Abstract
According to the two state receptor model, the β2-adrenergic receptor (β2-AR) isomerizes between an inactive state and a constitutively active state, which couples to the stimulatory G-protein in the absence of agonist. In bovine tracheal smooth muscle (BTSM), we investigated the effect of short and long term β2-AR activation by fenoterol on constitutive receptor activity.
Preincubation of the BTSM strips for 5 min, 30 min and 18 h with 10 μM fenoterol, followed by extensive washout (3 h, 37°C), caused a rapid and time-dependent inhibition of KCl-induced contraction, reaching 68±10, 51±6 and 46±4% of control, respectively, at 40 mM KCl (P<0.05 all). At all time points, the EC50 values to KCl were significantly reduced as well.
Preincubation of BTSM with 0.1, 1.0 and 10 μM fenoterol during 18 h caused a concentration-dependent decrease of the 40 mM KCl response to 70±5, 47±12 and 43±9% of control, respectively (P<0.05 all).
The reduced KCl contractions were reversed in the presence of 1 μM timolol. Moreover, the sensitivity to KCl in the presence of timolol was enhanced after fenoterol incubation. Inverse agonism was also found for other β-blockers, with a rank order of efficacy of pindolol ⩾timolol=propranolol>alprenolol⩾sotalol>labetalol.
At 25 mM KCl-induced tone, the contraction induced by cumulative timolol administration was competitively antagonized by the less efficacious inverse agonist labetalol, indicating that the fenoterol-induced effects cannot be explained by residual β-agonist binding.
In conclusion, fenoterol treatment of BTSM causes a time- and concentration-dependent development of constitutive β2-AR activity, which can be reversed by various inverse agonists. The β-agonist-induced changes could represent a novel regulation mechanism of β2-AR activity.
Keywords: β2-adrenergic receptor, constitutive receptor activity, inverse agonism, fenoterol, timolol, β-adrenergic receptor antagonists, bovine tracheal smooth muscle, contraction, KCl
Introduction
It has been demonstrated that some mutations in the C-terminal part of the third intracellular loop of the human β2-adrenoceptor (β2-AR) result in constitutive activation of the receptor (Samama et al., 1993). Besides agonist-independent activation of adenylyl cyclase, the mutant receptor displays a higher affinity for agonists, even in absence of G-proteins, an increased potency of agonists for stimulation of adenylyl cyclase, and an increased intrinsic activity for partial agonists as compared to the wild type receptor (Samama et al., 1993). Because the G-protein independent increase in agonist affinity for the mutant receptor cannot be explained by the classical Ternary Complex Model (De Lean et al., 1980) this model has been extended assuming isomerization of the receptor between an inactive and a constitutively active conformation. In this model, only the constitutively active conformation of the receptor can couple to the G-protein and elicit a biological response. Mutation of the human β2-AR would lead to a shift in the receptor equilibrium in favour of the constitutively active conformation by an impaired ability of the receptor to constrain peptide regions in the third intracellular loop from their interaction with the G-protein (Samama et al., 1993).
The constitutively active conformation of the wild type β2-AR can also be visualized by overexpression of the receptor, which is consistent with the concept that a small fraction of the total receptor pool may exist in the activated conformation even in the absence of agonist (Chidiac et al., 1994). Transgenic mice with cardiac-specific overexpression of the β2-AR showed an increased basal atrial contractility, which, in the highest overexpressing transgenic animals, was not responsive to further increase by isoprenaline (Milano et al., 1994). In myocardial membranes of these transgenic mice both basal and isoprenaline-stimulated adenylyl cyclase activity were increased as compared to controls, accompanied by an increased isoprenaline sensitivity in the transgenic membranes (Milano et al., 1994). The activity of both the overexpressed and mutant constitutively active β2-AR's can be inhibited by antagonists with negative intrinsic activity (inverse agonists), such as ICI 118551 and betaxolol for the mutant receptor (Samama et al., 1994) and for example timolol and propranolol for the overexpressed human wild type receptor (Chidiac et al., 1994).
