Abstract
The properties of the ATPase released during electrical field stimulation (EFS) (8 Hz, 25 s) of the sympathetic nerves of the superfused rabbit isolated vas deferens were investigated.
Superfusate collected during EFS rapidly metabolised exogenous ATP (100 μM) and 50% was broken down in 5.67±0.65 min. The main metabolite was ADP, virtually no AMP was produced and adenosine was absent. No enzyme activity was seen in samples collected in the absence of EFS.
Lineweaver-Burke analysis of the initial rates of ATP hydrolysis gave a KM of 40 μM and Vmax of 20.3 nmol ATP metabolized min−1 ml−1 superfusate. ATPase activity was unaffected by storage at room temperature for 24 h, but was abolished at pH4 or by heating at 80°C for 10 min.
ARL 67156 inhibited ATP breakdown in a concentration-dependent manner (IC50=25 μM (95% confidence limits=22–27 μM), Hill slope=−1.06±0.04).
When EFS was applied three times at 30 min intervals, ATP metabolism was 20–30% less in superfusate collected during the second and third stimulation periods compared with the first. ATPase activity was released in a frequency-dependent manner, with significantly greater activity seen after stimulation at 4 and 8 Hz than at 2 Hz.
In conclusion, EFS of the sympathetic nerves in the rabbit vas deferens causes release of substantial ATPase, but little ADPase activity into the extracellular space. This contrasts with the guinea-pig vas deferens, which releases enzymes that degrade ATP to adenosine. Thus, the complement of enzymes released by nerve stimulation is species-dependent.
Keywords: ARL 67156, ATP, ATPase, purinergic neurotransmission, vas deferens
Introduction
Electrical field stimulation (EFS) of the sympathetic nerves innervating the vas deferens of many species causes the release of adenosine 5′-triphosphate (ATP) and noradrenaline as excitatory cotransmitters. ATP and noradrenaline act on postjunctional P2X1 receptors and α1-adrenoceptors respectively, to evoke a biphasic contraction of the smooth muscle (see Sneddon et al., 1996 for review). Once released into the extracellular space, ATP is broken down, whilst noradrenaline is taken back up into the nerve by a specific transporter (Amara & Kuhar, 1993).
Burnstock, (1972) proposed that extracellular, membrane-bound ectonucleotidases sequentially degrade ATP to adenosine 5′-diphosphate (ADP), adenosine 5′-monophosphate (AMP) and adenosine, which may then be taken up by surrounding tissues. Such ectoATPases/apyrases have since been characterized extensively (see Plesner, 1995; Zimmermann, 1996) and a family of enzymes has been cloned recently (see Zimmermann, 1999; Zimmermann & Braun, 1999) and renamed ectonucleoside triphosphate diphosphohydrolases (eNTDPases, Zimmermann et al., 2000). They are unaffected by inhibitors of intracellular ATP-dependent ion pumps such as ouabain, but are inhibited by 6-N,N-diethyl-D-β,γ-dibromomethyleneATP (ARL 67156) (Crack et al., 1995; Khakh et al., 1995).
A second mechanism by which extracellular ATP can be metabolized was reported by Todorov and colleagues, who showed that EFS of sympathetic nerves in the guinea-pig vas deferens leads to the release of nucleotidases that degrade ATP to adenosine (Kennedy et al., 1997; Todorov et al., 1997). Like eNTDPases, the released ATPase is unaffected by inhibitors of intracellular ATPases, but is sensitive to ARL 67156. Recently, we showed that nucleotidase release was also evoked by EFS of sympathetic nerves in the rat and mouse isolated vas deferens and of parasympathetic nerves in the guinea-pig isolated urinary bladder (Westfall et al., 2000b), all tissues in which ATP is an excitatory cotransmitter. Thus, nucleotidase release is neither species-dependent, nor restricted to sympathetic nerves. In contrast, under the conditions of our experiments, no nucleotidase release was seen during stimulation of the enteric nerves of the guinea-pig isolated taenia coli, implying that the release of the nucleotidases is not a general property of all autonomic nerves or smooth muscle tissues.
