Abstract
Experiments were designed to investigate the role of cyclo-oxygenase isoforms in endothelial dysfunction in ageing. Aortic rings with endothelium of aged and young (24 vs 4 month-old) Wistar rats, were mounted in organ chambers for the recording of changes in isometric tension.
In young rats, acetylcholine (ACh) caused a complete relaxation which was not affected by indomethacin (0.3 μM), NS-398 (a preferential COX-2 inhibitor; 1 μM), SQ-29548 (a thromboxane-receptor antagonist; 1 μM), nor valeryl-salicylate (VAS, a preferential inhibitor of COX-1; 3 mM).
In aged rats, ACh caused a biphasic response characterized by a first phase of relaxation (0.01–1 μM ACh), followed by a contraction (3–100 μM ACh). Indomethacin, NS-398 and SQ-29548, but not VAS, augmented the first phase. Indomethacin, VAS, NS-398 and SQ-29548 decreased the contractions to high ACh concentrations. Then, the sensitivity to thromboxane receptor activation was investigated with U-46619. The results show comparable EC50 values in young and aged rats.
In aged rats, the ACh-stimulated release of prostacyclin, prostaglandin F2α and thromboxane A2 was decreased by either indomethacin, NS-398, VAS or endothelium removal. However, in young animals, the ACh-stimulated release of prostacyclin and prostaglandin F2α were smaller than in older animals and remained unaffected by NS-398.
Aortic endothelial cells from aged – but not young – rats express COX-2 isoform, while COX-1 labelling was observed in endothelial cells from both young and aged rats.
These data demonstrate the active contribution of COX-1 and -2 in endothelial dysfunction associated with ageing.
Keywords: ACh, endothelium-dependent relaxation, NS-398, senescence, thromboxane A2, thromboxane-endoperoxide receptor, valeryl salicylate, prostaglandins
Introduction
The endothelium modulates the response of vascular smooth muscle by producing not only relaxing factors [such as nitric oxide, the endothelium-dependent hyperpolarizing factor or prostacyclin] but also mediators which cause the contraction of the underlying smooth muscle cells (Furchgott & Vanhoutte, 1989). These contracting factors include endothelin-1, superoxide anions and arachidonic acid metabolites such as thromboxane (TX) A2 or prostaglandin (PG) H2. The endothelial dysfunction observed in ageing (as well as under pathological conditions) is characterized by impaired endothelium-dependent relaxations, which could be associated with either an alteration of the release or the effect of endothelial vasoactive substances (Belmin, 1999; Mombouli & Vanhoutte, 1999). However, the mechanisms accounting for the impairment of endothelium-dependent relaxations in ageing have not been completely elucidated. For instance, reduced endothelium-dependent hyperpolarizations could participate to the dysfunction (Nakashima & Vanhoutte, 1993). In addition, both a decreased bioavailability of endothelium-derived nitric oxide and an impaired expression of soluble guanylate cyclase could contribute to the blunted response to endothelium-dependent agonists in ageing (Moritoki et al., 1992; Tschudi et al., 1996; Cernadas et al., 1998; Klobeta et al., 2000). Furthermore, indomethacin, a non-selective inhibitor of cyclo-oxygenases, has a beneficial effect not only on endothelium-dependent relaxations in animal models of ageing, but also on the vasodilatation to acetylcholine in old patients (Koga et al., 1988; Taddei et al., 1995; 1997; Imaoka et al., 1999). This suggests that the release or the effect of cyclo-oxygenase-dependent vasoactive factors may also contribute to the endothelial dysfunction in ageing.
Cyclo-oxygenase (COX) is a rate-limiting enzyme in the metabolism of arachidonic acid. Different genes (Vane et al., 1998) encode the two COX isoenzymes (COX-1 and COX-2). COX-1 is expressed in a constitutive manner, while COX-2 expression can be induced following cytokine stimulation or after vascular injury (Vane et al., 1998; Rimarachin et al., 1994). The purpose of the present study was to further investigate the contribution of the cyclo-oxygenase pathway in endothelial dysfunction in ageing. We examined the relaxations to acetylcholine from isolated aortas from young and aged rats in order to determine the respective contribution of each COX isoform as well as that of thromboxane-endoperoxide receptors.
Methods
All experiments were performed on the thoracic aorta from rats (male Wistar; Iffa-Credo, Domaine des Oncins, St Germain sur l'Arbresle, France), aged 4 and 24 months. Systolic blood pressure was recorded by the tail-cuff method in unanaesthetized animals.
All procedures were in accordance with the guidelines of the European Community standards for animal care and euthanasia (French Ministére de l'Agriculture; authorization number 07430). The rats were anaesthetized with pentobarbitone sodium (50 mg kg−1; intraperitoneally) and were exsanguinated.
Organ chambers experiments
The thoracic aortas were dissected free, excised and placed in ice-cold modified Krebs-Ringer bicarbonate solution of the following composition (in mM): NaCl 118.3, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 2.5, NaHCO3 25.0, calcium disodium edetate (EDTA) 0.026 and glucose 5.0 (control solution). The blood vessels were cleaned of adherent connective tissue and cut into rings (5 mm long). In some preparations, the endothelium was mechanically removed by inserting the tips of a pair of forceps in the lumen and gently rubbing the ring back and forth on a piece of tissue wetted with ice-cold control solution. Rings were suspended horizontally between two stainless steel wires in organ chambers which contained 8 ml of control solution (37°C) aerated with 95% O2 and 5% CO2 and were connected to force transducers (Grass FT03) for recording of changes in isometric force. Prior to experimentation, the rings were stretched in a stepwise manner until the optimal point of the length-active force relationship was reached and then were allowed to equilibrate for 30 min. All rings were then exposed to KCl (120 mM) to determine their maximal responsiveness.
