Abstract
In the rat hepatic artery, the endothelium-derived hyperpolarizing factor (EDHF) was identified as potassium. Potassium hyperpolarizes the smooth muscles by gating inward rectified potassium channels and by activating the sodium-potassium adenosine triphosphatase (Na+-K+ATPase). Our goal was to examine whether potassium could explain the EDHF in porcine coronary arteries.
On coronary strips, the inhibition of calcium-dependent potassium channels with 100 nM apamin plus 100 μM charibdotoxin inhibited the endothelium-dependent relaxations, produced by 10 nM substance P and 300 nM bradykinin and resistant to nitro-L-arginine and indomethacin.
The scavenging of potassium with 2 mM Kryptofix 2.2.2 abolished the endothelium-dependent relaxations produced by the kinins and resistant to nitro-L-arginine and indomethacin.
Forty μM 18α glycyrrethinic acid or 50 μM palmitoleic acid, both uncoupling agents, did not inhibit these kinin relaxations. Therefore, EDHF does not result from an electrotonic spreading of an endothelial hyperpolarization.
Barium (0.3 nM) did not inhibit the kinin relaxations resistant to nitro-L-arginine and indomethacin. Therefore, EDHF does not result from the activation of inward rectified potassium channels.
Five hundred nM ouabain abolished the endothelium-dependent relaxations resistant to nitro-L-arginine and indomethacin without inhibiting the endothelium-derived NO relaxation.
The perifusion of a medium supplemented with potassium depolarized and contracted a coronary strip; however, the short application of potassium hyperpolarized the smooth muscles.
These results are compatible with the concept that, in porcine coronary artery, the EDHF is potassium released by the endothelial cells and that this ion hyperpolarizes and relaxes the smooth muscles by activating the Na+-K+ATPase.
Keywords: Substance P, bradykinin, EDHF, potassium, Na+-K+ ATPase, ouabain, inward rectified potassium channel, barium
Introduction
In porcine coronary arteries, the two kinins substance P (SP) and bradykinin (BK), relax the smooth muscles in an endothelium-dependent manner by releasing nitric oxide from the endothelium, and by triggering the phenomenon known as endothelium-derived hyperpolarizing factor (EDHF) (Bény & Brunet 1988; Pacicca et al., 1992). During these endothelium-dependent relaxations caused by kinins, the membrane potential of endothelial cell and of underlying smooth muscle cells simultaneously hyperpolarizes in the same manner (Bény et al., 1986; 1987; Brunet & Bény, 1989; Bény, 1990a,1990b). It is not known whether the two hyperpolarizations are cause-effect related. Two different hypothesis that link EDHF to the endothelial and the smooth muscle cell hyperpolarizations could explain the synchrony of these two electrical events. EDHF could be either the resultant of an electrotonic conduction of the endothelial cell hyperpolarization to the neighbouring smooth muscle cells or it could be potassium ions released by the endothelial cells during their hyperpolarizations (Chaytor et al., 1998; Edwards et al., 1998; Dora et al., 1999; Yamamoto et al., 1999). Depending upon the tissue, potassium ions would hyperpolarize the smooth muscle cells either by gating inward rectified potassium channels or by activating the sodium-potassium adenosine triphosphatase (Na+-K+ATPase), or both (Knot et al., 1996; Edwards et al., 1998; Prior et al., 1998).
Our goal is to determine which of these mechanisms explains the phenomenon of EDHF in porcine coronary arteries. Namely, whether EDHF is the manifestation of an electrical coupling between the endothelial and the smooth muscle cells or whether it is potassium ions.
Methods
Preparation of tissues
Anterior descending branches of domestic pig Sus scrofa coronary arteries were obtained at the slaughterhouse. The coronary lumen was rinsed by injection of cold, oxygenated (95% O2, 5% CO2) Krebs solution (mM: NaCl 118.7, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, NaHCO3 24.8, MgSO4 1,2, glucose 10.1, pH 7.3–7.4). Adherent tissue was removed from sections of the coronary artery. Rings of about 2 mm width obtained from these sections were cut longitudinally to give strips of about 5 mm in length parallel to the circular smooth muscles. For some experiments, the endothelium was removed by gently rubbing the internal surface of the strip with a cotton tip. To check that the endothelium had been removed by this procedure, the response of the strip to SP was tested (Pacicca et al., 1992).
Pharmacological experiments
When mechanical tension was measured to obtain concentration-response curves, ligatures were attached to both ends of the strips, and these were mounted with a resting isometric tension of about 5 mN in a 85 μl tissue bath as previously described (Pacicca et al., 1992). To establish the concentration-response curves for SP and BK, 0.3 μM U-46619 was added to the perfusion fluid throughout the experiment to produce a reproducible state of initial tension in the strip. The tension stabilized to about 5 mN. The strips were superfused with Krebs' solution containing each concentration of the peptide in an ascending, non-cumulative manner for a sufficient time to allow full relaxation. A 10–20 min washout period was allowed between successive concentrations. Peptides were administered to the preparations by diluting them directly in the plastic beaker that contained the perfusion solution. The inhibitors of nitric oxide and prostacyclin synthesis were administered to the strips in the perfusion fluid at concentrations of 100 μM nitro-L-arginine (L-NA) and 10 μM indomethacin for at least 25 min before the first application of the kinins.
When testing the effect of the potassium chelator cryptate 2.2.2, only one concentration (the endothelium-derived relaxing factor (EDRF) inhibitory concentration (IC50)) of each peptide was used. Preliminary experiments demonstrated tachyphylaxis in the presence of the chelator. Since tachyphylaxis would hide the peptide-induced relaxation shown in a typical concentration-response curve, it was necessary to test only one concentration of each kinin.
