Abstract
5-HT4 receptors mediate circular muscle relaxation in both human and canine large intestine, but this phenomenon alone can not explain the improvement in colonic motility induced by selective 5-HT4 receptor agonists in vivo. We set out to characterize 5-HT4 receptor-mediated effects in longitudinal muscle strips of canine and human large intestine.
Electrical field stimulation (EFS) was applied providing submaximal isotonic contractions. L-NOARG (0.1 mM) was continuously present in the organ bath to preclude nitric oxide-induced relaxation to EFS.
The selective 5-HT4 receptor agonist prucalopride (0.3 μM) enhanced EFS-evoked contractions, that were antagonized in both preparations by the selective 5-HT4 receptor antagonist GR 113808 (0.1 μM). The prucalopride-induced increase was present in canine ascending and descending colon, but absent in rectum. Regional differences in response to prucalopride were not observed in human ascending and sigmoid colon and rectum. Incubation with atropine (1 μM) or tetrodotoxin (0.3 μM) inhibited EFS-induced contractions, which were then unaffected by prucalopride (0.3 μM) in both tissues.
In the presence of methysergide (3 μM; both tissues) and granisetron (0.3 μM; only human tissues), 5-HT (0.3 μM) enhanced EFS-induced contractions, an effect that was antagonized by GR 113808 (0.1 μM). In the presence of atropine or tetrodotoxin, EFS-induced contractions were inhibited, leaving 5-HT (0.3 μM) ineffective in both preparations.
This study demonstrates for the first time that in human and canine large intestine, 5-HT4 receptors are located on cholinergic neurones, presumably mediating facilitating release of acetylcholine, resulting in enhanced longitudinal muscle contractility. This study and previous circular muscle strip studies suggest that 5-HT4 receptor agonism facilitates colonic propulsion via a coordinated combination of inhibition of circumferential resistance and enhancement of longitudinal muscle contractility.
Keywords: human, canine, 5-HT4, 5-HT receptor, contractility, large intestine, colon
Introduction
Since their discovery in mouse colliculi neurones (Dumuis et al., 1988), the study of 5-HT4 receptors in various tissues, cell lines and cell structures has provided a bulk of locations and actions of, and hypotheses around, 5-HT4 receptors. To start with, the 5-HT4 receptor subtype may be a generic term for up to 14 splice variants, that in theory may have different localization, transductional mechanisms and even operational characteristics (Gerald et al., 1995; Blondel et al., 1998; Bender et al., 2000). It remains to be determined, however, whether the observed differences at the protein level in cells and in distribution of splice variant mRNA in tissues correlate to functional responses across tissues or even across intact species. Nevertheless, if one considers 5-HT4 receptors in bioassays, their distributions and actions do differ markedly across species and across tissues.
In bioassays, it was demonstrated that 5-HT4 receptors are abundantly present in the gut (for review: Hegde & Eglen, 1996), but were also found in human brain (Reynolds et al., 1995; by means of binding studies), heart (Kaumann, 1993), adrenal cortex (Lefebvre et al., 1998) and bladder (Candura et al., 1996). In the gut, rat oesophagus muscularis mucosae contains smooth muscle 5-HT4 receptors mediating relaxation (Baxter et al., 1991), an effect suggested to be absent in the guinea-pig and dog (Cohen et al., 1994). In the human lower oesophageal sphincter, there is evidence for the presence of excitatory 5-HT4 receptors on the cholinergic nerves (Tomita et al., 1997). In the gastric area, contractile 5-HT4 receptors have been found on the cholinergic nerves in the guinea-pig (Buchheit & Buhl, 1994), dog (Bingham et al., 1995) and, based on preliminary data, man (Schuurkes et al., 1991). In the small intestine, 5-HT4 receptors mediate mucosal secretion (Budhoo et al., 1996) and smooth muscle relaxation (Kuemmerle et al., 1995). Coming down to the large intestine, 5-HT4 receptors were found on circular smooth muscle, mediating relaxation (Meulemans et al., 1995; McLean et al., 1995; Tam et al., 1994; Prins et al., 2000), and on the mucosa mediating secretion (Borman & Burleigh, 1996).
However, circular muscle relaxation alone can not only explain the improvement of propulsive large intestinal motility observed after administration of selective 5-HT4 receptor agonists in vivo. In the dog chronically equipped with force transducers on the serosal side of the large intestine, a model frequently used to study the influence of candidate drugs on gastrointestinal motility, 5-HT4 receptor stimulation resulted in enhancement of contractility in the ascending portion of the large intestine and an inhibition in the rectum (Briejer et al., 1998a,1998b). Recently, we reported this gradual increase in 5-HT4 receptor-mediated inhibition towards the distal large intestine to align our results with canine large intestinal circular muscle strips in vitro, where the relaxation was almost absent in the ascending colon and most pronounced in the rectal portion (Prins et al., 1999). However, the enhancement of proximal contractility observed in vivo was not explained. In view of the positive coupling of 5-HT4 receptors to adenylate cyclase, we hypothesized that an increase in colon muscle contractility could be due to 5-HT4 receptors facilitating release of a contractile neurotransmitter.