Experiments were performed in parallel rings with endothelium. The presence of functional endothelial cells was confirmed, unless otherwise stated, by the relaxation to thrombin (1 u ml−1) during contraction evoked by phenylephrine (0.1 to 3 μM) (Lüscher & Vanhoutte, 1986a; Ge et al., 1995). After extensive washout and equilibration, the preparations were exposed for 45 min with either indomethacin (0.3 μM; a non-selective COX inhibitor), valeryl salicylate (VAS; 3 mM; a preferential inhibitor of COX-1; Davidge & Zhang, 1998; Bhattacharayya et al., 1995), NS-398 (1 μM; a preferential inhibitor of COX-2; Futaki et al., 1994; Henrion et al., 1997), or SQ-29548 (1 μM; a thromboxane receptor antagonist; Ge et al., 1995). The preparations were then exposed to phenylephrine (30 nM to 10 μM; to obtain about 50% of the maximal contraction to KCl and to match the level of contraction between the different conditions). When the contraction was stable, increasing concentrations of acetylcholine (ACh; 1 nM to 100 μM) were added. The organ chamber medium (8 ml) was collected 30 min after the addition of the last concentration of ACh (100 μM) and was immediately frozen (−70°C) for further determination of the formation of TXA2, prostacyclin and PGF2α by the preparations. Pilot experiments revealed that a 30-min delay period was necessary to accumulate detectable amounts of metabolites from both unstimulated preparations and rings exposed to cyclo-oxygenases inhibitors.
Prostaglandins (PG) and thromboxane measurements
The release of either TXB2 (the stable metabolite of TXA2), prostacyclin (PGI2) or PGF2α was examined in the organ chamber incubation medium. Although TXB2 is produced by platelets, previous studies have shown that isolated rat aortic rings exposed to ACh could release this arachidonic acid metabolite in an endothelium-dependent manner (Ge et al., 1995). Organ chamber medium was collected and stored at −70°C. Samples were treated as described by Powell (Powell, 1982) with some modifications. Briefly, samples were centrifuged, acidified with formic acid and mixed with 2500 c.p.m. of [3H]-TXB2 (3.7–9.25 TBq mmol−1) for recovery estimation prior to solid phase extraction with a 3-ml C18 silica cartridge. The C18 silica cartridges were first treated with pure methanol (4 ml). The excess methanol was removed by passing water (4 ml). For extraction, samples were applied and the cartridges were washed once with 4 ml of water and further dried. PGs and TX were eluted with 4 ml methanol, dried using a Speed Vac dryer and resuspended in 1 ml of EIA (enzyme immunoassay) buffer (EIA buffer: 1 M phosphate buffer, pH 7.4 containing 0.15 M NaCl, 1 mM EDTA, 0.1% bovine serum albumin and 0.01% sodium azide). TXB2, the stable metabolite of TXA2, PGF2α or 6-keto-PGF1α, the stable metabolite of prostacyclin, were determined by EIA (Pradelles et al., 1985).
Immunohistochemistry
Aortic rings (3 mm long) were frozen in isopentane in liquid nitrogen immediately after euthanasia of the animal. Frozen transverse sections (7 μm) were incubated with 5% bovine serum albumin (BSA) in phosphate buffered saline solution (PBS) for 30 min at room temperature, washed once in PBS, then incubated with primary polyclonal antibodies against COX-1 and COX-2 (Créminon et al., 1995a,1995b) in 2% BSA in PBS for 2 h at room temperature. These antibodies were used at a dilution 1 : 400 for COX-1 and 1 : 200 for COX-2. After washing in PBS, the slides were incubated with a biotinylated goat anti-rabbit IgG at a dilution of 1 : 40 for 30 min at room temperature. Immunostains were visualized with the use of avidin-biotin HRP visualization system.
The specificity of the immunostaining was evaluated either by omission of the primary antibody, or replacement of the primary antibody by nonimmune rabbit IgG at equivalent protein concentration and processed as above. Under these conditions, no staining was observed in the vessel wall of either young or aged rats.
Materials
The following drugs were used in organ chamber studies: ACh HCl, indomethacin, phenylephrine, SQ-29548 ([1S]1α, 2β(5Z), 3β, 4α-7- (3-{2- [(phenylamino) carbonyl] hydrazino } methyl)-79 oxabicyclo [2.2.1] hept-2-yl-5-heptonic acid), superoxide dismutase and thrombin from Sigma-Aldrich (St Quentin Fallavier, France). Valeryl salicylate, NS-398 (N-(2-Cyclohexyloxy-4-nitrophenyl) methane sulphonamide) and U-46619 (15S)-hydroxy-11α, 9α-(epoxymethano) prosta-5Z, 13E-dienoic acid) were purchased from Cayman Chemical Company (Ann Arbor, MI, U.S.A). Drug concentrations are expressed as final molar concentrations in the bath solution. Modified Krebs-Ringer solution and drugs were prepared daily in distilled water except for valeryl salicylate which was dissolved directly into warm control solution and indomethacin which was dissolved in distilled water containing Na2CO3 (10 μM final bath concentration) and sonicated before use. Stock solutions of ACh and phenylephrine (10 mM) were prepared in distilled water and were frozen in aliquots (−20°C). Stock solutions of NS-398 (10 mM) and SQ-29548 (10 mM) were prepared in pure dimethylsulphoxide, frozen in aliquots (−20°C) and further dissolved in water. Stock solutions of U-46619 (10 mM) were obtained in pure alcohol, kept in aliquots (−80°C) and further dissolved in water. The final concentration of solvents used (dimethylsulphoxide or alcohol; 0.1%) did not have any significant effect.