Electrophysiological experiments
The methods used were described previously (Bény et al., 1986). Briefly, mechanical tension and transmembrane potential were measured simultaneously, the strip was incubated in a 100 μl Perspex bath continuously perfused with oxygenated Krebs (1.250 μl min−1) at 36°C with a peristaltic pump. An extremity of the strip was pinned on a silicon rubber surface with the intimal surface facing up. The other extremity was fixed horizontally to a force transducer. Changes in tension were measured isometrically with a transducer (Grass FT03C, U.S.A.), amplified with a carrier frequency bridge (Philips PR 9307, Netherlands) and recorded on a polygraph (W&W Electronics, U.S.A.). A force of 10 mN was first applied to the strip by pulling the transducer with a micromanipulator. In some experiments, the application of 0.3 μM U 46619 or 10 μM acetylcholine was then added to the perfusion fluid throughout the experiment to produce a reproducible state of initial tension in the strip. Acetylcholine was commonly used instead of U-46619 since the stable plateau phase of acetylcholine contraction is smaller than that caused by U-46619 thus reducing the amplitude of relaxations which could prevent cell recording with the microelectrode. Neither acetylcholine nor U-46619 change the smooth muscle cell membrane potential while they contract the coronary strip (Ito et al., 1979). The membrane potential was measured using a conventional glass microelectrode (60–80 MΩ) filled with 3 M KCl. The cells were impaled near the fixed points of the tissue in order to reduce problems associated with muscle movement. To impale an endothelial cell, the electrode was gradually moved towards the intima until the sudden appearance of a negative potential at the electrode (Bény, 1990a). KCl (0.5 to 3 M) was injected in 2 to 10 μl aliquots during 0.5 s directly into the perifusion fluid with a Gilson microman pipette. The tip of the pipette was applied to the surface of the incubation medium at different positions between the inlet and the outlet (aspiration tubing). These different positions changed the transit time of the ‘cloud' of KCl from about 2 to 5 s. The purpose of such applications of exogenous KCl was to mimic a transient release of this ion from the endothelial cells in localized areas, such as the myoendothelial bridges.
Preparation of peptides and chemicals
The peptides bradykinin and substance P were each prepared at a concentration of 1 mg ml−1 in 0.25% acetic acid, nifedipine at a concentration of 30 mM in water, cromakalin at a concentration of 10 mM in dimethylsulphoxide and acetylcholine at a concentration of 1 mg ml−1 in 0.9% NaCl. These solutions were stored as aliquots of 100 or 50 μl at −20°C until use. U-46619 was prepared at a concentration of 1 mg ml−1 in 75% ethanol. The inhibitors were prepared at a concentration of 10 mg ml−1 in 0.02% HCl for L-NA and at a concentration of 2 mg ml−1 in >99.8% ethanol for indomethacin. The U-46619, the peptides and the inhibitors were diluted subsequently to the desired concentrations with Krebs' solution. The other molecules were prepared directly in Krebs' solution.
Drugs
U46619 was obtained from Cayman chemical (Ann Arbor, U.S.A.). Ouabain, acetylcholine, nifedipine, cromakalin, 18α glycyrrethinic acid, palmitoleic acid, indomethacin were obtained from Sigma (St. Louis, MO, U.S.A.), SP and BK from Bachem Feinchemikalien (AG, Budendorf, Switzerland), L-NA from Aldrich (Steinheim, Germany). Nitroglycerin 0.2 mg ml−1 ethanol (Nitronal) from G. Pohl-Boskamp Gmbh & Co (Hohenlockstedt, Germany), Isoprenaline 0.2 mg ml−1 0.9% NaCl (Isuprel) from Sanofi Winthrop (Brussel, Belgium and cryptate 2.2.2 (Kryptofix 2.2.2) from Fluka (Buchs, Switzerland). Apamin and charibdotoxin were purchased from Alomone Labs (Jerusalem, Israel).
Statistical analysis
Data were calculated as the mean±standard error of the mean (s.e.mean). Student's test was used to compare results. A P value <0.05 was taken as significant.
Results
Effect of exogenous potassium ions
To test whether exogenous potassium relaxes contracted strips of porcine coronary arteries, strips without endothelium were continuously, tonically contracted by 0.3 μM U-46619. Potassium added to a Krebs incubation solution that already contains 5.9 mM K+ did not relax these strips but instead, increased the isometric force developed in a concentration dependent manner. Force increased by 21±9% in response to the addition of 18 mM K+ (Figure 1B). On the contrary, when the strips were incubated in the absence of potassium in the incubation medium and contracted by 0.3 μM U-46619, the addition of 0.24 mM K+ had no effect (four observations), but 1.2 mM K+ relaxed the strip by 3±1% (n=4) and 5.9 mM K+ by 30±7% (n=4). These relaxations were abolished by 1 mM ouabain (four observations).
Figure 1.
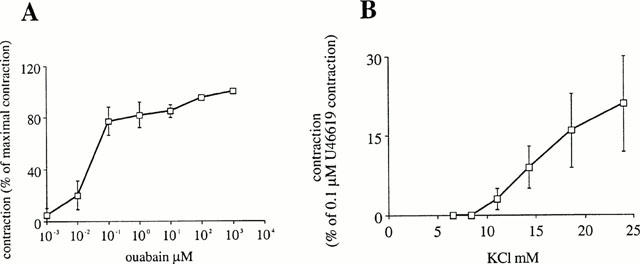
Concentration-response curve to (A) ouabain and (B) KCl. In (B), strips were tonically contracted by 0.1 μM U46619 P in the presence of 100 μM nitro-L-arginine (L-NA) and 10 μM indomethacin.
