rat hippocampal cells.
Both co- and post-treatments with either the stilbene resveratrol (5–25 μM) or the flavonoids quercetin (5–25 μM) and (+)-catechin (1–10 μM) were capable of attenuating hippocampal cell death and intracellular reactive oxygen species accumulation produced by SNP (100 μM and 1 mM, respectively). However, among the phenolic compounds tested, only the flavonoids afforded significant protection against 5 mM SIN-1-induced toxicity.
The effects of phenolic constituents were shared by Trolox (100 μM), a vitamin E analogue, but not by selective inhibitors of cyclo-oxygenases (COX) and lipoxygenases (LOX).
Among the phenolic compounds tested, only quercetin (10 μM) inhibited 100 μM SNP-stimulated protein kinase C (PKC) activation, whereas none of them were able to attenuate nitrite accumulation caused by SNP (100 μM).
Taken together, these data suggest that the neuroprotective abilities of quercetin, resveratrol, and (+)-catechin result from their antioxidant properties rather than their purported inhibitory effects on intracellular enzymes such as COX, LOX, or nitric oxide synthase. Quercetin, however, may also act via PKC to produce its protective effects.
Keywords: Neuroprotection, red wine, resveratrol, quercetin, (+)-catechin, nitric oxide, cyclo-oxygenase, lipoxygenase, protein kinase C
Introduction
The notion that red wine may have potential health benefits initially received a great deal of attention following reports that moderate wine consumption was linked to a lower incidence of cardiovascular disease – the so-called ‘French Paradox' (Renaud & De Lorgevil, 1992). A role for ethanol itself in the protective effects of red wine is however uncertain (for a review see Soleas et al., 1997; VanGolde et al., 1999). In addition to ethanol, red wine contains a broad range of polyphenols that are present in the skin and seeds of grapes (Hertog et al., 1993; Goldberg et al., 1996; Celotti et al., 1996; Sato et al., 1997; Soleas et al., 1997). Among them, the natural phytoalexin resveratrol (3,5,4′-trihydroxystilbene) and the flavonoids quercetin and (+)-catechin have been invoked in order to explain the beneficial effects of moderate red wine consumption against coronary heart disease (Formica & Regelson, 1995; Pace-Asciak et al., 1995; Andriambeloson et al., 1997; Hayek et al., 1997; Rotondo et al., 1998; Tsai et al., 1999). Possible mechanisms by which these phenolic compounds might exert their protective effects include their oxygen scavenging abilities and antioxidative properties (Belguendouz et al., 1997; Hayek et al., 1997; Plumb et al., 1998; Maccarronne et al., 1999) as well as their inhibitory effects on both the arachidonic acid cascade (Kimura et al., 1985; Formica & Regelson, 1995; Pace-Asciak et al., 1995; Maccarronne et al., 1999) and nitric oxide (NO) synthase activity (Kim et al., 1999; Tsai et al., 1999).
In addition to red wine's purported cardioprotective effects, recent evidence suggests that wine consumption may also protect against certain neurological disorders. For example, epidemiological studies have shown that moderate red wine consumption is significantly correlated with a reduction in both the incidence of age-related macular degeneration (Obisesan et al., 1998) and Alzheimer's disease (AD; Orgogozo et al., 1997). Furthermore, in vivo animal studies have demonstrated the protective effects of quercetin (Shutenko et al., 1999), resveratrol (Virgili & Contestabile, 2000), and (+)-catechin (Inanami et al., 1998) in various models of neurotoxicity. Taken together, these latest findings raise the possibility that red wine constituents may be beneficial in the prevention of age-related neurodegenerative disorders.
Considering that oxidative stress is an age-related process implicated in various neurodegenerative disorders (Floyd, 1999), we investigated the possible neuroprotective effects of the red wine constituents quercetin, resveratrol, and (+)-catechin on cell death induced by the well known nitric oxide (NO) free radical donors sodium nitroprusside (SNP) and 3-morpholinosydnonimine (SIN-1) (Dawson & Dawson, 1996). The cellular pathways that lead from the generation of NO to cell death include its reaction with superoxide anions to form the potent oxidant peroxynitrite (Dawson & Dawson, 1996), as well as NO's stimulatory effects on intracellular enzymes such as protein kinase C (PKC) (Maiese et al., 1993) and cyclo-oxygenase/lipoxygenase (Salvemini et al., 1993; Swierkosz et al., 1995, Holzhutter et al., 1997). The present experiments were performed in cultured cells of the hippocampus, an area severely affected in both AD and ischaemia (Mann et al., 1986; Schmidt-Kastner & Freund, 1991) and known to be particularly susceptible to oxidative stress (Furuta et al., 1995).
Methods
Reagents
Materials used for cell cultures were obtained from Gibco BRL (Burlington, Ontario, Canada). 2-(1-thienyl)ethyl 3,4-dihydroxybenzylidenecyanoacetate was purchased from Tocris (Ballwin, MO, U.S.A.), whereas MK-886 was obtained from Calbiochem (San Diego, CA, U.S.A.). Unless stated otherwise, all other compounds including quercetin, resveratrol, and (+)-catechin were purchased from Sigma Chemical Co. (St-Louis, MO, U.S.A.).
Hippocampal cell cultures
Mixed (glial/neuronal) hippocampal cultured cells were prepared from E19 fetuses obtained from Sprague-Dawley rats (Charles River Canada, St-Constant, Québec, Canada) as described previously (Bastianetto et al., 2000). Animal care was according to protocols and guidelines of the McGill University Animal Care Committee and the Canadian Council for Animal Care. Briefly, hippocampal cells were plated immediately after dissection at a density of about 5×104 viable cells per well in 96-well plates coated with poly-D-lysine (10 μg ml−1). Cells were grown in D-MEM high glucose medium containing 2 mM sodium pyruvate, 25 mM KCl, 15 mM HEPES and 10% (v v−1) foetal bovine serum (FBS, ImmunoCorp, Montréal, Québec, Canada). Mixed cell cultures were obtained by removing the original medium at day 3 and replacing it by medium of the same composition. Cultures were maintained at 37°C until day 7 in a humidified atmosphere (5% CO2 and 95% air).
Experimental treatments
On the day of the experiment, cells were co-treated for 20 h with either SNP (100 μM) or SIN-1 (5 mM) and the different test drugs that were dissolved in HEPES-buffered MEM high glucose medium without FBS (pH 7.4). Following this incubation period, cell viability was determined using both the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and 3-amino-7-dimethyl-amino-2-methylphenazine hydrochloride (Neutral Red, NR) colorimetric assays, whereas ROS production was evaluated using the 2′,7′ dichlorofluorescein (DCF) fluorescence assay.