For the measurement of prostaglandins and TXB2, [3H]-TXB2 and the C18 silica cartridge were purchased from NEN-Dupont (Paris, F) and from Bakerbond, Baker (Phillipsburg, NJ, U.S.A.), respectively. For immunohistochemistry studies, the secondary antibody (biotinylated goat anti-rabbit IgG), the non-immune rabbit IgG and the avidin-biotin HRP visualization system were purchased from SIGMA-Aldrich (St Quentin Fallavier, France).
Statistical analysis
Results are given as means±s.e.mean. When evaluating the relaxations to ACh, experiments were performed on preparations exposed to different concentrations of phenylephrine (30 nM to 30 μM) in order to reach the same relative degree of tone, defined as 50% of the response of each preparation to KCl (120 mM). Relaxations to ACh are expressed as per cent inhibition of the contraction evoked by phenylephrine. The IC50 values represent the concentration of ACh that elicits 50% of inhibition of the contraction evoked by phenylephrine. EC50 values represent the concentration of U-46619 that elicits 50% of the contraction. n represents the number of rats used.
Statistical analysis was performed using StatView 4.5 software (Abacus). An analysis of variance (ANOVA) for repeated measures was used to evaluate the effects of cyclo-oxygenases inhibitors and that of SQ-29548 on the concentration-dependent responses to ACh in either young or aged rats. An analysis of variance (ANOVA) was used, followed by Bonferroni as a post hoc test, when comparing in each group of animals the contractions to KCl, the response to phenylephrine, the release of arachidonic acid metabolites, the maximal relaxation to ACh or the IC50 values for Ach under the different experimental conditions. Statistical evaluation of rat body weight, systolic blood pressure and of EC50 values and maximal responses for U-46619 in young and aged rats was done by Student's t-test for unpaired observations. Means were considered significantly different when P was less than 0.05.
Results
At the time of the in vitro experiments, the body weight was 428±10 and 709±27 g in young (4 month-old) and aged (24 month-old) animals, respectively (n=8; P=0.001). The systolic blood pressure was 153±9 mmHg in young adults while it averaged 165±7 mmHg in old rats (n=8; P=0.5).
Organ chamber experiments
The amplitude of response to KCl (120 mM) was significantly larger in preparations from aged, as compared with those from young rats (P=0.001; Table 1). Within each animal group, the contractions to KCl and those to phenylephrine were not different between the different experimental conditions (Table 1). In both young and aged rats, all preparations were contracted with phenylephrine (30 nM to 30 μM) to reach a comparable relative degree of tone, defined as 50% of the response of each preparation to KCl (120 mM; Table 1).
Table 1.
Contractions to phenylephrine (Phe) and relaxation to ACh in aortic rings with endothelium from young and aged animals (n=8 each)
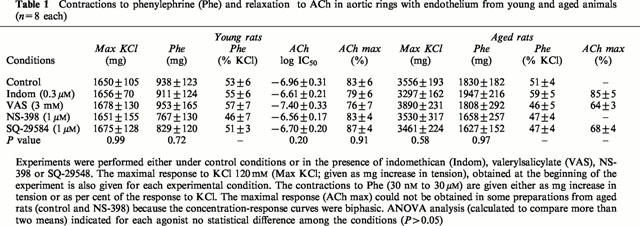
In the aged rat aorta, increasing concentrations of acetylcholine (ACh) caused biphasic responses, characterized by a first phase of relaxation at low concentrations (from 10 nM to 1 μM), and followed by a contractile response at higher concentrations (from 3 to 100 μM) (Figure 1). Indomethacin (0.3 μM) significantly augmented the first phase of relaxation to ACh (P=0.003) and abolished the contractions induced by high concentrations of ACh (P=0.0001; Figure 1A). If the preparations from aged rats were brought back to initial conditions and were then challenged again with ACh, indomethacin only impaired significantly the contractions induced by high concentrations of ACh (3 to 100 μM; P=0.02), while the relaxations observed at of lower ACh concentrations remained unaffected (10 nM to 1 μM; P=0.67) (Figure 1B). In aorta from young animals, ACh evoked a complete relaxation which was not affected by indomethacin (P=0.61; Figure 1C; Table 1).
Figure 1.
Effect of indomethacin (0.3 μM) on the relaxation evoked by ACh during contraction to phenylephrine in aortic rings with endothelium from aged (A, n=6; B, n=5) and young rats (C, n=6). (B) The preparations were challenged first with ACh before investigating again the full concentration response curve. The relaxations to ACh are expressed as percent inhibition of the contraction to phenylephrine and are given as mean±s.e.mean. The asterisk (*) indicates a significant difference in the response to ACh between control preparations and those exposed to indomethacin (p=0.001 for A and B).
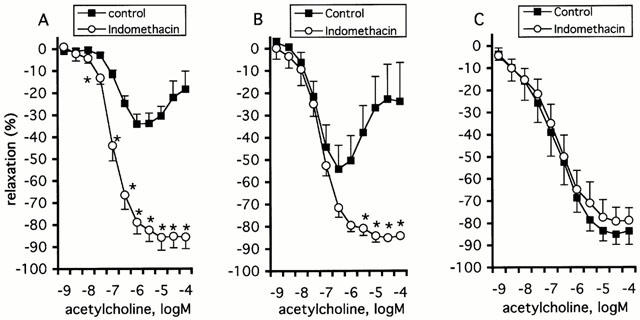
Next, the effects of preferential inhibitors of either COX-1 (valeryl salicylate, VAS, 3 mM), or COX-2 (NS-398, 1 μM) on the response to ACh were investigated (Figure 2; Table 1). In aged rats, VAS significantly impaired the contractions to high concentrations of ACh (3 to 100 μM; P=0.022), but had no effect on the relaxations evoked by low concentrations of the agonist (10 nM to 1 μM; P=0.58) (Figure 2A). Unlike VAS, NS-398 augmented the relaxation phase evoked by ACh (10 nM to 1 μM, P=0.04) and significantly decreased the contractions observed at higher concentrations of ACh (3 to 100 μM; P=0.027) (Figure 2A). In young animals, the relaxation to ACh was unaffected by VAS (P=0.82) and by NS-398 (P=0.71) (Figure 2B, Table 1).