For electrophysiological experiments, 0.5 to 3 M KCl was added as 2 to 10 μl aliquots during 0.5 s into the flow of the superfusion medium. In this case we calculated that a concentration of about 0.1 to 1.5 M was rapidly reached for a short period of time of about 2 to 5 s. This manner of applying potassium ions in a puff, probably mimics the situation of potassium released by an endothelial cells more than the perfusion with a solution enriched in potassium. In a first series of experiments, the strip was without endothelium and contracted by 10 μM acetylcholine to produce a reproducible state of initial tension in the strip. The membrane potential of the smooth muscle cells was −42.5±1 mV (n=6) before the addition of acetylcholine and −43±1 (n=6) during the perfusion with acetylcholine. In this tissue, acetylcholine does not cause endothelium-dependent relaxation nor smooth muscle or endothelial cell hyperpolarization (Ito et al., 1979). When 2 μl 1.5 M potassium were injected into the perifusion chamber close to the superfusion inlet, it depolarized the smooth muscle cells by 25±2 mV (n=4). This induced the strip to increases the force it develops by 12±3.5% (n=4). On the contrary, 2 μl 1.5 M KCl injected close to the outlet caused a transient hyperpolarization of 4±0.3 mV (n=4) (Figure 2C). These refer to the successful observations of hyperpolarizations out of many trials changing the pattern of potassium application. In another series of experiments, the coronary strip had an intact endothelium and was contracted by 0.3 μM U-46619. The membrane potential of the smooth muscle cells was −44±2 (n=8). This value was not significantly different from that recorded in the absence of the thromboxane analogue. In this case, when 10 μl 3 M potassium was injected into the perifusion chamber close to the superfusion inlet, it depolarized the smooth muscle cells by 23±1 mV (n=4) (Figure 2A). This induced the strip to contract by 13±1% (n=4) the tonic force developed in response to perifused U-46619. On the other hand, when 5 μl 0.5 M KCl injected close to the outlet caused a small, transient depolarization of 3±1 mV (n=4) followed by a hyperpolarization of 15±1 mV (n=4) (Figure 2B). These results are the four maximal hyperpolarizations we observed in this series of experiments (see Figure 2B). These results show that depending on their application pattern, exogenous potassium ions can mimic the EDHF effect on smooth muscle cells.
Figure 2.
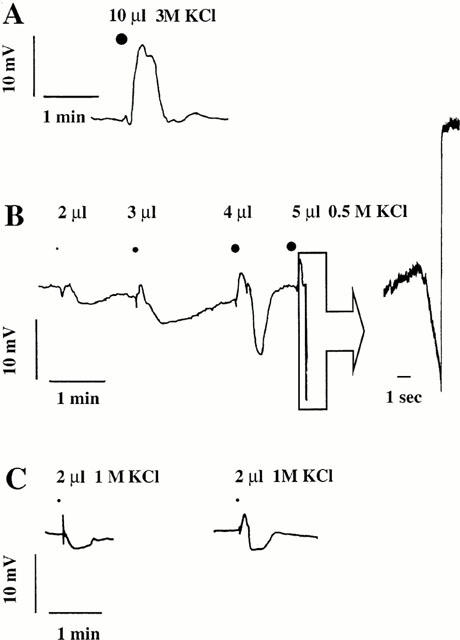
Original recording of the smooth muscle cell membrane potential on a strip with (A+B) or without (C) intact endothelium. Effect of a fast injection of KCl in the perfused tissue bath close to the perifusion inlet (A), and close to the outlet, which caused a shorter, localized application of the ion (B and C).
Source of endogenous potassium ions
If potassium is the EDHF, this implies that the endothelial cells release this ion through potassium channels. Calcium-dependent potassium channels sensitive to a mixture of Apamin and charibdotoxin have previously been demonstrated in endothelial cells (Edwards et al., 1998). Therefore we employed 100 nM Apamin+100 μM charibdotoxin to inhibit these channels to observe the effect on relaxation. On a strip tonically contracted by 0.3 μM U46619 and incubated with 10 μM indomethacin and 100 μM nitro-L-arginine, the relaxation caused by 10 nM SP was reduced to 22±8% and by 300 nM BK to 3±1.5% compared to the relaxations observed in the absence of the toxins. Thus the EDHF effect of SP is reduced by 72% and that of bradykinin by 96% when the potassium release was inhibited.
Role of endogenous potassium ions
If potassium released by the endothelial cells is the EDHF, the endothelium-dependent relaxation resistant to nitro-L-arginine plus indomethacin caused by bradykinin should be inhibited when this ion is removed from the extracellular medium by chelation. To scavenge the potassium ions, diazapolyoxa macrobicyclic complexes called cryptates 2.2.2 (Kryptofix 2.2.2) were used in Krebs solution and in a solution without potassium ions and where the NaCl was replaced by choline chloride to eliminate monovalent cations. The effect of Kryptofix was tested on the relaxing effect of 10 nM substance P and of 300 nM bradykinin on a coronary strip with intact endothelium contracted by 0.3 μM U-46619 and incubated with 10 μM indomethacin and 100 μM nitro-L-arginine to reveal the EDHF responses. These concentrations were used since they are close to the EDRF IC50 of these two peptides (Pacicca et al., 1992). Only one concentration of each peptide was tested to avoid tachyphylaxis. A concentration of 2 mM Kryptofix 2.2.2 did not change the pH of the Krebs solution. This is compatible with its stability constant (log Ks) of 9.6 for H+. In its complexed form Kryptofix 2.2.2 is charged and therefore cell does not penetrate into the cell.
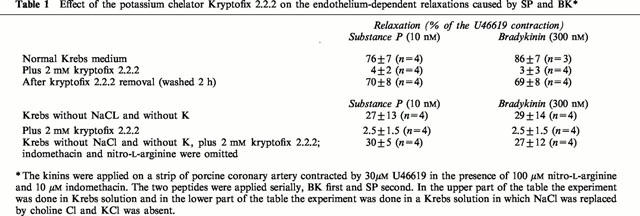
In Krebs solution with 10 μM indomethacin and 100 μM nitro-L-arginine, the strips contracted by 0.3 μM U-46619 were relaxed by 76 to 86% by the kinins (Figure 3A and Table 1). The application of 2 mM Kryptofix 2.2.2 to the strips contracted by U-46619 increased the isometric force by 11±1% (n=5). This concentration of Kryptofix 2.2.2 abolished the relaxations caused by bradykinin and substance P (Table 1 and Figure 3A). The effect of the potassium chelator is reversible. After an experiment with Kryptofix 2.2.2 the strips were washed in Krebs solution for 2 h and then the strips, contracted by 0.3 μM U-46619 in the presence of 10 μM indomethacin and 100 μM nitro-L-arginine but without the cryptate, were relaxed again by 69 to 70% by the kinins (Table 1).
