Abstract
The agonist-specific coupling properties of the three cloned human α2-adrenoceptor subtypes have been compared, when expressed at similar levels in Chinese hamster ovary (CHO) cell lines, using noradrenaline and (±)-meta-octopamine as agonists.
Noradrenaline can couple the receptor to both the inhibition and stimulation of forskolin-stimulated cyclic AMP production in all three receptor subtypes, with the relative strength of the coupling to the pathways varying for each of the receptor subtypes.
meta-Octopamine selectively couples the α2A-adrenoceptor only to the inhibition of forskolin-stimulated cyclic AMP production. However, meta-octopamine couples the α2B- and α2C-adrenoceptors to both the inhibition and stimulation of forskolin-stimulated cyclic AMP production.
The relative potency of meta-octopamine to noradrenaline varies between the different α2-adrenoceptor subtypes. The effects of meta-octopamine are around two orders of magnitude less potent than those of noradrenaline on both the α2A- and α2B-adrenoceptor subtypes. In contrast, in the case of the α2C-adrenoceptor, meta-octopamine is only one order of magnitude less potent than noradrenaline in the stimulation of forskolin-stimulated cyclic AMP production and, in addition, is equipotent with noradrenaline in the inhibition of forskolin-stimulated cyclic AMP production and has an increased maximal response. This raises the possibility that meta-octopamine may have physiologically important actions via α2C-adrenoceptors in vivo.
The results show that the modulation of cyclic AMP production occurs in both a subtype- and agonist-specific manner for α2A-adrenoceptors and in a subtype specific manner for α2B- and α2C-adrenoceptors.
Keywords: α2-Adrenoceptor subtypes, octopamine, cyclic AMP, adenylyl cyclase, noradrenaline
Introduction
Agonist-specific coupling (agonist trafficking) of G-protein coupled receptors to different second messenger systems has been demonstrated for a wide range of receptors (see Evans et al., 1995b; Kenakin, 1995). We have previously shown that the meta- and para- isomers of the biogenic amine, octopamine, which is a naturally occurring ligand of sympathetic α-adrenoreceptors, can couple a cloned human α2A-adrenoceptor to multiple second messenger systems when expressed in a Chinese hamster ovary (CHO) cell line (Evans et al., 1995a; Airriess et al., 1996). In contrast to the catecholamines, which couple the α2A-adrenoceptor to both a concentration-dependent decrease and increase in the rate of cyclic AMP production, the structural isomers of octopamine were only able to couple the receptor to a concentration-dependent decrease in cyclic AMP production (Airriess et al., 1997). These results suggest that the cloned human α2A-adrenoceptor can be coupled selectively, by different endogenous agonists, to G-protein pathways mediating the regulation of adenylyl cyclase activity. We have also shown the importance of conserved serine residues in transmembrane domain V (TMV) of the human α2A-adrenoceptor in agonist-specific coupling of the receptor to the inhibition and stimulation of adenylyl cyclase activity by noradrenaline and the structural isomers of octopamine (Rudling et al., 1997; 1999).
All mammalian species appear to express three separate α2-adrenoceptor subtypes (Bylund et al., 1994). In humans, the three genes encoding these receptor subtypes have been cloned. They are designated α2C10, α2C4 and α2C2 based on their human chromosomal location. The cloned α2C10 gene corresponds to the α2A-pharmacological subtype (Kobilka et al., 1987), whilst the cloned α2C4 gene corresponds to the α2C-pharmacological subtype (Regan et al., 1988) and the α2C2 gene corresponds to the α2B-pharmacological subtype (Lomasney et al., 1990).
α2-Adrenoceptors are widely distributed in peripheral tissues and in the central nervous system where they carry out a wide range of functions (McGrath et al., 1989; Bylund et al., 1994; MacDonald et al., 1997; Docherty, 1998). Recent results from knock-out mouse strains suggest that α2A-adrenoceptors are likely to mediate most, but not all, of the classical effects ascribed to α2-adrenoceptor agonists. Similar studies on α2B-adrenoceptors indicate that they have a more restricted distribution in the brain being exclusively found in the thalamus. However, in peripheral blood vessels they may be responsible for the initial hypertensive effects observed upon the intravenous injection of α2-adrenoceptor agonists. Studies on the distribution of α2C-adrenoceptors show that they are again widely distributed in both the brain and peripheral tissues. At present their functional role remains enigmatic and it has been suggested that they may act to modulate subtly the actions of the other two α2-adrenoceptor subtypes and be involved in the fine tuning of responses (see MacDonald et al., 1997).
Evidence is accumulating that the three α2-adrenoceptor subtypes, as well as being pharmacologically distinct, also exhibit differences in their rates of desensitization, in their trafficking properties, in their association with scaffolding proteins and in their coupling capacities to second messenger systems, such as those involving adenylyl cyclase, protein kinase C, arachidonic acid and calcium (e.g. See Kukkonen et al., 1998; Peltonen et al., 1998; Pihlavisto et al., 1998; Audubert et al., 1999; Olli-Lähdesmäki et al., 1999; Prezeau et al., 1999; Takesono et al., 1999). The ability of the three α2-adrenoceptor subtypes to regulate the cyclic AMP second messenger pathway, has been extensively studied in numerous cell lines (Duzic & Lanier, 1992; Eason et al., 1992; 1994; Jansson et al., 1994b; Näsman et al., 1997; Pohjanoksa et al., 1997). However, a great degree of variability has been observed in the results obtained from these studies, which has been attributed variously to the receptor density, the environment of the cell line and the agonist used. In the present study, we report on the agonist-specific properties of noradrenaline and (±)meta-octopamine to couple the three α2-adrenoceptor subtypes, expressed at similar levels in CHO cells, to the modulation of adenylyl cyclase activity. A brief account of some of this work has already been published in abstract form (Rudling & Evans, 1998).
Methods
Cell culture
Transfected Chinese hamster ovary cells with low expression levels of either the cloned human α2A- (592.4±58.6 fmol receptor mg−1 protein, n=8), α2B- (446.9±52.6 fmol receptor mg−1 protein, n=8) or α2C- (465.6±26.5 fmol receptor mg−1 protein, n=8) adrenoceptor subtype, were kindly made available to the laboratory by Prof R.J. Lefkowitz, Howard Hughes Medical Institute, Duke University, Durham, U.S.A. The cells were grown to near confluency in cell culture at 37°C with 10% CO2. The culture medium consisted of Ham's F-12 nutrient mixture supplemented with 10% bovine foetal calf serum, penicillin (50 units ml−1) and streptomycin (50 μg ml−1). G-418 sulphate (50 μg ml−1) was also included in the culture medium to ensure only cells expressing the α2-adrenoceptor subtypes were selected. Twenty-four hour pre-incubation of the cells in growth medium containing pertussis toxin (200 ng ml−1), uncoupled the Gi-mediated inhibition of cyclic AMP production (see Rudling et al., 1999). Inhibition of the agonist-dependent Gs-mediated stimulation of cyclic AMP production was achieved by 24 h pre-incubation of cells in growth medium containing 20 μg ml−1 cholera toxin. This concentration of cholera toxin has previously been shown to be sufficient for complete ADP-ribosylation of all substrates, without being detrimental to the cells (Eason et al., 1992)
Membrane preparation
Cells were washed by rinsing 150 mm culture dishes with 5 ml of Dulbecco's phosphate buffered saline three times, to remove the culture medium. The cells were scraped into 4 ml of ice-cold lysis buffer (50 mM, NaPO4, pH 7.4, 1 mM MgSO4) and then washed with a further 4 ml of ice-cold lysis buffer. After the cells had been incubated on ice for 15 min, they were lysed with a Dounce homogenizer by performing 20 complete strokes using a small clearance pestle. The cell lysate was centrifuged at 1000×g for 5 min to pellet whole cells and cell nuclei. The supernatant was further centrifuged at 28,000×g for 30 min at 4°C to pellet the crude membrane fraction. The resulting membrane pellet was resuspended in lysis buffer and stored at −70°C. Protein concentrations were determined using a protein assay kit (Merck), based on the method of Bradford (1976), with bovine serum albumin as a reference standard.
