Abstract
The aim of this study was to determine whether antimalarial agents inhibit ATP-sensitive potassium (KATP) channels and thereby contribute to the observed side-effects of these drugs.
Mefloquine (10–100 μM), but not artenusate (100 μM), stimulated insulin release from pancreatic islets in vitro.
Macroscopic KATP currents were studied in inside-out patches excised from Xenopus oocytes expressing cloned KATP channels.
Mefloquine (IC50 ∼3 μM), quinine (IC50 ∼3 μM), and chloroquine inhibited the pancreatic β-cell type of KATP channel Kir6.2/SUR1. Artenusate (100 μM) was without effect.
Mefloquine and quinine also blocked a truncated form of Kir6.2 (Kir6.2ΔC36) when expressed in the absence of SUR1. The extent of block was similar to that observed for Kir6.2/SUR1 currents.
Our results suggest that inhibition of the β-cell KATP channel accounts for the ability of quinoline-based antimalarial drugs to stimulate insulin secretion, and thereby produce hypoglycaemia.
The results also indicate that quinoline-based antimalarial agents inhibit KATP channels by interaction with the Kir6.2 subunit. This subunit is common to β-cell, neuronal, cardiac, skeletal muscle, and some smooth muscle KATP channels suggesting that KATP channel inhibition may contribute to the other side effects of these drugs, which include cardiac conduction abnormalities and neuropsychiatric disturbances.
Keywords: KATP channel, potassium channel, malaria, mefloquine, artenusate, antimalarial agents
Introduction
The quinoline drugs, which include quinine, quinidine, chloroquine, mefloquine and halofantrine are widely used as antimalarial agents. These drugs have a variety of side-effects, of which the most clinically important are hypoglycaemia, cardiac conduction abnormalities and neuropsychiatric disturbances (Assan et al., 1995; Davis, 1997; Davis et al., 1997; Jaspers et al., 1996; Nosten et al., 1993; Bateman & Dyson, 1996; Bhatia, 1991; Bem et al., 1992). When combinations of quinoline drugs are used, toxic effects may be accentuated (Nosten et al., 1993). The artemisinin derivatives are antimalarial drugs that are chemically unrelated to quinolines but which are also associated with neurological and cardiac toxicity in animal experiments (Brewer et al., 1994).
Quinine causes hypoglycaemia by stimulating insulin secretion, as a consequence of its ability to close ATP-sensitive potassium (KATP) channels in the plasma membrane of pancreatic β-cells (Bokvist et al., 1990). This induces a membrane depolarization that triggers calcium entry, a rise in intracellular calcium and, consequently, insulin release (Ashcroft & Rorsman, 1989). Sulphonylurea compounds used in the treatment of type 2 diabetes mellitus and imidazoline drugs such as phentolamine (Proks & Ashcroft, 1997) also close KATP channels. By contrast, a diverse class of drugs known as KATP-channel openers enhance KATP channel activity (Ashcroft & Ashcroft, 1992; Edwards & Weston, 1993). The effects of drugs that close or open KATP channels can be mediated through different types of KATP channel subunit (Ashcroft & Gribble, 1998; 1999).
Recent molecular studies have revealed that the KATP channel is an octameric complex of two types of subunit, Kir6.2 and SUR, which coassemble with 4:4 stoichiometry (Sakura et al., 1995; Inagaki et al., 1995; Clement et al., 1997). Kir6.2 forms the pore of the KATP channel and contains the ATP-inhibitory site, while the sulphonylurea receptor, SUR, acts as a regulatory subunit that endows the channel with sensitivity to sulphonylureas and KATP-channel openers (Aguilar-Bryan et al., 1995; Inagaki et al., 1996; Tucker et al., 1997). Although the wild-type KATP channel requires both types of subunit (Kir6.2 and SUR1) to make a functional channel, a mutant form of Kir6.2 with a C-terminal truncation of 26 or 36 amino acids (Kir6.2ΔC) is capable of independent expression (Tucker et al., 1997). Kir6.2ΔC therefore provides a useful tool for studying the effects of drugs on the pore-forming subunit of the KATP channel.
