Abstract
The cannabinoid (CB) receptor agonist WIN55,212-2 (500 nM) had no effect on the first of a pair of population spikes evoked in the CA1 region of hippocampal slices prepared from young adult (4–6 weeks old) rats, despite powerfully reducing paired-pulse depression. In contrast WIN55,212-2 caused a substantial depression of the single population spike (reduced to 43% control) and the field EPSP (reduced to 72% of control) recorded in slices prepared from neonatal (10–13 days old) rats. This effect was stereoselective and blocked by the CB1 receptor antagonist AM281 (500 nM). The results indicate that activation of CB1 receptors inhibits excitatory synaptic transmission in neonatal, but not adult rat hippocampus. This developmental regulation of CB1 receptor mediated control of excitatory transmission may help explain some, but not all, of the previous discrepancies in the literature.
Keywords: Cannabinoid; CB1 receptor; WIN55,212-2; synaptic transmission; hippocampus; developmental regulation; paired-pulse depression
Introduction
There is general agreement in the literature that cannabinoids acting through cannabinoid (CB1) receptors inhibit γ-aminobutyric acid (GABA) mediated synaptic transmission in rat hippocampal tissue. Thus, immunohistochemical studies have shown that CB1 receptors are primarily located on GABAergic neurones (Katona et al., 1999; Tsou et al., 1999; Irving et al., 2000), and the CB receptor agonist WIN55,212-2 (R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-(1-naphthalenyl) methanone mesylate) inhibits GABA release (Katona et al., 1999) and depresses pharmacologically isolated GABAA receptor mediated inhibitory postsynaptic currents (IPSCs) (Hoffman et al., 2000; Irving et al., 2000). This is supported by our previous observations that cannabinoids powerfully reduce paired-pulse depression of population spikes, which is thought to reflect the strength of GABAergic feedback inhibitory pathways (Paton et al, 1998; Al-Hayani & Davies, 1999). The effects of WIN55,212-2 on excitatory transmission are less clear cut with various groups reporting no effects (see Figure 1 in Terranova et al., 1995; Paton et al., 1998), or profound reduction (Shen et al., 1996; Misner & Sullivan., 1999; Sullivan, 1999; Ameri et al., 1999) of population spikes, field excitatory postsynaptic potentials (EPSPs) or whole-cell recorded excitatory postsynaptic currents (EPSCs). It is apparent from these studies that, in general, those groups using field potential recordings which are usually done in slices prepared from adult animals find little or no effect of the cannabinoids, whereas those groups which used patch recordings from neonatal or cultured tissue report a profound inhibition. The aim of this study was to investigate whether some of the discrepancies in these previous results can be explained by developmental differences in the regulation of excitatory transmission. We have therefore compared the effects of the CB receptor agonist WIN55,212-2 on excitatory synaptic transmission recorded in hippocampal slices prepared from neonatal and young adult rats.
Figure 1.
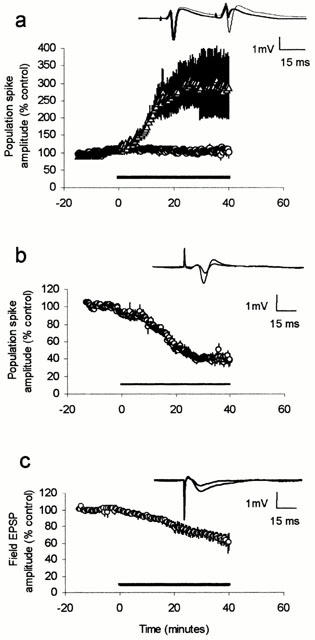
WIN55,212-2 reduces excitatory transmission in neonatal but not adult hippocampus. (a) Graph plots mean amplitude of first (PS1, ○) and second (PS2, ▵) population spikes evoked by paired pulse stimulation delivered 30 ms apart in five slices prepared from adult rats. Perfusion of 500 nM WIN55,212-2 for 40 min, indicated by bar, had no effect on PS1, but dramatically increased the amplitide of PS2, thus decreasing paired-pulse depression. Inset in this and other figures shows example synaptic responses recorded from a typical slice before and at the end of drug perfusion. (b) Graph plots mean amplitude of population spike evoked by single pulse stimulation in six slices prepared from neonatal rats. (c) Mean effect of WIN55,212-2 on the field EPSP recorded from slices prepared from neonatal rats (n=5).
