Abstract
The effects of intrastriatal infusion of 3-morpholinosydnonimine (SIN-1) or sodium nitroprusside (SNP) on dopamine (DA), 3-methoxytyramine (3-MT), dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), L-dihydroxyphenylalanine (L-DOPA), ascorbic acid and uric acid concentrations in dialysates from the striatum of freely moving rats were evaluated using microdialysis.
SIN-1 (1 mM) infusion for 180 min increased microdialysate DA and 3-MT concentrations, while L-DOPA, DOPCA+HVA, ascorbic acid and uric acid levels were unaffected. Co-infusion with ascorbic acid (0.1 mM) inhibited SIN-1-induced increases in DA and 3-MT dialysate concentration.
SNP (1 mM) infusion for 180 min increased greatly the dialysate DA concentration to a peak (2950% of baseline) at the end of the infusion, while increases in 3-MT were negligible. In addition, SNP decreased ascorbic acid and L-DOPA but increased uric acid concentration in the dialysate. Co-infusion with deferoxamine (0.2 mM) inhibited the late SNP-induced increase in DA dialysate concentration, but did not affect the decrease in ascorbic acid and increase uric acid dialysate concentrations.
SNP (1 mM) infusion for 20 min moderately increased uric acid, DA and 3-MT, but decreased L-DOPA levels in the dialysate. Ascorbic acid concentration increased at the end of SNP infusion. Co-infusion with ascorbic acid (0.1 mM) inhibited the SNP-induced increase in DA and 3-MT, but did not affect the decrease in L-DOPA and increase in uric acid dialysate concentrations.
These results suggest that NO released from SIN-1 may account for the increase in the dialysate DA concentration. NO released following decomposition of SNP may account for the early increase in dialysate DA, while late changes in microdialysate composition following SNP may result from an interaction between NO and the ferrocyanide moiety of SNP. Exogenous ascorbic acid inhibits the effect of exogenous NO on DA release probably by scavenging NO, suggesting that endogenous ascorbic acid may modulate the NO control of DA release from 300 striatal dopaminergic terminals.
Keywords: NO-donor, dopamine, in vivo release, ascorbic acid, iron, microdialysis, rat striatum
Introduction
Sodium nitroprusside (SNP) and 3-morpholinosydnonimine (SIN-1) have been widely employed in in vitro and in vivo experimental studies as nitric oxide (NO) donors in order to study the role of NO in dopamine (DA) release and metabolism in terminal dopaminergic fields. For instance, NO has been thought to facilitate calcium-dependent DA release, since NO-donors, such as SNP and hydroxylamine in striatal slices in vitro (Zhu & Luo, 1992; Lonart et al., 1993), or SNP and SIN-1 in the striatum of anaesthetized rats in vivo (West & Galloway, 1997; 1998), increased extracellular DA levels. In the striatum of anaesthetized rats, Guevara-Guzman et al. (1994) showed that SNP and another NO-donor, S-nitrosoglutathione, increased extracellular DA concentrations; in contrast, the NO-donor S-nitroso-N-acetylpenicillamine (SNAP) decreased extracellular DA concentration, as did NO gas, given directly by dissolution in degassed perfusion fluid. Segieth et al. (2000) showed that SNAP promoted DA release from the rat hippocampus in vivo at low concentrations, whilst high concentrations induced long-lasting DA decreases. Thus, the results of these studies have been conflicting, probably because different mechanisms underlie the NO generation from different NO-donors. For instance, SNP-1, besides NO, generates the superoxide anion, being thus a potential peroxynitrite generator (Menconi et al., 1998), while the release of NO from SNP occurs after reaction with various reducing agents, including ascorbic acid. Thereafter, the ferrocyanide moiety generates cyanide and Fe(II) (Bates et al., 1991); the latter may react with NO to form N2O and Fe(III) (Le Brun et al., 1997).
The close relationship between NO and ascorbic acid has been outlined in several biological systems (Millar, 1995; Lilley & Gibson, 1997). Ascorbic acid is not synthesized in the brain. However, it is found in high concentrations throughout the mammalian brain. Ascorbic acid undergoes active uptake at the choroid plexus (Spector, 1982) by a carrier-mediated saturable process (Lam & Daniel, 1986), but traverses the blood-brain barrier by simple diffusion (Lam & Daniel, 1986). A homeostatic mechanism regulates extracellular brain ascorbic acid (Shenk et al., 1982; Miele & Fillenz, 1996). Ascorbic exists primarily in the reduced form in vivo, since it can be recycled by enzymatic mechanisms, involving both semidehydroascorbate/dehydroascorbate reductases (Diliberto et al., 1982) and GSH-dependent glutaredoxin (Martenson & Meister, 1991). Neuronal ascorbic acid concentrations are 20–25 times greater than extracellular ascorbic acid concentration (Rice, 2000). Ascorbic acid may either protect NO from destruction by superoxide anion (Dudgeon et al., 1998; Jackson et al., 1998) or scavenge it (Whiteman & Halliwell, 1996). In this regard, Karanth et al. (2000) claimed that ascorbic acid may even act as an inhibitory transmitter in the hypothalamus by scavenging NO. Neal et al. (1999) showed that DA released from the retina is oxidized by NO and that endogenous ascorbic acid protects DA from oxidation by scavenging NO. Ascorbic acid, however, may induce hydroxyl radical generation from both freshly prepared and photodegraded SNP, an in vitro effect which was blocked by NO (Rauhala et al., 1998). The study of the role of endogenous ascorbic acid in SIN-1- or SNP-induced changes in dopamine release and metabolism in the striatum of freely moving rats was therefore deemed of interest.