In order to test these hypotheses in dogs and humans, we set out to characterize the responses to the endogenous ligand 5-HT and the selective 5-HT4 receptor agonist prucalopride in electrical field-stimulated longitudinal muscle strips of canine and human large intestine.
Methods
Preparation
Fifteen Beagle dogs (13 female, 2 male, weighing 7–14 kg) were used. Ten of these dogs, that had been previously used for gastrointestinal in vivo studies, were allowed a two-week recovery period, after which they were sacrificed on the day of the experiment by decerebration and immediate desanguination through the carotid artery. The other five dogs had been used in cardiovascular in vivo studies (solvent treatment) the day before the experiment and were anaesthetized with pentobarbital (30 mg kg−1 i.v.), and, subsequently, sacrificed with KCl (150 mg kg−1 i.v.). There were no differences in any responses observed between tissue from dogs sacrificed either the day before or on the day of the experiment. The canine entire large intestine was isolated and approximate 3-cm sections of ascending colon (1 cm distal to the ileocolonic junction), rectum (at the point where the urethra (male) or vagina (female) is adhered to the rectum) and descending colon (in the middle between the ascending colonic and rectal portion) were dissected and mucosa and mesentery was cut away. Tissue taken from dogs sacrificed the day before the experiment was stored overnight in Krebs-Henseleit solution (containing (in mM) glucose 11.1; CaCl2 2.51; NaHCO3 25; MgSO4 1.18; KH2PO4 1.18; KCl 4.69 and NaCl 118) at 4°C. Human large intestinal specimens were obtained from patients undergoing surgery for colonic cancer, untreatable constipation or diverticular disease (ascending colon n=4; sigmoid colon n=13; rectum n=6) with the approval of the local ethics committee. The specimens were cut open longitudinally and the luminal contents, if present, were rinsed out with Krebs-Henseleit solution. Mucosa and adhering mesentery were cut away and the remaining muscle tissue was stored overnight in Krebs-Henseleit solution at 4°C.
Muscle strips (of the taeniae coli in case of human tissue) were cut in the longitudinal direction, each measuring approximately 2–3 mm width and 2–3 cm length. The strips were mounted onto organ bath hooks equipped for electrical field stimulation (EFS) with coaxial, platina-wire electrodes, and placed in an organ bath set-up for measurement of isotonic displacement under a load of 2 g.
Protocol
The strips were allowed a stabilization period of 30 min, after which carbachol (10 μM) was added twice to contract the tissue. Each addition of carbachol was followed by a period of time necessary to reach the maximal contraction, followed by replacing the organ bath solution twice with fresh Krebs-Henseleit solution.
Preliminary experiments showed that EFS could induce relaxations as well as contractions in both preparations. These relaxations were not sensitive to addition of 5-HT (0.3 μM) or prucalopride (0.3 μM), but they were prevented by incubation of L-NOARG (0.1 mM). Additionally, L-NOARG improved reproducibility of contractions to EFS. Thus, for further experimentation L-NOARG (0.1 mM) was added to the organ bath solution to preclude nitric oxide-induced relaxation to isolate contractions to EFS. In the case where 5-HT was used, methysergide (3 μM) was included in the organ bath solution to prevent interactions of 5-HT with 5-HT1, 5-HT2, 5-ht5, 5-HT6 or 5-HT7 receptors. In the case where 5-HT was tested in human tissue, granisetron (0.3 μM) was present as well to preclude 5-HT3 receptor interactions.
After 30 min of incubation in the presence of L-NOARG and/or methysergide or granisetron, the strips were electrically stimulated by pulses of 1 ms width in trains of 10 s duration at an interval of 3 min with a frequency of 50 Hz and a voltage of 20 V (one pulse train). Then, the frequency was set to 20 Hz (canine tissue) or 12 Hz (human tissue) and the voltage was reduced until approximately 50% of the contraction due to EFS at 50 Hz and 20 V. The submaximal EFS-induced contractions were followed until at least five reproducible contractions (spanning a time period of 15 min) were observed, and then antagonist or solvent was added. Following an incubation period of 15 min, equal to five EFS pulse trains, single doses of agonists were applied and the response to EFS was followed for another five pulse trains (equal to 15 min). Each strip was used to assess the effect of a single treatment on the effect of an agonist. Some strips were used to test the effect of pre-incubation with prucalopride (0.3 μM) against the cumulative concentration–contraction curve to exogenously added acetylcholine (30 nM–1 mM)
Data analysis
For statistical analysis and graphical presentation, the average of the contractions due to five EFS pulse trains observed prior to the addition of antagonist was taken as 100% contraction (called the initial value for graphical presentation) and all contractions (15 in total per muscle strip) were expressed as a percentage of this. Repeated measures over time (longitudinal experimental design) were analysed using PROC MIXED (SAS PC v 6.12) for balanced data with an unstructured covariance matrix, with a level of P<0.05 considered to indicate significance. The number of specimens used was denoted by n.