Ligand binding studies
Radioligand binding assays using [3H]-yohimbine were carried out to determine the expression levels of the α2-adrenoceptor subtypes in the CHO cell lines. Membranes (100–150 μg protein) were incubated at 37°C for 20 min in the presence of 6 nM [methyl-3H]-yohimbine (91 Ci mmol−1) (Amersham Pharmacia Biotech) in binding assay buffer ((mM) NaCl 150, MgCl2 5, EDTA 20, Tris 50, pH 7.4) with varying concentrations of unlabelled yohimbine (expression level assays) or agonist (competitive displacement assays), in a final incubation volume of 500 μl. Non-specific binding was determined in the presence of 1 mM yohimbine (expression level assays) or 100 μM phentolamine (competitive displacement assays). The reaction was terminated by dilution with 500 μl of ice-cold buffer and centrifugation at 4°C at 20,800×g for 10 min. The pellets were washed with 1 ml of ice-cold buffer and recentrifuged. Finally, the pellets were resuspended in 100 μl of 0.1 M NaOH and added to 7 ml of scintillation fluid for counting.
Radioligand binding curves were analysed using a non-linear regression program of GraphPAD software. The binding data was best fitted by a one site model (see Rudling et al., 1999). The Bmax was then converted from c.p.m.s. to pmol mg−1 membrane protein. Data represent the means of three separate experiments performed in duplicate.
Cyclic AMP production
Cells were first washed, by rinsing culture plates (60 mm) with 3 ml of Dulbecco's phosphate buffered saline (PBS; Gibco), to remove culture medium. They were then incubated for 20 min at 37°C in PBS containing 100 μM 3-isobutyl-1-methyl-xanthine (IBMX; Sigma), a phosphodiesterase inhibitor. The cells were then exposed to solutions of agonists at specific concentrations in the presence of 10 μM forskolin (Sigma), a membrane permeant adenylyl cyclase activator, plus 100 μM IBMX. Solutions of 10 μM forskolin plus 100 μM IBMX alone were used to determine the control rate of forskolin-stimulated cyclic AMP production. Incubations were terminated after 20 min at 37°C, by removal of the PBS followed by the addition of 500 μl of ice-cold, acidified ethanol (60 ml absolute-EtOH : 1 ml 1 N HCl). The plates were scraped and pooled with two subsequent 250 μl washes with acidified ethanol. The cell debris was then removed by centrifugation at 17,900×g for 5 min. The supernatant was evaporated to dryness by means of a vacuum centrifuge (Savant) and the residue was re-suspended in 150 μl of Tris/EDTA buffer. Cyclic AMP levels were determined in duplicate using a [8-3H]-cyclic AMP assay kit (Biotrak TRK 432, Amersham Pharmacia Biotech).
Dose response curves for the various agonists, both with and without pertussis toxin or cholera toxin pre-treatment of the cells, were constructed for concentrations ranging from 0.1 nM to 1 mM. The concentration of cyclic AMP (pmol plate−1) in experimental dishes was expressed as a percentage of the [cyclic AMP] in the control dishes from the same group. Four control dishes were compared with each group of fourteen experimental dishes. pIC50 and/or pEC50 values were calculated for individual experiments where data sets reached a clear plateau by fitting to a sigmoidal dose-response (variable slope) equation. Means and s.e.mean were then calculated from the results of three or more individual experiments carried out in duplicate. For data sets that failed to reach a clear maximum over the concentration range tested only a maximum percentage of the control value reached is given.
The cyclic AMP levels obtained in the transfected CHO cell lines after forskolin-stimulation were 605±63 pmol/dish (n=12) for non-pretreated control cells, 368±31 pmol/dish (n=12) for PTX pretreated cells and 8093±444 pmol/dish (n=12) for CTX pretreated cells. Non-transfected CHO-K1 cells showed no response to treatment with either (−)-noradrenaline or (±)-meta-octopamine (data not shown).
Analysis of variance (ANOVA) was used to test for significant agonist-mediated effects in individual experiments. Significant ANOVA's were then further analysed by Tukey's HSD multiple comparison test, to determine at what concentration the levels of cyclic AMP production differed significantly from the forskolin-only control values. Unless otherwise stated, all data are shown as mean±s.e.mean.
Arachidonic acid release
Four hours prior to experimentation [3H]-arachidonic acid (214 Ci mmol−1, Amersham Pharmacia Biotech) was added to the CHO cell media (3 μCi/ml). The cells were washed with PBS containing 1% BSA and 10 mM glucose (PBG) and subsequently incubated with PBG containing 10 mM LiCl (PBG/Li) for 20 min at 37°C. The dishes were then stimulated with varying concentrations of agonist (dissolved in PBG/Li) for 20 min (37°C). At the end of the incubation period 200 μl aliquots of the incubation media were transferred to scintillation vials and counted for 5 min. Four independent experiments were conducted in triplicate for both (−)-noradrenaline and (±)-meta-octopamine for each receptor subtype.
Pharmaceutical compounds
(−)-Noradrenaline, IBMX and forskolin were from Sigma. Racemic meta-octopamine was from Aldrich. Pertussis toxin and cholera toxin were from Calbiochem.
Results
α2A-adrenoceptor cyclic AMP response
Our previous studies have shown an agonist specific coupling of the cloned human α2A-adrenoceptor to the modulation of cyclic AMP production when the receptor is expressed in Chinese hamster ovary (CHO) cells (Airriess et al., 1997; Rudling et al., 1999). However, suggestions have been made that such coupling properties may be dependent on the receptor expression level in clonal cell lines. Thus, to facilitate a comparison of these results with the other two cloned subtypes of the human α2-adrenoceptor, we have repeated our experiments on the human α2A-adrenoceptor using a cell line with similar expression levels to those expressing the α2B- and the α2C-adrenoceptor subtypes.