Figure 3.
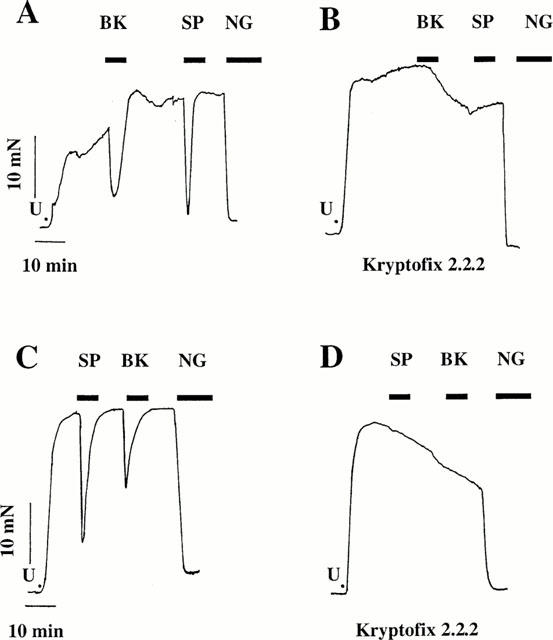
Original recordings of mechanical activity of strips tonically contracted with 0.3 μM U46619 P in the presence of 100 μM nitro-L-arginine (L-NA) and 10 μM indomethacin. Effect of 300 nM bradykinin (BK) and 10 nM substance P (SP) (IC50-concentrations) and 4 μM nitroglycerin (NG). The experiments were done in a medium without potassium in the panels C and D. In the panels B and D the experiments were done in the presence of the potassium chelator 2 mM kryptofix 2.2.2.
Table 1.
Effect of the potassium chelator Kryptofix 2.2.2 on the endothelium-dependent relaxations caused by SP and BK*
After more than 20 min in the same incubation solution (Krebs solution with 10 μM indomethacin, 100 μM nitro-L-arginine and 0.3 μM U-46619 plus 2 mM Kryptofix 2.2.2) the membrane potential of the endothelial cells was −40±1 mV (n=9). This potential is the same as that already published for the same cells incubated without Kryptofix (Bény, 1990a). In the above incubation solution the membrane potential of the smooth muscle cells was −41±1 (n=4) which is not significantly different than that measured in the same medium but without the potassium chelator (44±2 mV, n=8). In addition the hyperpolarization of the endothelial cell in response to the kinins was not inhibited by kryptofix 2.2.2 (not shown). This shows that cryptate did not affect the membrane potential of the endothelial and smooth muscle cells, nor the hyperpolarizing effect of the kinins on the endothelial cells.
The stability constant (log Ks) of Kryptofix 2.2.2 in water is 2 for lithium, 3.9 for sodium. 5.4 for potassium, 4.4 for calcium and 9.6 for protons (Dietrich et al., 1993). Two consequences are: (A) the concentration of the monovalent cations was changed by the chelator (Table 2) and (B) only a part of the Kryptate was available to scavenge the potassium responsible for the EDHF. Therefore, the same experiment was done in a solution without potassium and sodium chloride. In this medium, the strips still were contracted by U-46619 and the kinins relaxed these strips by 27 to 29% (Figure 3C and Table 1). These relaxations were reduced to between 2.5 and 2 mM Kryptofix 2.2.2 (Figure 3D and Table 1). In the same medium, but without indomethacin and nitro-L-arginine (synthesis of nitric oxide and prostacylin is not inhibited) substance P and bradykinin relaxed the strip by 27 to 30% (Table 1). This showed that in this medium, the endothelial cells are still able to produce nitric oxide.
Table 2.
Effect of the potassium chelator Kryptofix 2.2.2 on the concentration of free cations in the incubation medium*

These results are compatible with the concept that potassium released during kinin treatment are the EDHF in porcine coronary arteries.
Role of inward rectified potassium channels
Potassium ions hyperpolarize and consequently relax the smooth muscles by either gating the inward rectified potassium channels or by activating the electrogenic pump Na+-K+ ATPase (Edwards et al., 1998). In this context, we addressed the question: do the inward rectified potassium channels play a role in EDHF phenomenon in this artery? These channels are inhibited by 30 μm barium (Quayle et al., 1996). This concentration of barium did not change the concentration-relaxation curve of bradykinin on strips with intact endothelium contracted by 0.3 μM U-46619 and incubated with 10 μM indomethacin and 100 μM nitro-L-arginine to reveal the EDHF responses (Figure 4A). A concentration of 30 μM barium inhibited by 46±11% (n=4) the relaxation caused by 1 nM SP without changing the IC50 of the concentration-relaxation of strips with intact endothelium contracted by 0.3 μM U-46619 and incubated with 10 μM indomethacin and 100 μM nitro-L-arginine (Figure 4D). This experiment showed that the inhibition of the inward rectified potassium channels is not sufficient to abolish the EDHF produced in response to substance P and bradykinin in porcine coronary arteries.
Figure 4.
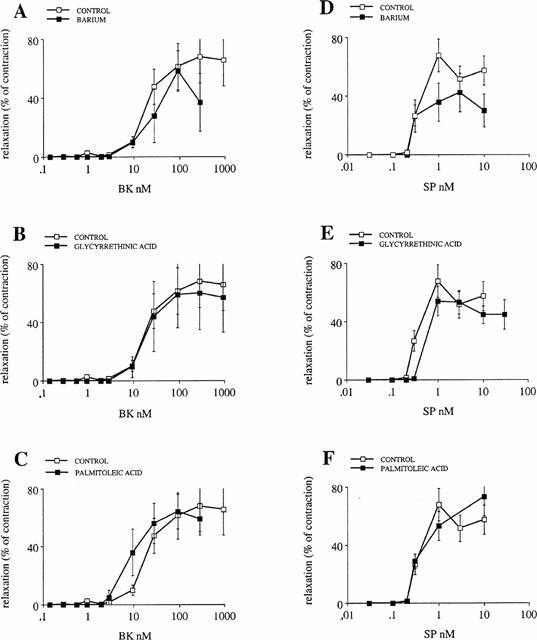
Concentration-response curves of bradykinin- and substance P-induced EDHF relaxations in the presence of 100 μM nitro-L-arginine and 10 μM indomethacin. The relaxations caused by the peptides are expressed in % of the tonic contraction produced throughout the experiment by 0.3 μM U 46619. Effect of 2 μM BaCl2, an inhibitor of inward rectified potassium channels (A and D); of 40 μM 18α glycyrrethinic acid (B and E) and of 50 μM palmitoleic acid, two inhibitors of cell to cell communication through gap junctions (C and F).