Compounds
The following compounds were used (with their abbreviations, if any, in italics, and respective suppliers given in parentheses): Tetrodotoxin (TTX), acetylcholine, 5-hydroxytryptamine creatinine sulphate (5-HT; Serva, Germany), atropine sulphate, NG-nitro-L-arginine (L-NOARG, Janssen Chimica, Belgium), [1-[2-[(methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl 1-methyl-1H-indole-3-carboxylate (GR 113808), granisetron HCl, 4-amino-5-chloro-2,3-dihydro-N-(1-[3-methoxypropyl]-4-piperidinyl)-7-benzofurancarboxamide HCl (prucalopride; R093877; Janssen Research Foundation), methysergide maleate (Sandoz, Switzerland). All compounds were dissolved and diluted freshly on the day of the experiment in 0.9% NaCl solution, except for GR 113808 and cisapride, which were dissolved in 0.9% NaCl acidified with tartaric acid in the stock solution. The solvents had no effect on the baseline length or the effect of agonists.
Results
Canine large intestine longitudinal muscle
Carbachol (10 μM) produced a stable contraction of ascending, descending and rectum longitudinal muscle strips. In ascending colon, prucalopride (0.3 μM) was without effect on basal muscle length, but in descending colon and rectum, prucalopride induced a small relaxation. Presence of L-NOARG (0.1 mM) did not affect these relaxations.
In the presence of L-NOARG (0.1 mM), electrical field stimulation resulted in stable (ascending and descending colon) and variable (rectum) contractions. The EFS-induced contractions of ascending and descending colon but not those of rectal muscle strips were enhanced by addition of prucalopride (0.3 μM) or 5-HT (0.3 μM) (Figures 1 and 2). As in dog ascending colon prucalopride did not induce a relaxation, and reproducible contractions to EFS were observed, we used ascending colon to perform further experimentation with.
Figure 1.
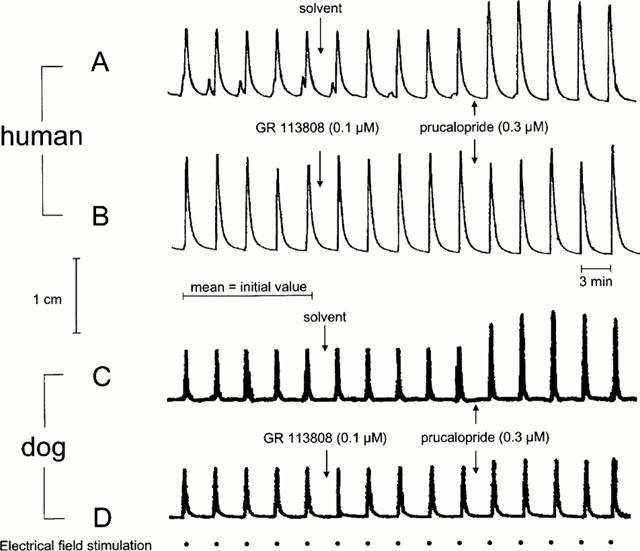
(A, B) Representative chart-recorder tracing of the effect of prucalopride on submaximal electrical field stimulation-induced contractions in the presence of L-NOARG (0.1 mM) of human isolated sigmoid colon (taeniae coli) longitudinal muscle and the effect of pre-incubation of the selective 5-HT4 receptor antagonist GR 113808 on the effect of prucalopride. (C, D) Representative chart-recorder tracing of the effect of prucalopride on submaximal electrical field stimulation-induced contractions in the presence of L-NOARG (0.1 mM) of canine isolated ascending colon longitudinal muscle and the effect of pre-incubation of the selective 5-HT4 receptor antagonist GR 113808 on the effect of prucalopride.
Figure 2.
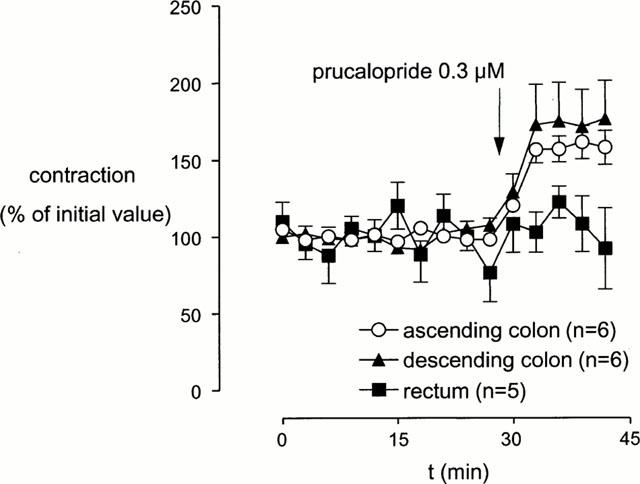
The effect of prucalopride on submaximal EFS-induced contractions in the presence of L-NOARG (0.1 mM) across various regions of the canine isolated large intestine longitudinal muscle. Data points are mean±s.e.mean of EFS-induced contractions.