Recently, it has been reported by Wang et al. (1994) that chronic activation of the Gi-coupled μ opioid receptor in human SH-SY neuroblastoma cells by the agonist morphine gradually caused a constitutively active receptor state. The evidence for this was based on the observation that after morphine exposure, followed by thorough washing, the antagonist naloxone acutely enhanced cyclic AMP accumulation. This inverse agonistic behaviour of naloxone has also been demonstrated in morphine-pretreated guinea-pig ileum preparations (‘abstinence response') whereas in control tissues naloxone was without effect (Cruz et al., 1996).
Based on these findings, we examined whether short and long term activation of native β2-ARs in bovine tracheal smooth muscle (BTSM), using the selective β2-agonist fenoterol, would induce constitutive receptor activity.
Methods
Tissue preparation and incubation
Fresh bovine tracheas were obtained from the slaughterhouse and transported to the laboratory in Krebs-Henseleit (KH) buffer (mM: NaCl 117.50, KCl 5.60, MgSO4 1.18, NaH2PO4 1.28, CaCl2 2.52, NaHCO3 25.00 and D-glucose 5.55), pregassed with 95% O2 and 5% CO2; pH 7.4. The tracheal smooth muscle was dissected carefully, prepared free of mucosa and connective tissue, and cut into strips with an average weight of 10 mg, while incubated in KH-buffer, gassed with 95% O2 and 5% CO2 at room temperature. Subsequently, all strips were incubated for 18 h in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 mM NaHCO3, 20 mM HEPES, 100 u ml−1 penicillin, 100 μg ml−1 streptomycin and 10% foetal calf serum at 37°C, in the absence or presence of various concentrations of fenoterol, as indicated. Short term incubations (5 and 30 min) with fenoterol were performed by adding the agonist near the end of the total 18 h incubation period, as appropriate.
Isometric contraction studies
After washing in several volumes of KH-buffer, gassed with 95% O2 and 5% CO2, pH 7.4 at 37°C, the BTSM strips were mounted in 20 ml organ baths containing gassed KH-buffer (37°C) for isometric recording on Kipp BD41 flat bed recorders using Grass FT03 force displacement transducers connected to Grass low level DC amplifiers. During a 90 min equilibration period, with washings every 30 min, the resting tension was gradually adjusted to 3 g. Subsequently, the strips were precontracted with 20 and 40 mM isotonic KCl and, after two changes to fresh KH-buffer, precontracted by cumulative administration of methacholine (0.1, 1, 10 μM), followed by washout (three changes of bath volume) for 30 min. Under isoprenaline (0.1 μM) induced basal tone the strips were adjusted to 3 g resting tension, immediately followed by two changes of bath volume. The entire procedure resulted in a thorough washout of fenoterol (3 h, 37°C). Subsequently, the first concentration response curve (CRC) to stepwise increasing concentrations (6–40 mM) of isotonic KCl was recorded. After washout (two changes of bath volume) a second CRC to KCl was recorded, following 30 min of preincubation in the absence or presence of 1 μM timolol.
Subsequently, the inverse agonism by different β-adrenergic receptor antagonists were studied in BTSM strips preincubated with 10 μM fenoterol for 18 h. Following the procedure described above, two subsequent KCl CRCs were recorded in the absence and presence of the antagonist under investigation, respectively. The antagonists and the concentrations used are mentioned in the text.
Competition experiments for inverse agonism by timolol were performed on strips preincubated for 18 h with 10 μM fenoterol, at a 25 mM KCl-induced tone. The competing antagonists were incubated 30 min before starting a cumulative timolol CRC.
Relaxation studies
BTSM strips were mounted for isotonic recording in KH-buffer gassed with 95% O2 and 5% CO2, pH 7.4 at 37°C, using a preload of 0.5 g. A 60 min equilibration period was allowed, after which the strips were precontracted twice by cumulative administration of methacholine (0.1, 1, 10 and 0.1, 1, 10, 100 μM, respectively), with washing periods of 60 min. To establish basal tone 0.1 μM isoprenaline was administered between the two contractions, immediately followed by a 30 min washing period. After preincubation for 30 min in the absence or presence of the β-AR antagonist, smooth muscle tone was raised with methacholine to 50–70% of the maximal contraction, as measured with 100 μM methacholine in the second precontraction. Cumulative concentration-relaxation-curves were constructed using (−)-isoprenaline, added in 0.5 log increments.