ATP is also an excitatory neurotransmitter in the rabbit isolated vas deferens (Sneddon et al., 1984; Sneddon & Machaly, 1992), but in preliminary studies on this tissue, we found that the complement of enzyme activity released during EFS appeared to differ greatly from that seen in the guinea-pig vas deferens. Therefore, the aims of this study were to characterize in more detail the nucleotidases released from the rabbit isolated vas deferens. A preliminary account has been published (Westfall et al., 2000a).
Methods
Tissue preparation
New Zealand White male rabbits (3–4.2 kg) were killed by an overdose of sodium pentobarbitone (150 mg kg−1) and then exsanguination. The vasa deferentia were removed and the prostatic half (∼ 3 cm) cleaned of connective tissue, bisected and cut open along the longitudinal axis, exposing the lumen. One vas deferens (∼ 60 mg wet tissue weight) was loaded into a Brandel perfusion chamber (total volume 200 μl). Whatman 541 filters were cut to fit both ends of the chamber, which was then inserted vertically into a thermostatic block with a platinum screen electrode at either end. The tissues were perfused from bottom to top at 2 ml min−1 with a modified Krebs solution of the following composition (mM): NaCl 118.4, NaHCO3 25, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 2.5 and glucose 11; heated to 37°C and bubbled with 95% O2, 5% CO2.
Collection and measurement of ATPase activity
Unless stated otherwise, EFS was applied to the tissues via the screen electrodes at 8 Hz, 0.1 ms pulse width and supramaximal voltage for 25 s with a Grass S88 stimulator, connected to a Grass SIU5F stimulus isolation unit. The superfusate was collected throughout the stimulation period (∼ 800 μl) and divided into 80 μl aliquots. Ten μl of a stock ATP solution was added to each aliquot (final concentration=100 μM ATP), along with 10 μl of H2O, hydrochloric acid or ARL 67156, giving a total incubation volume of 100 μl. All assays were performed at room temperature (15–20°C).
In most experiments the ATP content of the assay samples at the end of the incubation was determined by adding the total sample volume (100 μl) to 100 μl of luciferin-luciferase assay mix and the light emitted recorded on a Chrono-log Lumivette luminometer for 20 s. A standard curve using known amounts of ATP was constructed before each experiment and from this the amount of ATP in the test samples calculated.
In several experiments, samples were assayed for ATP, ADP, AMP and adenosine using a gradient HPLC system. Purines were separated on a Supelcosil™ LC-18-T column attached to a Beckman System Gold HPLC (two 110B Solvent Delivery Modules, 507 Autosampler, 406 Analog Interface Module and 166 Programmable Detection Module). The amount of each purine was quantified using the 166 μV detection module at a wavelength of 254 nm. Buffer solutions consisted of 0.1 M KH2PO4, 4 mM tetrabutylammonium hydrogen sulphate, pH 6.0 (buffer A) and 70% buffer A, 30% methanol, pH 7.2 (buffer B). The nucleotides were separated using a gradient in which the concentration of buffer B was increased from 0 to 100% over 20 min. Identification of individual peaks was by comparison with the retention times of known purine standards. The concentration of individual purines was determined by the peak area per pmol compared with standards.
Experimental protocols
In each experiment at least two identical samples were prepared. The ATP content of one was assayed immediately (time=zero), whilst the remainder were assayed at various intervals up to 20 min later. The amount of ATP metabolized at each time point was calculated by subtracting the amount present in that sample from the value at time=zero. As this value represents the amount of ATP metabolized by 80 μl of superfusate, it was multiplied by 12.5 to give the amount of ATP metabolized per ml of superfusate.
To characterize the stability of the releasable enzyme, two sets of aliquots were prepared from the superfusate of one tissue. The ATPase activity of one set was measured immediately and served as a control. The other set was stored at room temperature for 24 h, then ATPase activity was measured. The effect of ARL 67156 on ATPase activity was investigated by incubating superfusate samples with ATP (100 μM) for up to 10 min in the absence or presence of a range of concentrations of ARL 67156. ARL 67156 was added to the superfusate 2 min before ATP. The role of nerve stimulation in enzyme release was studied by including tetrodotoxin (1 μM) in the solution superfusing the tissues for 10 min before, and during EFS application.