KATP channels are also found in a wide variety of other tissues including cardiac, smooth and skeletal muscle and some neurones of the central nervous system (Ashcroft & Ashcroft, 1992; Ashcroft & Gribble, 1999). There is considerable variation in the sensitivity of KATP channels in different tissues to both sulphonylureas and K-channel openers. Both of these drugs bind to the SUR subunit and the observed variability results from the presence of different types of SUR subunit in different tissues (Inagaki et al., 1995; 1996; Isomoto et al., 1996). In contrast, phentolamine does not interact with the sulphonylurea receptor, and it is equally effective at blocking Kir6.2ΔC26 channels as Kir6.2/SUR1 channels (Proks & Ashcroft, 1997). The aim of this study was to determine whether the antimalarial drugs mefloquine and artenusate functionally interact with one or other type of KATP channel subunit, and thereby contribute to the observed side-effects of these drugs.
Methods
Molecular biology
Mouse Kir6.2 (GenBank D50581) and rat SUR1 (Genbank L40624) were used in this study. A 36 amino acid C-terminal deletion of mouse Kir6.2 (Kir6.2ΔC36) was made by introduction of a stop codon at the appropriate residue using site-directed mutagenesis (Tucker et al., 1997). Constructs were subcloned into the pBF expression vector and synthesis of capped mRNA was carried out using the mMessage mMachine large-scale in vitro transcription kit (Ambion, Austin, TX, U.S.A.).
Electrophysiology
Female Xenopus laevis were anaesthetized with MS222 (2 g l−1 added to the water). One ovary was removed via a mini-laparotomy, the incision sutured and the animal allowed to recover. Once the wound had completely healed, the second ovary was removed in a similar operation and the animal was then killed by decapitation whilst under anaesthesia. Immature stage V-VI Xenopus oocytes were manually defolliculated, injected with ∼2 ng of mRNA encoding Kir6.2ΔC36 or coinjected with mRNAs encoding Kir6.2 (∼0.05 ng) and SUR1 (∼2 ng), and studied 1–4 days after injection (Gribble et al., 1997).
Macroscopic currents were recorded from giant inside-out patches using an EPC7 patch-clamp amplifier (List Electronik, Darmstadt, Germany) at 20–24°C (Gribble et al., 1997). The holding potential was 0 mV and currents were evoked by repetitive 3 s voltage ramps from −110 mV to +100 mV. Currents were filtered at 0.2 kHz, digitized at 0.5 kHz using a Digidata 1200 Interface and analysed using pClamp software (Axon Instruments, Burlingame, U.S.A.). The pipette solution contained (mM): KCl 140, MgCl2 1.2, CaCl 2 2.6, and HEPES 10 (pH 7.4 with KOH). The internal (bath) solution contained (mM): KCl 110, MgCl2 1.4, KOH 30, EGTA 10, and HEPES 10 (pH 7.2 with KOH). Drugs were prepared as 1000× stock solutions in DMSO (mefloquine; Larium®, Roche, Basel, Switzerland), ethanol (artesunate; Guilin no.2 Pharmaceutical Factory, Giangxi, China) or water (quinine and chloroquine; Sigma) and added to the internal solution as required.
Insulin secretion
Islets were isolated from mouse pancreas by collagenase digestion (type 11, Sigma) and hand-picking. They were cultured overnight in RPMI medium containing 8.3 mM glucose (1 ml per dish) at 37°C in a humidified atmosphere of 95% air/5% CO2. Next day, the medium was replaced with fresh RPMI medium with or without (for control) the test compound and islets were cultured for up to 6 days. Aliquots of the medium were collected at 48, 96 and 140 h for insulin assay, and replaced with fresh media. Insulin was assayed using a Pharmacia Insulin RIA 100 kit, which shows 100% cross-reactivity with rodent insulin, and was quantitated as pmol of insulin release per islet per 48 h. Secretion was compared with that of control islets isolated from the same batch, incubated in RPMI containing 8.3 mM glucose.
Results
We first tested the effects of the quinoline-based antimalarial drugs quinine and mefloquine, and the structurally unrelated artemisinin derivative artenusate, on insulin secretion. The basal rate of insulin secretion from islets incubated in control solution was 2.3±1.2 pmol islet−1 24 h−1 (n=18). In the presence of 10 μM mefloquine, insulin secretion was approximately double that of control islet cultures. Islets treated with 100 μM mefloquine, however, showed a ∼5 fold increase in insulin secretion (P=0.002; Figure 1). This degree of stimulation was similar to that produced by 0.1 mM IBMX plus 20 mM glucose, which generally induces near-maximal insulin secretion. Artesunate (100 μM) did not affect insulin secretion.