Data analysis
Isometrically recorded contractile responses were expressed in grams developed tension. Isoprenaline-induced relaxation responses, in the absence or presence of the antagonist, were expressed as a percentage of the maximal methacholine-induced contraction. pKB-values of the antagonists were determined using the formula pKB=−log[antagonist]+log (DR−1) (MacKay, 1978). Results are presented as means±s.e.mean of the indicated number of experiments. Treatment-groups were compared by the Student's t-test for paired or unpaired observations, as mentioned.
Materials
Tissue culture supplies were purchased from Gibco BRL Life Technologies (Paisley, U.K.). DMEM and methacholine were obtained from ICN Biomedicals (Costa Mesa, CA, U.S.A.). Timolol, labetalol and (−)-isoprenaline were from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Fenoterol (Boehringer Ingelheim, Ingelheim, Germany), acebutolol (Rhone Poulenc Rorer, Amstelveen, The Netherlands), pindolol (Novartis, Basel, Switzerland), alprenolol (Astra, Rijswijk, The Netherlands), propranolol (Zeneca, Macclesfield, U.K.) and sotalol (Bristol-Myers Squibb, Woerden, The Netherlands) were kind gifts from the manufacturers.
Results
Time- and concentration-dependence of fenoterol-induced constitutive β-adrenergic receptor activity
Preincubation of BTSM strips with 10 μM fenoterol for 5 min, 30 min and 18 h resulted in a time-dependent reduction of KCl-induced contraction (Figure 1). Paired analysis of the data indicated a decrease of the 40 mM KCl-induced contraction to 68±10 (P<0.05), 51±6 (P<0.005) and 46±4% (P<0.0001) of control after 5 min, 30 min and 18 h incubation with fenoterol, respectively. The time-dependent depression of the contraction was accompanied by a significant decrease in sensitivity towards the contractile agent; the EC50 value of KCl shifting from 25.4±0.6 mM (control) to 30.8±0.3, 32.0±0.2 and 31.2±0.3 mM after 5 min, 30 min and 18 h of incubation, respectively (P<0.0001 for all conditions, paired analysis).
Figure 1.
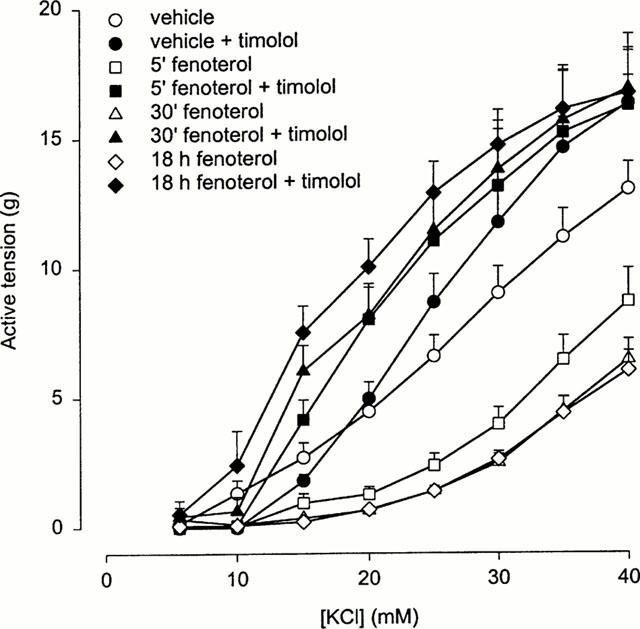
Isometric contraction of BTSM strips by increasing concentrations of KCl in the absence and subsequent presence of 1 μM timolol, after pre-incubation of the strips with vehicle or 10 μM fenoterol for 5 min, 30 min and 18 h, followed by thorough washout of the agonist. Results are means±s.e.mean of seven experiments.