Drugs
ATP (disodium salt, Sigma) and ARL 67156 (provided by Astra Charnwood) were dissolved in distilled water and stored as 10 mM stocks. The luciferin-luciferase assay (Sigma) contained firefly luciferase, luciferin, MgSO4, DTT, EDTA, bovine serum albumin and tricine buffer salts. KH2PO4, tetrabutylammonium hydrogen sulphate and tetrodotoxin were also obtained from Sigma.
Statistics
Values in the text refer to mean±s.e.mean or mean±95% confidence limits for IC50 values. Concentration-response curves were fitted to the data by logistic (Hill equation), non-linear regression analysis (Graphpad Prism, San Diego, U.S.A.). Statistical significance of the results was tested either by Student's paired t-test or one way analysis of variance and Tukey's comparison as appropriate. Differences were considered significant when P<0.05.
Results
Metabolism of ATP by superfusate
EFS (8 Hz, 25 s) of the sympathetic nerves innervating the rabbit isolated vas deferens evoked release of an ATPase into the superfusate, as exogenous ATP (100 μM) was broken down rapidly when added to superfusate samples (Figure 1). No enzyme activity was seen in samples collected before EFS (not shown). The time taken for 50% of ATP to be broken down in samples from different animals ranged from 2.5 min to 9 min (mean=5.67±0.65 min, n=10). The main metabolite was ADP, which accumulated in direct proportion to the disappearance of ATP. Small amounts of AMP were produced, but adenosine was absent. As little ADPase and no 5′-nucleotidase activity was apparent over this time-scale, subsequent experiments characterized the properties of the releasable ATPase.
Figure 1.
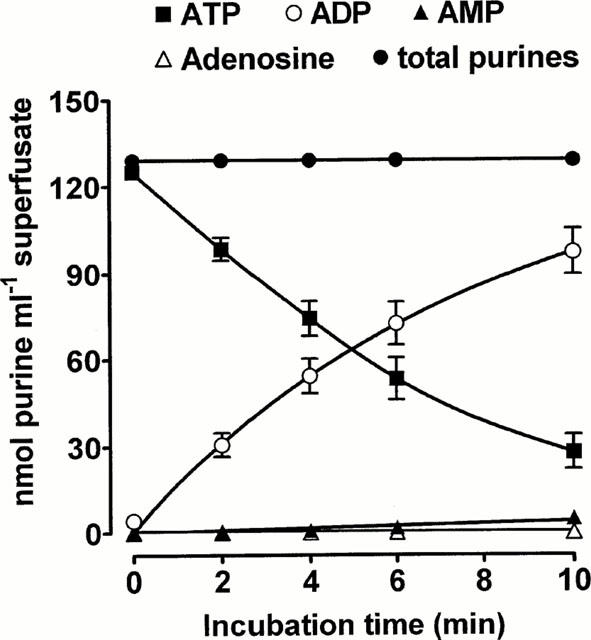
Metabolism of ATP by secreted ATPase. EFS (8 Hz, 25 s) was applied to the superfused rabbit isolated vas deferens. The ability of superfusate collected during stimulation to degrade exogenous ATP (100 μM) was then monitored using HPLC. Each point is the mean of 10 experiments. Vertical bars indicate s.e.mean.
To obtain apparent kinetic parameters for the ATPase, we measured the initial rates of hydrolysis of ATP (10–100 μM). Analysis of the data using the Lineweaver-Burke equation gave a KM value of 40 μM and a Vmax of 20.3 nmol ATP metabolized min−1 ml−1 superfusate (Figure 2).
Figure 2.