Figure 1.
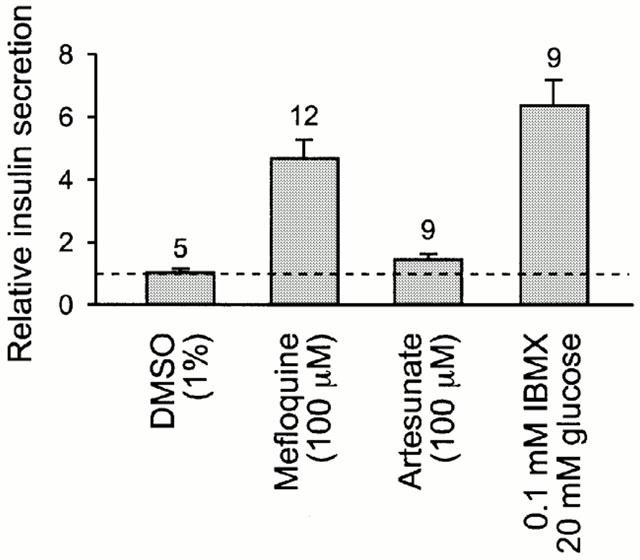
Effect of antimalarial drugs on insulin secretion. Batches of islets were incubated for 6 days in the presence of the test solution, and the culture medium was collected every 48 h for insulin assay. The rate of insulin secretion is expressed relative to that of control islets from the same batch. Data collected over the 6 day period is pooled, and the number of insulin assays is given above each bar. The dashed line indicates the rate of insulin secretion from control islets exposed to 8.3 mM glucose but no drug. P=0.002 vs DMSO, for mefloquine; and P=0.1 vs DMSO for artenusate.
To investigate whether the stimulatory effect of antimalarial drugs is mediated via inhibition of KATP channel activity, we tested their effect on the cloned β-cell KATP channel (Kir6.2/SUR1) expressed in Xenopus oocytes. These channels show properties typical of native β-cell KATP channels (Gribble et al., 1997). Macroscopic currents were recorded in excised membrane patches, and test solutions were applied to the intracellular membrane surface. Figure 2 shows that Kir6.2/SUR1 currents were blocked by quinine, mefloquine, chloroquine and quinidine, but were only slightly affected by the structurally unrelated compound artesunate, consistent with the effects of the drugs on insulin secretion from islets in vitro. Half-maximal channel inhibition was produced by ∼3 μM quinine or mefloquine, and <100 μM chloroquine. The block was voltage independent and was slowly reversible. The ability of quinoline drugs to inhibit the β-cell KATP channel may explain their ability to stimulate insulin secretion.
Figure 2.
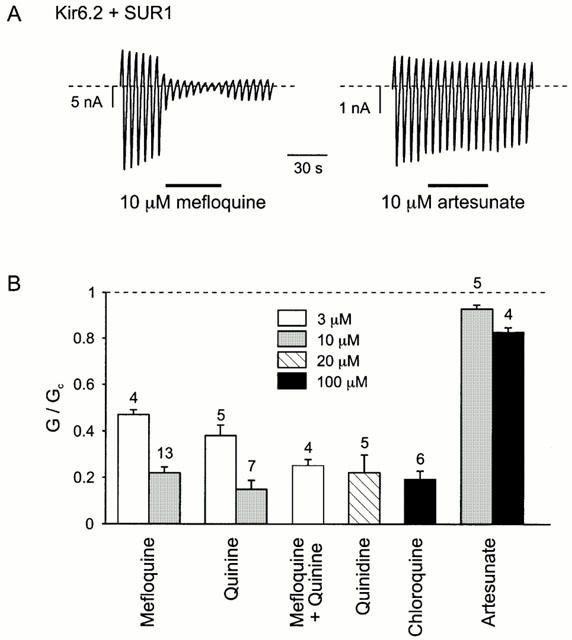
Effect of antimalarial drugs on Kir6.2-SUR1 currents. (A) Macroscopic currents recorded in inside-out patches from oocytes coinjected with Kir6.2 and SUR1 mRNAs. Drugs were applied as indicated by the bars. The voltage was ramped repetitively from −110 to +100 mV from a holding potential of 0 mV. The dashed line indicates the zero current level. (B) Mean effect of antimalarial agents, at the concentrations indicated, on Kir6.2/SUR1 currents. The conductance in the presence of the drug (G) is expressed as a fraction of the mean conductance in control solution before and after exposure to the drug (Gc). The number of patches is given above the bars. The dashed line indicates the control conductance level in the absence of drug.