Methods
Animals
Male Wistar rats (Morini, R. Emilia, Italy), weighing between 280–330 g were used in all experiments. The rats were maintained under standard animal care conditions (12 : 12 h light/dark cycle, lights coming on at 07.00 h; room temperature 21°C), with food and water ad libitum. Prior to the start of any experiment, the health of the rat was assessed according to published guidelines (Morton & Griffiths, 1985). All procedures were specifically licensed under the European Community directive 86/609 included in Decreto No. 116/1992 of the Italian Ministry of Public Health.
Drugs
SNP, SIN-1, deferoxamine, ascorbic acid, and potassium ferrocyanide (K4FeCN6.3H2O) were purchased from Sigma-Aldrich (Milan, Italy).
Drug administration
Drug concentrations were chosen as follows: SNP and SIN-1, according to Guevara-Guzman et al. (1994) and West & Galloway (1998); deferoxamine, according to Desole et al. (1998).
Microdialysis probe construction
The striatal probe combined two independent microdialysis probes of concentric design with two separate inlets and a shared outlet, as previously described (Miele et al., 2000; Serra et al., 2000). The probes were constructed using two sections of plastic-coated silica tubing (diameter 0.15 mm; Scientific Glass Engineering, Milton Keynes, U.K.) each placed in the centre of a semi-permeable polyacrylonitrile dialysis fibres (molecular cut-off weight of 12 KD, Filtral 16 Hospal Industrie, France). Each probe had a final diameter of 0.22 mm. The tips of the dialysis fibres were sealed and joined using quick-drying epoxy glue. The two sections of silica tubing served as inlets; the outlet was made also with a section of plastic-coated silica tubing, positioned in the centre of polythene tubing. The semi-permeable membrane was coated with epoxy leaving an active length of 4 mm. The diameter of the final probe was approximately 0.50 mm. The striatal probe combining two microdialysis probes of concentric design with two separate inlets and a shared outlet, allowed separate co-infusion of drugs (Serra et al., 2000).
Surgery
Stereotaxic surgery was performed under chloral hydrate (400 mg kg−1 i.p.) anaesthesia. The microdialysis probes were implanted in the right striatum using the following co-ordinates from the atlas of Paxinos & Watson (1986): A/P +0.5 mm from bregma, +2.5 mm M/L, and −6.0 mm D/V from dura. Body temperature during anaesthesia was maintained at 37°C by means of an isothermal-heating pad (Harvard Apparatus, Kent, U.K.). Following surgery the animals were placed in large plastic bowls (50×55 cm), and maintained in a temperature- and light-controlled environment, with free access to food and water. Experiments were carried out 24 h after probe implantation with the animal in its home bowl. This arrangement allowed the rats free movement.
Microdialysis procedure
The composition of the Ringer solution used was as follows, in mM: NaCl 147, KCl 4, CaCl2 1.2, MgCl2 1 (pH 6.0). A microinfusion pump (CMA/100, Microdialysis, Sweden) pumped Ringer solution at a flow rate of 1.0 μl min−1 using two separate syringes connected to the inlets by a length of polythene tubing; every 20 min, 60 μl dialysate samples were collected manually in 250 μl micro-centrifuge tubes (Alpha Laboratories, U.K.) attached to the outlet. Subsequently, a 20 μl aliquot of collected dialysate was injected into the analytical system. Drugs were added to the Ringer solution and infused via the striatal probe implanted in the striatum.
Chromatographic analysis
DA, L-DOPA, dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA) 3-methoxytyramine (3-MT), ascorbic acid and uric acid were quantified by high performance liquid chromatography with electrochemical detection (HPLC-EC) as previously described (Miele et al., 2000; Serra et al., 2000), using an Alltech 426 HPLC pump equipped with a Rheodyne injector, column 15 cm×4.6 mm i.d. Alltech Adsorbsphere C18 5U, electrochemical detector Antec CU-04-AZ and Varian Star Chromatographic Workstation. The mobile phase was citric acid 0.5 M, Na acetate 1 M, EDTA 12.5 mM, MeOH 10% and sodium octylsulphate 650 mg l−1 (pH 3.0); the flow rate was 1.3 ml min−1. The first sample was collected after 60 min of stabilization (time 0), then dialysates were collected, at 20 min intervals, for 40 min prior to the start of experiments.
Histology
Following the experiments, rats were killed with an overdose of chloral hydrate (800 mg kg−1 i.p.). The location of each microdialysis probe was confirmed by post-mortem histology. Brains were fixed in formal saline and 50 μm coronal sections were made with a cryostat. The slices were stained with cresyl violet and examined under a microscope.
Statistical analysis
The concentrations in the dialysate were expressed in nM (DA, L-DOPA, 3-MT) or μM (DOPAC, HVA, ascorbic acid, uric acid) and given as mean±s.e.mean. Drug effects on neurochemicals were statistically evaluated in terms of changes in absolute dialysate concentrations. Statistical significance was assessed using analysis of variance (ANOVA) for difference between groups and over time. Difference within or between groups were determined by paired or unpaired t-tests with Bonferroni multiple comparison adjustment.
Results
Effects of intrastriatal SIN-1 or SNP infusion on dialysate concentrations of DA, 3-MT, L-DOPA, DOPAC+HVA, ascorbic acid and and uric acid
Intrastriatal SIN-1 infusion (1 mM for 180 min, n=3) significantly increased DA (Figure 1) and 3-MT (data not shown) dialysate concentrations, compared with preceding control values which returned to baseline within 60 min after SIN-1 discontinuation. SIN-1 infusion did not affect ascorbic (Figure 2A), L-DOPA (Figure 2B), or uric acid (Figure 2C) dialysate concentrations. DOPAC+HVA concentrations (baseline values 3.02±0.32 μM) were unaffected (data not shown).
Figure 1.