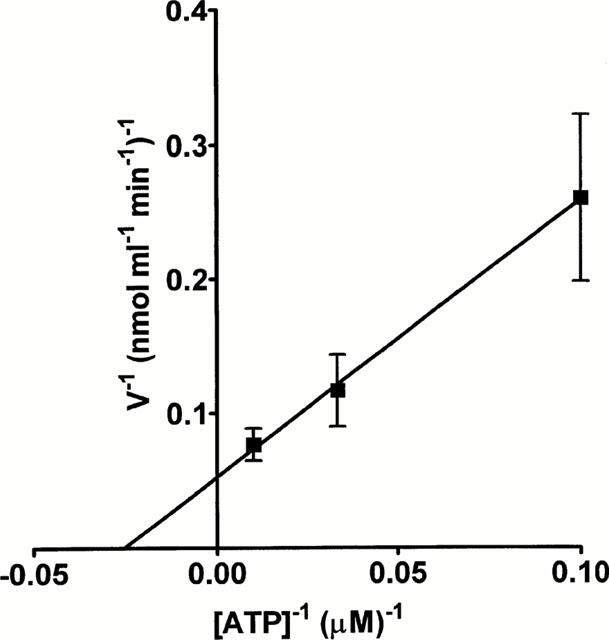
Kinetic analysis of the ATPase activity. EFS (8 Hz, 25 s) was applied to the superfused rabbit isolated vas deferens and the superfusate collected. The initial rates of hydrolysis of a range of concentrations of ATP (10–100 μM) were determined and the data analysed using the Lineweaver-Burke equation. Each point is the mean of three experiments. Vertical bars indicate s.e.mean.
The ATPase is stable at room temperature as there was no difference in the time-course or extent of ATP (100 μM) breakdown by the superfusate whether it was measured immediately or after the superfusate had been stored at room temperature for 24 h (Figure 3a). However, ATPase activity was essentially abolished by heating the samples at 80°C for 10 min or by acidifying the incubation medium to pH 4 with concentrated hydrochloric acid (Figure 3b).
Figure 3.
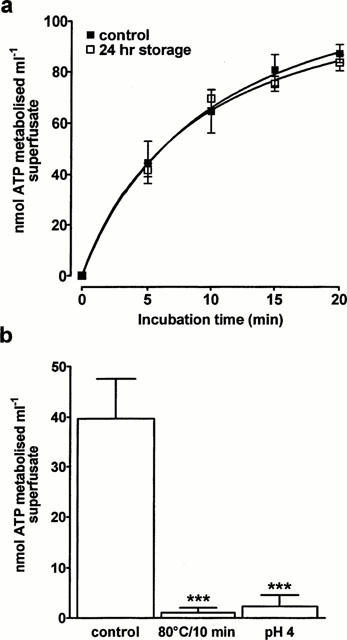
The effect of storage, heating and pH on the releasable ATPase. EFS (8 Hz, 25 s) was applied to the superfused rabbit isolated vas deferens. (a) The ability of superfusate collected during stimulation to degrade exogenous ATP (100 μM) was then determined either immediately or after the superfusate had been stored for 24 h at room temperature. (b) Samples of superfusate collected during stimulation were heated at 80°C for 10 min or acidified to pH 4 by adding concentrated hydrochloric acid. Exogenous ATP (100 μM) was then added and the amount of ATP remaining after 4 min incubation measured. n=4 for each experiment. Vertical bars indicate s.e.mean. ***P<0.001 compared to control.
Effect of ARL 67156 on ATPase activity
The eNTPDase inhibitor ARL 67156 (1–300 μM) inhibited the breakdown of ATP in a concentration-dependent manner and at 300 μM virtually abolished ATP metabolism (Figure 4a). Higher amounts of ARL 67156 could not be used due to its limited availability. A log concentration-inhibition curve for ARL 67156 at the 4 min time point gave an IC50 value of 25 μM (95% confidence limits=22–27 μM), and a Hill slope of −1.06±0.04 (Figure 4b). Similar analysis at each time-point showed that the inhibitory effect of ARL 67156 was the same throughout the assay (not shown).
Figure 4.

Inhibition of the releasable ATPase by ARL 67156. EFS (8 Hz, 25 s) was applied to the superfused rabbit isolated vas deferens. (a) The ability of superfusate collected during stimulation to degrade exogenous ATP (100 μM) was then determined in the absence or presence of a range of concentrations of ARL 67156. (b) The inhibitory effect of ARL 67156 was quantified at the 4 min incubation time-point. Each point is the mean of four experiments. Vertical bars indicate s.e.mean.