There is currently controversy about whether it is safe to use combinations of quinolines, including mefloquine and quinine, in a clinical setting, because of the possibility of drug interactions (Nosten et al., 1993; Palmer et al., 1993; World Health Organization, 1996). We therefore tested the effect of this drug combination on KATP channel activity. As shown in Figure 2, when quinine and mefloquine were applied together, the extent of block was consistent with a simple model in which both drugs compete for the same site.
To determine whether the site at which quinoline-related drugs interact lies on Kir6.2 or on SUR1, we tested the drugs on a truncated form of Kir6.2 (Kir6.2ΔC36) which expresses functional channels in the absence of SUR1. Figure 3 shows that Kir6.2ΔC36 currents were inhibited by quinine and mefloquine in a similar manner to Kir6.2/SUR1 currents. This result indicates that the inhibitory effect of these drugs is not mediated by SUR1, and further suggests that the quinoline antimalarial agents interact either with Kir6.2ΔC36 or (possibly) with a third protein, endogenously expressed in Xenopus oocytes, that modulates the activity of Kir6.2ΔC36. In this respect, the quinoline antimalarial agents resemble imidazoline drugs such as phentolamine (Proks & Ashcroft, 1997).
Figure 3.
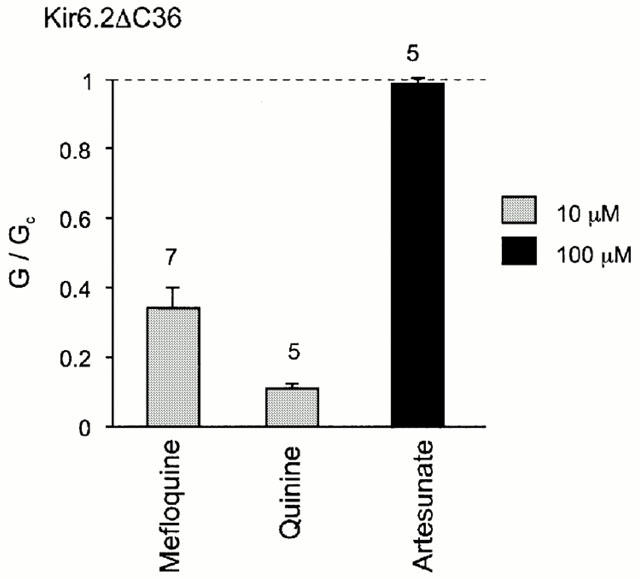
The mean effect of antimalarial drugs on Kir6.2ΔC36 currents. The conductance in the presence of the drug (G) is expressed as a fraction of the mean conductance in control solution before and after exposure to the drug (Gc). The number of patches is given above the bars. The dashed line indicates the control conductance in the absence of drug.
Discussion
Our results demonstrate that the quinoline-based drug mefloquine inhibits the β-cell KATP channel and stimulates islet insulin secretion, and confirm previous findings that this is also the case for quinine (Bokvist et al., 1990). They further show that inhibition is mediated by interaction of the drugs with the Kir6.2 subunit of the KATP channel, rather than with the regulatory SUR subunit. Thus, quinoline drugs resemble imidazolines, rather than sulphonylureas, in their mode of action. The structurally unrelated compound artesunate was without effect on KATP channel activity.
The raised serum insulin levels found in human subjects given quinoline antimalarial agents may, therefore, be due to the inhibition of β-cell KATP channels. Serum concentrations of mefloquine achieved during treatment are 2–6 μM and the range for quinine is 30–60 μM. Mefloquine is 98% or more protein bound (free serum concentration ≈amp;0.1 μM), while 88–93% of quinine is bound to plasma proteins (free serum concentration ≈amp;5 μM). Thus the free serum concentrations of mefloquine, but not those of quinine, are low compared to the media concentrations that produce half-maximal KATP channel inhibition in our in vitro experiments (∼3 μM). In addition, the media mefloquine concentrations needed to stimulate measurable insulin secretion by islet cell cultures were greater than the predicted free drug concentrations in vivo.