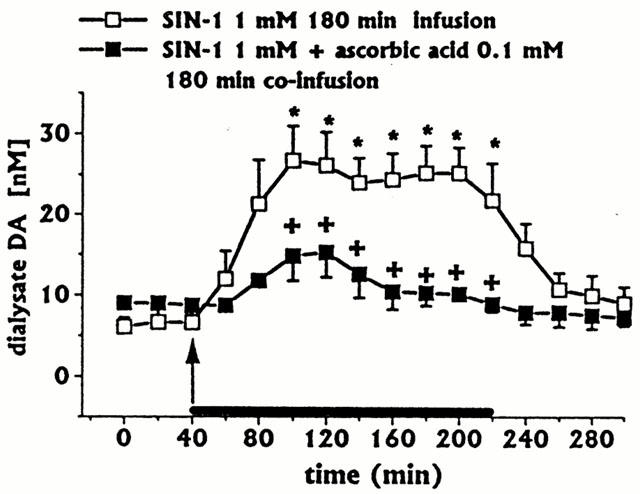
Effect of intrastriatal infusion of SIN-1 on DA dialysate concentrations, and effect of ascorbic acid co-infusion on SIN-1-induced changes. Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bar) and for 80 min after discontinuation of drug infusion. Values are given as mean±s.e.mean (n=3). *P<0.05 compared with baseline values. +P<0.05 compared with SIN-1 group.
Figure 2.
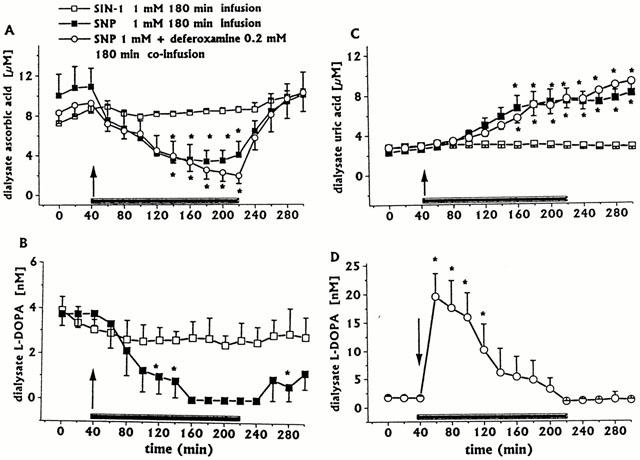
Effect of intrastriatal infusion of SIN-1 (1 mM, n=3), SNP (1 mM, n=3) or deferoxamine 0.2 mM co-infusion with SNP 1 mM (n=4), on ascorbic acid (A), L-DOPA (B, D) and uric acid (C) dialysate concentrations. Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bar) and for 80 min after discontinuation of drug infusion. Values are given as mean±s.e.mean (n=3). *P<0.05 compared with baseline values.
Continuous intrastriatal SNP infusion (1 mM for 180 min, n=3) induced a great increase in the concentration of DA in the dialysate, with a peak (2950% of baseline) at the end of the infusion period (Figure 3A). The increase in 3-MT concentration, albeit statistically significant, was negligible, if compared with changes in DA (data not shown); in addition, SNP induced decreases in ascorbic acid (Figure 2A) and L-DOPA (Figure 2B) and increases in uric acid (Figure 2C) dialysate concentrations; changes in DOPAC+HVA concentrations (baseline values 2.46±0.40 μM) were negligible (data not shown). The increase in uric acid was progressive, without a tendency to return to baseline after SNP discontinuation.
Figure 3.
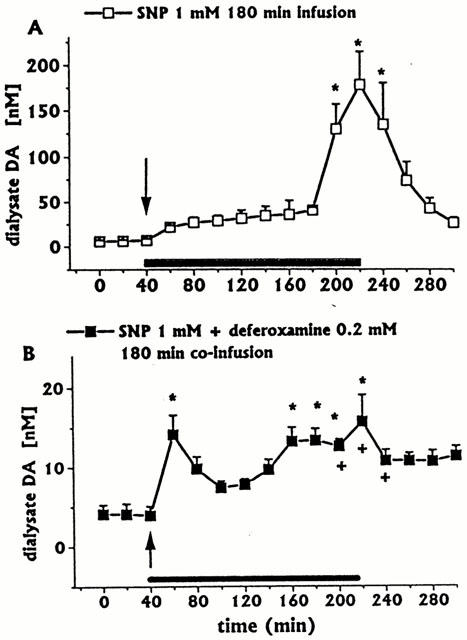
Effect of intrastriatal infusion of SNP (1 mM, n=3) on DA dialysate concentrations (A), and effect of deferoxamine (0.2 mM, n=4) co-infusion on SNP-induced change (B). Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bar) and for 80 min after discontinuation of drug infusion. Values are given as mean±s.e.mean. *P<0.05 compared with baseline values. +P<0.05 compared with SNP group.
A short-lasting SNP infusion (1 mM for 20 min, n=3) induced a moderate early increase in DA (Figure 4) and 3-MT (data not shown), an early, although not significant decrease, and a late significant increase in ascorbic acid dialysate concentrations (Figure 5A). In addition, SNP induced decreases in L-DOPA (Figure 5B) and increases in uric acid concentrations (Figure 5C). DOPAC+HVA concentrations (baseline values 2.96±0.30 μM) were unaffected (data not shown).
Figure 4.