In the presence of L-NOARG (0.1 mM), the selective 5-HT4 receptor agonist prucalopride (0.3 μM) enhanced submaximal EFS-induced contractions in ascending colon (Figure 3A). The selective 5-HT4 receptor antagonist GR 113808 (0.1 μM) did not affect the EFS-induced contractions, but antagonized the effect of prucalopride. Atropine (1 μM) and tetrodotoxin (TTX; 0.3 μM) reduced the EFS-induced contractions to a level not significantly different from zero and prucalopride (0.3 μM) did not modify this reduction. The effects of prucalopride under the conditions described are summarized in Table 1. In an additional set of experiments, prucalopride (0.3 μM) failed to affect the concentration–response curve to exogenously administered acetylcholine (n=3; data not shown).
Figure 3.
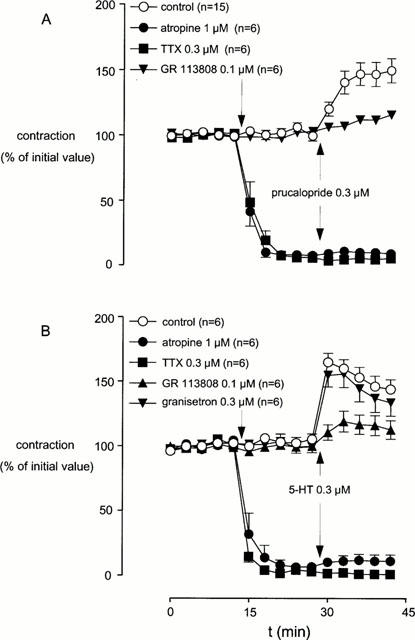
The effect of atropine, tetrodotoxin, granisetron and GR 113808 on the prucalopride-induced (A) or 5-HT-induced (B) enhancement of submaximal electrical field stimulation-induced contractions of canine isolated ascending colon longitudinal muscle. L-NOARG (0.1 mM) was present in the organ bath solution routinely, whereas methysergide (3 μM) was present when 5-HT was used as an agonist. Data points are mean±s.e.mean of EFS-induced contractions.
Table 1.
The effect of various treatments on the 5-HT (0.3 μM) or prucalopride (0.3 μM)-induced effect on electrical field stimulated longitudinal muscle strips of canine ascending colon tissue and human taeniae coli.
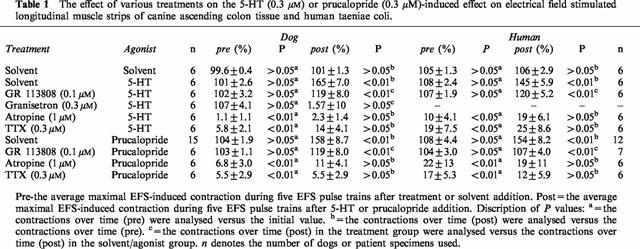
In the presence of L-NOARG (0.1 mM) and the mixed 5-HT1, 5-HT2, 5-ht5, 5-HT6 and 5-HT7 receptor antagonist methysergide (3 μM), 5-HT induced an enhancement of EFS-induced contractions, which was unaffected by pre-incubation with the selective 5-HT3 receptor antagonist granisetron (0.3 μM; Figure 3B). Selective 5-HT4 receptor blockade induced by GR 113808 (0.1 μM) did not affect EFS-induced contractions, but this pre-treatment inhibited the enhancement evoked by 5-HT (0.3 μM). Atropine (1 μM) and TTX (0.3 μM) blocked the EFS-induced contractions and 5-HT (0.3 μM) did not modify this reduction. The 5-HT-induced effects under the conditions applied are depicted in Table 1.
Human taeniae coli longitudinal muscle
The selective 5-HT4 receptor agonist prucalopride (0.3 μM) did not affect basal muscle length. In the presence of L-NOARG (0.1 mM), prucalopride (0.3 μM) enhanced submaximal EFS-induced contractions, any effect that was antagonized by the selective 5-HT4 receptor antagonist GR 113808 (0.1 μM; Figure 4A). Atropine (1 μM) and TTX (0.3 μM) reduced the EFS-induced contractions to a level not significantly different from zero and prucalopride (0.3 μM) failed to modify these reductions. The effects of prucalopride under the conditions described are summarized in Table 1. Similar to that observed in canine tissue, prucalopride (0.3 μM) did not modify the concentration–contraction curve to exogenously added acetylcholine (n=3; data not shown). In contrast to that observed in dog tissue, regional differences in response to prucalopride were not observed in human large intestine.
Figure 4.