Release of ATPase
Having characterized basic biochemical properties of the releasable ATP, subsequent studies were designed to investigate the release of the enzyme. No ATPase activity was seen in samples when tetrodotoxin (1 μM) was included in the solution superfusing the tissue (Figure 5a). To determine if the ATPase could be released in a reproducible manner, EFS (8 Hz, 25 s) was applied three times at 30 min intervals. ATPase activity was present in each sample, but ATP metabolism was 20–30% less (P<0.05) at each time point in superfusate collected during the second stimulation period when compared with the first (Figure 5b). Stimulating the tissue for a third time did not produce any further decrease in ATPase activity.
Figure 5.
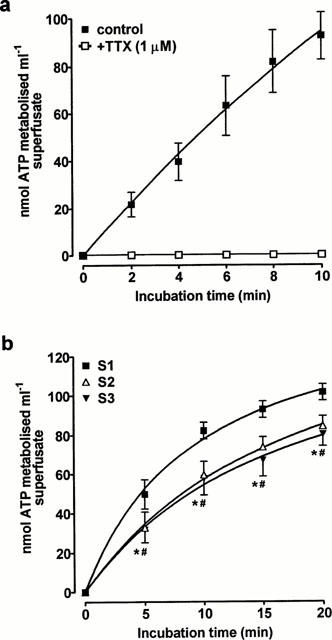
Sensitivity to tetrodotoxin and reproducibility of the release of ATPase activity. (a) EFS (8 Hz, 25 s) was applied to the superfused rabbit isolated vas deferens in the presence of tetrodotoxin (1 μM). (b) EFS (8 Hz, 25 s) was applied to the rabbit isolated vas deferens three times (S1, S2, S3) at 30 min intervals. In each case, the ability of samples of superfusate collected during stimulation to degrade exogenous ATP (100 μM) was then determined. Each point is the mean of (a) four or (b) eight experiments. Vertical bars indicate s.e.mean. *P<0.05, for S1 vs S2, #P<0.05 for S1 vs S3.
ATPase activity was released in a frequency-dependent manner as the breakdown of ATP was significantly slower in superfusate collected during stimulation at 2 Hz than in samples collected at 4 and 8 Hz (P<0.01) (Figure 6). Increasing the stimulation frequency from 4 Hz to 8 Hz did not further increase the ATPase activity.
Figure 6.
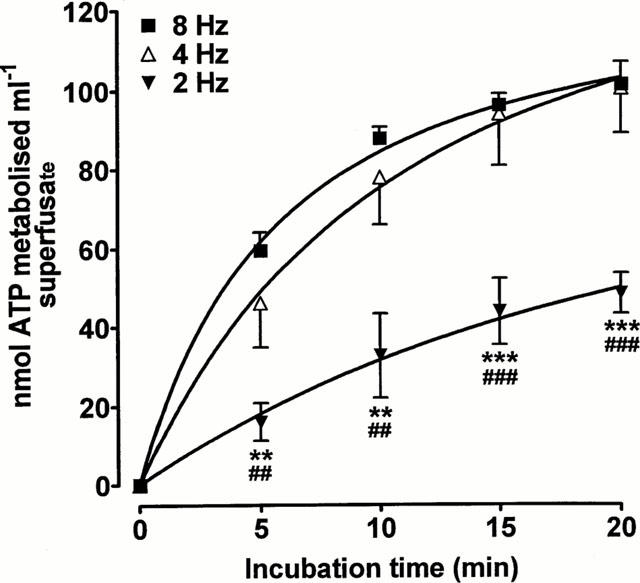
Frequency-dependence of the release of ATPase activity. EFS (2, 4 or 8 Hz, 25 s) was applied to the rabbit isolated vas deferens. The ability of samples of superfusate collected during stimulation to degrade exogenous ATP (100 μM) was then determined. Each tissue was only stimulated once and each point is the mean of six experiments. Vertical bars indicate s.e.mean. **P<0.01, ***P<0.001 for 2 Hz vs 4 Hz. ##P<0.01, ###P<0.001 for 2 Hz vs 8 Hz.
Discussion
This study has shown that sympathetic nerve stimulation of the rabbit isolated vas deferens causes release of substantial ATPase activity, which rapidly removed the γ-phosphate of exogenous ATP to produce ADP. However, ADP was metabolized slowly and accumulated in the incubation medium. Thus, the enzyme has a high selectivity for the triphosphate over the diphosphate, i.e. it is an ATPase rather than an apyrase or phosphorylase.