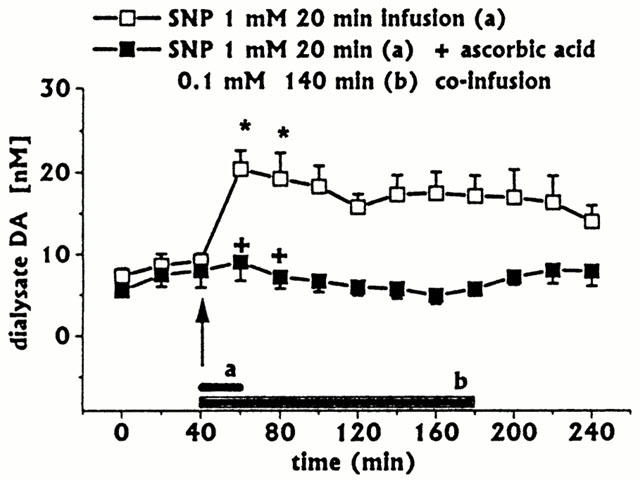
Effect of a short-lasting intrastriatal infusion of SNP (short horizonatal black bar) on DA dialysate concentrations, and effect of ascorbic acid 0.1 mM co-infusion (horizontal black bar) on SNP-induced changes. Dialysates were collected, at 20 min intervals: (a) during SNP infusion and for 200 min after SNP discontinuation; (b) during SNP (20 min)+ascorbic acid 140 min co-infusion, and for 60 min after ascorbic acid discontinuation. Values are given as mean±s.e.mean (n=3). *P<0.05 compared with baseline values; +P<0.05 compared with SNP group.
Figure 5.
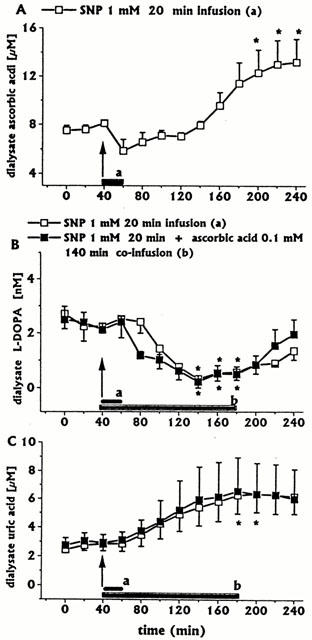
Effect of a short-lasting (20 min) intrastriatal infusion of SNP (1 mM, short horizontal black bar) on ascorbic acid (A), L-DOPA (B) and uric acid (C) dialysate concentrations. The effects of ascorbic acid 0.1 mM co-infusion for 140 min (horizontal black bar) on SNP-induced changes in L-DOPA or uric acid dialysate concentrations are shown in (B) and (C), respectively. Dialysates were collected, at 20 min intervals: (a) during SNP infusion and for 200 min after SNP discontinuation; (b) during SNP (20 min)+ ascorbic acid (140 min) co-infusion, and for 60 min after ascorbic acid discontinuation. Values are given as mean±s.e.mean (n=3). *P<0.05 compared with baseline values.
Effects of intrastriatal ascorbic acid co-infusion on SIN-1- or SNP-induced neurochemical changes
The finding that SNP infusion significantly decreased endogenous ascorbic acid prompted us to evaluate the effect of exogenous ascorbic acid on SNP- and SIN-1-induced changes in neurochemical dialysate concentrations. Ascorbic acid 0.1 mM was co-infused either with SIN-1 (1 mM) or SNP (1 mM, short-lasting infusion).
Ascorbic acid (0.1 mM) 180 min co-infusion with SIN-1 (n=3) fully inhibited SIN-1-induced increases in DA (Figure 1) and 3-MT (data not shown) concentrations. All other striatal neurochemical parameters were unaffected (data not shown). When ascorbic acid 0.1 mM (infusion time 140 min) was co-infused with SNP 1 mM (infusion time 20 min, n=3), the SNP-induced increase in DA (Figure 4) and 3-MT (data not shown) was inhibited, whilst decreases in L-DOPA (Figure 5B) and increases in uric acid (Figure 5C) concentrations were unaffected. Also, DOPAC+HVA concentrations (baseline values 2.19±0.18 μM) were unaffected (data not shown).
Effects of intrastriatal deferoxamine co-infusion on SNP-induced neurochemical changes
In a previous study (Desole et al., 1998), we showed that deferoxamine, a well-known iron chelator, antagonized SNP-induced apoptosis in PC12 cells. The study of the effect of intrastriatal infusion of deferoxamine on SNP-induced changes in dialysate neurochemicals was therefore deemed of interest.
Deferoxamine 0.2 mM co-infused for 180 min with SNP 1 mM (n=4) greatly antagonized the late SNP-induced increases in DA (Figure 3B) and 3-MT dialysate concentrations (data not shown)., but did not significantly affect the early increase in DA or 3-MT. In addition, deferoxamine induced an early great increase in dialysate L-DOPA (Figure 2D). In contrast, the SNP-induced decrease in ascorbic acid (Figure 2A) and increase in uric acid concentrations were unaffected (Figure 2C). In addition, the DOPAC+HVA concentration (baseline values 1.98±0.31 μM) was unaffected (data not shown).
The infusion of ferrocyanide moiety of SNP (1 mM for 180 min, n=3) induced only a moderate early increase in DA (Figure 6) and 3-MT (data not shown) dialysate concentrations. It was not possible to measure the dialysate concentrations of ascorbic acid, L-DOPA or uric acid because of an overlapping ferrocyanide peak. Detection was possible following discontinuation of drug infusion: ascorbic acid, L-DOPA and uric acid were in the range of baseline values.
Figure 6.