Although the effects of quinoline antimalarial agents are considered to reflect the free drug concentrations, other factors also have to be considered. In conventional pharmacodynamic analysis, the rate constant for elimination of drug from the ‘effect' compartment is the prime determinant of the response (Sheiner et al., 1979). We found that KATP channel block was only slowly reversible on the time scale of our electrophysiological experiments, which may contribute to an enhanced efficacy in vivo. The biological activity of mefloquine may also be influenced by its association with lipoproteins (Foley & Tilley, 1997). Detailed analysis of β-cell function and free serum concentrations of quinine, its diastereoisomer quinidine, and mefloquine given in conventional doses to healthy volunteers suggests a drug-specific concentration-effect relationship (Davis, 1997). At therapeutic doses, quinine has the most potent stimulatory effect on β-cell function but mefloquine has the greatest hyperinsulinaemic effect for a given free serum concentration. Thus, direct comparison between quinoline drugs, and of our in vitro data to the in vivo situation, must be made with caution.
It is possible that mefloquine has other effects on glucose handling, in addition to inhibition of β-cell KATP channel activity. Thus, mefloquine, like quinine (Bokvist et al., 1990), might block other types of K+ channels in the β-cell, thereby enhancing insulin secretion. Furthermore, mefloquine is metabolized to quinoline-carboxylic acid, which is structurally related to quinolinic acid, a well-known inhibitor of gluconeogenesis (Blackshear et al., 1975). The hypothesis that this mefloquine metabolite inhibits gluconeogenesis in vivo was not assessed in the present study.
We found no evidence for a synergistic interaction between mefloquine and quinine on Kir6.2/SUR1 currents. When both drugs were present, the extent of block was simply the sum of that expected for the two compounds individually, consistent with competition for the same binding site on the KATP channel. This finding adds weight to the hypothesis that the hyperinsulinaemic effect of mefloquine is mediated by KATP channel inhibition, in common with other quinoline drugs.
Kir6.2 serves as a common pore-forming subunit for KATP channels in β-cells, cardiac and skeletal muscle and neurones of the hippocampus, cerebellum and substantia nigra (Ashcroft & Gribble, 1999; Inagaki et al., 1996; Karschin et al., 1998; Liss et al., 1999). Our results therefore suggest that KATP channels in all these tissues may also be affected by therapeutic concentrations of quinoline antimalarial drugs. Studies on native tissues are now required to confirm our findings. In cardiac muscle, KATP channel opening during ischaemia reduces the QT interval of the electrocardiogram (Nichols & Lederer, 1991). It is uncertain whether cardiac KATP channels also play a role in determining the QT interval under physiological conditions, but it is possible that their closure by quinoline drugs, especially quinine (Davis et al., 1990), prolongs the QT interval during treatment of acute malaria. Likewise, although the roles of KATP channels in the hippocampus and other brain regions are uncertain, our results suggest the possibility that quinoline drugs, most notably mefloquine (Bem et al., 1992), might cause neurotoxicity through a direct interaction with neuronal KATP channels.
The artemisinin-associated neurological and cardiac toxicity in animal experiments (Brewer et al., 1994) has not been found in humans despite widespread and increasing use of this relatively safe class of drugs in human malaria (Hien & White, 1993). Our data confirm that concentrations of artesunate that are in excess of therapeutic levels (peak plasma concentrations after intravenous injection ∼30 μM; Batty et al., 1998) have no effect on KATP channel activity in vitro. The QT prolongation seen in animals given high doses of artemisinin drugs for long periods is, therefore, unlikely to be due to KATP channel closure. However, our identification of Kir6.2 as a molecular target for commonly-used quinoline drugs may provide a specific mechanism for a range of clinically significant side-effects in humans.
Acknowledgments
We thank the Novo Nordisk UK grant, the Wellcome Trust, the MRC and the British Diabetic Association for support.