(−)-Noradrenaline
Following 20 min of incubation with (−)-noradrenaline, a maximum significant inhibition of cyclic AMP production to 75.1±7.6% (n=9) of the control value (F=2.46; d.f.=6,43; P<0.05) was seen in α2A-adrenoceptor expressing CHO cells exposed to an agonist concentration of 1 μM (Figure 1A). This inhibitory response had a pIC50 of 7.92±0.46. At higher concentrations of (−)-noradrenaline the inhibitory effects of the agonist were not significantly altered. In contrast to some previous studies (Fraser et al., 1989; Eason et al., 1992; Airriess et al., 1997; Rudling et al., 1999) in the transfected cell line used in this study, with a lower expression level, the coupling of the α2A-adrenoceptor to cyclic AMP production did not display a biphasic appearance. However, after 24 h pre-incubation with PTX, the inhibition of cyclic AMP production was abolished and replaced by a significant stimulation (F=7.24; d.f.=6,31; P<0.001) with a maximum effect at an agonist concentration of 1 mM (161.1±10.8% of control value; n=5) (Figure 1A). pEC50 values of 4.72 and 5.84 were obtained for this stimulatory phase in two independent experiments which reached a clear plateau, indicating that this occurred at a higher concentration than the pIC50 of the inhibitory phase. When α2A-adrenoceptor-transfected CHO cells were pre-incubated with CTX for 24 h, to persistently activate the stimulatory G-proteins (Gs), the dose response curve reached a maximum inhibitory level of 65.5±9.0% of control value (n=7) and had a pIC50 of 7.76±0.12 which did not significantly differ from that obtained in the absence of CTX pre-treatment (Figure 1A).
Figure 1.
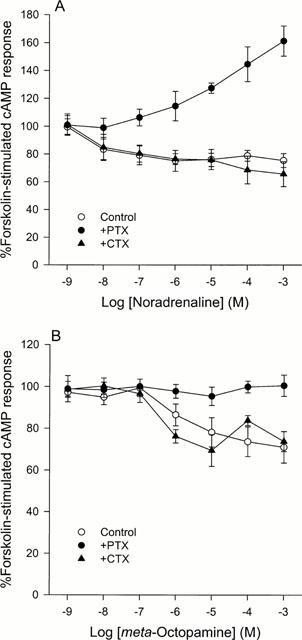
A comparison of the effects of (−)-noradrenaline (A) and (±)-meta-octopamine (B) on forskolin-stimulated cyclic AMP production in CHO cells stably expressing the cloned human α2A-adrenoceptor after no pretreatment or after pretreatment with either pertussis toxin or cholera toxin. (A) Noradrenaline inhibits forskolin-stimulated cyclic AMP production with a threshold occurring between 1 and 10 nM in control cells and in cells pretreated with cholera toxin but after pertussis toxin pretreatment it stimulates cyclic AMP production with a threshold occurring between 0.10 and 1 μM. Data represent means and vertical lines show s.e. mean, n=4–12. (B) (±)-meta-Octopamine is around two orders of magnitude less potent than noradrenaline at inhibiting forskolin-stimulated cyclic AMP production in control cells and in cells pretreated with cholera toxin, with a threshold occurring between 0.10 and 1 μM. However, after pertussis toxin pretreatment it did not couple the receptor to a stimulation of cyclic AMP production at any of the concentrations tested up to 1 mM. Data represent means and vertical lines show s.e. mean, n=5–11.
(±)-meta-Octopamine
Similar results, to those obtained using a CHO cell line expressing high receptor levels (Airriess et al., 1997; Rudling et al., 1999), were observed when CHO cells expressing low levels of α2A-adrenoceptors were exposed to (±)-meta-octopamine. Significant inhibition of cyclic AMP production occurred at (±)-meta-octopamine concentrations of 10 μM and above (F=4.17; d.f.=6,58; P<0.05) (Figure 1B). The inhibitory phase reached a maximum of 71.0±7.5% (n=8) of the control value and had a pIC50 of 5.30±0.39. However, the maximum inhibition of cyclic AMP production by (±)-meta-octopamine in this transfected cell line was about 10% less than that occurring with the cell line with the higher expression level (Airriess et al., 1997; Rudling et al., 1999). After pre-incubation with PTX, no stimulation of cyclic AMP production was revealed after exposure to (±)-meta-octopamine (Figure 1B). This is in contrast to the results obtained with (−)-noradrenaline after PTX pre-treatment. In addition, after CTX pre-treatment of the cells, (±)-meta-octopamine did not produce an enhanced inhibition of cyclic AMP production, compared with data obtained in the absence of CTX (Figure 1B).
α2B-adrenoceptor cyclic AMP response
(−)-Noradrenaline
Incubation of CHO cells stably expressing the cloned human α2B-adrenoceptor with (−)-noradrenaline, in the absence of either PTX or CTX pre-treatment, resulted in the agonist causing a maximum significant stimulation of cyclic AMP production when administered at 10 μM and above (695.1±172.0% of the control value; n=5) (F=4.38; d.f.=6,31; P<0.05) (Figure 2A). pEC50 values of 7.17 and 7.00 were obtained for this stimulatory phase in two independent experiments which reached a clear plateau. This dramatic stimulation was enhanced, by 2–3 fold, after pre-treating the cells with PTX, with the maximum significant effect occurring at an agonist concentration of 10 μM (1605.9±223.9% of the control value; n=7) (F=6.11; d.f.=6,58; P<0.001) (Figure 2A). pEC50 values of 6.41 and 6.85 were obtained for this stimulatory phase in two independent experiments which reached a clear plateau. However, although this implies that in the absence of PTX pre-treatment maximum stimulation was being prevented, CTX pre-treatment of the cells failed to reveal any significant inhibitory responses over the concentration range tested (P<0.05) (Figure 2A).
Figure 2.
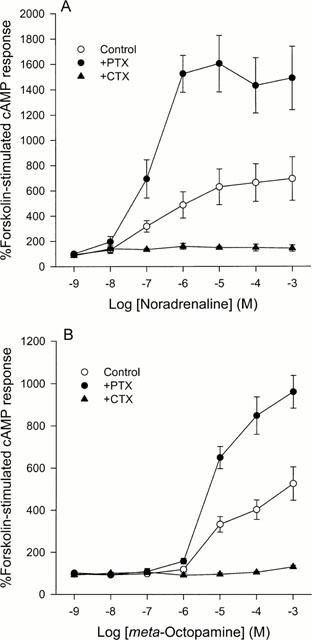
A comparison of the effects of (−)-noradrenaline (A) and (±)-meta-octopamine (B) on forskolin-stimulated cyclic AMP production in CHO cells stably expressing the cloned human α2B-adrenoceptor after no pretreatment or after pretreatment with either pertussis toxin or cholera toxin. (A) Noradrenaline potentiates forskolin-stimulated cyclic AMP production in control cells with a threshold occurring between 10 and 100 nM. After pertussis toxin pretreatment this effect is increased. After cholera toxin pretreatment, no inhibition of cyclic AMP production could not be demonstrated. Data represent means and vertical lines show s.e.mean, n=5–8. (B) (±)-meta-Octopamine produces similar effects to noradrenaline but is around two orders of magnitude less potent. Data represent means and vertical lines show s.e.mean, n=5–10.
(±)-meta-Octopamine
Stimulation of α2B-adrenoceptor transfected CHO cells with (±)-meta-octopamine, revealed similar dose response curves, under all three conditions, to those obtained with (−)-noradrenaline. A significant maximal stimulation of cyclic AMP production to 526.2±79.3% of control (n=11) occurred at agonist concentrations of 1 mM (F=19.27; d.f.=6,62; P<0.01) (Figure 2B). This stimulatory phase had a pEC50 of 4.99±0.20. However, the maximum stimulation caused by (±)-meta-octopamine was slightly less than that observed with (−)-noradrenaline. Again, this stimulation of cyclic AMP production was enhanced after PTX pre-treatment, with maximum significant effects being observed at a concentration of 1 mM (960.4±77.6% of the control value; n=6) (F=84.14; d.f.=6,38; P<0.001) (Figure 2B). pEC50 values of 5.27 and 5.43 were obtained for this stimulatory phase in two independent experiments which reached a clear plateau. In addition, CTX pre-treatment of the cells failed to reveal any significant inhibitory responses over the concentration range tested (P<0.05) (Figure 2B).