Figure 2.
Effect of valeryl salicylate (VAS; 3 mM) and NS-398 (1 μM) on the relaxation induced by ACh in aortic rings with endothelium from aged (A) and young rats (B). The relaxations to ACh are expressed as per cent inhibition of the contraction to phenylephrine and are given as mean±s.e.mean. The # sign indicates a significant difference in aged rats between the response to ACh (3 to 100 μM) under control preparations and that after exposure to VAS (P=0.022). The asterisks (*) indicates a significant difference in aged rats between the response to ACh (10 nM to 100 μM) under control preparations and that after exposure to NS-398 (P=0.008).
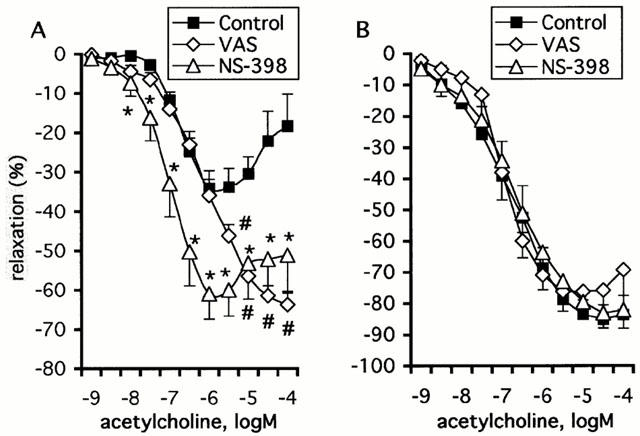
The thromboxane-endoperoxide receptor antagonist SQ-29548 (1 μM) significantly augmented the relaxations to ACh (10 nM to 1 μM) in aortic rings from aged rats (P=0.03; Figure 3A). In addition, SQ-29548 abolished the contractions observed at higher concentrations of ACh (3 to 100 μM; Figure 3A). On the contrary, SQ-29548 did not affect the response to ACh in young rats (P=0.43; Figure 3B; Table 1). Since the endothelial dysfunction in ageing was improved following blockade of thromboxane-endoperoxide receptors, the response of vascular smooth muscle to the thromboxane analogue U-46619 (0.1 nM to 3 μM) was examined in rings without endothelium from young and aged rats. The EC50 values obtained for U-46619 were comparable between preparations from young and aged rats (P=0.37; Table 2). The maximal response to U-46619 was significantly greater in old rats as compared with young animals (P=0.0001), but when expressed as per cent of the response to KCl (120 mM) there was no longer a significant difference between the two groups (P=0.12; Table 2).
Figure 3.
Effect of SQ-29548 (1 μM) on the relaxation to ACh in aortic rings with endothelium from aged (A; n=6) and young rats (B; n=6). The relaxations to ACh are expressed as per cent inhibition of the contraction to phenylephrine and are given as mean±s.e.mean. The asterisks (*) indicate a statistically significant difference in aged rats between the response to ACh (10 nM to 100 μM) under control preparations and that following exposure to SQ-29548 (P=0.001).
2.
Contractions to the thromboxane analogue U-46619 in aortic rings without endothelium from young and aged rats (n=6 each)

Prostaglandins and thromboxane B2 measurement
ACh (100 μM) stimulated the release of prostacyclin in aortic rings of aged (P=0.002) and young (P=0.006) rats (Figure 4A). The prostacyclin release was greater in rings from aged animals (P=0.01) (Figure 4A). In both aged and young animals, the ACh-stimulated prostacyclin release was endothelium-dependent (P=0.0002; P=0.029) and was significantly decreased by indomethacin (0.3 μM; P=0.007; P=0.04) or valeryl salicylate (3 mM; P=0.007; P=0.04), respectively. However, NS-398 (1 μM) decreased the ACh-stimulated prostacyclin release in preparations from aged animals (P=0.01), but not in those from young rats (P=0.45) (Figure 4A). After stimulation with ACh the release of prostacyclin from aged preparations exposed to NS-398 was comparable to that of control aortas from young rats (P=0.89).
Figure 4.
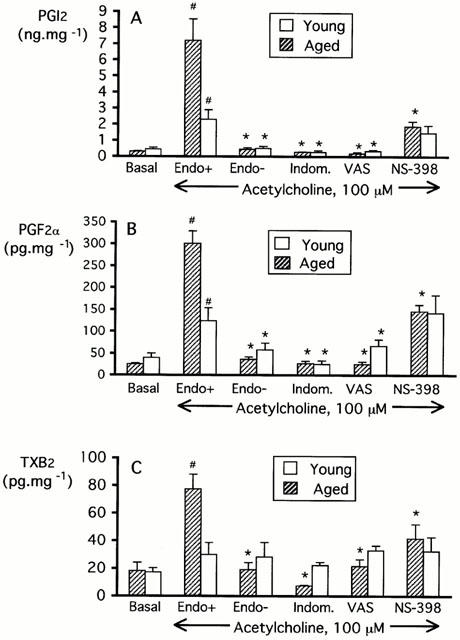
Release of prostacyclin (A), prostaglandin F2α (B) and thromboxane A2 (C) in the organ chamber medium under basal conditions or following stimulation with ACh (100 μM; 30 min) in aortic rings with or without endothelium of aged (n=5–8) and young rats (n=4–8). Experiments on rings with endothelium exposed to ACh were performed either under control conditions, or in the presence of indomethacin (0.3 μM), valeryl salicylate (VAS; 3 mM) or NS-398 (1 μM). The asterisk indicates a statistically significant difference when compared to the ACh-stimulated release of PGl2 (PGF2α or TXB2) from control preparations with endothelium of young and aged rats. The # sign indicates a statistically significant effect of ACh when compared to the release of PGl2 (PGF2α or TXB2) observed in unstimulated control preparations with endothelium of young or aged rats.