Abbreviations
- KATP channel
ATP-sensitive potassium channel
- SUR
sulphonylurea receptor
References
- AGUILAR-BRYAN L., NICHOLS C.G., WECHSLER S.W., CLEMENT J.P., BOYD A.E., GONZALEZ G., HERRERA-SOSA H., NGUY K., BRYAN J., NELSON D.A. Cloning of the β-cell high-affinity sulphonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–425. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- ASHCROFT F.M., ASHCROFT S.J.H. The sulphonylurea receptor. Biochim. Biophys. Acta. 1992;1175:45–59. doi: 10.1016/0167-4889(92)90008-y. [DOI] [PubMed] [Google Scholar]
- ASHCROFT F.M., GRIBBLE F.M. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 1998;21:288–294. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- ASHCROFT F.M., GRIBBLE F.M. ATP-sensitive K+ channels in health and disease. Diabetologia. 1999;42:903–919. doi: 10.1007/s001250051247. [DOI] [PubMed] [Google Scholar]
- ASHCROFT F.M., RORSMAN P. Electrophysiology of the pancreatic β-cell. Prog. Biophys. Mol. Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- ASSAN R., PERRONNE C., CHOTARD L., LARGER E., VILDE J.L. Mefloquine-associated hypoglycaemia in a cachectic AIDS patient. Diabete Metab. 1995;21:54–58. [PubMed] [Google Scholar]
- BATEMAN D.N., DYSON E.H. Quinine toxicity. Adverse Drug React. Acute Poison Rev. 1996;4:215–233. [PubMed] [Google Scholar]
- BATTY K.T., THU L.T., DAVIS T.M., MAI T.X., HUNG N.C., TIEN N.P., POWELL S.M., THIEN H.V., BINH T.Q., KIM N.V. A pharmacokinetic and pharmadynamic study of intravenous vs oral artensunate in uncomplicated falciparum malaria. Br. J. Clin. Pharmacol. 1998;45:123–129. doi: 10.1046/j.1365-2125.1998.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEM J.L., KERR L., STURCHLER D. Mefloquine prophylaxis: an overview of spontaneous reports of severe psychiatric reactions and convulsions. J. Trop. Med. Hyg. 1992;95:167–179. [PubMed] [Google Scholar]
- BHATIA M.S. Chloroquine-induced psychiatric complications. Br. J. Psych. 1991;159:735. doi: 10.1192/bjp.159.5.735. [DOI] [PubMed] [Google Scholar]
- BLACKSHEAR P.J., HOLLOWAY A.H., ALBERTI K.G.M.M. The effects of inhibition of gluconeogenesis on ketogenesis in starved and diabetic rats. Biochem. J. 1975;148:353–362. doi: 10.1042/bj1480353b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOKVIST K., RORSMAN P., SMITH P.A. Block of ATP-regulated and Ca2+-activated K+ channels in mouse pancreatic β-cells by external tetraethylammonium ions and quinine. J. Physiol. 1990;423:311–325. doi: 10.1113/jphysiol.1990.sp018025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREWER T.G., GRATE S.J., PEGGINS J.O., WEINA P.J., PETRAS J.M., LEVINE B.S., HEIFFER M.H., SHUSTER B.G.. Fatal neurotoxicity of arteether and artemether. Am. J. Trop. Med. Hyg. 1994;51:251–259. doi: 10.4269/ajtmh.1994.51.251. [DOI] [PubMed] [Google Scholar]
- CLEMENT J.P., KUNJILWAR K., GONZALEZ G., SCHWANSTECHER M., PANTEN U., AGUILAR-BRYAN L., BRYAN J. Association and stoichiometry of KATP channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- DAVIS T.M.E. Antimalarial drugs and glucose metabolism. Br. J. Clin. Pharmacol. 1997;44:1–7. doi: 10.1046/j.1365-2125.1997.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS T.M.E., DEMBO L.G., KAYE-EDDIE S.A., HEWITT B.J., HISLOP R.G., BATTY K.T. Neurological, cardiovascular and metabolic effects of mefloquine in healthy volunteers: a double-blind, placebo-controlled trial. Br. J. Clin. Pharmacol. 1997;43:665. doi: 10.1046/j.1365-2125.1996.04745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS T.M.E., SUPANARANOND W., PUKRITTAYAKAMEE S., KARBWANG J., MOLUNTO P., MEKTHON S., WHITE N. A safe and effective consecutive-infusion regimen for rapid quinine loading in severe falciparum malaria. J. Infect. Dis. 1990;161:1305–1308. doi: 10.1093/infdis/161.6.1305. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., WESTON A.H. The pharmacology of ATP-sensitive potassium channels. Ann. Rev. Pharm. Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- FOLEY M., TILLEY L. Quinoline antimalarials: mechanisms of action and resistance. Int. J. Parasitol. 1997;27:231–240. doi: 10.1016/s0020-7519(96)00152-x. [DOI] [PubMed] [Google Scholar]