Methods
Neonatal Sprague-Dawley rats aged between 10 and 13 days or young adult rats between 4 and 6 weeks were used. After general anaesthesia with halothane, they were decapitated and the brain was removed from the skull and submerged in oxygenated cold (under 5°C) artificial cerebrospinal fluid (aCSF). The hippocampus was dissected out and chopped transversely on a McIIwain tissue chopper forming slices 400 μm thick. The slices were placed onto a moist filter paper in a petri dish and maintained in an well-oxygenated and humidified chamber. After at least 1 h, slices were transferred to an interface-type recording chamber which was continuously perfused with artificial cerebrospinal fluid at a rate of 1.5 ml min−1. The temperature was maintained between 28–29°C. A bipolar stimulating electrode was used to stimulate the Schaffer collateral commissural fibres and evoked population spikes or field EPSPs were recorded from the cell body or dendritic layer of the CA1 region using a glass capillary microelectrode filled with 3 M NaCl. Half-maximal population spikes or field EPSPs were then evoked at 15-s intervals until a stable baseline of at least 15 min was established. Data was stored and analysed using the LTP program (Anderson & Collingridge, 1997; www.LTP-program.com). Drugs were applied by addition to the perfusion medium. Stock solutions of WIN55,212-2, and WIN55,212-3 (S(−)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-(1-naphthalenyl)methanone mesylate, the inactive isomer), were made up in alcohol and stored at 4°C. When required they were mixed with Tween 80 (two parts Tween 80 to one part of drug) and the ethanol was evaporated by using steam of nitrogen gas. Saline was then added in aliquots of 0.05 ml and the solution diluted with aCSF to obtain the required concentration. AM281 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide) was made up as a 10 mM stock solution in DMSO and diluted in aCSF as required. In all cases statistical analysis was performed using the INSTAT program to measure significance using the paired Student's t-test. Each slice included in the results came from a different rat. A P value of 0.05 or less was considered statistically significant. WIN55,212-2 and WIN55,212-3 were obtained from RBI/Sigma (Gillingham, U.K.) and AM281 from Tocris (Bristol, U.K.).
Results
Paired-pulse stimulation with an interstimulus interval of 30 ms was applied to slices prepared from young adult rats. Using this protocol the second population spike (PS2) is evoked during the phase of GABAergic inhibition evoked by the first stimulus (PS1) and is therefore depressed (mean amplitude of PS2 was 56±9% of PS1). WIN55,212-2 (500 nM) perfused for at least 40 min had no effect on the amplitude of PS1 but dramatically increased the amplitude of PS2 (299±69% of control after 30 min perfusion, Figure 1a, P<0.02, n=5), thus reducing paired-pulse depression. However, in slices prepared from neonatal animals the same concentration of WIN55,212-2 caused a profound inhibition of PS1, reducing the amplitude to 43±7% of control as measured after 30 min perfusion (Figure 1b, P<0.01, n=6). Since the amplitude of PS1 affects the strength of paired-pulse depression no further paired-pulse experiments were performed. In a separate set of neonate slices WIN55,212-2 showed a slightly smaller effect on the fEPSP, reducing the peak amplitude to 72±5% of control when measured at the same time point (Figure 1c, P<0.05, n=5). Both effects were very long lasting, showing little or no recovery after 1 h wash (data not shown). The inactive stereoisomer, WIN55,212-3 (500 nM) had no effect on the population spike recorded from neonate slices (Figure 2a, n=4). The CB1 receptor antagonist AM281 (500 nM) also had no effect on the population spike when perfused alone for 30 min, but completely blocked the effect of subsequently perfused WIN55,212-2 (n=5).
Figure 2.
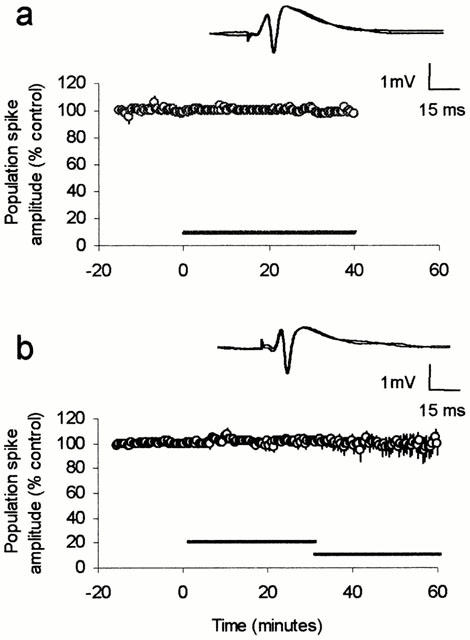
The effect of WIN55,212-2 in slices prepared from neonatal rats is receptor-mediated. (a) The inactive isomer, WIN55,212-3 (500 nM) perfused for 20 min had no effect on the population spike amplitude (n=4). (b) Perfusion of the CB1 receptor antagonist AM281 (500 nM) alone for 30 min had no effect on the population spike amplitude, but blocked the effect of the subsequently perfused WIN55,212-2 (500 nM for 30 min, n=5).