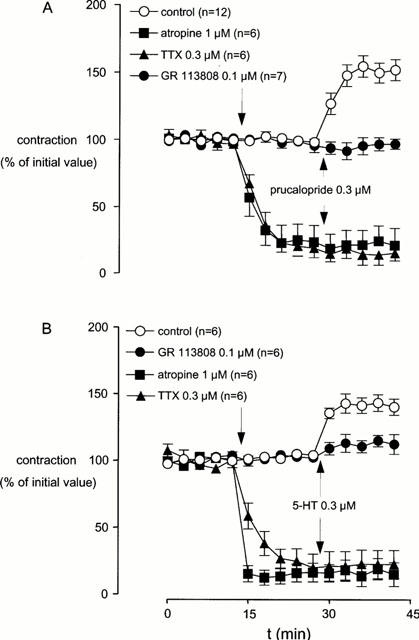
The effect of atropine, tetrodotoxin, and GR 113808 on the prucalopride-induced (A) and 5-HT-induced (B) enhancement of submaximal electrical field stimulation-induced contractions of human isolated large intestine (taeniae coli) longitudinal muscle (from ascending and sigmoid colon and rectum). L-NOARG (0.1 mM) was present in the organ bath solution routinely, whereas methysergide (3 μM) and granisetron (0.3 μM) were present as well in the case where 5-HT was used as an agonist. Data points represent mean±s.e.mean of EFS-evoked contractions.
Prucalopride induced in four rectum specimens a maximum effect (expressed as maximal increase of initial value) of 150±6%, in five sigmoid colon specimens 145±5% and in three ascending colon specimens 155±6%. These prucalopride-induced effects were not significantly different.
In the presence of L-NOARG (0.1 mM) and a cocktail of methysergide (3 μM) and granisetron (0.3 μM), 5-HT induced an increase in EFS-induced contractions (Figure 4B), without alteration of basal muscle length. Again, GR 113808 (0.1 μM) did not affect EFS-induced contractions, but antagonized the enhancement by 5-HT (0.3 μM). Atropine (1 μM) and TTX (0.3 μM) blocked the EFS-induced contractions and 5-HT (0.3 μM) could not modify these reductions. The 5-HT-induced effects under the conditions described are given in Figure 4B and Table 1.
Discussion
The data presented in this study clearly demonstrate that in canine and human large intestinal longitudinal muscle, 5-HT4 receptors mediate enhancement of contractions due to electrical field stimulation. These 5-HT4 receptors mediating contractility are most likely located on the cholinergic nerves. This is the first report demonstrating 5-HT4 receptors on cholinergic nerves of the large intestine of higher animal species, a phenomenon that has long been suggested to be absent.
The effect of the selective 5-HT4 receptor agonist prucalopride in both preparations pointed clearly to interactions with 5-HT4 receptors. Prucalopride expresses nanomolar affinity for 5-HT4 receptors and possesses approximately 1000 fold selectivity for 5-HT4 receptors over other 5-HT receptors (Briejer et al., 1998c; Janssen Research Foundation; data on file). Thus, the concentration used in this study (0.3 μM) producing an effect, strongly suggested 5-HT4 receptor involvement.
In canine ascending colon, involvement of 5-HT receptors other than 5-HT4 receptors in the increase in contractions due to 5-HT was ruled out by the blockade of 5-HT1, 5-HT2, 5-ht5, 5-HT6 and 5-HT7 receptors by methysergide and the lack of effect of the selective 5-HT3 receptor antagonist granisetron on the 5-HT-evoked effect. In experiments using human taeniae coli, the organ bath solution already contained a combination of methysergide and granisetron at concentrations expected to block every 5-HT receptor with the exception of 5-HT4 receptors (for which both methysergide and granisetron, at the concentrations used, do not express significant affinity). Therefore, it was expected that the 5-HT-induced amplification of contractions due to EFS was due to activation of 5-HT4 receptors. Indeed, the observation that the selective 5-HT4 receptor antagonist GR 113808 (Gale et al., 1994) was able to antagonize the enhancement induced by 5-HT in both preparations, strongly suggested involvement of 5-HT4 receptors, as was also corroborated by the blockade of the effect of prucalopride by GR 113808. GR 113808 expresses 5-HT4 receptor antagonist affinity at nanomolar concentrations (pKB estimates in human and canine tissues ranging from 8.8 to 9.4; Gale et al., 1994; Kaumann, 1993; Prins et al., 1999; 2000) and does not produce significant binding to all other 5-HT receptors up to 0.3 μM (Gale et al., 1994). Thus, the GR 113808-induced antagonism of the 5-HT- and prucalopride-induced responses indicates involvement of 5-HT4 receptors.