The rescuing ability of the different compounds was determined by pre-treating hippocampal cells with a HEPES-buffered MEM high glucose medium containing SNP (1 mM) for a duration of 2 h. After the incubation period, the medium was removed and replaced with a similar one (without FBS and SNP) in the presence or absence of the different drugs tested. Cell viability (MTT and NR assays) and ROS production (DCF fluorescence assay) were determined 24 h later.
Resveratrol (10−1 M) was freshly dissolved on the day of the experiment in 100% DMSO whereas all other drugs were freshly prepared as 2.5×10−2 M stock solutions in 100% ethanol. These solvents had no effect by themselves on cell survival (data not shown).
Assessment of cell viability
Cell survival was evaluated 20 h later with the commonly used dye MTT, an indicator of mitochondrial respiratory chain activity (Liu et al., 1997). Because free radicals are able to alter the activity of mitochondrial enzymes that are involved in MTT reduction (Sims, 1996), we also used Neutral Red, a dye which is taken up by lysosomes of living cells, as an additional marker of hippocampal cell integrity (Bastianetto et al., 2000). MTT reduction and NR uptake into living cells were quantified at 570 nm and 540 nm, respectively, using a micro-plate reader (Bio-Tek Instruments® Inc., Ville St-Laurent, Québec, Canada).
Assessment of intracellular free radicals
Intracellular free radical levels were estimated in parallel to cell survival using the DCF fluorescent dye, as previously described (Mattson et al., 1995). Application of 2,7-dichlorofluorescein diacetate (25 μM; Molecular Probes Inc., Eugene, OR, U.S.A.) at the onset of experimental treatment results in a rapid conversion into 2′,7′-dichlorofluorescein which is then able to interact with intracellular free radicals to form the fluorescent dye DCF. DCF fluorescence was quantified at the end of the treatment period (excitation=485 nm, emission=530 nm) using a fluorescence multiwell plate reader.
Measurement of protein kinase C activity
The activity of protein kinase C (PKC) was assessed on 7 day-old mixed hippocampal cells using a PKC assay kit (Signa TECT™ PKC Assay System, Promega, Madison, WI, U.S.A.) according to the protocol described previously (Bastianetto et al., 2000). Briefly, cells were exposed for 5 min to either vehicle or SNP (100 μM, 5 min) in the presence or absence of 10 μM of each red wine constituent. Treated cells were immediately put on ice and rinsed with pre-cooled Hank's buffer. Pre-cooled extraction buffer was then added to each well and cells were scraped and homogenized in a pre-cooled dounce homogenizer. Cell lysates were incubated on ice for 20 min and centrifuged at 14,000×g for 10 min at 4°C to remove cellular debris. Resulting supernatant was used to determine the activity of PKC. Specific activity of PKC was obtained by subtracting the radioactivity of the reaction in the presence of 100 μM of a PKC peptide inhibitor (Promega) from that of the reaction conducted without the PKC peptide inhibitor. All results were presented as per cent of the activity from cells exposed to vehicle (control group).
Nitrite assay
Accumulation of nitrite (NO2−), an indicator of NO synthase activity, was measured in the culture medium by the Griess reaction using the Calbiochem NO colorimetric assay Kit (San Diego, CA, U.S.A.) adapted for a 96-well plate reader (Swierkosz et al., 1995). Hippocampal cells were exposed to SNP (100 μM) and either quercetin (10 μM), resveratrol (10 μM), or (+)-catechin (10 μM) in phenol red-free MEM. Concentrations of nitrite, the end-product of NO production, were quantified 20 h later by adding 100 μl of Griess reagent to 40 μl samples of cell culture medium according to the manufacturer's protocol. The optical density at 540 nm was measured with the micro-plate reader. Nitrite concentrations were calculated by comparison with the optical density of a nitrate standard prepared in culture medium. The detection limit for nitrite measurement in culture medium was 0.5 μM.
Statistical analysis
Survival of vehicle-treated control groups not exposed to either SNP or the different drugs alone was defined as 100%. A one-way ANOVA followed by Newman Keuls' multiple comparisons or unpaired Student's t-tests were used to compare control and treated groups, with P values <0.05 being considered statistically significant.
Results
Inhibitory effect of co-treatment with red wine constituents on SNP-induced toxicity and reactive oxygen species production
Treatment of hippocampal cell cultures with the NO donor SNP (100 μM) resulted in cell damage of between 41–50% (MTT values) and 39–57% (NR values) (Figure 1A–C). Both the MTT and NR assays indicated that quercetin (5–25 μM) strongly attenuated SNP-induced toxicity, producing a maximal effect on MTT and NR values at 25 μM (Figure 1A). This difference between the two assays is likely explained by the fact that the MTT values of groups treated with quercetin (25 μM) alone were significantly increased compared to vehicle-treated groups (100±1% (control) vs 130±4% (control+quercetin), P<0.01), whereas the NR values remained unchanged (100±2% (control) 95±5% (control+ quercetin)). Resveratrol (5–25 μM) was also able to increase cell survival, and this protective effect was significant at 5 μM and maximal at the highest (25 μM) concentration tested (Figure 1B). As for (+)-catechin (1–10 μM), it significantly protected hippocampal cells at a concentration of 1 μM and had a maximal protective effect at the highest concentration tested here (10 μM) (Figure 1C). The MTT values, but not the NR values, of control groups treated with either resveratrol or (+)-catechin were significantly higher compared to those of vehicle-treated control groups, and these effects were maximal at 5 μM of resveratrol (100±1% (control) vs 117±5% (control+resveratrol), P<0.01) and 10 μM of (+)-catechin (100±2% (control) vs 115±4% (control+(+)-catechin), P<0.01) (Figure 1B,C). However, none of these constituents induced a significant increase in the MTT values compared to untreated cells when they were administered directly into the initial media (i.e. MEM in presence of FBS and without SNP), indicating that this increase is not related to neutrophic actions but might be due instead to changes and/or washes of the medium (data not shown).
Figure 1.