Role of Na+-K+ ATPase
We next addressed the question of whether the Na+-K+ ATPase are implicated in the EDHF phenomenon?
Ouabain slowly contracted coronary strips in a concentration dependent manner (Figure 1A). The concentration-response curve reached a plateau of 74±10% (n=4) (compared to a supramaximal phasic contraction produced by 10 μM acetylcholine) from a concentration of 100 nM ouabain. At a maximum, 10 μM ouabain contracted the strip by 96±2% (n=4) of a supramaximal phasic contraction produced by 10 μM acetylcholine.
We verified that ouabain contracted the smooth muscle only by inhibiting the Na+-K+ ATPase electrogenic pump and not by another phenomenon. Voltage gated calcium channels were inhibited with 10 μM nifedipine or by chelating extracellular calcium with 2 mM EGTA, to produce a concentration of calcium <0.1 pM. In both cases the contractions produced by 1 mM ouabain were abolished (four observations for both protocols). This demonstrated that the ouabaïn induced contraction depends on the opening of voltage-gated calcium channels and that the entry of extracellular calcium by that way is essential for the contractile effect of ouabain. We also verified that a hyperpolarization of the smooth muscle cells can still relax a strip without endothelium contracted by ouabain by using the potassium channel opener cromakalin. Ten μM cromakalin relaxed coronary strips contracted by 1 mM ouabain by 105±4% (n=4). Such a contracted strip of artery was also relaxed by 98±7% (n=4) by 4 μM nitroglycerin and by 24±10% (n=4) by 1 μM isoprenalin. The results of these experiments are compatible with the fact that ouabain contracts the coronary strips only by inhibiting the Na+-K+ ATPase. Three intracellular pathways can relax such a contracted strip: (A) the decrease of calcium concentration, and the synthesis of (B) cyclic guanosine triphosphate (cyclic GMP) or (C) cyclic adenosine triphosphate (cyclic AMP). It has been demonstrated previously that 1 mM ouabain does not inhibit the endothelial cell hyperpolarization caused by acetylcholine in rat hepatic artery (Edwards et al., 1998) and that 500 nM ouabain does not inhibit that produced by substance P and bradykinin in porcine coronary arteries (Edwards et al., 2000).
A concentration of 500 nM ouabain abolished the endothelium-dependent relaxations produced by substance P and bradykinin resistant to indomethacin and nitro-L-arginine (four observations) (Figure 5A,B). However, this concentration of ouabain did not abolish the relaxation caused by substance P and bradykinin in the absence of indomethacin and nitro-L-arginine. In that case, the endothelium dependent relaxations produced by nitric oxide were still present. They reached a maximum of 23±9% (n=4) for 1 nM substance P and 38±8% (n=4) for 10 nM bradykinin (Figure 5A,B).
Figure 5.
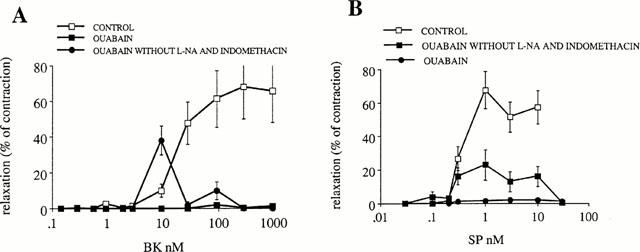
Concentration-response curves of bradykinin- and substance P-induced EDHF relaxations in the presence of 10 μM indomethacin and 100 μM nitro-L-arginine. The relaxations caused by the peptides are expressed in % of the tonic contraction produced throughout the experiment by 0.3 μM U 46619. Effect of 500 nM ouabain. The effect of ouabain is also shown in experiments done without indomethacin and nitro-L-arginine to show the role of nitric oxide.
Role of gap junctions
In another set of experiments, we observed that 1 mM ouabain inhibits dye coupling between cultured endothelial cells. Therefore, we tested 18α glycyrrethinic acid as an uncoupling agent. This molecule has been used previously to demonstrate that the phenomenon of EDHF results from an electrotonic spreading of the endothelial cell hyperpolarizations to the smooth muscle cells (Chaytor et al., 1998; Yamamoto et al., 1999). We also tested palmitoleic acid because this molecule abolishes the dye coupling between cultured endothelial cells (Domenighetti et al., 1998). A concentration of 40 μM 18α glycyrrethinic acid or of 50 μM palmitoleic acid did not inhibit the effect of bradykinin and substance P (Figure 4B,C,E and F respectively). This shows that since an uncoupling agent does not inhibit the EDHF in this artery, EDHF does not result from an electrical coupling between the endothelial and the smooth muscle cells. In addition, this observation also shows that the possible uncoupling action of ouabain does not explain its inhibition of EDHF in porcine coronary artery. We conclude therefore, that in porcine coronary arteries, EDHF hyperpolarizes the smooth muscle cells by activating the Na+-K+ ATPase pump of the smooth muscle membrane.