ACh also stimulated the release of prostaglandin F2α in aortic rings of aged (P<0.001) and young (P=0.04) rats (Figure 4B). This effect was also greater in rings from aged animals as compared with young (P=0.001) (Figure 4B). Both in aged and young animals, the ACh-stimulated release of prostaglandin F2α was endothelium-dependent (P=0.0001; P=0.05) and was significantly reduced by indomethacin (0.3 μM; P=0.0001; P=0.05) or VAS (3 mM; P=0.0001; P=0.04), respectively. However, NS-398 (1 μM) decreased the ACh-stimulated release of prostaglandin F2α in aortic rings of aged animals (P=0.002), but not in those from young rats (P=0.86) (Figure 4B). Following stimulation with ACh, the release of prostaglandin F2α from aged rat preparations exposed to NS-398 was comparable to that of control aortas from young rats (P=0.98).
ACh (100 μM) increased the release of thromboxane in aortic rings of aged rats (P=0.005) (Figure 4C). This effect was endothelium-dependent (P=0.0004) and significantly was reduced by indomethacin (0.3 μM; P=0.0001), valeryl salicylate (3 mM; P=0.005) or NS-398 (1 μM; P=0.03) (Figure 4C). In young animals, there was no difference between thromboxane release obtained under basal conditions and that obtained following ACh stimulation (P=0.49).
Immunohistochemistry
Immunohistochemistry studies were performed to confirm the results obtained with the preferential COX-1 and COX-2 inhibitors in organ chambers and to gather substantial information regarding the cellular location of the COX isoforms in the vessel wall of aged and young animals.
In aortas from young rats, COX-1 expression was observed mainly in endothelial cells (Figure 5A) but COX-2 isoform was undetectable (Figure 5C). In aortas from aged rats, COX-1 was expressed in both endothelial and smooth muscle cells (Figure 5B), while COX-2 expression was detected in endothelial cells only (Figure 5D). COX-1 staining appeared to be spread throughout the cellular compartment in endothelial cells from both young and aged animals, while COX-2 labelling seemed to be preferentially localized in a limited region of aortic endothelial cells from aged rats.
Figure 5.
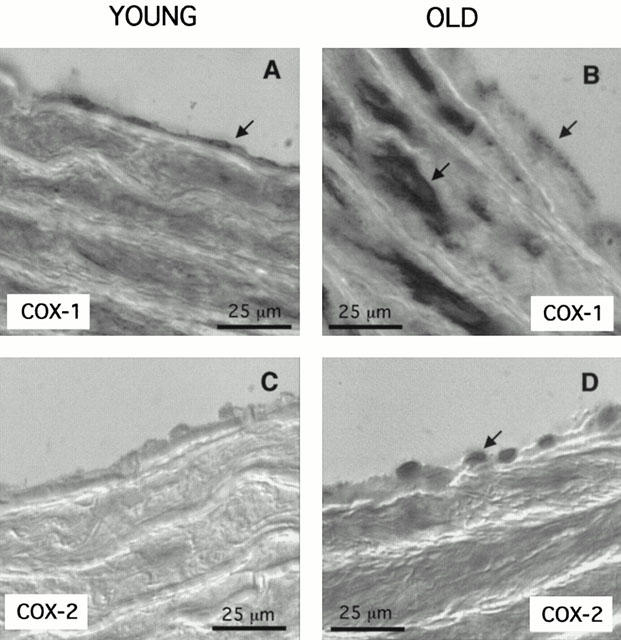
Representative immunohistochemistry (of three separate experiments) investigating the expression of Cox-1 (A,B) and Cox-2 (C,D) in aortas from young (A,C) and aged (B,D) rats. The arrows indicate the cellular location of the labelled cyclo-oxygenase isoforms.
Discussion
The present study demonstrates the contribution of COX-1 and COX-2 in endothelial dysfunction observed in ageing and the subsequent activation of thromboxane-endoperoxide receptors.