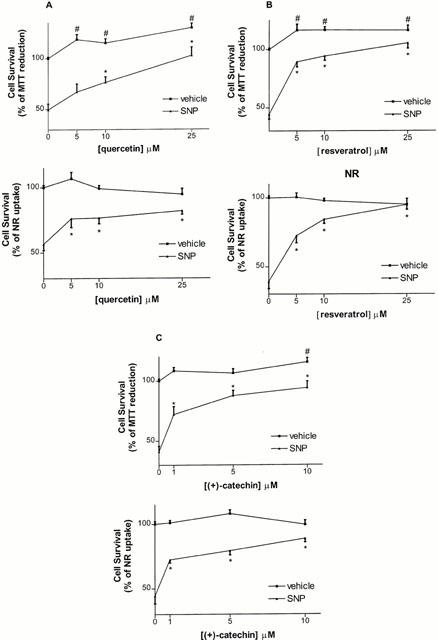
Effect of co-treatment with quercetin (5–25 μM) (A), resveratrol (5–25 μM) (B) and (+)-catechin (1–10 μM) (C) against toxicity induced by SNP (100 μM), as estimated by the MTT and NR assays in rat hippocampal cell cultures. Values represent mean±s.e.mean from 3–5 independent experiments. #P<0.01 compared to vehicle-treated groups, *P<0.01 compared to groups treated with SNP alone.
Protective concentration-dependent effects were also observed when the cells were treated with either quercetin (5–25 μM) or (+)-catechin (1–10 μM) in the presence of SIN-1 (5 mM), a NO donor that also produces superoxide anions (Figure 2A,C). These effects appeared to be maximal, and almost complete, at 10 μM (Figure 2A,C). In contrast, a one-way ANOVA indicated that resveratrol (5–25 μM) failed to dose-dependently protect against SIN-1-induced toxicity, although it tended to increase cell survival at the highest concentration tested (25 μM) (from 45 to 67% (MTT), from 41 to 59% (NR) (Figure 2B).
Figure 2.
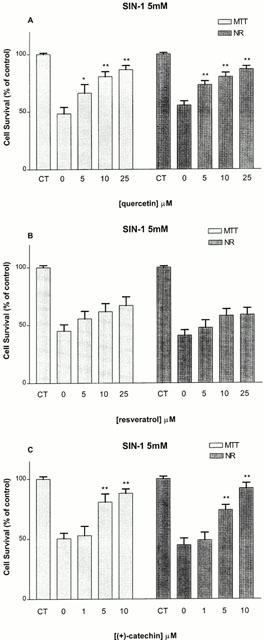
Effect of co-treatment with quercetin (5–25 μM) (A), resveratrol (5–25 μM) (B) and (+)-catechin (1–10 μM) (C) against toxicity induced by SIN-1 (5 mM), as estimated by the MTT and NR assays in rat hippocampal cell cultures. Values represent mean±s.e.mean from 3–4 independent experiments. *P<0.05, **P<0.01 compared to groups treated with SIN-1 alone.
We have previously reported that SNP-induced toxicity is associated with a stimulation of reactive oxygen species (ROS) (Bastianetto et al., 2000). As expected, the DCF assay indicated that SNP (100 μM) caused a significant increase (*P<0.01) in ROS accumulation (226–288% above control values) 20 h following addition to the culture medium (Figure 3A–C). This stimulatory effect of SNP on ROS production was significantly attenuated by either quercetin (10–25 μM), resveratrol (5–25 μM), or (+)-catechin (5–10 μM) in the same range of concentrations that protected hippocampal cells against SNP-induced toxicity (Figure 3A–C). The attenuating abilities of phenolic compounds were significant at 5 μM and maximal at the highest concentrations tested (10 μM or 25 μM) (Figure 3A–C). ROS accumulation in cell cultures treated with either quercetin (5 and 25 μM), resveratrol (25 μM), or (+)-catechin (1–10 μM) alone was also slightly, but significantly, decreased compared to vehicle-treated control groups, suggesting that these red wine constituents can lower ROS accumulation in the absence of any toxic-inducing stimulus (Figure 3A–C).
Figure 3.
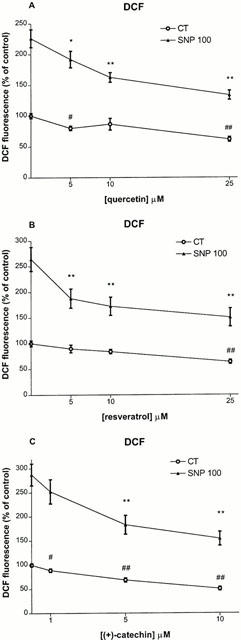
Effect of co-treatment with quercetin (5–25 μM) (A), resveratrol (5–25 μM) (B) and (+)-catechin (1–10 μM) (C) on either 100 μM SNP-induced intracellular ROS in rat hippocampal cell cultures. Phenolic compounds were applied at the onset of SNP exposure. ROS were determined 20 h after by quantification of DCF fluorescence as described in Methods. Values represent mean±s.e.mean from 3–5 independent experiments. #P<0.05, ##P<0.01 compared to groups treated with vehicle, *P<0.05, **P<0.01 compared to groups treated SNP.
The inhibitory effects of these red wine constituents against toxic events initiated by SNP were shared by Trolox (100 μM), a vitamin E analogue that displays antioxidant activities (42±9 (SNP) vs 96±3 (SNP+Trolox), P<0.01, (MTT); 38±2 (SNP) vs 96±2 (SNP+Trolox), P<0.01 (NR); 239±26 (SNP) vs 112±9 (SNP+Trolox), P<0.01, (DCF)).
Inhibitory effect of post-treatment with red wine constituents on SNP-induced toxicity and ROS production
Exposure of hippocampal cell cultures to SNP (1 mM) significantly reduced cell viability (80% of MTT values and 84% of NR values, P<0.01 and 0.05, respectively) when measured 2 h following application. When cell survival was assessed 24 h later, the MTT and NR values had decreased to 31–56% and 33–58%, respectively, and were associated with an increase in ROS production (148–178% above control values).
We evaluated whether post-treatment with the different red wine constituents were capable of protecting hippocampal cells that were pre-exposed to SNP for a 2 h period. Interestingly, application of quercetin (5–10 μM) resulted in an increase in MTT (from 43 to 65%) and NR values (from 43 to 74%) compared to control, as estimated 24 h later (Figure 4A). Resveratrol (5–10 μM) also potently rescued hippocampal cells, with a maximal effect at the highest concentration tested (10 μM) (Figure 4B). (+)-Catechin (1–5 μM) appeared to be the most efficacious and most potent constituent, with a significant effect at 1 μM and an almost complete protective effect at 5 μM (from 44 to 98% of MTT values and from 48 to 88% of NR values) (Figure 4C). The rescuing effects of quercetin, resveratrol and (+)-catechin were accompanied by their abilities to reverse, in the same concentration range, intracellular ROS levels (Figure 5).
Figure 4.