This profile contrasts with that of the soluble enzymes released by the guinea-pig vas deferens, which degrade ATP to adenosine (Todorov et al., 1997). It is not yet known if this reflects release of an ADPase as well as the ATPase, or if a single enzyme capable of dephosphorylating both ATP and ADP is released in the guinea-pig. Regardless, it is clear that whilst soluble nucleotidase release has been detected in all species studied to date, the complement of enzymes released is species-dependent.
In sympathetically-innervated tissues the initial dephosphorylation of ATP to ADP is the most important step in metabolism of ATP to adenosine, as ADP is much less potent than ATP at P2X1 receptors (Evans et al., 1995) and so makes little or no contribution to purinergic neurotransmission. It may be that ADP produced in situ in the rabbit vas deferens is broken down to AMP by an eNTPDase. At present it is not known if a 5′-nucleotidase is also released in the rabbit vas deferens and further experiments are required to address this point.
In the present experiments the breakdown of ATP was very fast and its average half-life was less than 6 min. This is much faster than ATP breakdown by the ATPase released from the guinea-pig, rat and mouse vas deferens and guinea-pig urinary bladder (half life ⩾ 20 min) and the Vmax of the rabbit ATPase was about 20 times greater than that of the guinea-pig enzyme (Westfall et al., 2000b). The difference is even greater when the amount of tissue used is taken into account, that is, three guinea-pig vasa deferentia with a total wet tissue weight of about 150 mg, compared with only 60 mg of a single rabbit tissue. It is not yet clear if the greater ATPase activity reflects differences in the density of innervation of these tissues or if other factors, such as enzyme cofactors, are involved.
We found that the rabbit ATPase metabolised ATP with a KM of 40 μM, similar to the values for the ATPase released by the guinea-pig vas deferens (34 μM, Mihaylova-Todorova et al., 1998; 39 μM, Westfall et al., 2000b). Three related eNTPDases have been cloned and functionally expressed (see Zimmermann, 1999; Zimmermann & Braun, 1999) and each has a KM for ATP that is only a little higher than this; 75 μM for rat CD39 (also known as ecto-apyrase or eNTPDase1; Wang et al., 1998), 394 μM for human ecto-ATPase (also known as CD39-L1 or eNTPDase2; Mateo et al., 1999) and 128 μM for HB6 (also known as CD39-L3 or eNTPDase3; Smith & Kirley, 1999). A soluble form of recombinant human CD39, created by removing the two transmembrane domains, has a much lower KM for ATP (2.1 μM; Gayle et al., 1998), although a similar rat CD39 mutant has a KM of 220 μM (Wang et al., 1998). At present, the possibility that the soluble ATPase released from the vas deferens might be a truncated form of eNTPDase cannot be discounted.
This study showed that ARL 67156 inhibited the releasable ATPase in a concentration-dependent manner and at 300 μM it nearly abolished the activity. The IC50 for ARL 67156 was 25 μM, similar to its potency in inhibiting the ATPase released from the guinea-pig isolated vas deferens (50% inhibition at approximately 30 μM, Westfall et al., 2000b) and for inhibition of ATP breakdown by eNTPDases in human blood (IC50=25 μM; Crack et al., 1995) and the rat vas deferens (IC50=7.9 μM; Khakh et al., 1995). At similar concentrations ARL 67156 also increases contractions to exogenous ATP in the guinea-pig isolated vas deferens and urinary bladder and potentiates the neurotransmitter actions of ATP in these tissues (Westfall et al., 1996; 1997).
In the present study we found that when EFS was applied at 30 min intervals, ATP breakdown was 20–30% less in superfusate collected during the second stimulation period compared with the first, but there was no further decrease with a third stimulation. This implies that there is a large store of the enzyme in the tissue, though why there is an initial decrease in release is not clear. We also found that release was a frequency-dependent phenomenon, as EFS at 2 Hz released significantly less enzyme than EFS at 4 and 8 Hz, although release at 4 Hz and 8 Hz was similar. How these enzyme release profiles relate to changes in neurogenic contractions is not known, as the number of functional studies in this tissue is very limited (Sneddon et al., 1984; Sneddon & Machaly, 1992) and the corresponding contractile experiments have not been performed.