Due to the positive coupling of 5-HT4 receptors to adenylate cyclase smooth muscle relaxation would have been expected if these receptors were located on the smooth muscle. In contrast, the 5-HT4 receptor-mediated responses in the current study were contractile, which pointed to an indirect 5-HT4 receptor-mediated effect via depolarization of nerves releasing a contractile neurotransmitter. Indeed, involvement of nerves was demonstrated by the blockade of EFS-induced contractions in both preparations by TTX. The contractile neurotransmitter was shown to be acetylcholine, as indicated by the blockade induced by the non-selective muscarinic antagonist atropine, thus suggesting 5-HT4 receptors are located on the cholinergic nerves. Furthermore, the observation that prucalopride (0.3 μM) failed to affect the curve to exogenously administered acetylcholine ruled out the possibility of a post-junctional 5-HT4 receptor-mediated potentiation mechanism of acetylcholine-induced contraction or an acetylcholine esterase inhibition property of prucalopride at this concentration. Thus, in canine and human large intestine longitudinal muscle, pre-junctional 5-HT4 receptors located on cholinergic nerves mediated enhanced contractility provided these cholinergic nerves were operational. Earlier, 5-HT4 receptors on cholinergic nerves were found to mediate contraction of guinea-pig colonic segments (Briejer & Schuurkes, 1996). This study indicates that also non-rodent animal species including humans are endowed with 5-HT4 receptors on cholinergic nerves of large intestine longitudinal muscle.
Previously, it was assumed that the 5-HT4 receptor-mediated effects in the large intestine of these animal species were limited to circular muscle, where smooth muscle 5-HT4 receptors mediate relaxation. It was even suggested that a 5-HT4 receptor-mediated mechanism of release of acetylcholine was absent in the human colon, on basis of the observation that the 5-HT4 receptor agonist cisapride failed to modify unstimulated or EFS-stimulated release of 3H-acetylcholine from human colonic taenial muscle (Burleigh & Trout, 1985). Interestingly, the endogenous ligand 5-HT was not tested in that study. In comparison with 5-HT, cisapride has been reported a weak partial 5-HT4 receptor agonist in the guinea-pig colon (5-HT4 receptors on cholinergic nerves; Elswood et al., 1991) but also on smooth muscle 5-HT4 receptors in human colon (Tam et al., 1994). Thus, the 5-HT4 receptor agonism produced by cisapride might be insufficient to evoke release of acetylcholine from human colonic cholinergic nerves. In agreement with Burleigh & Trout's study, cisapride (1 μM) failed to induce an effect on EFS-induced contractions in the bioassay with human colon specimens as reported here (n=5; unpublished results). Assessment of the efficacy of 5-HT, prucalopride and cisapride in 3H-acetylcholine release experiments with human colonic tissue could further clarify this issue.
In conscious dogs chronically equipped with circularly orientated force transducers on the serosal side of the large intestine, prucalopride enhanced proximal and inhibited distal large intestinal contractility (Briejer et al., 1998a) through a 5-HT4 receptor-mediated mechanism (Briejer et al., 1998b). The current in vitro study demonstrates the presence of 5-HT4 receptors mediating contractility in the proximal to mid, but not the distal region of the canine large intestine longitudinal muscle. Additionally, we conducted some EFS experiments with circular muscle strips of canine ascending colon, and found that GR 113808 (0.1 μM) prevented the prucalopride (0.3 μM)-induced increase in EFS-induced contractions (n=6, unpublished results). Thus, the enhanced contractility due to 5-HT4 receptor activation is most likely not limited to the longitudinal muscle portion of dog large intestine but also affects the circular muscle. In this manner, the proximal enhancement of contractility in conscious dogs might correspond to the enhanced contractility observed in ascending colon (both muscle layers) in vitro. Further, our previous study with canine large intestine demonstrated smooth muscle 5-HT4 receptors mediating relaxation of circular muscle, which was almost absent in the ascending colon, but increased towards the rectum (Prins et al., 1999). As such, 5-HT4 receptors may influence canine large intestinal contractility by combination of proximal enhanced contractility and distal circular muscle inhibited contractility. Interestingly, in human tissue, regional differences in amplitude of responses after 5-HT4 receptor stimulation have not been found, neither in circular muscle relaxation (previous studies) nor in longitudinal muscle enhanced contractility (this study). Although the number of specimens from each region of human large intestine available to us was limited and unequally distributed along the large intestine, 5-HT4 receptor-mediated effects, indistinguishable in amplitude, were observed in specimens spanning from the proximal (ascending colon) to the distal (rectum) region. Thus, it seems unlikely that the regional differences in 5-HT4 receptor-mediated contractility in large intestine longitudinal muscle are similar in dogs and humans.
We conclude that in human large intestinal taenial longitudinal muscle strips, pre-junctional 5-HT4 receptors on cholinergic neurones facilitate contractility, presumably through release of acetylcholine. Regional differences in 5-HT4 receptor-mediated responses in human longitudinal muscle were not found in this study. In canine ascending colon and descending colon, but not in rectum longitudinal muscle strips pre-junctional 5-HT4 receptors on cholinergic nerves mediate enhancement of contractility. The stimulating properties of selective 5-HT4 receptor agonists on colonic motor function in vivo may be explained by inhibition of circular muscle resistance (previous studies) and stimulation of longitudinal muscle contractility (this study). Furthermore, the canine large intestine, which is frequently used in in vivo models to study motility, was found to have appreciable predictive value for the human large intestine with respect to 5-HT4 receptor function.