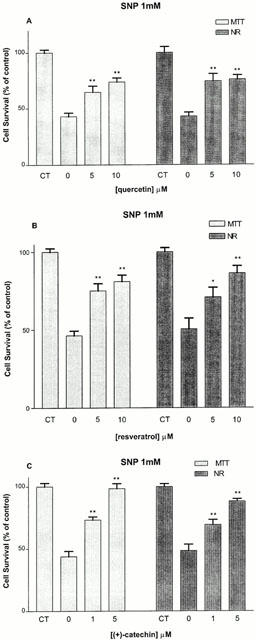
Effect of 2 h post-treatment with quercetin (5–10 μM) (A), resveratrol (5–10 μM) (B) and (+)-catechin (1–5 μM) (C) against 1 mM SNP-induced toxicity, as estimated by the MTT and NR assays in rat hippocampal cell cultures. Values represent mean±s.e.mean from 3–5 independent experiments. *P<0.05, **P<0.01 compared to groups treated with SNP alone.
Figure 5.
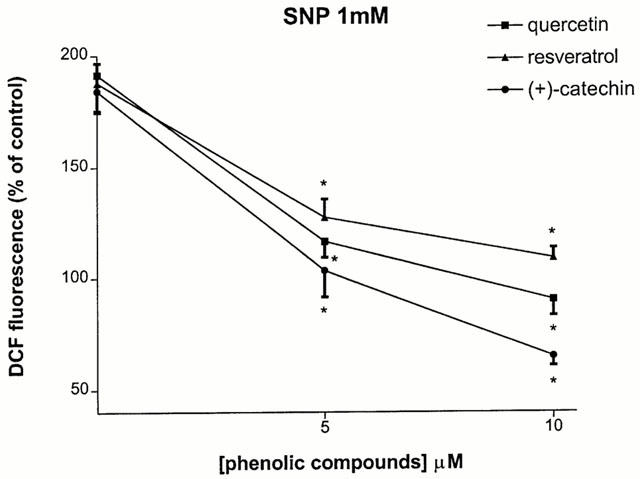
Effect of 2 h post-treatment with quercetin (5–10 μM), resveratrol (5–10 μM) and (+)-catechin (1–5 μM) on against 1 mM SNP-induced intracellular ROS in rat hippocampal cell cultures. Phenolic compounds were applied 2 h after the onset of SNP exposure. ROS were determined 24 h after by quantification of DCF fluorescence as described in Methods. Values represent mean±s.e.mean from 3–5 independent experiments. *P<0.01 compared to groups treated with either SNP.
Similarly, a 2 h post-administration with Trolox (100 μM) resulted in an increase in cell survival that was accompanied by a significant decrease in ROS production (32±4 (SNP) vs 69±4 (SNP+Trolox), P<0.01 (MTT); 37±4 (SNP) vs 68±5 (SNP+Trolox), P<0.01 (NR); 148±8 (SNP) vs 85±6 (SNP+Trolox), P<0.01 (DCF)).
Lack of effect of selective lipoxygenase- and cyclo-oxygenase-inhibitors against SNP-induced toxicity
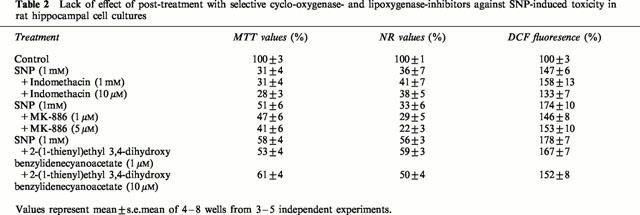
Prior results have shown that quercetin, resveratrol and (+)-catechin inhibit eicosanoid synthesis by inhibiting, at low micromolar concentration, either cyclo-oxygenase or lipoxygenase activities (Kimura et al., 1985; Pace-Asciak et al., 1995; Formica & Regelson, 1995; Maccarrone et al., 1999). We therefore determined whether selective lipoxygenase- and cyclo-oxygenase-inhibitors offered protective effects against SNP-induced toxicity. Table 1 indicates that co-treatment with indomethacin (1–5 μM), an inhibitor of cyclo-oxygenases COX-1 (IC50=740 nM) and COX-2 (IC50=970 nM) (Futaki et al., 1994), did not protect hippocampal cells against SNP-induced toxicity, even at concentrations up to 10 μM (data not shown). Similarly, MK-886 (1–5 μM), a potent and specific inhibitor of leukotriene biosynthesis (Menard et al., 1990), failed to display any protective effects at concentrations known to prevent the activation of 5-lipoxygenase (Dixon, 1990) as did 2-(1-thienyl)ethyl 3,4-dihydroxybenzylidenecyanoacetate (1–10 μM), a caffeic acid derivative with potent lipoxygenase inhibitory activities (Cho et al., 1991) (Table 1). Similarly, post-treatment with cyclo-oxygenase or lipoxygenase inhibitors did not rescue hippocampal cells pre-exposed to SNP (1 mM) (Table 2). These inhibitors also failed to attenuate ROS accumulation (Tables 1 and 2).
Table 1.
Lack of effect of co-treatment with selective cyclo-oxygenase- and lipoxygenase-inhibitors against SNP-induced toxicity in rat hippocampal cell cutures
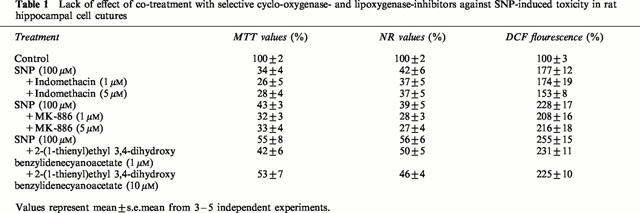
Table 2.
Lack of effect of post-treatment with selective cyclo-oxygenase- and lipoxygenase-inhibitors against SNP-induced toxicity in rat hippocampal cell cultures
Effects of red wine constituents on SNP-stimulated PKC activity and nitrite concentration
As described previously (Bastianetto et al., 2000), application of SNP (5 min, 100 μM) induced PKC activation in cultured hippocampal cells. Treatments with quercetin (10 μM) almost completely inhibited SNP-stimulated PKC activity (Figure 6). In contrast, resveratrol (10 μM) and (+)-catechin (10 μM) failed to modulate SNP-stimulated PKC activity (Figure 6).
Figure 6.