α2C-adrenoceptor cyclic AMP response
(−)-Noradrenaline
In marked contrast to the other α2-adrenoceptor subtypes after exposure to (−)-noradrenaline, the α2C-adrenoceptor did not show any significant effect on cyclic AMP production (P<0.05) in the absence of PTX or CTX pre-treatment (Figure 3A). However, after 24 h pre-treatment with either of the toxins, a change in the modulation of cyclic AMP production was revealed over the concentration range tested. After pre-incubation of the transfected CHO cells with PTX, activation of the α2C-adrenoceptors by (−)-noradrenaline revealed a significant stimulation of cyclic AMP production at concentrations of 1 μM and above (F=6.11; d.f.=6,58; P<0.001) (Figure 3A). A maximum effect of 159.2±7.6% (n=8) of the control value was observed, with a pEC50 of 6.58±0.12. Exposure of (−)-noradrenaline to cells pre-treated with CTX, resulted in an inhibition of cyclic AMP production being observed over the same concentration range as the stimulation was revealed after PTX pre-treatment (Figure 3A). This inhibition of cyclic AMP production reached a maximum significant level of 83.0±2.4% of control levels (n=9) at 1 μM (F=5.32; d.f.=6,42; P<0.01) and had a pIC50 of 6.66±0.15. The appearance of a significant stimulation and inhibition of cyclic AMP production at (−)-noradrenaline concentrations of 1 μM and above, after PTX and CTX pre-treatment, respectively, may explain why no net change in cyclic AMP production is observed in the absence of both toxins over the concentration range tested.
Figure 3.
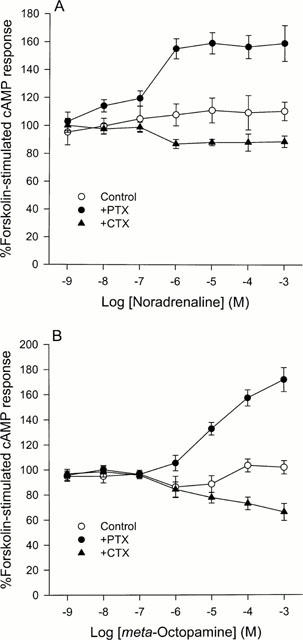
A comparison of the effects of (−)-noradrenaline (A) and (±)-meta-octopamine (B) on forskolin-stimulated cyclic AMP production in CHO cells stably expressing the cloned human α2C-adrenoceptor after no pretreatment or after pretreatment with either pertussis toxin or cholera toxin. (A) Noradrenaline does not appear to significantly alter forskolin-stimulated cyclic AMP levels in control cells up to a concentration of 1 mM. However, after pertussis toxin pretreatment it can produce a weak stimulation of cyclic AMP production and after cholera toxin it can produce a weak inhibition of cyclic AMP production. The threshold for both these responses occurs between 0.10 and 1 μM. Data represent means and vertical lines show s.e.mean, n=5–14. (B) (±)-meta-Octopamine produces similar effects to noradrenaline showing a concentration-dependent weak coupling to both the inhibition and stimulation of forskolin-stimulated cyclic AMP production. Data represent means and vertical lines show s.e.mean, n=5–12.
(±)-meta-Octopamine
After incubation with (±)-meta-octopamine, a maximum significant inhibition of cyclic AMP production was observed at an agonist concentration of 1 μM (F=4.02; d.f.=6,56; P<0.05) (Figure 3B). This inhibitory phase reached a maximum of 86.2±8.59% of control values (n=6) and had a pIC50 of 6.60±0.12. At higher concentrations of (±)-meta-octopamine the inhibitory effects decreased such that by 100 μM there was no significant variation (P<0.05) from the basal level of cyclic AMP production (101.8±2.2% of the control value). This stimulatory phase of the biphasic curve had a pEC50 of 4.93±0.20. However, after 24 h of pre-incubation with PTX, this inhibition was abolished and replaced by a dramatic stimulation with a maximum value of 171.5±9.6% (n=8) of control at 1 mM (±)-meta-octopamine (F=24.17; d.f.=6,42; P<0.001) (Figure 3B). Although (−)-noradrenaline was about an order of magnitude more potent than (±)-meta-octopamine at stimulating this response under these conditions, the maximum level of stimulation produced by the two agonists is comparable over the concentration range tested. However, (±)-meta-octopamine is equipotent with (−)-noradrenaline at coupling the α2C-adrenoceptor to the inhibition of cyclic AMP production. After CTX pretreatment of the cells, exposure to (±)-meta-octopamine concentrations of 1 μM and above results in a significant inhibition of cyclic AMP production with a maximal inhibition to 66.0±6.7% of control values at 1 mM (F=8.88; d.f.=6,42; P<0.001) (Figure 3B). pIC50 values of 6.81 and 5.68 were obtained for this inhibitory phase in two independent experiments. The maximum inhibition of cyclic AMP production by (±)-meta-octopamine was about 15% greater than that brought about by (−)-noradrenaline acting via the α2C-adrenoceptor.
Does increased cyclic AMP accumulation involve the activation of phospholipase A2?
The involvement of the activation of phospholipase A2 (PLA2) in the α2-adrenoceptor-mediated increases in cellular cyclic AMP levels in the presence of PTX appears to be controversial. Fraser et al. (1989) suggested that PLA2 activation could potentiate the agonist-mediated increases in cyclic AMP levels in the presence of PTX and that the effect depended on the receptor density in the transfected CHO cells. However, Jones et al. (1991) suggested that in CHO cells that this effect was not altered by the PLA2 inhibitor, quinacrine, and that further, the activation of PLA2 in these cells was blocked by PTX. The latter observations have recently been confirmed by Audubert et al. (1999). Thus, to examine whether PLA2 activation could underlie any of the agonist-mediated increases in cyclic AMP levels reported in the present investigation, we have assessed the ability of both (−)-noradrenaline and (±)-meta-octopamine to activate PLA2 activity by measuring the agonist-induced release of arachidonic acid from each of the three CHO cell lines expressing the α2-adrenoceptor subtypes at low expression levels. No significant agonist-stimulated release of arachidonic acid was observed for any of the cell lines studied at agonist concentrations between 1 nM and 1 mM (data not shown).
Ligand binding
The differences in the relative potencies of (−)-noradrenaline and (±)-meta-octopamine between the different α2-adrenoceptor subtypes in the functional assays on cyclic AMP accumulation could be explained by differences in the affinities of the agonists between the different α2-adrenoceptor subtypes. This possibility was tested directly by comparing the ability of (−)-noradrenaline and (±)-meta-octopamine to displace the binding of [3H]-yohimbine, an α2-adrenoceptor antagonist (see Table 1).
Table 1.