Acknowledgments
The authors wish to thank W. de Ridder for skilfull statistical evaluation of the results and the surgeons Dr V. Nieuwenhuijs (Diakonessenhuis Utrecht, The Netherlands) and Dr P. Cheyns (St. Jozef's General Hospital, Turnhout, Belgium) for providing human tissue.
Abbreviations
- EFS
electrical field stimulation
- TTX
tetrodotoxin
References
- BAXTER G.S., CRAIG D.A., CLARKE D.E. 5-Hydroxytryptamine4 receptors mediate relaxation of the rat oesophageal tunica muscularis mucosae. Naunyn-Schmiedebergs' Arch. Pharmacol. 1991;343:439–446. doi: 10.1007/BF00169544. [DOI] [PubMed] [Google Scholar]
- BENDER E., PINDON A., VAN OERS I., ZHANG Y.-B., GOMMEREN W., VERHASSELT P., JURZAK M., LEYSEN J., LUYTEN W. Structure of the human serotonin 5-HT4 receptor gene and cloning of a novel 5-HT4 splice variant. J. Neurochem. 2000;74:478–489. doi: 10.1046/j.1471-4159.2000.740478.x. [DOI] [PubMed] [Google Scholar]
- BINGHAM S., KING B.F., RUSHANT B., SMITH M.I., GASTER L., SANGER G.J. Antagonism by SB 204070 of 5-HT-evoked contractions in the dog stomach: an in-vivo model of 5-HT4 receptor function. J. Pharm. Pharmacol. 1995;47:219–222. doi: 10.1111/j.2042-7158.1995.tb05782.x. [DOI] [PubMed] [Google Scholar]
- BLONDEL O., GASTINEAU M., DAHMOUNE Y., LANGLOIS M., FISCHMEISTER R. Cloning, expression, and pharmacology of four human 5-hydroxytryptamine4 receptor isoforms produced by alternative splicing in the carboxyl terminus. J. Neurochem. 1998;70:2252–2261. doi: 10.1046/j.1471-4159.1998.70062252.x. [DOI] [PubMed] [Google Scholar]
- BORMAN R.A., BURLEIGH D.E. Human colonic mucosa possesses a mixed population of 5-HT receptors. Eur. J. Pharmacol. 1996;309:271–274. doi: 10.1016/0014-2999(96)00466-9. [DOI] [PubMed] [Google Scholar]
- BRIEJER M.R., SCHUURKES J.A.J. 5-HT3 and 5-HT4 receptors and cholinergic and tachykininergic neurotransmission in the guinea-pig proximal colon. Eur. J. Pharmacol. 1996;308:173–180. doi: 10.1016/0014-2999(96)00297-x. [DOI] [PubMed] [Google Scholar]
- BRIEJER M.R., GHOOS E., EELEN J., SCHUURKES J.A.J. Serotonin 5-HT4 receptors mediate the R093877-induced changes in contractile patterns in the canine colon. Gastroenterology. 1998b;112:A705. [Google Scholar]
- BRIEJER M.R., MEULEMANS A.L., BOSMANS J.-P., VAN DAELE P., SCHUURKES J.A.J. In vitro pharmacology of the novel enterokinetic R093877. Gastroenterology. 1998c;112:A704. [Google Scholar]
- BRIEJER M.R., VAN DAELE P., BOSMANS J-P., GHOOS E., EELEN J., SCHUURKES J.A.J. Dose-dependent effects after oral and intravenous administration of R093877 on colonic motility in conscious dogs. Gastroenterology. 1998a;112:A705. [Google Scholar]
- BUCHHEIT K.H., BUHL T. Stimulant effects of 5-hydroxytryptamine on guinea pig stomach preparations in vitro. Eur. J. Pharmacol. 1994;262:91–97. doi: 10.1016/0014-2999(94)90031-0. [DOI] [PubMed] [Google Scholar]
- BUDHOO M.R., HARRIS R.P., KELLUM J.M. The role of the 5-HT4 receptor in Cl- secretion in human jejunal mucosa. Eur. J. Pharmacol. 1996;314:109–114. doi: 10.1016/s0014-2999(96)00474-8. [DOI] [PubMed] [Google Scholar]
- BURLEIGH D.E., TROUT S.J. Evidence against an acetylcholine releasing action of cisapride in the human colon. Br. J. Clin. Pharmacol. 1985;20:475–478. doi: 10.1111/j.1365-2125.1985.tb05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANDURA S.M., MESSORI E., FRANCESCHETTI G.P., D'AGOSTINO G., VICINI D., TAGLIANI M., TONINI M. Neural 5-HT4 receptors in the human isolated detrusor muscle: effects of indole, benzimidazolone and substituted benzamide agonists and antagonists. Br. J. Pharmacol. 1996;118:1965–1970. doi: 10.1111/j.1476-5381.1996.tb15631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN M.L., SUSEMICHEL A.D., BLOOMQUIST W., ROBERTSON D.W. 5-HT4 receptors in rat but not guinea pig, rabbit or dog esophageal smooth muscle. Gen. Pharmacol. 1994;25:1143–1148. doi: 10.1016/0306-3623(94)90130-9. [DOI] [PubMed] [Google Scholar]