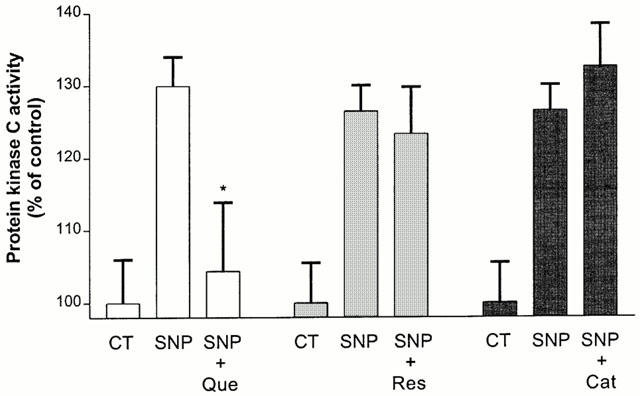
Effect of quercetin (Que, 10 μM), resveratrol (Res, 10 μM) and (+)-catechin (Cat, 10 μM) on (100 μM, 5 min) SNP-induced PKC activity in rat hippocampal mixed cell cultures. PKC activity was measured as described in Methods. Values represent mean±s.e.mean from five independent experiments. *P<0.01 compared to SNP-treated groups.
As previously reported (Koshimura et al., 1998), an exposure to SNP (100 μM) increased nitrite levels in the culture medium (from 1–21 μM of nitrite, P<0.01). All red wine derived-phenolic compounds failed to attenuate the NO production at the same concentration (10 μM) that protected hippocampal cells against 100 μM SNP-induced toxicity, as estimated by the MTT assay (Figure 7A,B).
Figure 7.
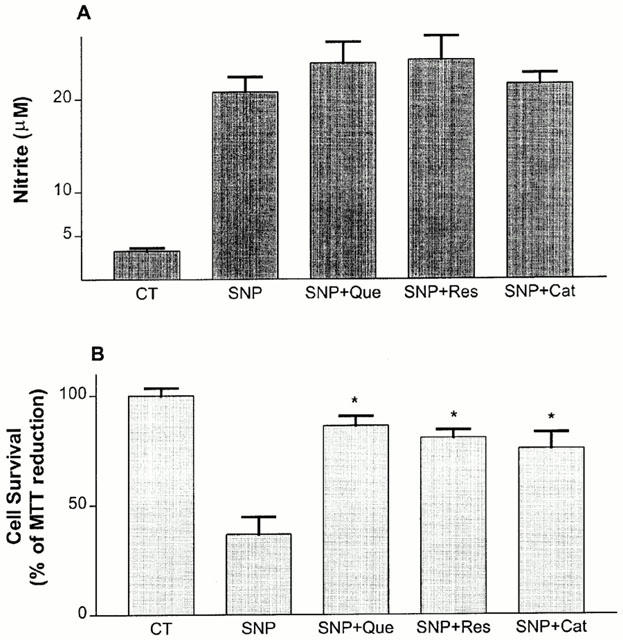
Effect of quercetin (Que, 10 μM), resveratrol (Res, 10 μM) and (+)-catechin (Cat, 10 μM) on (100 μM, 20 h) SNP-stimulated nitrite accumulation into the culture medium of rat hippocampal mixed cells. At the end of incubation time, nitrite accumulation (A) and cell survival (B) were estimated as described in Methods. Values represent mean±s.e.mean from four independent experiments. *P<0.01 compared to SNP-treated groups.
Discussion
The present study indicates that the flavonoids quercetin and (+)-catechin, as well as the stilbene resveratrol, are able to protect, and more interestingly, to rescue hippocampal cells against toxicity induced by NO. These data, and those obtained in other cell types such as fibroblasts (Subirade et al., 1995) and HT22 cells (Moosmann & Behl, 1999), demonstrate that red wine-derived phenolic constituents can inhibit the deleterious events of oxidative stress produced by NO generation, a process that may be relevant to neurodegenerative events occurring during chronic inflammation, cerebral ischaemia (for a review see Hobbs et al., 1999), or excitotoxicity (Dawson et al., 1991).
Several studies have shown that NO-induced toxicity is engendered by its reaction with the superoxide anion radical (O2−) to yield the highly cytotoxic ROS peroxynitrite. Peroxynitrite then decomposes to form nitrogen dioxide and the radical hydroxyl, leading to lipid peroxidation, protein oxidation, and DNA damage (Dawson & Dawson, 1996). Both the purported antioxidant activities, as well as the ROS scavenging properties, of quercetin, resveratrol and (+)-catechin are likely to explain, at least in part, their protective and rescuing abilities. Indeed, all of these compounds shared with Trolox, a well known antioxidant and oxygen free radical scavenger, the ability to attenuate increases in SNP-stimulated ROS accumulation in the same concentration range for which they have been reported to inhibit free radicals- or metal ion-induced lipid peroxidation (Plumb et al., 1998; Belguendouz et al., 1997; Maccarrone et al., 1999), or to scavenge free radicals such as superoxide anions (Robak & Gryglewski, 1988; Huk et al., 1998; Kostyuk & Potapovich, 1998) and peroxynitrite (Pannala et al., 1997; Nanjo et al., 1999).
In addition to their antioxidant properties, red wine constituents have been reported to attenuate, in the same concentration range, levels of cyclo-oxygenase (prostaglandins) and lipoxygenase (leukotrienes, eicosatetraenoic acids) metabolites (Kimura et al., 1985; Formica & Regelson, 1995; Subbaramaiah et al., 1998; Maccarrone et al., 1999) known to be increased during ischaemia-reperfusion events (Rao et al., 1999) or after NO exposure (Tetsuka et al., 1994; Swierkosz et al., 1995). However, neither indomethacin, nor MK-886 or 2-(1-thienyl)ethyl 3,4-dihydroxybenzylidenecyanoacetate were able to block events elicited by SNP, indicating that the protective/rescuing properties of red wine constituents are not likely triggered by their purported inhibitory action of the arachidonic acid cascade (Kimura et al., 1985; Formica & Regelson, 1995; Pace-Asciak et al., 1995; Subbaramaiah et al., 1998; Maccarrone et al., 1999).