In conclusion, sympathetic nerve stimulation in the rabbit isolated vas deferens causes release of substantial ATPase activity, which is stable at room temperature and rapidly metabolizes exogenous ATP to ADP. In contrast, little ADPase activity was detected. This differs greatly from the guinea-pig vas deferens, which releases enzymes that degrade ATP to adenosine. Thus, although the release of soluble nucleotidases appears to be species-independent, the complement of enzymes released is species-dependent. Nonetheless, in both rabbit and guinea-pig, the releasable ATPase has biochemical and pharmacological similarities to eNTPDases and may play a role in the termination of the neurotransmitter actions of ATP.
Acknowledgments
This work was supported by grants from the Wellcome Trust, Carnegie Trust, Nuffield Foundation and Scottish Hospitals Endowment Research Trust (C. Kennedy, P. Sneddon) and the National Institute of Health (D.P. Westfall). The authors acknowledge the valuable input from Svetlana Mihaylova-Todorova and Latchezar Todorov.
Abbreviations
- ADP
adenosine 5′-diphosphate
- AMP
adenosine 5′-monophosphate
- ARL 67156
6-N,N-diethyl-D-β,γ-dibromomethyleneATP
- ATP
adenosine 5′-triphosphate
- EFS
electrical field stimulation
- eNTDPase
ectonucleoside triphosphate diphosphohydrolase
References
- AMARA S.G., KUHAR M.J. Neurotransporters: recent progress. Ann. Rev. Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Purinergic nerves. Pharmacol. Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- CRACK B.E., POLLARD S.E., BEUKERS M.W., ROBERTS S.M., HUNT S.F., INGALL A.H., MCKECHNIE K.C.W., IJZERMAN A.P., LEFF P. Pharmacological and biochemical analysis of FPL 67156, a novel, selective inhibitor of ecto-ATPase. Br. J. Pharmacol. 1995;114:475–481. doi: 10.1111/j.1476-5381.1995.tb13251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS R.J., LEWIS C., BUELL G., VALERA S., NORTH R.A., SURPRENANT A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2X purinoceptors) Mol. Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- GAYLE R.B., MALISZEWSKI C.R., GIMPEL S.D., SCHOENBORN M.A., CASPARY G., RICHARDS C., BRASEL K., PRICE V., DROSOPOULOS J.H.F., ISLAM N., ALYONYCHEVA T.N., BROEKMAN M.J., MARCUS A.J. Inhibition of platelet function by recombinant soluble ecto-ADPase/CD39. J. Clin. Invest. 1998;101:1851–1859. doi: 10.1172/JCI1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY C., TODOROV L.D., MIHAYLOVA-TODOROVA S., SNEDDON P. Release of soluble nucleotidases: a novel mechanism for neurotransmitter inactivation. Trends in Pharmacol. Sci. 1997;18:263–266. doi: 10.1016/s0165-6147(97)01088-2. [DOI] [PubMed] [Google Scholar]
- KHAKH B.S., MICHEL A.D., HUMPHREY P.P.A. Inhibition of ecto-ATPase and Ca2+-ATPase in rat vas deferens by P2-purinoceptor antagonists. Br. J. Pharmacol. 1995;115:P2. doi: 10.1111/j.1476-5381.1995.tb16336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATEO J., HARDEN T.K., BOYER J.L. Functional expression of a cDNA encoding a human ecto-ATPase. Br. J. Pharmacol. 1999;128:396–402. doi: 10.1038/sj.bjp.0702805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIHAYLOVA-TODOROVA S., TODOROV L., GERTHOFFER W.T., WESTFALL D.P. Characterization and initial purification of soluble neuronally released nucleotidase from guinea-pig vas deferens. FASEB J. 1998;12:A768. [Google Scholar]
- PLESNER L. Ecto-ATPases: identities and functions. Int. Rev. Cytol. 1995;158:141. doi: 10.1016/s0074-7696(08)62487-0. [DOI] [PubMed] [Google Scholar]