Discussion
Source of endogenous potassium ions
The endothelial cells from porcine coronary artery in primary culture are hyperpolarized by the kinins (Brunet & Bény, 1989; Bény, 1990a,1990b). This hyperpolarization reaches a maximum close to the equilibrium potential of potassium ion when EK is changed by manipulating extracellular potassium concentration (Brunet & Bény, 1989). In addition, whole cell and single channel patch clamp experiments showed that the kinins gate calcium-dependent potassium channels and that the transient current elicited by the peptides is a potassium current (Baron et al., 1996; 1997). Therefore the stimulation of the endothelial cells by the kinins leads to the opening of potassium channels and consequently to a release of potassium ions. Our observation that a mixture of apamin plus charibdotoxin inhibited by 72% the substance P produced relaxation and almost abolished the bradykinin caused relaxation resistant to nitro-L-arginine plus indomethacin is compatible with these results. In addition, the charibdotoxin and apamin sensitive channel does not seem to be directly activated by EDHF on smooth muscle cells (Edwards et al., 1998). Therefore, the observation of a significant reduction of EDHF response when the endothelial cells are inhibited to release potassium ions is crucial to demonstrate that potassium could be the EDHF.
Role of endogenous potassium ions
If potassium released by the endothelial cells is the EDHF, the scavenging of this ion should abolish the EDHF caused arterial strip relaxation. This experiment is difficult to perform since the physiological solution already contains potassium ions. In addition, to our knowledge, the existing potassium chelators are not perfectly specific. The stability constant (log Ks) of the kryptofix 2.2.2 in water is 2 for lithium, 3.9 for sodium. 5.4 for potassium, 4.4 for calcium and 9.6 for protons (Dietrich et al., 1993). Thus, the chelator changes all the cation concentrations as computed using the affinity and mass equilibrium equation (Table 2). Nevertheless, kryptofix 2.2.2 strongly inhibited the kinin induced EDHF relaxations. Since the concentration of cations was not physiological in that case, we did experiments in a medium without potassium and where NaCl was replaced by choline Cl. In these non-physiologic conditions, bradykinin and substance P were still able to cause an EDHF response and this response was abolished by cryptate. Additionally, when, the synthesis of nitric oxide was not inhibited by nitro-L-arginine, cryptate did not abolish the relaxation of the strip caused by the peptides, demonstrating that cryptate did not alter the mechanisms of endothelium-dependent smooth muscle cell relaxation implicating nitric oxide. These results are compatible with the idea that endogenous potassium ions could be the EDHF in porcine coronary arteries.
Effect of exogenous potassium ions
Clearly, if potassium ions released by the endothelial cells are the EDHF, exogenous ions should mimic the EDHF effect. They should hyperpolarize the arterial smooth muscle cells and relax the coronary strip without endothelium.
The observations that the addition of potassium to a medium depleted of this ion caused a relaxation of the coronary strip was already extensively studied (Bonaccorsi et al., 1977; Webb & Bohr, 1978; Bukoski et al., 1983). It is compatible with the interpretation that when potassium ions are absent from the medium the Na+-K+ ATPase is blocked. When the ion is added, the pump is then activated, leading to a hyperpolarization that closes voltage-dependent calcium channels, lowers cytosolic free calcium concentration and consequently relaxes the strip. The fact that this particular relaxation was inhibited by ouabain supports this idea. On the contrary, when potassium ions were added to a physiological medium that already contained 5.9 mM potassium, they induced a contraction because the increase in extracellular K+ concentration depolarized the smooth muscle cells by changing the equilibrium potential of this ion. Gating voltage dependent calcium channels therefore produces contraction. These results seem to contradict the hypothesis that EDHF could be the potassium ions in porcine coronary artery (Quignard et al., 1999). However, in the arteries, potassium ions are most likely released in discrete areas between the endothelial and the smooth muscle cells and with a particular time course. This hypothesis is supported by the observation that it was more difficult to observe the hyperpolarizing effect of potassium in de-endothelialized strips. Since, these discrete areas would be destroyed by the procedure of endothelium removal. Nevertheless, our observation that exogenous potassium applied in short pulses at relatively high concentrations to strips without endothelium hyperpolarizes the smooth muscle cells fulfils a necessary condition in favour of the hypothesis that the potassium is the EDHF. The observation that potassium ions can hyperpolarize the smooth muscle cells even better in strips with intact endothelium confirms this idea.
In this context, it could be argued that potassium ions applied to a strip with intact endothelium, it induces the release of nitric oxide and EDHF. However, this is unlikely because the endothelial cells are not excitable. The increase in cytosolic free calcium in these cells is associated with a hyperpolarization and not with a depolarization (Brunet & Bény, 1989; Schilling, 1989; Frieden et al., 1999). Indeed an increase in extracellular potassium concentration induces a depolarization of these cells, therefore to a decrease in cytosolic free calcium concentration. This will result in an inhibition of vasoactive factor synthesis.
To date potassium is the only candidate molecule for the EDHF in porcine coronary artery that mimics the time course and magnitude of the endothelium-dependent hyperpolarizations.
A possible interpretation of our observations is that the myoendothelial junctions must be preserved to allow exogenous potassium ions to hyperpolarize the smooth muscle cells since the Na+-K+ pump could be concentrated in this structure. The potassium channels could also be concentrated in the same structure but on the endothelial side. Therefore, the removal of the endothelial cells would result in a destruction of this hypothetical ‘potassium synapse'. The observation of a depolarizing effect of potassium ions on the porcine coronary arteries led Quignard et al. (1999) to the opposite conclusion that these ions cannot be the EDHF in this vessel. Similar to our results, these authors observed very few cases of hyperpolarizations of smooth muscle cells on strips without endothelium in response to potassium ions; however, these authors did not attempt to do this experiment on strips with intact endothelium.
The observation that exogenous potassium ions can hyperpolarize and relax the smooth muscle cells is a necessary demonstration in support of the hypothesis that potassium ions are the EDHF. Our observations that scavenging of the endogenous potassium ions and in addition, inhibition of the channels that release potassium ions from the endothelial cells, significantly inhibit the EDHF relaxations allow us to conclude that potassium ions in this artery play a major role as EDHF.