It has been suggested that the blockade of PKC activity is involved in the protection of NO-mediated toxicity (Maiese et al., 1993; Bastianetto et al., 2000) and that inhibition of nitrite oxide synthase (NOS) may be a potential therapeutic target in the treatment of pathophysiological states related to NO overproduction (Hobbs et al., 1999). In the present study, only quercetin was able to block SNP-stimulated PKC activation, pointing to a possible involvement of this enzyme in the protective effects mediated by quercetin. These findings are in agreement with previous studies showing that quercetin (10 μM), but not (+)-catechin (10 μM), inhibits TPA-induced PKC activation in fibroblast cells (Lee & Lin, 1997). We also found that resveratrol (10 μM) failed to modulate SNP-stimulated PKC activity in our model. Although it has been reported that resveratrol (2.5–30 μM) can inhibit TPA-induced PKC activity (Subbaramaiah et al., 1998), our data are in accordance with other studies showing that resveratrol is a poor inhibitor of PKC when this enzyme is activated by either Ca2+/phosphatidylserine (IC50=90 μM) (Stewart et al., 1999) or phosphatidylcholine/phosphatidylserine (IC50=30 μM) (Garcia-Garcia et al., 1999). Moreover, none of the phenolic compounds tested were able to modulate the activity of NOS elicited by SNP (as measured by nitrite accumulation), indicating that the protective effects of red wine constituents are not likely associated to the inhibition of NOS. These findings are in agreement with the previous report that quercetin has only a weak inhibitory effect (IC50=107 μM) on NO production, whereas (+)-catechin has none at all (Kim et al., 1999). However, Tsai et al. (1999) found that resveratrol (10 μM) inhibited NO generation and suppressed inducible-NOS (iNOS) in lipopolysaccharide (LPS)-activated macrophages. This discrepancy may be due to differences in experimental conditions, including cell types and toxic agents (LPS vs SNP).
Concentrations of quercetin (4–16 mg ml−1 i.e. 12–48 μM), resveratrol (0.1–4.9 mg ml−1 i.e. 0.4–21 μM), and (+)-catechin (1.5 mg ml−1 i.e. 5 μM) in red wine, depend upon vintage and type of grapes (Hertog et al., 1993; Goldberg et al., 1996; Celotti et al., 1996; Sato et al., 1997; Soleas et al., 1997), and can reach those required to produce their purported in vitro protective effects as seen in the present study. Furthermore, Shutenko et al. (1999) demonstrated that a peripheral administration of quercetin increases the level of NO during rat brain ischaemia-reperfusion, suggesting that in vivo quercetin treatment can prevent the deleterious accumulation of superoxide occurring during ischaemia-reperfusion injury. However, its relatively poor absorptive properties in humans renders its potential therapeutic usefulness less likely (Formica & Regelson, 1995).
Interestingly, it has recently been shown that a chronic administration of resveratrol partially protects rat hippocampal neurons against kainic acid-induced damage in vivo (Virgili & Contestabile, 2000). Although the intake of red wine (per os) resulted in the detection of resveratrol concentrations in the plasma and other tissues of the rat that were in a pharmacologically active range (Bertelli et al., 1996; 1998), the physiological significance of resveratrol to daily human red wine consumers remains to be established (Soleas et al., 1997). Nonetheless, the oral administration of the (−) racemer of catechin, which seems to appear the most efficient protective red wine constituent in our model, has been reported to protect hippocampal CA1 pyramidal cells against neurotoxicity induced by transient focal brain ischaemia in gerbils (Inanami et al., 1998). This protective effect of the flavanone catechin was also accompanied by an increase in superoxide scavenging activity in the brain of catechin-treated gerbils, suggesting that it is able to cross the blood-brain barrier (Inanami et al., 1998). It is therefore conceivable that moderate, daily red wine consumption can provide sufficient amounts of active phenolic compounds – in particular resveratrol and catechin – to offer neuroprotection. This assumption is supported by two recent and exhaustive epidemiological studies revealing the existence of a negative correlation between moderate red wine drinking and the occurrence of AD (Orgogozo et al., 1997) and age-related macular degeneration (Obisesan et al., 1998).
In summary, we have shown that red wine phenolic constituents are capable of both protecting and rescuing rat hippocampal cultured cells against NO-induced toxicity. These effects are likely mediated by antioxidant activities and do not appear to involve purported inhibitory effects on intracellular enzymes such as COX/LOX, NOS and, with the exception of quercetin, PKC. These data also support the hypothesis of beneficial effects of daily red wine consumption against the occurrence of neuropathological diseases of either a chronic or acute nature, as well as on the natural process of aging in which over-production of free radicals is likely to play a deleterious role.
Acknowledgments
This work was supported by a research grant from the Medical Research Council of Canada (MRCC). W.H. Zheng holds a studentship award from MRCC. The authors would like to thank T. Kornecook, F. Mennicken and D. Auld for helpful discussion and advice.
Abbreviations
- DCF
2′,7′ dichlorofluorescein
- D-MEM
Dulbecco's modified Eagles medium
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NR
Neutral red (3-amino-7-dimethyl-amino-2-methylphenazine hydrochloride)
- ROS
reactive oxygen species
- SIN-1
3-morpholinosydnonimine
- SNP
sodium nitroprusside
References
- ANDRIAMBELOSON E., KLESCHYOV A.L., MULLER B., BERETZ A., STOCLET J.C., ANDRIANTSITOHAINA R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br. J. Pharmacol. 1997;120:1053–1058. doi: 10.1038/sj.bjp.0701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASTIANETTO S., ZHENG W.H., QUIRION R. The ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicity: involvement of its flavonoid constituents and protein kinase C. J. Neurochem. 2000;74:2268–2277. doi: 10.1046/j.1471-4159.2000.0742268.x. [DOI] [PubMed] [Google Scholar]