- GRIBBLE F.M., ASHFIELD R., AMMALA C., ASHCROFT F.M. Properties of cloned ATP-sensitive K-currents expressed in Xenopus oocytes. J. Physiol. 1997;498.1:87–98. doi: 10.1113/jphysiol.1997.sp021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIEN T.T., WHITE N.J. Qinghaosu. Lancet. 1993;341:603–608. doi: 10.1016/0140-6736(93)90362-k. [DOI] [PubMed] [Google Scholar]
- INAGAKI N., GONOI T., CLEMENT J.P., IV, NAMBA N., INAZAWA J., GONZALEZ G., AGUILAR-BRYAN L., SEINO S., BRYAN J. Reconstitution of IKATP: an inward rectifier subunit plus the sulphonylurea receptor. Science. 1995;270:1166–1169. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- INAGAKI N., GONOI T., CLEMENT J.P., IV, WANG C.Z., AGUILAR-BRYAN L., BRYAN J., SEINO S. A family of sulfonylurea receptors determines the properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- ISOMOTO S., KONDO C., YAMADA M., MATSUMOTO S., HORIO Y., MATSUZAWA Y., KURACHI Y. A novel sulphonylurea receptor forms with BIR (Kir6.2) a smooth muscle type of ATP-sensitive K+ channel. J. Biol. Chem. 1996;271:24321–24325. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- JASPERS C.A., HOPPERUS BUMA A.P., VAN THIEL P.P., VAN HULST R.A., KAGER P.A. Tolerance of mefloquine chemoprophylaxis in Dutch military personnel. Am. J. Trop. Med. Hyg. 1996;55:230–234. doi: 10.4269/ajtmh.1996.55.230. [DOI] [PubMed] [Google Scholar]
- KARSCHIN A., BROCKHAUS J., BALLANYI K. KATP channel formation by the sulphonylurea receptor SUR1 with Kir6.2 subunits in rat dorsal vagal neurons in situ. J. Physiol. 1998;509.2:339–346. doi: 10.1111/j.1469-7793.1998.339bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISS B., BRUNS R., ROEPER J. Alternative sulphonylurea receptor expression defines metabolic sensitivity of K-ATP channels in dopaminergic midbrain neurones. EMBO J. 1999;18:833–884. doi: 10.1093/emboj/18.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLS C.G., LEDERER W.J. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am. J. Physiol. 1991;261:H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- NOSTEN F., TER KUILE F.O., LUXEMBURGER C., WOODROW C., KYLE D.E., CHONGSUPH AJAISIDDHI T., WHITE N.J. Cardiac effects of antimalarial treatment with halofantrine. Lancet. 1993;341:1054–1056. doi: 10.1016/0140-6736(93)92412-m. [DOI] [PubMed] [Google Scholar]
- PALMER K.J., HOLLIDAY S.M., BROGDEN R.N. Mefloquine. A review of its antimalarial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1993;45:430–475. doi: 10.2165/00003495-199345030-00009. [DOI] [PubMed] [Google Scholar]
- PROKS P., ASHCROFT F.M. Phentolamine block of KATP channels is mediated by Kir6.2. Proc. Nat. Acad. Sci. 1997;94:11716–11720. doi: 10.1073/pnas.94.21.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKURA H., AMMALA C., SMITH P.A., GRIBBLE F.M., ASHCROFT F.M. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel expressed in pancreatic β-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- SHEINER L.B., STANSKI D.R., VOZEH S., MILLER R.D., HAM J. Simultaneous modelling of pharmacokinetics and pharmacodynamics: applications to d-tubocurarine. Clin. Pharmac. Ther. 1979;25:358–371. doi: 10.1002/cpt1979253358. [DOI] [PubMed] [Google Scholar]
- TUCKER S.J., GRIBBLE F.M., ZHAO C., TRAPP S., ASHCROFT F.M. Truncation of Kir6.2 produces ATP-sensitive K-channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- WORLD HEALTH ORGANIZATION Mefloquine: update on safety issues. WHO Drug Info. 1996;10:58–61. [Google Scholar]