Discussion
Our results show that in the CA1 region of hippocampal slices prepared from young adult rats WIN55,212-2 had no effect on PS1 and therefore no apparent effect on excitatory synaptic transmission. The finding that in the very same slices, WIN55,212-2 perfused at the same concentration and for the same time caused a powerful reduction in paired-pulse depression shows that there was no problem with drug access in the adult tissue. In contrast, WIN55,212-2 did depress excitatory transmission in hippocampal slice prepared from neonatal rats. The fact that this inhibition is stereoselective indicates that it is receptor-mediated, and the observation that it is sensitive to AM281 suggests that specifically CB1 receptors are involved.
The finding that cannabinoid modulation of excitatory transmission in the hippocampus is developmentally regulated might explain some discrepancies regarding the published effects of WIN55,212-2. The previous work that has reported little or no effect of WIN55,212-2 on CA1 excitatory transmission has generally been performed in slices prepared from adults rats (Terranova et al, 1995; Paton et al, 1998). In contrast, experiments reporting that cannabinoid receptor activation reduced EPSC size by approximately 50% were performed on slices prepared from 13–19-day-old animals (Misner & Sullivan, 1999), or in cultured hippocampal cells (Shen et al, 1996; Sullivan, 1999; Misner & Sullivan, 1999).
It should be noted that there are exceptions which may require some other explanation. Most notably Ameri et al. (1999) report a profound inhibition of fEPSPs recorded from slices prepared from rats weighing 150–180 g. However, our experiments show that when interpreting the effects of cannabinoids on excitatory transmission, account must be taken of the developmental stage of the tissue used, and that one might expect to see a greater effect in tissue prepared from neonate as compared to adult animals.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AM281
1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide
- CB
cannabinoid
- EPSC
excitatory postsynaptic current
- field EPSP
excitatory postsynaptic potential
- GABA
γ-aminobutyric acid
- IPSC
inhibitory postsynaptic current
- PS1
population spike 1
- PS2
population spike 2
- WIN55,212-2
R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-(1-naphthalenyl)methanone mesylate
- WIN55,212-3
S(−)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-(1-naphthalenyl)methanone mesylate
References
- AL-HAYANI A., PATON G., PERTWEE R.G., DAVIES S.N. Cannabinoid effects on paired pulse depression in the rat hippocampal slice. Soc. Neurosci. Abst. 1999;25:585. [Google Scholar]
- AMERI A., WILHELM A., SIMMET T. Effects of the endogeneous cannabinoid, anandamide, on neuronal activity in rat hippocampal slices. Br. J. Pharmacol. 1999;126:1831–1839. doi: 10.1038/sj.bjp.0702478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON W.W., COLLINGRIDGE G.L. A data acquisition program for on-line analysis of long-term potentiation and long-term depression. Soc. Neurosci. Abst. 1997;23:665. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- HOFFMAN A.F., LUPICA C.R. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J. Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRVING A.J., COUTTS A.A., HARVEY J., RAE M.G., MACKIE K., PERTWEE R.G. Functional expression of cell surface cannabinoid CB1 receptors on presynaptic inhibitory terminals in rat cultured hippocampal neurons. Neurosci. 2000;98:253–262. doi: 10.1016/s0306-4522(00)00120-2. [DOI] [PubMed] [Google Scholar]
- KATONA I., SPERLAGH B., SIK A., KAFALVI A., VIZI E.S., MACKIE K., FREUND T.F. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axonal terminals of specific hippocampal interneurons. J. Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISNER D.L., SULLIVAN J.M. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J. Neurosci. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON G.S., PERTWEE R.G., DAVIES S.N. Correlation between cannabinoid mediated effects on paired pulse depression and induction of long term potentiation in the rat hippocampal slice. Neuropharmacol. 1998;37:1123–1130. doi: 10.1016/s0028-3908(98)00096-3. [DOI] [PubMed] [Google Scholar]
- SHEN M.X., PISER T.M., SEYBOLD V.S., THAYER S.A. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J. Neurosci. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULLIVAN J.M. Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J. Neurophysiol. 1999;82:1286–1294. doi: 10.1152/jn.1999.82.3.1286. [DOI] [PubMed] [Google Scholar]
- TERRANOVA J.-P., MICHAUD J.-C., LE FUR G., SOUBRIE P. Inhibition of long-term potentiation in rat hippocampal slices by anadamide and WIN55,212-2: reversal by SR141716A, a selective antagonist of CB1 cannabinoid receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:576–579. doi: 10.1007/BF00169393. [DOI] [PubMed] [Google Scholar]
- TSOU K., MACKIE K., SANUDOPENA M.C., WALKER J.M. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing gabaergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]