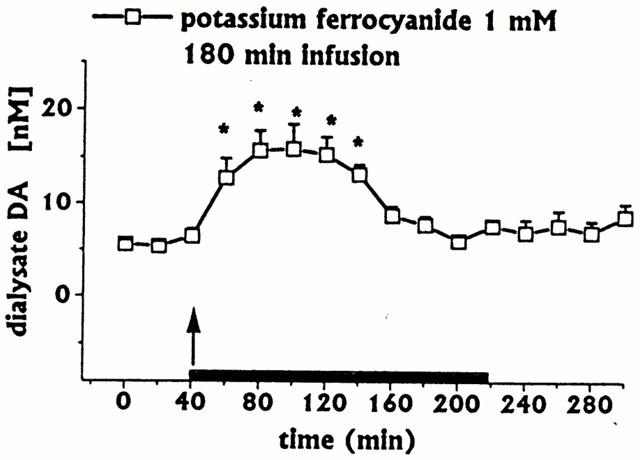
Effect of intrastriatal infusion of potassium ferrocyanide on DA dialysate concentration. Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bar) and for 80 min after drug discontinuation. Values are given as mean±s.e.mean (n=3). *P<0.05 compared with baseline values.
Discussion
The present study was designed to analyse the mechanism by which the NO-donors SNP and SIN-1 affect release and metabolism of striatal DA in vivo. The results showed that prolonged intrastriatal infusion of either SNP or SIN-1 increased dialysate DA and 3-MT concentrations. The effect of SNP was several times greater than SNP-1; in addition, the peak of the SNP-induced increase occurred at the end of the infusion period, while that of SIN-1 occurred earlier. Furthermore, SNP decreased both ascorbic acid and L-DOPA and increased uric acid dialysate concentrations, while SIN-1 did not affect any of these parameters. Neither drug affected dialysate DOPAC+HVA level. A shorter SNP infusion induced a moderate early increase in DA and a sustained late increase in uric acid concentration; in addition, SNP produced decreases in L-DOPA and biphasic changes in ascorbic acid dialysate concentrations: an early, although not statistically significant decrease, and a late significant increase. Bates et al. (1991) showed that SNP decomposition occurs after SNP has undergone reduction. In the present study, it is likely that the SNP reducing agents are both ascorbic acid and L-DOPA. The lasting decrease in L-DOPA following SNP short infusion may be explained by the fact that L-DOPA is found in nanomolar concentrations in dialysates and, to our knowledge, no homeostatic mechanism for its extracellular concentrations has been so far described. On the contrary, ascorbic acid is found in micromolar concentrations and an efficient homeostatic mechanism, demonstrated in vivo (Miele & Fillenz, 1996), regulates its striatal extracellular concentrations. It is not surprising, therefore, if a sustained decrease in dialysate ascorbic acid concentrations occurs only following prolonged SNP infusion. The late increase in ascorbic acid dialysate concentrations following the short SNP infusion might be a consequence of the activation of the glutamate-ascorbate heteroexchange mechanism, which modulates extracellular brain ascorbic acid (Miele et al., 1994; Rice, 2000). Studies are in progress to verify this hypothesis.
The increase in dialysate DA is probably due to NO released following either SIN-1 decomposition or, in the early phase of the experiment, SNP decomposition. In fact, co-infusion with ascorbic acid inhibited increases in DA and 3-MT concentrations induced either by SIN-1 or by the short-lasting infusion of SNP. Ascorbic acid scavenges NO (Whiteman & Halliwell, 1996; Neal et al., 1999; Karanth et al., 2000) and prevents, at very high physiological concentrations, the interaction of NO and superoxide (Jackson et al., 1998). Infusion of SIN-1, however, did not decrease dialysate concentrations of endogenous ascorbic. These findings may be explained by the efficiency of homeostatic and recycling mechanisms which regulate extracellular brain ascorbic acid (Shenk et al., 1982; Miele & Fillenz, 1996) and by the fact that NO itself releases ascorbic acid from neurons (Lilley & Gibson, 1997; Neal et al., 1999; Karanth et al., 2000). In a study in progress, we found that intrastriatal infusion of 7-nitroindazole (a neuronal nitric oxide sinthase inhibitor) significantly increased dialysate concentrations of ascorbic acid (unpublished observations). These findings further support the hypothesis that endogenous ascorbic acid plays a modulatory role in NO-induced striatal DA release.
The late increase in DA dialysate concentrations following prolonged SNP infusion is probably due to Fe(II) release from the ferrocyanide moiety following SNP decomposition. In turn, Fe(II) may react with NO to form N2O+Fe(III) (Le Brun et al., 1997). Some convergent findings indicate that the above sequence may be the mechanism of SNP-induced late increases in DA dialysate concentrations: (1) infusion of ferrocyanide induced only a moderate early increase in dialysate DA; (2) the late SNP-induced great increase in DA dialysate was inhibited by the iron chelator deferoxamine; (3) co-infusion of SIN-1 (NO-donor) with ferrocyanide, which is released following SNP decomposition, resulted in a late increase in dialysate DA (unpublished observations); (4) intrastriatal infusion of Fe(II) 1 mM induces a late DA increase in dialysates from the striatum of freely moving rats (Han et al., 1999). Studies are in progress to further clarify the role of NO, iron and ascorbic acid in SNP-induced increase in striatal Da release.
Both prolonged and short-lasting infusion of SNP produced decreases (below the detection limit) of dialysate L-DOPA; in contrast, SIN-1 infusion did not induce changes in dialysate L-DOPA. Surprisingly, co-infusion of deferoxamine with SNP resulted in a great early increase in dialysate L-DOPA. Like DA, L-DOPA is found in nanomolar concentration in baseline dialysates. It is a catechol-containing molecule, and thus may undergo autoxidation either in vitro or in vivo to generate L-DOPA-semiquinone (Serra et al., 2000). However, as a catechol-containing agent, L-DOPA may protect DA from non-enzymatic oxidation (Kerry & Rice-Evans, 1999). In a study in progress, we found that intrastriatal infusion of 7-nitroindazole (a neuronal nitric oxide sinthase inhibitor) significantly increased dialysate concentrations of L-DOPA (unpublished observations). These findings support the hypothesis that L-DOPA might actively co-operate with ascorbic acid in modulating NO-induced DA release from striatal dopaminergic terminals.