The present results confirm the impairment of endothelium-dependent relaxations to ACh in the aorta of normotensive aged rats, as observed earlier (Hongo et al., 1988; Moritoki et al., 1986). In addition, the effect of the cyclo-oxygenase inhibitor indomethacin observed in the present study is in agreement with previous data obtained in aged (Koga et al., 1988) or in spontaneously hypertensive rats (Lüscher & Vanhoutte, 1986b; Mombouli & Vanhoutte, 1993). The present results show that both isoforms of cyclo-oxygenase (the constitutive COX-1 and the inducible COX-2) contribute to the endothelial dysfunction in the aorta of aged rats. This conclusion is based on the different effects observed for indomethacin and the preferential inhibitors of each isoform (valeryl salicylate and NS-398). The detection of COX-2 in endothelial cells from aged – but not from young – animals (this study) and in senescent cultured endothelial cells (Garfinkel et al., 1994) further reinforces this interpretation. The present study shows that both indomethacin and the COX-2 inhibitor NS-398, but not the COX-1 inhibitor valeryl salicylate, significantly augmented the relaxation to low concentrations of ACh. The rebound in the ACh response observed at larger concentrations is abolished either by indomethacin or the COX-1 inhibitor valeryl salicylate. However, although the COX-2 inhibitor NS-398 impaired the contractions evoked by ACh, the full concentration-response curve to the agonist remained biphasic under these conditions. Therefore, COX-2 products are likely to contribute to the endothelial dysfunction at low concentrations of ACh (below 1 μM) while COX-1 products would be involved in the second phase following exposure to high concentrations of ACh (larger than 3 μM). Furthermore, the contribution of COX-2 to the endothelium-dependent response to ACh in ageing is consistent with previous observations showing that COX-2 activation could be involved in immediate biosynthesis of arachidonic acid metabolites following receptor activation (Murakami et al., 1999; Saunders et al., 1999). The subtle difference in subcellular localization of the cyclo-oxygenase isoforms reported earlier (Spencer et al., 1998; Vane et al., 1998) may also contribute to their separate involvement in the response to ACh observed in the present study. An additional possibility is that the two isoforms expressed in ageing may be coupled to distinct phospholipases A2 and thus, to different arachidonic acid pools, as suggested by previous studies from Reddy & Herschman, (1997) and Murakami et al. (1999). Alternatively, the distinct involvement of COX-2 and COX-1 in the ACh response may be explained by the fact that COX-2 is more active when arachidonic acid concentrations are low, while COX-1 is active when substrate concentrations are high, as demonstrated by previous results obtained with CHO cells transfected with COX-1 and COX-2 (Murakami et al., 1999).
Activation of the thromboxane-endoperoxide receptor contributes to the endothelial dysfunction in ageing, as evidenced by the significant effect of SQ-29548 on both phases of the response to ACh. This result contrasts with the lack of effect of another thromboxane receptor antagonist (SQ-30741) in the aorta of aged Wistar-Kyoto rats (Küng & Lüscher, 1995). The discrepancy between these two studies may be related to the fact that the Wistar-Kyoto rats were 17 month-old, as compared to the 24 month-old rats of the present results. However, differences in the experimental protocol cannot be ruled out. Indeed, we observed that the endothelial dysfunction to ACh in ageing was not as severe when the preparations were challenged twice with ACh. The fact that both isoforms are susceptible to suicide inactivation (Smith et al., 1996) suggests that in aged aortic endothelial cells, cyclo-oxygenases may be less able to process the arachidonic acid substrate during the second ACh challenge and subsequently release lower amounts of metabolites. This interpretation would not only fit with the smaller indomethacin-sensitive component of the relaxation observed in aortic rings challenged twice with ACh but it could also help to explain the lack of effect of a thromboxane receptor antagonist in the study by Küng & Lüscher (1995).
In the present study, the thromboxane receptor antagonist augmented the relaxation to low concentrations of ACh and also abolished the contractions observed at higher concentrations of the agonist. This effect was associated with increased release of arachidonic acid metabolites (thromboxane A2 and prostaglandin F2α) following ACh stimulation of aortas from aged rats. The present data and those by Dorn et al. (1992) suggest that these metabolites may contribute to the impairment of endothelium-dependent responses in aged rats. A possible increased sensitivity of ageing aortic vascular smooth muscle to thromboxane-endoperoxide receptor activation can be ruled out since the characteristics of response to the thromboxane analogue U-46619 were similar in preparations from young and aged animals. However, the present study does not exclude the possibility that other ligands of thromboxane receptors, such as the endoperoxide prostaglandin H2, may contribute to the endothelial dysfunction. In addition, the augmented aortic release of prostacyclin observed in the present study could also participate in the thromboxane receptor activation and to the endothelial dysfunction in ageing, as suggested earlier in the aorta from hypertensive rats (Williams et al., 1994; Rapoport & Williams, 1996). Furthermore, despite the obligatory role of the endothelium in the ACh-stimulated release of arachidonic acid metabolites, we cannot rule out the possibility of a transcellular production of these metabolites within the vessel wall (Karim et al., 1996).
In young rats, the endothelium-dependent relaxations to ACh were unaffected by indomethacin or VAS, while these compounds significantly decreased the production of prostaglandins (PGI2 and PGF2α) under these conditions. This discrepancy may be explained by the fact that the amount of prostaglandins released in the incubation medium does not reach a concentration high enough to mediate a detectable vasoactive effect.
In conclusion, the present study shows that inhibition of cyclo-oxygenase-1 and cyclo-oxygenase-2 in aortas from aged animals improves the endothelium-dependent response to ACh. These data suggest that both isoforms may have a deleterious effect on endothelial function in ageing.
Acknowledgments
This study was supported by the Institut National de la Santé et de la Recherche Médicale (Paris, France). Dr Yang's fellowship was financed by an educational grant from the Institut de Recherche International Servier (Courbevoie, France).