Effect of EDHF on the smooth muscle cells
The inward rectified potassium channels
A concentration of 30 μM barium specifically inhibits the inward rectified potassium channels (Quayle et al., 1996). Our observation that the effect of EDHF produced by bradykinin was not changed in 30 μM Ba2+ shows that EDHF does not function by gating these channels in porcine coronary arteries, on the other hand the small inhibition caused by barium on the EDHF produced by substance P shows that the inward rectified channels are not important for substance P EDHF. It could be that these channels are implicated in the hyperpolarizations of the endothelial cells produced by substance P but not by bradykinin (Frieden et al., 1999). In this way barium would act on the endothelial cells stimulated by substance P and not on the smooth muscle. In any case, EDHF produced by either peptide does not work by only gating inward rectified potassium channels of smooth muscle, in this artery. This conclusion is compatible with the finding that in porcine coronary arteries, the smooth muscle cell endothelium-dependent hyperpolarization caused by bradykinin is not affected by barium (Quignard et al., 1999).
The Na+-K+ ATPase
The observation that the contraction effect of ouabain is abolished by incubation of the strip in a medium without calcium and by inhibition of voltage gated calcium channels, is compatible with the idea that ouabain, by the inhibition of electrogenic Na+-K+ ATPase, depolarizes the smooth muscle cells. This depolarization by gating voltage-dependent calcium channels that permit extracellular calcium to enter in the cells, causes the contraction. We assume that the contraction of the coronary strips by ouabain was a consequence of the Na+-K+ ATPase inhibition and not caused by a non-specific effect. In this context, the fact that small concentration of ouabain (500 nM) abolished the EDHF response indicates that in porcine coronary arteries, EDHF is mainly an activator of the Na+-K+ ATPase. On the contrary, in rat hepatic artery, the inhibition of inward rectified potassium channels is necessary to abolish EDHF effect in the presence of ouabain (Edwards et al., 1998). Consequently, in this artery, the activation of both inward rectified channels and of electrogenic pump explains EDHF effects on the smooth muscle cells. In porcine coronary arteries, when the synthesis of nitric oxide was not inhibited, an endothelium-dependent relaxation was still present. The amplitude of this remnant relaxation is compatible with the effect of nitric oxide released by both peptides (Pacicca et al., 1992). This also indicates that ouabain did not inhibit the endothelial cell response to the kinins. Our conclusions contradict those of Quignard et al. (1999), which show that in porcine coronary arteries, ouabain does not significantly affect the endothelium-dependent hyperpolarization caused by bradykinin. This discrepancy could be explained by the latent period necessary to ouabain to develop its full inhibitory effect, which could not have been respected.
The gap junctions
We observed that 1 mM ouabain inhibits dye coupling between cultured endothelial cells. Since EDHF can result from the passive electrotonic spreading of the endothelial cell hyperpolarization to the smooth muscle cells through gap junction, we tested the effect of two uncoupling agents distinct from ouabain (Chaytor et al., 1998; Yamamoto et al., 1999). The observation that neither 18α glycyrrethinic nor palmitoleic acid inhibited the EDHF in porcine coronary arteries indicates that EDHF does not result from electrical coupling and that blocking of EDHF by ouabain is not due to uncoupling.
The epoxyeicosatrienoic acids (EETs)
The conclusion that EDHF may be the potassium in porcine coronary arteries is not compatible with the concept that EDHF is a product of cytochrome P450 epoxygenase (Fisslthaler et al., 1999). In our hands, the products of cytochrome P450 namely the epoxyeicosatrienoic acids (EETs) (14,15-EET; 5,6-EET; 11,12-EET; 8,9EET) neither hyperpolarize nor relax the de-endothelialized strip of porcine coronary arteries. In addition, 17-octadecynoic acid, an inhibitor of the cytochrome P450 epoxygenase, had no effect on the concentration-response curve of substance P, but it shifts the concentration-response curve of bradykinin to the left (Frieden et al., 1999). The latter effect can be explained by the observation that the sensitivity to calcium of high conductance calcium-dependent potassium channels is increased by the different EETs (Baron et al., 1997). Therefore, compatibility of our results with the observation that modulations in cytochrome P450 epoxygenase expression are reflected in EDHF relaxations, may be found at the level of the mechanism of endothelial cell potassium channel gating instead of at the level of the identity of EDHF. As a matter of fact, Edwards et al. (2000) concluded that the EETs are not the EDHF in porcine coronary arteries.
Conclusion
These results indicate that in porcine coronary artery, the EDHF released in response to bradykinin and substance P may be the potassium ion. This ion would hyperpolarize and therefore relax the smooth muscle cells by activating the Na+-K+ ATPase.
Acknowledgments
This work was supported by the Swiss National Science Foundation, grant 3100-49163.96. We gratefully thank Françoise Gribi for her excellent technical assistance and Dr R. Peck for helping to improve the manuscript.