Uric acid dialysate concentrations progressively increased during both prolonged and short-lasting SNP infusion, and after drug discontinuation. In contrast, SIN-1 infusion did not affect uric acid concentration. Uric acid is the end-product of the purine metabolism, and its enzymatic formation from xanthine/xanthine oxidase reaction is a well known superoxide generating system (Becker, 1993). This system is involved in some cytopthaic effects of NO-donor SNAP (Menconi et al., 1998). Uric acid, however, is an active component of the neuronal antioxidant pool (Becker, 1993). It is capable of inhibiting free-radical initiated lipid peroxidation and DNA damage, and it forms strong complexes with iron ions, particularly Fe(III) (Cohen et al., 1984). Uric acid is a scavenger of peroxynitrite, but not of NO (Hooper et al., 1998). In addition, uric acid attenuates in vitro cytopathic effects of SNAP (Menconi et al., 1998) or SIN-1 (Menconi et al., 1998, Lepore et al., 1999). According to Sevanian et al. (1991), one of the scavenging activities of uric acid is to maintain ascorbic acid in its reduced form in biological fluids. We showed that inhibition in vivo of uric acid production by means of allopurinol greatly decreases extracellular ascorbic concentration in the rat striatum (Enrico et al., 1997). In the present study, SIN-1 infusion did not affect uric acid levels. Indeed, it has been shown (Houston et al., 1998) that neither NO gas nor drug-derived NO affect xanthine oxidase activity. Thus, SNP-induced increases in dialysate uric acid underlie a mechanism independent of NO generation. Some convergent findings indicate that the cyanide moiety of SNP might be responsible for the increase in dialysate uric acid: (1) during ferrocyanide infusion, dialysate uric acid concentration could not be detected because of an overlapping ferrocyanide peak, however, detection was possible following discontinuation of drug infusion and uric acid was in the range of baseline values; (2) cyanide inhibits urate oxidase (E.C. 1.7.3.3) (Ohe & Watanabe, 1981); (3) cyanide inhibits cellular uric acid uptake (Cacini, 1982); (4) cyanide poisoning causes ATP degradation and plasma oxypurines increases (Katsumata et al., 1983). It is only a matter of speculation whether extracellular uric acid accumulation might have permitted the fast recovery of dialysate ascorbic acid following SNP discontinuation. The results of the present study, in fact, do not allow us to hypothesize any other active role for uric acid in SNP-induced changes in striatal DA release.
The results of the present study do not allow us to hypothesize the mechanism of NO-induced effect on DA release. The mechanism is supposed to be either a direct effect of NMDA glutamatergic receptor-evoked NO on striatal dopaminergic neurons, or mediated by striatal cholinergic or GABAergic interneurones (Silva et al., 1995; West & Galloway, 1997). NO-induced release in striatal DA appears to be largely dependent on calcium (Lonart et al., 1993; West & Galloway, 1998), but may occur by a calcium-independent mechanism (Stewart et al., 1996). The results of this study suggest that NO released following decomposition of SIN-1 may account for SIN-1-induced increases in dialysate DA. NO released following decomposition of SNP may account for the early increase in DA. The SNP-induced late increase in DA and uric acid appear to be a consequence of an interaction between NO and the ferrocyanide moiety of SNP with a consequent release of Fe(II) and cyanide. Exogenous ascorbic acid inhibits exogenous NO effect on DA release probably by scavenging NO, thus suggesting a modulatory role for endogenous ascorbic acid in the NO control of DA release from striatal dopaminergic terminals.
Acknowledgments
The research was supported by University of Sassari (ex 60% fund).
Abbreviations
- ANOVA
analysis of variance
- DA
dopamine
- DOPAC
dihydroxyphenylacetic acid
- HVA
homovanillic acid
- i.p.
intraperitoneally
- L-DOPA
L-dihydroxyphenylanine
- 3-MT
3-methoxytyramine
- NO
nitric oxide
- SIN-1
3-morpholinosydnonimine
- SNAP
S-nitroso-N-acetylpenicillamine
- SNP
sodium nitroprusside
References
- BATES J.N., BAKER M.T., GUERRA R., JR, HARRISON D.G. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem. Pharmacol. 1991;42:S157–S165. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- BECKER B.B. Towards the physiological function of uric acid. Free Rad. Biol. Med. 1993;14:615–631. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- CACINI W. Comparative accumulation of uric acid and hypoxanthine by slices of avian renal cortex. J. Pharmacol. Exp. Ther. 1982;220:86–90. [PubMed] [Google Scholar]
- COHEN A.M., ABERDROTH R.E., HOCHSTEIN P. Inhibition of free radical-induced DNA damage by uric acid. FEBS Lett. 1984;174:147–150. doi: 10.1016/0014-5793(84)81094-7. [DOI] [PubMed] [Google Scholar]