Abbreviations
- ACh
acetylcholine
- BSA
bovine serum albumin
- COX-1
cyclo-oxygenase-type 1 isoform
- COX-2
cyclo-oxygenase-type 2 isoform
- Indo
indomethacin
- PBS
phosphate buffered saline solution
- PG
prostaglandin
- Phe
phenylephrine
- TX
thromboxane
- VAS
valeryl salicylate
References
- BELMIN J.Vascular Ageing Biology of the Arterial Wall 1999Dordrecht/ Boston/ London: Kluwer Academic Publishers; 129–147.ed. Lévy, B.I. & Tedgui, A. pp [Google Scholar]
- BHATTACHARAYYA D.K., LECOMTE M., DUNN J., MORGANS D.J., SMITH W.L. Selective inhibition of prostaglandin endoperoxide synthase-1 (cyclooxygenase-1) by valeryl salicylic acid. Arch. Biochem. Biophys. 1995;317:19–24. doi: 10.1006/abbi.1995.1130. [DOI] [PubMed] [Google Scholar]
- CERNADAS M.R., SANCHEZ DE MIGUEL L., GARCIA-DURAN M., GONZALES-FERNANDES F., MILLAS I., MONTON M., RODRIGO J., RICO L., FERNANDEZ P., DE FROTOS T., RODRIGUEZ-FEO J.A., GUERRA J., CARAMELO C., CASADO S., LOPEZ-FARRE A. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and ageing rats. Circ. Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- CREMINON C., FROBERT Y., HABIB A., MACLOUF J., PRADELLES P., GRASSI J. Immunological studies of human constitutive cyclooxygenase (COX-1) using enzyme immunometric assay. Biochim. Biophys. Acta. 1995a;1254:333–340. doi: 10.1016/0005-2760(94)00196-6. [DOI] [PubMed] [Google Scholar]
- CREMINON C., HABIB A., MACLOUF J., PRADELLES P., GRASSI J., FROBERT Y. Differential measurement of constitutive (COX-1) and inducible (COX-2) cyclooxygenase expression in human umbilical vein endothelial cells using specific immunometric enzyme immunoassays. Biochim. Biophys. Acta. 1995b;1254:341–348. doi: 10.1016/0005-2760(94)00197-7. [DOI] [PubMed] [Google Scholar]
- DAVIDGE S.T., ZHANG Y. Estrogen replacement suppresses a prostaglandin H synthase-dependent vasoconstrictor in rat mesenteric arteries. Circ. Res. 1998;83:388–395. doi: 10.1161/01.res.83.4.388. [DOI] [PubMed] [Google Scholar]
- DORN G.W., II, BECKER M.W., DAVIS M.G. Dissociation of the contractile and hypertrophic effects of vasoconstrictor prostanoids in vascular smooth muscle. J. Biol. Chem. 1992;267:24897–24905. [PubMed] [Google Scholar]
- FURCHGOTT R.F., VANHOUTTE P.M. Endothelial relaxing and contracting factors. FASEB. J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- FUTAKI N., TAKAHASHI S., YOKOYAMA M., ARAI I., HIGUCHI S., IIZUKA H., OTOMO S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994;47:55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- GARFINKEL S., BROWN S., WESSENDORF J.H., MACIAG T. Post-transcriptional regulation of interleukin 1 alpha in various strains of young and senescent human umbilical vein endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1559–1563. doi: 10.1073/pnas.91.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GE T., HUGHES H., JUNQUERO D.C., WU K.K., VANHOUTTE P.M., BOULANGER C.M. Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H-synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circ. Res. 1995;76:1003–1010. doi: 10.1161/01.res.76.6.1003. [DOI] [PubMed] [Google Scholar]
- HENRION D., DECHAUX E., DOWELL F.J., MACLOUF J., SAMUEL J.L., LEVY B.I., MICHEL J.B. Alteration of flow-induced dilatation in mesenteric resistance arteries of L-NAME treated rats and its partial association with induction of cyclo-oxygenase-2. Br. J. Pharmacol. 1997;121:83–90. doi: 10.1038/sj.bjp.0701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONGO K., NAKAGOMI T., KASSELL N.F., SASAKI T., LEHMAN M., VOLLMER D.G., TSUKAHARA T., OGAWA H., TORNER J. Effects of ageing and hypertension on endothelium-dependent vascular relaxation in rat carotid artery. Stroke. 1988;19:892–897. doi: 10.1161/01.str.19.7.892. [DOI] [PubMed] [Google Scholar]
- IMAOKA Y., OSANAI T., KAMADA T., MIO Y., SATOH K., OKUMARA K. Nitric oxide-dependent vasodilator mechanism is not impaired by hypertension but is diminished with ageing in the rat aorta. J. Cardiovasc. Pharmacol. 1999;33:756–761. doi: 10.1097/00005344-199905000-00012. [DOI] [PubMed] [Google Scholar]
- KARIM S., HABIB A., LEVY-TOLEDANO S., MACLOUF J. Cyclooxygenase-1 and -2 of endothelial cells utilize exogenous or endogenous arachidonic acid for transcellular production of thromboxane. J. Biol. Chem. 1996;271:12042–12048. doi: 10.1074/jbc.271.20.12042. [DOI] [PubMed] [Google Scholar]
- KLOBETA S., BOULOUMIE A., MULSCH A. Ageing and chronic hypertension decrease expression of rat aortic soluble guanylyl cyclase. Hypertension. 2000;35:43–47. [PubMed] [Google Scholar]
- KOGA T., TAKATA Y., KOBAYASHI K., TAKISHITA S., YAMASHITA Y., FUJISHIMA M. Ageing suppresses endothelium-dependent relaxation and generates contraction mediated by the muscarinic receptors in vascular smooth muscle of normotensive Wistar-Kyoto and spontaneously hypertensive rats. J. Hypertens. 1988;6:S243–S245. doi: 10.1097/00004872-198812040-00073. [DOI] [PubMed] [Google Scholar]