Ligand-binding properties of α2-adrenoceptor subtypes
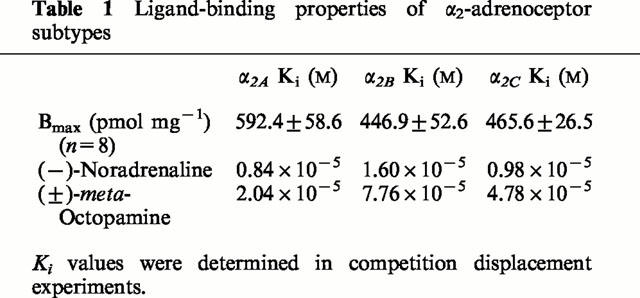
Figure 4 indicates that the binding affinity of each of the α2-adrenoceptor subtypes is greater for (−)-noradrenaline than for (±)-meta-octopamine. However, there are no substantial differences in binding affinity for either agonist at each of the α2-adrenoceptor subtypes. This suggests that differences in binding affinity are unlikely to underlie the differences in potency observed for the agonists tested in the functional assays on the accumulation of cyclic AMP by the cells expressing the α2-adrenoceptor subtypes.
Figure 4.
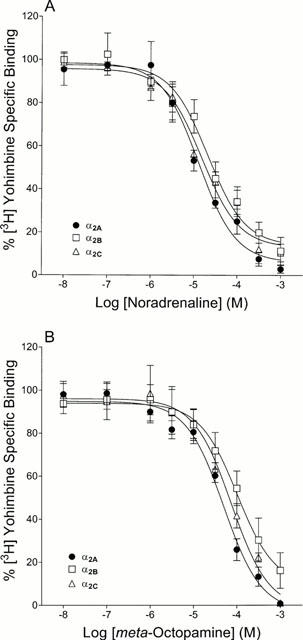
Competition binding curves of (−)-noradrenaline (A) and (±)-meta-octopamine (B) to the cloned human α2-adrenoceptor subtypes. Competition displacement experiments were performed on membranes prepared from transfected CHO cells expressing the different α2-adrenoceptor subtypes. Data represent means of three experiments performed in duplicate and vertical lines show s.e.mean.
Discussion
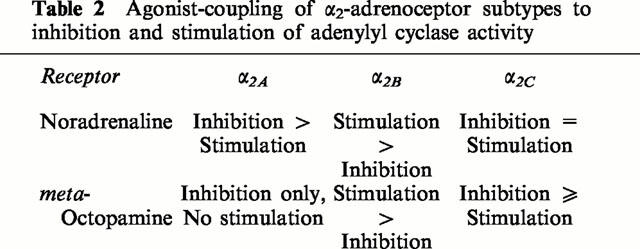
The cloning of the human genes encoding the three separate α2-adrenoceptor subtypes (Kobilka et al., 1987; Regan et al., 1988; Lomasney et al., 1990) has allowed the generation of stable cell lines expressing single receptor subtypes. These have allowed subtype-specific α2-adrenoceptor ligands to be screened, and provided the opportunity for α2-adrenoceptor-mediated signalling pathways to be studied in detail, without interference from other receptor subtypes. In the present study, CHO cells transfected to separately express the α2-adrenoceptor subtypes, at similar expression levels, were used to examine the ability of the agonists, noradrenaline and meta-octopamine, to couple the three receptor subtypes to the regulation of cyclic AMP production. It is apparent from the results obtained in this study (see Summary Table 2), that the modulation of cyclic AMP production occurs in both a subtype- and agonist-specific manner for α2A-adrenoceptors and in a subtype specific manner for α2B- and α2C-adrenoceptors.
Table 2.
Agonist-coupling of α2-adrenoceptor subtypes to inhibition and stimulation of adenylyl cyclase activity
In the present study, activation of the α2A-adrenoceptor expressed in CHO cells, by noradrenaline, results in the receptor coupling to an inhibition in forskolin-stimulated cyclic AMP production, which is similar to results obtained previously in several other cell lines including NIH-3T3 cells, Sf9 cells, Chinese hamster lung fibroblasts and S115 cells (Cotecchia et al., 1990; Duzic & Lanier, 1992; Marjamäki et al., 1992; Jansson et al., 1994a,1994b; 1995; Näsman et al., 1997). However, the results differ to previous studies involving CHO, HEK293, PC-12 and JEG-3 cells, where activation of α2A-adrenoceptors by noradrenaline resulted in a biphasic response in cyclic AMP production (Fraser et al., 1989; Duzic & Lanier, 1992; Eason et al., 1992; Pepperl & Regan, 1993; Svensson et al., 1996; Airriess et al., 1997; Jasper et al., 1998; Rudling et al., 1999). The receptor-mediated stimulation of adenylyl cyclase activity in CHO cells has previously been attributed to high receptor expression levels (Eason et al., 1992). Thus, since the expression level of the α2A-adrenoceptors in the CHO cells used in the present study was about 30 fold lower than in our previous studies with another CHO cell line (Rudling et al., 1999), this hypothesis may explain the lack of a biphasic dose-response curve in our present experiments. However, pertussis toxin pre-treatment of the cells resulted in a significant 60% increase in forskolin-stimulated cyclic AMP production being observed. This demonstrates that when the α2A-adrenoceptor is expressed at relatively low levels in a CHO cell line it is predominantly coupled to an inhibition in cyclic AMP production, but that it is also capable of producing a stimulatory response when the inhibitory pathway is blocked.
The differential agonist-specific coupling properties of the human α2A-adrenoceptor observed after exposure to noradrenaline and meta-octopamine in CHO cells expressing the receptor at high levels (Rudling et al., 1999), were also maintained at lower expression levels. This indicates that the phenomenon is not an artifact due to high expression levels.
In contrast to the α2A-subtype, the α2B-adrenoceptor could be coupled to the stimulation of forskolin-stimulated cyclic AMP production by both noradrenaline and meta-octopamine. However, meta-octopamine was around two orders of magnitude less potent than noradrenaline at producing this stimulatory response. These results agree with previous studies using PC-12, Sf9 and CHO cells, where activation of the α2B-adrenoceptor by various agonists caused a stimulatory effect on adenylyl cyclase activity (Duzic & Lanier, 1992; Jansson et al., 1995; Eason & Liggett, 1993). Similar findings have also been demonstrated by measuring the coupling of the α2B-subtype to cyclic AMP-dependent reporter gene expression in transiently transfected JEG-3 cells (Pepperl & Regan, 1993). However, when the α2B-subtype was expressed in S115 cells, it was able to couple to the stimulation of cyclic AMP production only after the cells had been pre-exposed to pertussis toxin, an effect not observed with the other two α2-adrenoceptor subtypes (Jansson et al., 1994b). In contrast, expression of the α2B-adrenoceptor in NIH-3T3 cells (Duzic & Lanier, 1992), CHO cells (Eason et al., 1992), astroglia cells (Enkvist et al., 1996), DDT1MF-2 cells (Duzic & Lanier, 1992), and NG108-15 cells (Sabol & Nirenberg, 1979) resulted in an inhibitory effect being observed upon activation of the receptor.