- BELGUENDOUZ L., FREMONT L., LINARD A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem. Pharmacol. 1997;53:1347–1355. doi: 10.1016/s0006-2952(96)00820-9. [DOI] [PubMed] [Google Scholar]
- BERTELLI A., BERTELLI A.A., GOZZINI A., GIOVANNINI L. Plasma and tissue resveratrol concentrations and pharmacological activity. Drugs Under Exp. Clin. Res. 1998;24:133–138. [PubMed] [Google Scholar]
- BERTELLI A.A., GIOVANNINI L., STRADI R., URIEN S., TILLEMENT J.P., BERTELLI A. Kinetics of trans- and cis-resveratrol (3,4′,5-trihydroxystilbene) after red wine oral administration in rats. Int. J. Clin. Pharmacol. Res. 1996;16:77–81. [PubMed] [Google Scholar]
- CELOTTI E., FERRARINI R., ZIRONI R., CONTE L.S. Resveratrol content of some wines obtained from dried Valpolicella grapes: Recioto and Amarone. J. Chromato. 1996;730:47–52. doi: 10.1016/0021-9673(95)00962-0. [DOI] [PubMed] [Google Scholar]
- CHO H., UEDA M., TAMAOKA M., HAMAGUCHI M., AISAKA K., KISO Y., INOUE T., OGINO R., TATSUOKA T., ISHIHARA T., NOGUCHI T., MORITA I., MUROTA S. Novel caffeic acid derivatives: extremely potent inhibitors of 12-lipoxygenase. J. Med. Chem. 1991;34:1503–1505. doi: 10.1021/jm00108a039. [DOI] [PubMed] [Google Scholar]
- DAWSON V.L., DAWSON T.M. Nitric oxide neurotoxicity. J. Chem. Neuroanat. 1996;10:179–190. doi: 10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- DAWSON V.L., DAWSON T.M., LONDON E.D., BREDT D.S., SNYDER S.H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc. Natl. Acad. Sci. U.S.A. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON R.A. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- FLOYD R.A. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc. Soc. Exp. Biol. Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- FORMICA J.V., REGELSON W. Review of the biology of quercetin and related bioflavonoids. Food & Chem. Toxicol. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- FURUTA A., PRICE D.L., PARDO C.A., TRONCOSO J.C., XU Z.S., TANIGUCHI N., MARTIN L.J. Localization of superoxide dismutases in Alzheimer's disease and Down's syndrome neocortex and hippocampus. Am. J. Pathol. 1995;146:357–367. [PMC free article] [PubMed] [Google Scholar]
- FUTAKI N., TAKAHASHI S., YOKOYAMA M., ARAI I., HIGUCHI S., OTOMO S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994;47:55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- GARCIA-GARCIA J., MICOL V., DE GODOS A., GOMEZ-FERNANDEZ J.C. The cancer chemopreventive agent resveratrol is incorporated into model membranes and inhibits protein kinase C alpha activity. Arch. Biochem. Biophys. 1999;372:382–388. doi: 10.1006/abbi.1999.1507. [DOI] [PubMed] [Google Scholar]
- GOLDBERG D., TSANG E., KARUMANCHIRI A., DIAMANDIS E., SOLEAS G., NG E. Method to assay the concentrations of phenolic constituents of biological interest in wines. Anal. Chem. 1996;68:1688–1694. doi: 10.1021/ac951083i. [DOI] [PubMed] [Google Scholar]
- HAYEK T., FUHRMAN B., VAYA J., ROSENBLAT M., BELINKY P., COLEMAN R., ELIS A., AND AVIRAM M. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arteriosclerosis Thromb. Vasc. Biol. 1997;17:2744–2752. doi: 10.1161/01.atv.17.11.2744. [DOI] [PubMed] [Google Scholar]
- HERTOG M.G.L., HOLLMAN P.C.H., VAN DE PUTTE B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines and fruit juices. J. Agric. Food Chem. 1993;41:1242–1246. [Google Scholar]
- HOBBS A.J., HIGGS A., MONCADA S. Inhibition of nitric oxide synthase as a potential therapeutic target. Annu. Rev. Pharmacol. Toxicol. 1999;39:191–220. doi: 10.1146/annurev.pharmtox.39.1.191. [DOI] [PubMed] [Google Scholar]
- HOLZHUTTER H.G., WIESNER R., RATHMANN J., STOSSER R., KUHN H. A kinetic model for the interaction of nitric oxide with a mammalian lipoxygenase. Eur. J. Biochem. 1997;245:608–616. doi: 10.1111/j.1432-1033.1997.00608.x. [DOI] [PubMed] [Google Scholar]
- HUK I., BROVKOVYCH V., NANOBASH VILI J., WEIGEL G., NEUMAYER C., PARTYKA L., PATTON S., MALINSKI T. Bioflavonoid quercetin scavenges superoxide and increases nitric oxide concentration in ischaemia-reperfusion injury: an experimental study. Br. J. Surg. 1998;85:1080–1085. doi: 10.1046/j.1365-2168.1998.00787.x. [DOI] [PubMed] [Google Scholar]
- INANAMI O., WATANABE Y., SYUTO B., NAKANO M., TSUJI M., KUWABARA M. Oral administration of (−)catechin protects against ischemia-reperfusion-induced neuronal death in the Gerbil. Free Rad. Res. 1998;29:359–365. doi: 10.1080/10715769800300401. [DOI] [PubMed] [Google Scholar]
- KIM H.K., CHEON B.S., KIM Y.H., KIM S.Y., KIM H.P. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem. Pharmacol. 1999;58:759–765. doi: 10.1016/s0006-2952(99)00160-4. [DOI] [PubMed] [Google Scholar]
- KIMURA Y., OKUDA H., ARICHI S. Effects of stilbenes on arachidonate metabolism in leukocytes. Biochim. Biophys. Acta. 1985;834:275–278. [PubMed] [Google Scholar]
- KOSHIMURA K., MURAKAMI Y., TANAKA J., KATO Y. Self-protection of PC12 cells by 6R-tetrahydrobiopterin from nitric oxide toxicity. J. Neurosci. Res. 1998;54:664–672. doi: 10.1002/(SICI)1097-4547(19981201)54:5<664::AID-JNR11>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- KOSTYUK V.A., POTAPOVICH A.I. Antiradical and chelating effects in flavonoid protection against silica-induced cell injury. Arch. Biochem. & Biophys. 1998;355:43–48. doi: 10.1006/abbi.1998.0708. [DOI] [PubMed] [Google Scholar]
- LEE S.F., LIN J.K. Inhibitory effects of phytopolyphenols on TPA-induced transformation, PKC activation, and c-jun expression in mouse fibroblast cells. Nutrition Cancer. 1997;28:177–183. doi: 10.1080/01635589709514572. [DOI] [PubMed] [Google Scholar]
- LIU Y., PETERSON D.A., KIMURA H., SCHUBERT D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-22,5-diphenyltetrazoliumbromide (MTT) reduction. J. Neurochem. 1997;69:581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- MACCARRONE M., LORENZON T., GUERRIERI P., AGRO A. Resveratrol prevents apoptosis in K562 cells by inhibiting lipoxygenase and cyclooxygenase activity. Eur. J. Biochem. 1999;265:27–34. doi: 10.1046/j.1432-1327.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- MAIESE K., BONIECE I., SKURAT K., WAGNER J.A. Protein kinases modulate the sensitivity of hippocampal neurons to nitric oxide toxicity and anoxia. J. Neurosci. Res. 1993;36:77–87. doi: 10.1002/jnr.490360109. [DOI] [PubMed] [Google Scholar]