- DESOLE M.S., SCIOLA L., SIRCANA S., GODANI C., MIGHELI R., DELOGU M.R., PIRAS G., DE NATALE G., MIELE E. Protective effect of deferoxamine on sodium nitroprusside-induced apoptosis in PC12 cells. Neurosci. Lett. 1998;247:1–4. doi: 10.1016/s0304-3940(98)00260-2. [DOI] [PubMed] [Google Scholar]
- DILIBERTO E.J., JR, DEAN G., CARTER C., ALLEN P.L. Tissue, subcellular, and submitochondrial distribution of semidehydroascorbate reductase: possible role of semidehydroascorbate reductase in cofactor regeneration. J. Neurochem. 1982;39:563–568. doi: 10.1111/j.1471-4159.1982.tb03982.x. [DOI] [PubMed] [Google Scholar]
- DUDGEON S., BENSON D.P., MACKENZIE A., PAISLEY-ZYSZKIEWICZ K., MARTIN W. Recovery by ascorbate of impaired nitric-oxide dependent relaxation resulting from oxidant stress in rat aorta. Br. J. Pharmacol. 1998;125:782–786. doi: 10.1038/sj.bjp.0702120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENRICO P., ESPOSITO G., MURA M.A., MIGHELI R., SERRA P.A., DESOLE M.S., MIELE E., DE NATALE G., MIELE M. Effects of allopurinol on striatal dopamine, ascorbate and uric acid during an acute morphine challenge: ex vivo and in vivo studies. Pharmacol. Res. 1997;35:577–585. doi: 10.1006/phrs.1997.0193. [DOI] [PubMed] [Google Scholar]
- GUEVARA-GUZMAN R., EMSON P.C., KENDRICK K.M. Modulation of in vivo striatal transmitter release by nitric oxide and cyclic GMP. J. Neurochem. 1994;62:807–810. doi: 10.1046/j.1471-4159.1994.62020807.x. [DOI] [PubMed] [Google Scholar]
- HAN J., CHENG F.C., YANG Z., DRYHURST G. Inhibitors of mitochondrial respiration, iron (II), and hydroxyl radical evoke release and extracellular hydrolysis of glutathione in rat striatum and substantia nigra: potential implications to Parkinson's disease. J. Neurochem. 1999;73:1683–1695. doi: 10.1046/j.1471-4159.1999.731683.x. [DOI] [PubMed] [Google Scholar]
- HOOPER D.C., SPITSIN S., KEAN R.B., CHAMPION J.M., DICKSON D.M., CHAUDHRY I., KOPROWSKI H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUSTON M., CHUMLEY P., RADI R., RUBBO H., FREEMAN B.A. Xanthine oxidase reaction with nitric oxide and peroxynitrite. Arch. Biochem. Biophys. 1998;355:1–8. doi: 10.1006/abbi.1998.0675. [DOI] [PubMed] [Google Scholar]
- JACKSON T.S., XU A., VITA A.J., KEANEY J.F., JR Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ. Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- KARANTH S., YU W.H., WALCZEWSKA A., MASTRONARDI C., MCCANN S.M. Ascorbic acid acts as inhibitory transmitter in the hypothalamus to inhibit stimulated luteinizing hormone-releasing hormone release by scavenging nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 2000;15:1891–1896. doi: 10.1073/pnas.97.4.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATSUMATA Y., SATO K., YADA S., SUZUKI O., YOSHINO M. Kinetics analysis of anaerobic metabolism in rats during acute cyanide poisoning. Life Sci. 1983;33:151–155. doi: 10.1016/0024-3205(83)90407-1. [DOI] [PubMed] [Google Scholar]
- KERRY N., RICE-EVANS C. Inhibition of peroxynitrite-mediated oxidation of dopamine by flavonoid and phenolic antioxidants and their structural relationship. J. Neurochem. 1999;73:247–253. doi: 10.1046/j.1471-4159.1999.0730247.x. [DOI] [PubMed] [Google Scholar]
- LAM D.K.C., DANIEL P.M. The influx of ascorbic acid into the rat brain. Quart. J. Exp. Physiol. 1986;71:483–490. doi: 10.1113/expphysiol.1986.sp003007. [DOI] [PubMed] [Google Scholar]
- LE BRUN N.E., ANDREWS S.C., MOORE G.R., THOMSON A.J. Interaction of nitric oxide with non-haem iron sites of escherichia coli bacterioferritin: reduction of nitric oxide to nitrous oxide and oxidation of iron(II) to iron(III) Biochem. J. 1997;326:173–179. doi: 10.1042/bj3260173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEPORE D.A., STEWART A.G., TOMASI A., ANDERSON A.J., HURLEY J.V., MORRISON W.A. The survival of skeletal muscle myoblast in vitro is sensitive to a donor on nitric oxide and superoxide, SIN-1, but not to nitric oxide or peroxynitrite alone. Nitric Oxide. 1999;3:273–280. doi: 10.1006/niox.1999.0239. [DOI] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Release of ascorbate and urate from a nitrergically-innervated smooth muscle. Br. J. Pharmacol. 1997;122:1746–1752. doi: 10.1038/sj.bjp.0701571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONART G., CASSELS K.L., JOHNSON K.M. Nitric oxide induces calcium-dependent [3H]dopamine release from striatal slices. J. Neurosci. Res. 1993;35:192–198. doi: 10.1002/jnr.490350210. [DOI] [PubMed] [Google Scholar]
- MARTENSON J., MEISTER A. Glutathione deficiency decreases tissue ascorbate levels in newborn rats: ascorbate spares glutathione and protects. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4656–4660. doi: 10.1073/pnas.88.11.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENCONI M.J., UNNO N., SMITH M., AGUIRRE D.E., FINK M.P. Nitric oxide donor-induced hyperpermeability of cultured intestinal epithelial monolayers: role of superoxide radical, hydroxyl radical, and peroxynitrite. Biochim. Biophys. Acta. 1998;1425:189–203. doi: 10.1016/s0304-4165(98)00072-5. [DOI] [PubMed] [Google Scholar]