The results observed in the present study, after pre-treatment of the cells with pertussis toxin, suggest that the agonists tested are also capable of coupling the α2B-adrenoceptor to an inhibition of cyclic AMP production. After pertussis toxin treatment of the cells to block the inhibitory pathway, the stimulatory effect was magnified by 2–3 fold. This is likely to be due to pertussis toxin-insensitive, stimulatory G-proteins being able to couple the receptor to the stimulation of cyclic AMP production without competition from pertussis toxin-sensitive, inhibitory G-proteins (Duzic & Lanier, 1992). However, when cholera toxin was used to block the agonist-mediated stimulatory effect, no significant increases in the inhibition of cyclic AMP production were observed. Thus, these results suggest that the α2B-subtype is more strongly coupled to the stimulation of cyclic AMP production but also has the capability of producing a weak inhibitory effect on cyclic AMP production in the transfected CHO cells which can reduce the stimulatory effect.
The most likely mechanism for α2B-mediated stimulation of cyclic AMP production is a direct coupling to Gs (Eason et al., 1992; Jansson et al., 1995). The results from the present study support a direct coupling to Gs, as cholera toxin pretreatment of the cells resulted in the abolition of the agonist-mediated stimulation of cyclic AMP production and agonist stimulation did not lead to any increases in arachidonic acid production. In addition, the stimulatory effect was not blocked by pertussis toxin therefore excluding the possibility that adenylyl cyclase is stimulated by the βγ-subunits of activated inhibitory G-proteins.
Noradrenaline-mediated stimulation of the α2C-adrenoceptor, in the absence of toxin pre-treatment, does not appear to couple this subtype to either a net stimulation or inhibition of cyclic AMP production (present study). However, after the transfected CHO cells had been incubated with pertussis toxin or cholera toxin to prevent agonist-mediated coupling to Gi or Gs, respectively, subsequent cyclic AMP studies showed monophasic curves with either a stimulation or inhibition of cyclic AMP production. This suggests that in the absence of toxin pretreatment that the stimulatory and inhibitory effects at each concentration balance each other out. Previous studies examining the ability of α2C-adrenoceptors, when expressed in CHO cells, to regulate cyclic AMP production, have produced biphasic curves which can be dissected into stimulatory and inhibitory components after toxin treatment of the cells (Eason et al., 1992; Eason & Liggett, 1993). In these previous studies the expression levels of the receptor in the CHO cells was 5–15 fold greater than in the present study. Thus, variations in receptor expression levels may explain the differences observed between the present and previous studies. In addition, the fact that the α2C-subtype is equally coupled to the inhibition and stimulation of cyclic AMP production, is consistent with the idea that its true physiological role may be to modulate the effects mediated by the other two α2-adrenoceptor subtypes (MacDonald et al., 1997). However, it should be noted that when the α2C-adrenoceptor was expressed in cell types such as Chinese hamster lung fibroblasts, JEG-3 cells and S115 cells, the receptor coupled only to the inhibition of cyclic AMP production when activated by an agonist (Cotecchia et al., 1990; Marjamäki et al., 1992; Pepperl & Regan, 1993; Jansson et al., 1994b).
The effects of meta-octopamine are around two orders of magnitude less potent than those of noradrenaline on both the α2A- and α2B-adrenoceptors. In contrast, in the case of the α2C-adrenoceptor, meta-octopamine is equipotent with noradrenaline at inhibiting cyclic AMP production and only about one order of magnitude less potent at stimulating cyclic AMP production. In addition, a greater maximal inhibition of cyclic AMP production occurs with meta-octopamine than with noradrenaline. Therefore, the results from the current study, suggest that meta-octopamine may have a physiological role in activating the α2C-adrenoceptor. It is interesting to note that octopamine has also been suggested recently to possibly be an endogenous physiologically selective β3-adrenoceptor agonist (Carpéné et al., 1999).
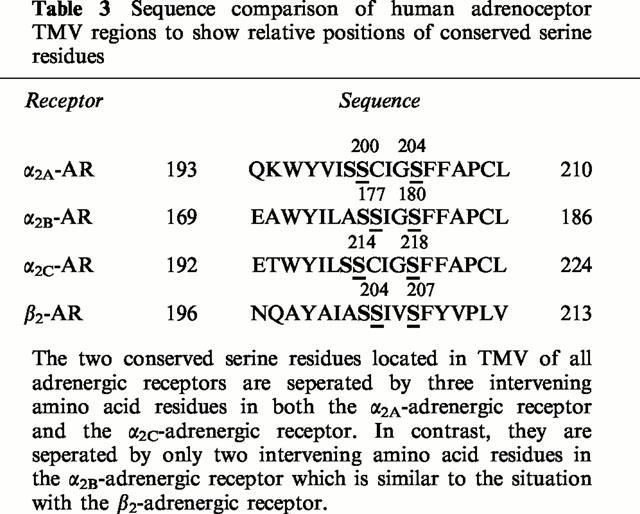
The subtype-selective differences in the modulation of cyclic AMP production observed in the present study, by expressing the α2-adrenoceptor subtypes at comparable levels in the same CHO cell line, may occur as a result of the conformational changes adopted by the receptor subtypes upon ligand binding since there appear to be no significant differences in agonist binding affinity between the different receptor subtypes. The conserved serine residues in transmembrane V (TMV) of adrenoceptors have been suggested to be involved in the interactions with the ring hydroxyl groups of catecholamine agonists (Strader et al., 1989; Wang et al., 1991; Hwa & Perez, 1996; Rudling et al., 1999; Sato et al., 1999). These serine residues are separated by three intervening amino acid residues (Cys, Ile, Gly) in both the human α2A- and α2C-adrenoceptors, but by only two intervening amino acid residues (Ile, Gly) in the human α2B-adrenoceptor, which is similar to the β2-adrenoceptor (Table 3). This similarity between the α2B- and β2-adrenoceptors may explain the ability of the α2B-subtype to produce an agonist-induced conformation of the receptor which strongly couples to the stimulation of forskolin-stimulated cyclic AMP production, in a similar way to the β2-adrenoceptor. Alternatively, the absence of the cysteine residue in the α2B-subtype, may either allow the receptor to produce an agonist-induced conformation which facilitates coupling to Gs, or inhibits the effective coupling of the receptor to Gi. Wang et al. (1991) have previously suggested that Cys201 of the α2A-adrenoceptor may have a role in interacting with the hydroxyl groups present on the catecholamine ring. Further experimentation is required to resolve this point.
Table 3.
Sequence comparison of human adrenoceptor TMV regions to show relative positions of conserved serine residues
Acknowledgments
We thank Prof R.J. Lefkowitz, Howard Hughes Medical Institute, Duke University, Durham, U.S.A. for making the transfected CHO cell lines expressing the cloned human α2-adrenoceptor subtypes available to us for this study. This work was funded by the BBSRC through the Babraham Institute. J.E. Rudling was funded by a BBSRC Postgraduate Studentship and J. Richardson by an MRC Postgraduate Studentship.