- KUNG C.F., LUSCHER T.F. Different mechanisms of endothelial dysfunction with ageing and hypertension in rat aorta. Hypertension. 1995;25:194–200. doi: 10.1161/01.hyp.25.2.194. [DOI] [PubMed] [Google Scholar]
- LUSCHER T.F., VANHOUTTE P.M. Endothelium-dependent responses to platelets and serotonin in spontaneously hypertensive rat. J. Hypertension. 1986a;8:-55 II–II-60. doi: 10.1161/01.hyp.8.6_pt_2.ii55. [DOI] [PubMed] [Google Scholar]
- LUSCHER T.F., VANHOUTTE P.M. Endothelium-dependent contractions to ACh in the aorta of the spontaneously hypertensive rat. Hypertension. 1986b;8:344–348. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- MOMBOULI J.V., VANHOUTTE P.M. Purinergic endothelium-dependent and-independent contractions in rat aorta. Hypertension. 1993;22:577–583. doi: 10.1161/01.hyp.22.4.577. [DOI] [PubMed] [Google Scholar]
- MOMBOULI J.V., VANHOUTTE P.M. Endothelial dysfunction: from physiology to therapy. J. Mol. Cell. Cardiol. 1999;31:61–74. doi: 10.1006/jmcc.1998.0844. [DOI] [PubMed] [Google Scholar]
- MORITOKI H., HOSOKI E., ISHIDA Y. Age-related decrease in endothelium-dependent dilator response to histamine in rat mesenteric artery. Eur. J. Pharmacol. 1986;126:61–67. doi: 10.1016/0014-2999(86)90738-7. [DOI] [PubMed] [Google Scholar]
- MORITOKI H., YOSHIKAWA T., HISAYAMA T., TAKEUCHI S. Possible mechanisms of age-associated reduction of vascular relaxation caused by atrial natriuretic peptide. Eur. J. Pharmacol. 1992;210:61–68. doi: 10.1016/0014-2999(92)90652-k. [DOI] [PubMed] [Google Scholar]
- MURAKAMI M., KAMBE T., SHIMBARA S., KUDO I. Functional coupling between various phospholipase A2 s and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J. Biol. Chem. 1999;274:3103–3115. doi: 10.1074/jbc.274.5.3103. [DOI] [PubMed] [Google Scholar]
- NAKASHIMA M., VANHOUTTE P.M. Age-dependent decrease in endothelium-dependent hyperpolarizations to endothelin-3 in the rat mesenteric artery. J. Cardiovasc. Pharmacol. 1993;22:S352–S354. doi: 10.1097/00005344-199322008-00092. [DOI] [PubMed] [Google Scholar]
- POWELL W.S. Rapid extraction of arachidonic acid metabolites from biological samples using octadecylsilyl silica. Methods Enzymol. 1982;86:467–477. doi: 10.1016/0076-6879(82)86218-6. [DOI] [PubMed] [Google Scholar]
- PRADELLES P., GRASSI J., MACLOUF J. Enzyme immunoassays of eicosanoids using ACh esterase as label: an alternative to radioimmunoassay. Anal. Chem. 1985;57:1170–1173. doi: 10.1021/ac00284a003. [DOI] [PubMed] [Google Scholar]
- RAPOPORT R.M., WILLIAMS S.P. Role of prostaglandins in acetylcholine-induced contraction of aorta from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 1996;28:64–75. doi: 10.1161/01.hyp.28.1.64. [DOI] [PubMed] [Google Scholar]
- REDDY S.T., HERSCHMAN H.R. Prostaglandin synthase-1 and prostaglandin synthase-2 are coupled to distinct phospholipases for the generation of prostaglandin D2 in activated mast cells. J. Biol. Chem. 1997;272:3231–3237. doi: 10.1074/jbc.272.6.3231. [DOI] [PubMed] [Google Scholar]
- RIMARACHIN J.A., JACOBSON J.A., SZABO P., MACLOUF J., CREMINON C., WEKSLER B.B. Regulation of cyclooxygenase-2 expression in aortic smooth muscle cells. Arterioscler. Thromb. 1994;14:1021–1031. doi: 10.1161/01.atv.14.7.1021. [DOI] [PubMed] [Google Scholar]
- SAUNDERS M.A., BELVISI M.G., CIRINO G., BARNES P.J., WARNER T.D., MITCHELL J.A. Mechanisms of prostaglandin E2 release by intact cells expressing cyclooxygenase-2: evidence for a ‘two-component' model. J. Pharmacol. Exp. Ther. 1999;288:1101–1106. [PubMed] [Google Scholar]
- SMITH W.L., GARAVITO R.M., DEWITT D.L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- SPENCER A.G., WOODS J.W., ARAKAWA T., SINGER I.I., SMITH W.L. Subcellular localization of prostaglandin endoperoxide H synthases-1 and -2 by immunoelectron microscopy. J. Biol. Chem. 1998;273:9886–9893. doi: 10.1074/jbc.273.16.9886. [DOI] [PubMed] [Google Scholar]
- TADDEI S., VIRDIS A., MATTEI P., GHIADONI L., FASOLO C.B., SUDANO I., SALVETTI A. Hypertension causes premature ageing of endothelial function in humans. Hypertension. 1997;29:736–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- TADDEI S., VIRDIS A., MATTEI P., GHIADONI L., GENNARI A., FASOLO C.B., SUDANO I., SALVETTI A. Ageing and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- TSCHUDI M.R., BARTON M., BERSINGER N.A., MOREAU P., COSENTINO F., NOLL G., MALINSKI T., LÜSCHER T.F. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J. Clin. Invest. 1996;98:899–905. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANE J.R., BAKHLE Y.S., BOTTING R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- WILLIAMS S.P., DORN G.W., II, RAPOPORT R.M. Prostaglandin 12 mediates contraction and relaxation of vascular smooth muscle. Am. J. Physiol. 1994;267:H796–H803. doi: 10.1152/ajpheart.1994.267.2.H796. [DOI] [PubMed] [Google Scholar]