In the presence of 1 μM timolol a strong potentiation of the reduced KCl-induced contraction was observed (Figure 1), associated by an increase in sensitivity towards the contractile agent, with a shift in EC50 from 24.9±0.9 mM in the control condition to 20.8±1.0, 20.3±1.0 and 17.4±1.2 mM after 5 min, 30 min and 18 h of incubation with fenoterol, respectively (P<0.05 for all conditions, paired analysis).
The contraction of the control strips was also slightly enhanced in the presence of timolol, with no significant effect on the EC50-value of KCl, however. This effect appeared not to be due to inverse agonism of basal constitutive activity by timolol. This is indicated by Figure 2, which shows that a second control CRC of KCl in the absence of timolol was increased to the same extent as a second control CRC of KCl in the presence of timolol. A similar increase of KCl-induced contraction was noticed in a second CRC, measured in the absence of timolol in BTSM strips after 18 h incubation with fenoterol, indicating that the pronounced potentiation of KCl-induced contraction by timolol under this condition (Figure 1) was caused by the preincubation with the β-agonist.
Figure 2.
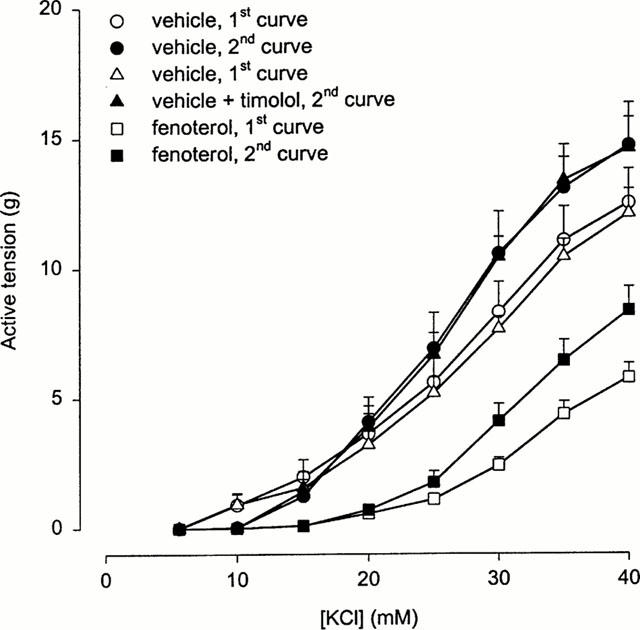
Repeated KCl-induced contractions of BTSM strips incubated for 18 h with vehicle or 10 μM fenoterol. A first vehicle-treated control group was subjected to two successive contractions in the absence of timolol. A second control group was first contracted in the absence of timolol, followed by a contraction in the presence of 1 μM timolol. Fenoterol-incubated strips were subjected to two successive contractions in the absence of timolol. Results are means±s.e.mean of 5–6 experiments.
Incubation of BTSM strips with 0.1, 1.0 and 10 μM of fenoterol during 18 h resulted in a concentration-dependent decrease of the 40 mM KCl response to 70±5, 47±12 and 43±9% of control, respectively (P<0.05 for all conditions, paired analysis) (Figure 3).
Figure 3.