Abbreviations
- CHO
Chinese hamster ovary cells
- CTX
cholera toxin
- cyclic AMP
adenosine 3′:5′-cyclic monophosphate
- EDTA
ethylenediaminetetraacetic acid
- G-protein
guanosine 5′-triphosphate binding protein
- IBMX
3-isobutyl-1-methylxanthine
- PTX
pertussis toxin
- TMV
transmembrane domain five of G-protein coupled receptor
- Tris
Tris(hydroxymethyl)aminomethane
References
- AIRRIESS C.N., RUDLING J.E., CHEEK T.R., MIDGLEY J.M., EVANS P.D. Specific coupling of a cloned human α2A-adrenergic receptor to a phosphoinositide pathway by low doses of octopamine. Soc. Neurosci. Abstr. 1996;22:1317. [Google Scholar]
- AIRRIESS C.N., RUDLING J.E., MIDGLEY J.M., EVANS P.D. Selective inhibition of adenylyl cyclase by octopamine via a human cloned α2A-adrenoceptor. Br. J. Pharmacol. 1997;122:191–198. doi: 10.1038/sj.bjp.0701348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUDUBERT F., KLAPISZ E., BERGUERAND M., GOUACHE P., JOUNIAUX A.-M, , BÉRÉZIAT G., MASLIAH J. Differential potentiation of arachidonic acid release by rat α2-adrenergic receptor subtypes. Biochim. Biophys. Acta. 1999;1437:265–276. doi: 10.1016/s1388-1981(99)00018-9. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid sensitive method for the quantitation of protein utilizing the principle of dye-protein binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BYLUND D.B., EIKENBERG D.C., HIEBLE J.P., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., MOLINOFF P.B., RUFFOLO R.R., Jr, TRENDELENBURG U. IV. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- CARPÉNÉ C., GALITZKY J., FONTANA E., ATGIÉ C., LAFONTAN M., BERLAN M. Selective activation of β3-adrenoceptors by octopamine: comparative studies in mammalian fat cells. Naunyn-Shmiedeberg's Arch. Pharmacol. 1999;359:310–321. doi: 10.1007/pl00005357. [DOI] [PubMed] [Google Scholar]
- COTECCHIA S., KOBILKA B.K., DANIEL K.W., NOLAM R.D., LAPETINA E.Y., CARON M.G., LEFKOWITZ R.J., REGAN J.W. Multiple second messenger pathways of α-adrenergic receptor subtypes expressed in eukaryotic cells. J. Biol. Chem. 1990;265:63–69. [PubMed] [Google Scholar]
- DOCHERTY J.R. Subtypes of functional α1- and α2-adrenoceptors. Eur. J. Pharmacol. 1998;361:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- DUZIC E., LANIER S.M. Factors determining the specificity of signal transduction by guanine nucleotide-binding protein-coupled receptors. J. Biol. Chem. 1992;267:24045–24052. [PubMed] [Google Scholar]
- EASON M.G., LIGGETT S.B. Functional α2A-adrenergic receptor-Gs coupling undergoes agonist-promoted desensitization in a subtype-selective manner. Biochem. Biophys. Res. Comm. 1993;193:318–323. doi: 10.1006/bbrc.1993.1626. [DOI] [PubMed] [Google Scholar]
- EASON M.G., JACINTO M.T., LIGGETT S.B. Contribution of ligand structure to activation of α2-adrenergic receptor subtype coupling to Gs. Mol. Pharmacol. 1994;45:696–702. [PubMed] [Google Scholar]
- EASON M.G., KUROSE H., HOLT B.D., RAYMOND J.R., LIGGETT S.B. Simultaneous coupling of α2-adrenergic receptors to two G-proteins with opposing effects. J. Biol. Chem. 1992;267:15795–15801. [PubMed] [Google Scholar]
- ENKVIST M.O.K., HÄMÄLÄINEN H., JANSSON C.C., KUKKONEN J.P., HAUTALA R., COURTNEY M.J., ÅKERMAN K.E.O. Coupling of astroglial α2-adrenoceptors to second messenger pathways. J. Neurochem. 1996;66:2394–2401. doi: 10.1046/j.1471-4159.1996.66062394.x. [DOI] [PubMed] [Google Scholar]
- EVANS P.D., AIRRIESS C.N., SWALES L.S., CHEEK T.R., MIDGLEY J.M. Agonist-specific coupling of a cloned human α2A-adrenergic receptor to multiple second messengers. Soc. Neurosci. Abstr. 1995a;21:1613. [Google Scholar]
- EVANS P.D., ROBB S., CHEEK T.R., REALE V., HANNAN F.L., SWALES L.S., HALL L.M., MIDGLEY J.M. Agonist-specific coupling of G-protein-coupled receptors to second messenger systems. Prog. Brain Res. 1995b;106:259–268. doi: 10.1016/s0079-6123(08)61222-4. [DOI] [PubMed] [Google Scholar]
- FRASER C.M., ARAKAWA S., MCCOMBIE W.R., VENTER J.C. Cloning, sequence analysis, and permanent expression of a human α2-adrenergic receptor in Chinese hamster ovary cells. J. Biol. Chem. 1989;264:11754–11761. [PubMed] [Google Scholar]
- HWA J., PEREZ D.M. The unique nature of the serine interactions for α1-adrenergic receptor agonist binding and activation. J. Biol. Chem. 1996;271:6322–6327. doi: 10.1074/jbc.271.11.6322. [DOI] [PubMed] [Google Scholar]
- JANSSON C.C., KARP M., OKER-BLOM C., NÄSMAN J., SAVOLA J.M., ÅKERMAN K.E.O. Two human α2-adrenoceptor subtypes α2A-C10 and α2B-C2 expressed in Sf9 cells couple to transduction pathways resulting in opposite effects on cAMP production. Eur. J. Pharm. 1995;290:75–83. doi: 10.1016/0922-4106(95)90019-5. [DOI] [PubMed] [Google Scholar]
- JANSSON C.C., MARJAMÄKI A., LUOMALA K., SAVOLA J.M., SCHEININ M., ÅKERMAN K.E.O. Coupling of human α2-adrenoceptor subtypes to regulation of cAMP production in transfected S115 cells. Eur. J. Pharm. 1994b;266:165–174. doi: 10.1016/0922-4106(94)90106-6. [DOI] [PubMed] [Google Scholar]
- JANSSON C.C., SAVOLA J.M., ÅKERMAN K.E.O. Different sensitivity of α2A-C10 and α2C-C4 receptor subtypes in coupling to inhibition of cAMP accumulation. Biochem. Biophys. Res. Comm. 1994a;199:869–875. doi: 10.1006/bbrc.1994.1309. [DOI] [PubMed] [Google Scholar]
- JASPER J.R., LESNICK J.D., CHANG L.K., YAMANISHI S.S., CHANG T.K., HSU S.A.O., DAUNT D.A., BONHAUS D.W., EGLEN R.M. Ligand efficacy and potency at recombinant α2-adrenergic receptors. Biochem. Pharmacol. 1998;55:1035–1043. doi: 10.1016/s0006-2952(97)00631-x. [DOI] [PubMed] [Google Scholar]
- JONES S.B., HALENDA S.P., BYLUND D.B. Alpha 2-adrenergic receptor stimulation of phospholipase A2 and of adenylate cyclase in transfected Chinese hamster ovary cells is mediated by different mechanisms. Mol. Pharmacol. 1991;39:239–245. [PubMed] [Google Scholar]