- MIELE M., BOUTELLE M.G., FILLENZ M. The physiologically induced release of ascorbate in rat brain is dependent on impulse traffic, calcium influx and glutamate uptake. Neuroscience. 1994;62:87–91. doi: 10.1016/0306-4522(94)90316-6. [DOI] [PubMed] [Google Scholar]
- MIELE M., FILLENZ M. In vivo determination of extracellular brain ascorbate. J. Neurosci. Methods. 1996;69:21–24. doi: 10.1016/S0165-0270(96)00094-5. [DOI] [PubMed] [Google Scholar]
- MIELE M., MURA M.A., ENRICO P., ESPOSITO G., SERRA P.A., MIGHELI R., ZANGANI D., MIELE E., DESOLE M.S. On the mechanism of d-amphetamine-induced changes in glutamate, ascorbic acid and uric acid release in the striatum of freely moving rats. Br. J. Pharmacol. 2000;129:582–588. doi: 10.1038/sj.bjp.0703066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLAR J. The nitric oxide/ascorbate cycle: how neurones may control their own oxygen supply. Med. Hypoth. 1995;45:21–26. doi: 10.1016/0306-9877(95)90194-9. [DOI] [PubMed] [Google Scholar]
- MORTON D.B., GRIFFITHS P.H.M. Guidelines on the recognition of pain, distress and discomfort in experimental animals and a hypothesis for assessment. Vet. Rec. 1985;116:431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- NEAL M.J., CUNNINGHAM J.M., MATTHEWS K.L. Release of endogenous ascorbic acid preserves extracellular dopamine in the mammalian retina. Invest. Ophtalmol. Vis. Sci. 1999;40:2893–2897. [PubMed] [Google Scholar]
- OHE T., WATANABE Y. Purification and properties of urate oxidase from Streptomyces cyanogenus. J. Biochem. (Tokyo) 1981;89:1769–1776. doi: 10.1093/oxfordjournals.jbchem.a133376. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. Rat Brain in Stereotaxic Coordinates. Academic Press, San Diego; 1986. [Google Scholar]
- RAUHALA P., KHALDI A., MOHANAKUMAR K.P., CHIUEH C.C. Apparent role of hydroxyl radicals in oxidative brain injury induced by sodium nitroprusside. Free Rad. Biol. Med. 1998;24:1065–1073. doi: 10.1016/s0891-5849(97)00386-9. [DOI] [PubMed] [Google Scholar]
- RICE M.E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- SEGIETH J., FOWLER L., WHITON P., PEARCE B. Nitric-oxide mediated regulation of dopamine release in the hippocampus in vivo. Neuropharmacology. 2000;39:571–577. doi: 10.1016/s0028-3908(99)00178-1. [DOI] [PubMed] [Google Scholar]
- SERRA P.A., ESPOSITO G., ENRICO P., MURA M.A., MIGHELI R., DELOGU M.R., MIELE M., DESOLE M.S., GRELLA G., MIELE E. Manganese increases L-DOPA autoxidation in the striatum of freely moving rats: potential implications to long-term L-DOPA therapy of Parkinson's disease. Br. J. Pharmacol. 2000;130:937–945. doi: 10.1038/sj.bjp.0703379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVANIAN A., DAVIES K.J.A., HOCHSTEIN P. Serum urate as an antioxidant for ascorbic acid. Amer. J. Clin. Nutr. 1991;54:129S–134S. doi: 10.1093/ajcn/54.6.1129s. [DOI] [PubMed] [Google Scholar]
- SHENK J.O., MILLER E., GADDIS E., ADAMS R.N. Homeostatic control of ascorbate concentrations in CNS extracellular fluid. Brain Res. 1982;253:353–356. doi: 10.1016/0006-8993(82)90709-0. [DOI] [PubMed] [Google Scholar]
- SILVA M.T., ROSE S., HINDMARSH J.G., AISLAITNER G., GORROD J.W., MOORE P.K., JENNER P., MARSDEN C.D. Increased striatal dopamine efflux in vivo following inhibition of cerebral nitric oxide synthase by the novel monosodium salt of 7-nitro indazole. Br. J. Pharmacol. 1995;114:257–258. doi: 10.1111/j.1476-5381.1995.tb13219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPECTOR R. Penetration of ascorbic acid from cerebrospinal fluid into brain. Exp. Neurol. 1982;72:645–653. doi: 10.1016/0014-4886(81)90013-3. [DOI] [PubMed] [Google Scholar]
- STEWART T.L., MICHEL A.D., BLACK M.D., HUMPHERY P.P. Evidence that nitric oxide causes calcium-independent release of [3H] dopamine from rat striatum in vitro. J. Neurochem. 1996;66:131–137. doi: 10.1046/j.1471-4159.1996.66010131.x. [DOI] [PubMed] [Google Scholar]
- WEST A.R., GALLOWAY M.P. Endogenous nitric oxide facilitates striatal dopamine and glutamate efflux in vivo: role of ionotropic glutamate receptor-dependent mechanisms. Neuropharmacology. 1997;36:1571–1581. doi: 10.1016/s0028-3908(97)00148-2. [DOI] [PubMed] [Google Scholar]
- WEST A.R., GALLOWAY M.P. Nitric oxide and potassium chloride-facilitated striatal dopamine efflux in vivo: role of calcium-dependent release mechanism. Neurochem. Int. 1998;33:493–501. doi: 10.1016/s0197-0186(98)00054-0. [DOI] [PubMed] [Google Scholar]
- WHITEMAN M., HALLIWELL B. Protection against peroxynitrite-dependent tyrosine nitration and α1-antiproteinase inactivation by ascorbic acid. A comparison with other biological antioxidants. Free Radic. Res. 1996;25:275–283. doi: 10.3109/10715769609149052. [DOI] [PubMed] [Google Scholar]
- ZHU X.Z., LUO L.G. Effect of nitroprusside (nitric oxide) on endogenous dopamine release from rat striatal slices. J. Neurochem. 1992;59:932–935. doi: 10.1111/j.1471-4159.1992.tb08332.x. [DOI] [PubMed] [Google Scholar]