- MANN D.M., YATES P.O., MARCYNIUK B. A comparison of nerve cell loss in cortical and subcortical structures in Alzheimer's disease. J. Neurol., Neurosurg. Psych. 1986;49:310–312. doi: 10.1136/jnnp.49.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTSON M.P., BARGER S.W., BEGLEY J.G., MARK R.J. Calcium, free radicals, and excitotoxic neuronal death in primary cell culture. Meth. Cell Biol. 1995;46:187–216. doi: 10.1016/s0091-679x(08)61930-5. [DOI] [PubMed] [Google Scholar]
- MENARD L., PILOTE S., NACCACHE P.H., LAVIOLETTE M., BORGEAT P. Inhibitory effects of MK-886 on arachidonic acid metabolism in human phagocytes. Br. J. Pharmacol. 1990;100:15–20. doi: 10.1111/j.1476-5381.1990.tb12044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOOSMANN B., BEHL C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NANJO F., MORI M., GOTO K., HARA Y. Radical scavenging activity of tea catechins and their related compounds. Biosci. Biotech. Biochem. 1999;63:1621–1623. doi: 10.1271/bbb.63.1621. [DOI] [PubMed] [Google Scholar]
- OBISESAN T.O., HIRSH R., KOSOKO O., CARLSON L., PARROTT M. Moderate wine consumption is associated with decreased odds of developing age-related macular degeneration in NHANES-1. J. Am. Ger. Soc. 1998;46:1–7. doi: 10.1111/j.1532-5415.1998.tb01005.x. [DOI] [PubMed] [Google Scholar]
- ORGOGOZO J.M., DARTIGUES J.F., LAFONT S., LETENNEUR L., COMMENGES D., SALOMON R., RENAUD S., BRETELER M.B. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Revue Neurologique. 1997;153:185–192. [PubMed] [Google Scholar]
- PACE-ASCIAK C.R., HAHN S., DIAMANDIS E.P., SOLEAS G., GOLDBERG D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin. Chim. Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- PANNALA A.S., RICE-EVANS C.A., HALLIWELL B., SINGH S. Inhibition of peroxynitrite-mediated tyrosine nitration by catechin polyphenols. Biochem. Biophys. Res. Comm. 1997;232:164–168. doi: 10.1006/bbrc.1997.6254. [DOI] [PubMed] [Google Scholar]
- PLUMB G.W., DE PASCUAL-TERESA S., SANTOS-BUELGA C., CHEYNIER V., WILLIAMSON G. Antioxidant properties of catechins and proanthocyanidins: effect of polymerisation, galloylation and glycosylation. Free Rad. Res. 1998;29:351–358. doi: 10.1080/10715769800300391. [DOI] [PubMed] [Google Scholar]
- RAO A.M., HATCHER J.F., KINDY M.S., DEMPSEY R.J. Arachidonic acid and leukotriene C4: role in transient cerebral ischemia of gerbils. Neurochem. Res. 1999;24:1225–1232. doi: 10.1023/a:1020916905312. [DOI] [PubMed] [Google Scholar]
- RENAUD S., DE LORGERIL M. Wine, alcohol, platelets and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- ROBAK J., GRYGLEWSKI R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988;37:837–841. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- ROTONDO S., RAJTAR G., MANARINI S., CELARDO A., ROTILLO D., DE GAETANO G., EVANGELISTA V., CERLETT C. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br. J Pharmacol. 1998;123:1691–1699. doi: 10.1038/sj.bjp.0701784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., MISKO T.P., MASFERRER J.L., SEIBERT K., CURRIE M.G., NEEDLEMAN P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO M., SUZUKI Y., OKUDA T., YOKOTSUKA K. Contents of resveratrol, piceid, and their isomers in commercially available wines made from grapes cultivated in Japan. Biosci. Biotech. Biochem. 1997;61:1800–1805. doi: 10.1271/bbb.61.1800. [DOI] [PubMed] [Google Scholar]
- SCHMIDT-KASTNER R., FREUND T.F. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- SHUTENKO Z., HENRY Y., PINARD E., SEYLAZ J., POTIER P., BERTHET F., GIRARD P., SERCOMBE R. Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem. Pharmacol. 1999;57:199–208. doi: 10.1016/s0006-2952(98)00296-2. [DOI] [PubMed] [Google Scholar]
- SIMS N.R. Energy metabolism, oxidative stress and neuronal degeneration in Alzheimer's disease. Neurodegeneration. 1996;5:435–440. doi: 10.1006/neur.1996.0059. [DOI] [PubMed] [Google Scholar]
- SOLEAS G.J., DIAMANDIS E.P., GOLDBERG D.M. Resveratrol: a molecule whose time has come? And gone. Clin. Biochem. 1997;30:91–113. doi: 10.1016/s0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
- STEWART J.R., WARD N.E., IOANNIDES C.G., O'BRIAN C.A. Resveratrol preferentially inhibits protein kinase C-catalyzed phosphorylation of a cofactor-independent, arginine-rich protein substrate by a novel mechanism. Biochemistry. 1999;38:13244–13251. doi: 10.1021/bi990875u. [DOI] [PubMed] [Google Scholar]
- SUBBARAMAIAH K., CHUNG W.J., MICHALUART P., TELANG N., TANABE T., INOUE H., JANG M., PEZZUTO J.M., DANNENBERG A.J. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J. Biol. Chem. 1998;273:21875–21882. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- SUBIRADE I., FERNANDEZ I., FERNANDEZ Y., PERIQUET A., MITJAVILA S. Catechin protection of 3T3 Swiss fibroblasts in culture under oxidative stress. Biol. Trace Element Res. 1995;47:313–319. doi: 10.1007/BF02790132. [DOI] [PubMed] [Google Scholar]
- SWIERKOSZ T.A., MITCHELL J.A., WARNER T.D., BOTTING R.M., VANE J.R. Co-induction of nitrite oxide synthase and cyclo-oxygenase: interactions between nitric oxide and prostanoids. Br. J. Pharmacol. 1995;114:1335–1342. doi: 10.1111/j.1476-5381.1995.tb13353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TETSUKA T., DAPHNA-IKEN D., SRIVASTAVA S.K., BAIER L.D., DUMAINE J., MORRISON A.R. Cross-talk between cyclooxygenase and nitric oxide pathways: prostaglandin E2 negatively modulates induction of nitric oxide synthase by interleukin 1. Proc. Natl. Acad. Sci. U.S.A. 1994;91:112168–12172. doi: 10.1073/pnas.91.25.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAI S.H., LIN-SHIAU S.Y., LIN J.K. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br. J. Pharmacol. 1999;126:673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN GOLDE P.H., SLOOTS L.M., VERMEULEN W.P., WIELDERS J.P., HART H.C., BOUMA B.N., VAN DE WIEL A. The role of alcohol in the anti low density lipoprotein oxidation activity of red wine. Atherosclerosis. 1999;147:365–370. doi: 10.1016/s0021-9150(99)00206-3. [DOI] [PubMed] [Google Scholar]
- VIRGILI M., CONTESTABILE A. Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine antioxidant agent, trans-resveratrol in rats. Neurosci. Lett. 2000;281:123–126. doi: 10.1016/s0304-3940(00)00820-x. [DOI] [PubMed] [Google Scholar]