- KENAKIN T. Agonist-receptor efficacy II: agonist trafficking of receptor signals. Trends Pharmacol. Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- KOBILKA B.K., MATSUI H., KOBILKA T.S., YANG-FENG T.L., FRANCKE U., CARON M.G., LEFKOWITZ R.J., REGAN J.W. Cloning, sequencing and expression of the gene coding for the human platelet α2-adrenergic receptor. Science. 1987;238:650–656. doi: 10.1126/science.2823383. [DOI] [PubMed] [Google Scholar]
- KUKKONEN J.P., RENVAKTAR A., SHARIATMADARI R., ÅKERMAN K.E.O. Ligand-and subtype-selective coupling of human alpha-2 adreneoceptors to Ca++ elevation in chinese hamster ovary cells. J. Pharm. Exp. Therap. 1998;287:667–671. [PubMed] [Google Scholar]
- LOMASNEY J.W., LORENZ W., ALLEN L.F., KING K., REGAN J.W., YANG-FENG T.L., CARON M.G., LEFKOWITZ R.J. Expansion of the α2-adrenergic receptor family: Cloning and characterization of a human kidney α2-adrenergic receptor subtype, the gene for which is located on chromosome 2. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5094–5098. doi: 10.1073/pnas.87.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONALD E., KOBILKA B.K., SCHEININ M. Gene targeting–homing in on α2-adrenoceptor-subtype function. Trends Pharmacol. Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- MARJAMÄKI A., ALA-UOTILA S., LUOMALA K., PERÄLÄ M., JANSSON C., JALKANEN M., REGAN J.W., SCHEININ M. Stable expression of recombinant human α2-adrenoceptor subtypes in two mammalian cell lines: characterization with [3H] rauwolscine binding, inhibition of adenylate cyclase and RNase protection assay. Biochim. Biophys. Acta. 1992;1134:169–177. doi: 10.1016/0167-4889(92)90041-9. [DOI] [PubMed] [Google Scholar]
- MCGRATH J.C., BROWN C.M., WILSON V.G. Alpha-adrenoceptors: A critical review. Med. Res. Rev. 1989;9:407–533. doi: 10.1002/med.2610090403. [DOI] [PubMed] [Google Scholar]
- NÄSMAN J., JANSSON C.C., ÅKERMAN K.E.O. The second intracellular loop of the α2A-adrenergic receptor determines subtype-specific coupling to cAMP production. J. Biol. Chem. 1997;272:9703–9708. doi: 10.1074/jbc.272.15.9703. [DOI] [PubMed] [Google Scholar]
- OLLI-LÄHDESMÄKI T., KALLIO J., SCHEININ M. Receptor subtype-induced targeting and subtype-specific internalization of human α2A-adrenoceptors in PC12 cells. J. Neuroscience. 1999;19:9281–9288. doi: 10.1523/JNEUROSCI.19-21-09281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELTONEN J.M., PIHLAVISTO M., SCHEININ M. Subtype-specific stimulation of [35S]GTPγS binding by recombinant α2-adrenoceptors. Eur. J. Pharmacol. 1998;355:275–279. doi: 10.1016/s0014-2999(98)00518-4. [DOI] [PubMed] [Google Scholar]
- PEPPERL D.J., REGAN J.W. Selective coupling of α2-adrenergic receptor subtypes to cyclic AMP-dependent reporter gene-expression in transiently transfected JEG-3 cells. Mol. Pharmacol. 1993;44:802–809. [PubMed] [Google Scholar]
- PIHLAVISTO M., SJÖHOLM B., SCHEININ M., WURSTER S. Modulation of agonist binding to recombinant human α2-adrenoceptors by sodium ions. Biochim. Biophys. Acata. 1998;1448:135–146. doi: 10.1016/s0167-4889(98)00118-9. [DOI] [PubMed] [Google Scholar]
- POHJANOKSA K., JANSSON C.C., LUOMALA K., MARJAMÄKI A., SAVOLA J.M., SCHEININ M. α2-Adrenoceptor regulation of adenylyl cyclase in CHO cells: dependence on receptor density, receptor subtype and current activity of adenylyl cyclase. Eur. J. Pharmacol. 1997;335:53–63. doi: 10.1016/s0014-2999(97)01154-0. [DOI] [PubMed] [Google Scholar]
- PREZEAU L., RICHMAN J.G., EDWARDS S.E., LIMBIRD L.E. The ζ isoform of 14-3-3 proteins interacts with the third intracellular loop of different α2-adrenergic receptor subtypes. J. Biol. Chem. 1999;274:13462–13469. doi: 10.1074/jbc.274.19.13462. [DOI] [PubMed] [Google Scholar]
- REGAN J.W., KOBILKA T.S., YANG-FENG T.L., CARON M.G., LEFKOWITZ R.J., KOBILKA B.K. Cloning and expression of a human kidney cDNA for an α2-adrenergic receptor subtype. Proc. Natl. Acad. Sci. U.S.A. 1988;85:6301–6305. doi: 10.1073/pnas.85.17.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDLING J.E., AIRRIESS C.N., EVANS P.D. The effect of site-directed mutagenesis on agonist-specific coupling of a cloned human α2A-adrenergic receptor. Soc. Neurosci. Abstr. 1997;23:2323. [Google Scholar]
- RUDLING J.E., EVANS P.D. A comparison of agonist-specific coupling of cloned human α2-adrenergic receptor subtypes. Soc. Neurosci. Abstr. 1998;24:596. [Google Scholar]
- RUDLING J.E., KENNEDY K., EVANS P.D. The effect of site-directed mutagenesis of two transmembrane serine residues on agonist-specific coupling of a cloned human α2A-adrenoceptor to adenylyl cyclase. Br. J. Pharmacol. 1999;127:877–886. doi: 10.1038/sj.bjp.0702614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABOL L.S., NIRENBERG M. Regulation of adenylate cyclase of neuroblastoma x glioma hybrid cells by α-adrenergic receptors. J. Biol. Chem. 1979;254:1913–1920. [PubMed] [Google Scholar]
- SATO T., KOBAYASHI H., NAGAO T., KUROSE H. Ser203 as well as Ser204 and Ser207 in fifth transmembrane domain of the human β2-adrenoceptor contributes to agonist binding and receptor activation. Br. J. Pharmacol. 1999;128:272–274. doi: 10.1038/sj.bjp.0702813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRADER C.D., CANDELORE M.R., HILL W.S., SIGAL I.S., DIXON R.A.F. Identification of two serine residues involved in agonist activation of the β-adrenergic receptor. J. Biol. Chem. 1989;23:13572–13578. [PubMed] [Google Scholar]
- SVENSSON S.P.S., BAILEY T.J., PORTER A.C., RICHMAN J.G., REGAN J.W. Heterologous expression of the cloned guinea pig α2A, α2B and α2C-adrenoceptor subtypes. Biochem. Pharmacol. 1996;51:291–300. doi: 10.1016/0006-2952(95)02179-5. [DOI] [PubMed] [Google Scholar]
- TAKESONO A., ZAHNER J., BLUMER K.J., NAGAO T., KUROSE H. Negative regulation of α2-adrenergic receptor-mediated G1 signalling by a novel pathway. Biochem. J. 1999;343:77–85. [PMC free article] [PubMed] [Google Scholar]
- WANG C.-D., BUCK M.A., FRASER C.M. Site-directed mutagenesis of α2A-adrenergic receptors: Identification of amino acids involved in ligand binding and receptor activation by agonists. Mol. Pharmacol. 1991;40:168–179. [PubMed] [Google Scholar]