Abbreviations
- BK
bradykinin
- cyclic AMP
cyclic adenosine triphosphate
- cyclic GMP
cyclic guanosine triphosphate
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-derived relaxing factor
- EET
epoxyeicosatrienoic acid
- IC50
inhibitory concentration (e.g. 50% inhibition of maximum)
- L-NA
nitro-L-arginine
- Na+-K+ ATPase
sodium-potassium adenosine triphosphatase
- SP
substance P
References
- BARON A., FRIEDEN M., BENY J.-L. Epoxyeicosatrienoic acids activate a high-conductance, Ca2+-dependent K+ channel on pig coronary artery endothelial cells. J. Physiol. 1997;504:537–543. doi: 10.1111/j.1469-7793.1997.537bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARON A., FRIEDEN M., CHABAUD F., BENY J.-L. Ca2+-dependent non-selective cation and potassium channels activated by bradykinin in pig coronary artery endothelial cells. J. Physiol. 1996;493:691–706. doi: 10.1113/jphysiol.1996.sp021415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÉNY J.-L. Endothelial and smooth muscle cells hyperpolarized by bradykinin are not dye coupled. Am. J. Physiol. 1990a;258:H836–H841. doi: 10.1152/ajpheart.1990.258.3.H836. [DOI] [PubMed] [Google Scholar]
- BÉNY J.-L. Effect of substance P on the membrane potential of coronary arterial endothelial cells in situ. Agents and Actions. 1990b;31:317–320. doi: 10.1007/BF01997626. [DOI] [PubMed] [Google Scholar]
- BÉNY J.-L., BRUNET P.C. Neither nitric oxyde nor nitroglycerin accounts for all the characteristics of the endothelially mediated vasodilatation of pig coronary arteries. Blood Vessels. 1988;25:308–311. [PubMed] [Google Scholar]
- BÉNY J.-L., BRUNET P.C., HUGGEL H. Effect of mechanical stimulation, substance P and vasoactive intestinal polypeptide on the electrical and mechanical activities of circular smooth muscles from pig coronary arteries contracted with acetylcholine: role of endothelium. Pharmacology. 1986;33:61–68. doi: 10.1159/000138202. [DOI] [PubMed] [Google Scholar]
- BÉNY J.-L., BRUNET P.C., HUGGEL H. Interaction of bradykinin and des-Arg9-bradykinin with isolated pig coronary arteries: mechanical and electrophysiological events. Regulat. Pept. 1987;17:181–190. doi: 10.1016/0167-0115(87)90061-9. [DOI] [PubMed] [Google Scholar]
- BONACCORSI A., HERMSMEYER K., APRIGLIANO O., SMITH C.B., BOHR D.F. Mechanism of potassium relaxation of arterial muscle. Blood Vessels. 1977;14:261–276. doi: 10.1159/000158133. [DOI] [PubMed] [Google Scholar]
- BRUNET P.C., BÉNY J.-L. Substance P and bradykinin hyperpolarize pig coronary artery endothelial cells in primary culture. Blood Vessels. 1989;26:228–234. doi: 10.1159/000158770. [DOI] [PubMed] [Google Scholar]
- BUKOSKI R.D., SEIDEL C.L., ALLEN J.C. Differences in K+-induced relaxation of canine femoral and renal arteries. Am. J. Physiol. 1983;245:H604–H609. doi: 10.1152/ajpheart.1983.245.4.H598. [DOI] [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Central role of heterocellular gap junctional communication in endothelium-dependant relaxations of rabbit arteries. J. Physiol. 1998;508:562–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIETRICH B., VIOUT P., LEHN J.-M.Aspects of organic and inorganic supramolecular chemistry Macrocyclic chemistry 1993Germany: VCH Weinheim; p. 342 [Google Scholar]
- DOMENIGHETTI A.A., BÉNY J.-L., CHABAUD F., FRIEDEN M. An intercellular regenerative calcium wave in porcine coronary artery endothelial cells in primary culture. J. Physiol. 1998;513:103–116. doi: 10.1111/j.1469-7793.1998.103by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORA K.A., MARTIN P.E.M., CHAYTOR A.T., EVANS W.H., GARLAND C.J., GRIFFITH T.M. Role of heterocellular gap junctional communication in endothelium-dependent smooth muscle hyperpolarization: inhibition by a connexin-mimetic peptide. Biochem. Biophys. Res. Commun. 1999;254:27–31. doi: 10.1006/bbrc.1998.9877. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., DORAT K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–271. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., THOLLON C., GARDENER M.J., FELETOU M., VILAINE J., VANHOUTTE P.M., WESTON A.H. Role of gap junctions and EETs in endothelium-dependent hyperpolarization of porcine coronary artery. Br. J. Pharmacol. 2000;129:1145–1154. doi: 10.1038/sj.bjp.0703188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISSLTHALER B., POPP R., KISS L., POTENTE M., HARDER D.R., FLEMING I., BUSSE R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- FRIEDEN M., SOLLINI M., BÉNY J.-L. Substance P and bradykinin activate different types of KCa channels to hyperpolarize porcine coronary artery endothelial cells. J. Physiol. 1999;519:361–371. doi: 10.1111/j.1469-7793.1999.0361m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO Y., KITAMURA K., KURIYAMA H. Effects of acetylcholine and catecholamines on the smooth muscle cell of the porcine coronary artery. J. Physiol. 1979;294:595–611. doi: 10.1113/jphysiol.1979.sp012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOT H.J., ZIMMERMANN P.A., NELSON M.T. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J. Physiol. 1996;492:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACICCA C., VON DER WEID P.-Y., BÉNY J.-L. Effect of Nitro-1-Arginine on endothelium-dependent hyperpolarizations and relaxations of pig coronary arteries. J. Physiol. 1992;457:247–256. doi: 10.1113/jphysiol.1992.sp019376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIOR H.M., WEBSTER N., QUINN D.J., BEECH D.J., YATES M.S. K+-induced dilation of a small renal artery: no role for inward rectifier K+ channels. Cardiovasc. Res. 1998;37:780–790. doi: 10.1016/s0008-6363(97)00237-x. [DOI] [PubMed] [Google Scholar]
- QUAYLE J.M., DART C., STANDEN N.B. The properties and distribution of inward rectifier potassium currents in pig coronary arterial smooth muscle. J. Physiol. 1996;494:715–726. doi: 10.1113/jphysiol.1996.sp021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUIGNARD J.F., FELETOU M., THOLLON C., VILAINE J.P., DUHAULT J., VANHOUTTE P.M. Potassium ions and endothelium-derived hyperpolarizing factor in guinea-pig carotid and porcine coronary arteries. Br. J. Pharmacol. 1999;127:27–34. doi: 10.1038/sj.bjp.0702493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILLING W.P. Effect of membrane potential on cytosolic calcium of bovine aortic endothelial cells. Am. J. Physiol. 1989;257:H778–H784. doi: 10.1152/ajpheart.1989.257.3.H778. [DOI] [PubMed] [Google Scholar]
- WEBB R.C., BOHR D.F. Potassium-induced relaxation as an indicator of Na+-K+ ATPase activity in vascular smooth muscle. Blood Vessels. 1978;15:198–207. doi: 10.1159/000158166. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO I., IMADEA K., SUZUKI H. Endothelium-dependent hyperpolarization and intercellular electrical coupling in guinea-pig mesenteric arterioles. J. Physiol. 1999;514:505–513. doi: 10.1111/j.1469-7793.1999.505ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