- DUMUIS A., BOUHELAL R., SEBBEN M., CORY R., BOCKAERT J. A nonclassical 5-hydroxytryptamine receptor positively coupled with adenylate cyclase in the central nervous system. Mol. Pharmacol. 1988;34:880–887. [PubMed] [Google Scholar]
- ELSWOOD C.J., BUNCE K.T., HUMPHREY P.P. Identification of putative 5-HT4 receptors in guinea-pig ascending colon. Eur. J. Pharmacol. 1991;196:149–155. doi: 10.1016/0014-2999(91)90421-l. [DOI] [PubMed] [Google Scholar]
- GALE J.D., GROSSMAN C.J., WHITEHEAD J.W., OXFORD A.W., BUNCE K.T., HUMPHREY P.P. GR 113808: a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br. J. Pharmacol. 1994;111:332–338. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERALD C., ADHAM N., KAO H.T., OLSEN M.A., LAZ T.M., SCHECHTER L.E., BARD J.A., VAYSSE P.J., HARTIG P.R., BRANCHEK T.A. The 5-HT4 receptor: molecular cloning and pharmacological characterization of two splice variants. EMBO J. 1995;14:2806–2815. doi: 10.1002/j.1460-2075.1995.tb07280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEGDE S.S., EGLEN R.M. Peripheral 5-HT4 receptors. FASEB. 1996;10:1398–1407. doi: 10.1096/fasebj.10.12.8903510. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. Blockade of human atrial 5-HT4 receptors by GR 113808. Br. J. Pharmacol. 1993;110:1172–1174. doi: 10.1111/j.1476-5381.1993.tb13937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUEMMERLE J.F., MURTHY K.S., GRIDER J.R., MARTIN D.C., MAKHLOUF G.M. Coexpression of 5-HT2A and 5-HT4 receptors coupled to distinct signaling pathways in human intestinal muscle cells. Gastroenterology. 1995;109:1791–1800. doi: 10.1016/0016-5085(95)90745-9. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE H., CONTESSE V., DELARUE C., VAUDRY H., KUHN J.M. Serotonergic regulation of adrenocortical function. Hormone Metab. Res. 1998;30:398–403. doi: 10.1055/s-2007-978904. [DOI] [PubMed] [Google Scholar]
- MCLEAN P.G., COUPAR I.M., MOLENAAR P. A comparative study of functional 5-HT4 receptors in human colon, rat oesophagus and rat ileum. Br. J. Pharmacol. 1995;115:47–56. doi: 10.1111/j.1476-5381.1995.tb16318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEULEMANS A.L., GHOOS E., CHEYNS P., SCHUURKES J.A.J. 5-HT-induced relaxations of human sigmoid colon are mediated via 5-HT4 receptors. Pflüger's Arch. Eur. J. Physiol. 1995;429:R9. [Google Scholar]
- PRINS N.H., SHANKLEY N.P., WELSH N.J., BRIEJER M.R., LEFEBVRE R.A., AKKERMANS L.M.A., SCHUURKES J.A.J. An improved in vitro bioassay for the study of 5-HT4 receptors in the human isolated large intestinal circular muscle. Br. J. Pharmacol. 2000;129:1601–1608. doi: 10.1038/sj.bjp.0703254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS N.H., VAN HASELEN J.F.W.R., LEFEBVRE R.A., BRIEJER M.R., AKKERMANS L.M.A., SCHUURKES J.A.J. Pharmacological characterization of 5-HT4 receptors mediating relaxation of canine isolated recturn circular smooth muscle. Br. J. Pharmacol. 1999;127:1431–1437. doi: 10.1038/sj.bjp.0702665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS G.P., MASON S.L., MELDRUM A., DE KECZER S., PARNES H., EGLEN R.M., WONG E.H. 5-Hydroxytryptamine (5-HT)4 receptors in post mortem human brain tissue: distribution, pharmacology and effects of neurodegenerative diseases. Br. J. Pharmacol. 1995;114:993–998. doi: 10.1111/j.1476-5381.1995.tb13303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUURKES J.A.J., MEULEMANS A.L., OBERTOP H., AKKERMANS L.M.A. 5-HT4 receptors on the human stomach. J. Gastrointest. Mot. 1991;3:199. [Google Scholar]
- TAM F.S., BUNCE K.T., HILLIER K., GROSSMAN C. Characterization of the 5-hydroxytryptamine receptor type involved in inhibition of spontaneous activity of human isolated colonic circular muscle. Br. J. Pharmacol. 1994;113:143–150. doi: 10.1111/j.1476-5381.1994.tb16186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMITA R., TANJOH K., MUNAKATA K. The role of motilin and cisapride in the enteric nervous system of the lower esophageal sphincter in humans. Surgery Today. 1997;27:985–992. doi: 10.1007/BF02385776. [DOI] [PubMed] [Google Scholar]