- SMITH T.M., KIRLEY T.L. Site-directed mutagenesis of a human brain ecto-apyrase: evidence that the E-type ATPases are related to the actin/heat shock 70/sugar kinase superfamily. Biochem. 1999;38:321–328. doi: 10.1021/bi9820457. [DOI] [PubMed] [Google Scholar]
- SNEDDON P., MACHALY M. Regional variation in purinergic and adrenergic responses in isolated vas deferens of rat, rabbit and guinea-pig. J. Auton. Pharmacol. 1992;12:421–428. doi: 10.1111/j.1474-8673.1992.tb00390.x. [DOI] [PubMed] [Google Scholar]
- SNEDDON P., MCLAREN G.J., KENNEDY C. Purinergic cotransmission: sympathetic nerves. Seminars in Neurosci. 1996;8:201–206. [Google Scholar]
- SNEDDON P., WESTFALL D.P., COLBY J., FEDAN J.S. A pharmacological investigation of the biphasic nature of the contractile response of rabbit and rat vas deferens to field stimulation. Life Sci. 1984;35:1903–1912. doi: 10.1016/0024-3205(84)90470-3. [DOI] [PubMed] [Google Scholar]
- TODOROV L.D., MIHAYLOVA-TODOROVA S., WESTFALL T.D., SNEDDON P., KENNEDY C., BJUR R.A., WESTFALL D.P. Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature. 1997;387:76–79. doi: 10.1038/387076a0. [DOI] [PubMed] [Google Scholar]
- WANG T.F., OU Y., GUIDOTTI G. The transmembrane domains of ectoapyrase (CD39) affect its enzymatic activity and quaternary structure. J. Biol. Chem. 1998;273:24814–24821. doi: 10.1074/jbc.273.38.24814. [DOI] [PubMed] [Google Scholar]
- WESTFALL T.D., KENNEDY C., SNEDDON P. Enhancement of sympathetic purinergic neurotransmission in the guinea-pig isolated vas deferens by the novel ecto-ATPase inhibitor ARL 67156. Br. J. Pharmacol. 1996;117:867–872. doi: 10.1111/j.1476-5381.1996.tb15273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESTFALL T.D., KENNEDY C., SNEDDON P. The ecto-ATPase inhibitor ARL 67156 enhances parasympathetic neurotransmission in the guinea-pig urinary bladder. Eur. J. Pharmacol. 1997;329:169–173. [PubMed] [Google Scholar]
- WESTFALL T.D., LIBERMAN R., WATERSTON S., WESTFALL D.P., SNEDDON P., KENNEDY C. Release of ATPase from the rabbit vas deferens during sympathetic nerve stimulation. Br. J. Pharmacol. 2000a;129:P178. doi: 10.1038/sj.bjp.0703662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESTFALL T.D., SARKAR S., RAMPHIR N., WESTFALL D.P., SNEDDON P., KENNEDY C. Characterisation of the ATPase released during sympathetic nerve stimulation of the guinea-pig isolated vas deferens. Br. J. Pharmacol. 2000b;129:1684–1688. doi: 10.1038/sj.bjp.0703271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMANN H. Biochemistry, localisation and functional roles of ecto-nucleotidases in the nervous system. Prog. Neurobiol. 1996;49:589–618. doi: 10.1016/0301-0082(96)00026-3. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN H. Two novel families of ectonucleotidases: molecular structures, catalytic properties and a search for function. Trends in Pharmacol. Sci. 1999;20:231–236. doi: 10.1016/s0165-6147(99)01293-6. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN H., BEAUDOIN A.R., BOLLEN M., GODING J.W., GUIDOTTI G., KIRLEY T.L., ROBSON S.C., SANO K.Proposed nomenclature for two novel nucleotide hydrolysing enzyme families expressed on the cell surface In: Ecto-ATPases and related ectonucleotidases 2000Shaker Publishing; 1–8.ed. Vanduffel, L., Lemmens, R. pp [Google Scholar]
- ZIMMERMANN H., BRAUN N. Ecto-nucleotidases–molecular structures, catalytic properties, and functional roles in the nervous system. Prog. Brain Res. 1999;120:371–385. [PubMed] [Google Scholar]