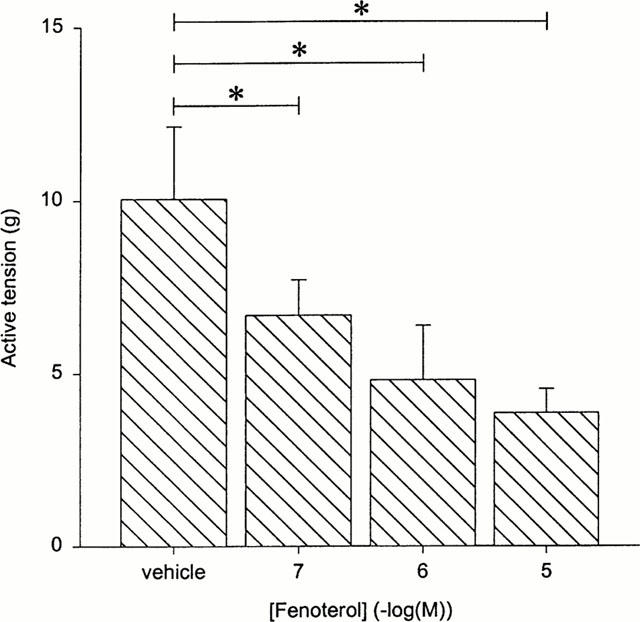
Isometric contraction of BTSM strips towards 40 mM KCl, after 18 h preincubation with vehicle or various concentrations of fenoterol, followed by thorough washout of the agonist. Results are means±s.e.mean of five experiments. *P<0.05 (paired Student's t-test).
Inverse agonist activity by β-adrenoceptor antagonists
Table 1 shows the pKB-values of six different β-adrenergic receptor antagonists for competitive antagonism of (−)-isoprenaline-induced BTSM relaxation. To study inverse agonistic activity of these antagonists on BTSM treated for 18 h with 10 μM fenoterol, concentrations were selected occupying 99.51 to 99.77% of the receptors (except sotalol which, at the 0.1 mM concentration used, has an occupancy of 98.44%). All antagonists showed significant inverse agonist activity as indicated by inhibition of fenoterol-induced constitutive β-AR activity (Figure 4). Based on the results at 40 mM KCl-induced tone, a rank order of inverse agonistic potency of pindolol⩾timolol=propranolol>alprenolol⩾sotalol>labetalol was found for the antagonists at the concentrations used. This rank order differed from the order of pKB values for competitive antagonism of (−)-isoprenaline, being pindolol>propranolol=alprenolol=timolol>labetalol>sotalol.
Table 1.
pKB-values and receptor occupancies (at the indicated concentrations) of β-AR antagonists on isoprenaline-induced BTSM relaxation
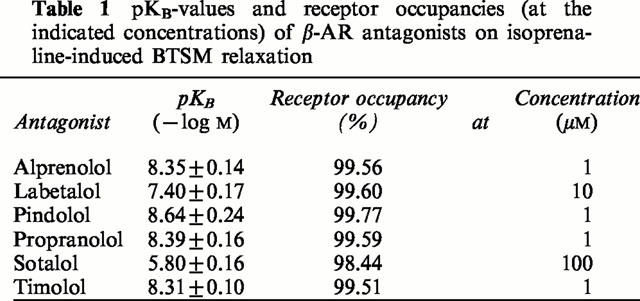
Figure 4.
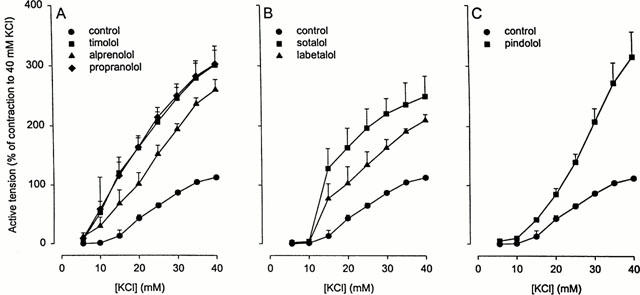
Isometric contraction of BTSM strips by increasing concentrations of KCl in the absence or presence of (A) 1 μM timolol, 1 μM propranolol, 1 μM alprenolol, (B) 10 μM labetalol, 100 μM sotalol, and (C) 1 μM pindolol after 18 h pre-incubation with 10 μM fenoterol, followed by thorough washout of the agonist. Both for the control in the absence of antagonist and for the different antagonists, the effects are expressed as per cent of the 40 μM KCl tone, measured in a preceding control response curve in the absence of antagonist. Results are means±s.e.mean of 3–7 experiments.
At a 25 mM KCl tone, the contraction induced by cumulative administration of timolol was competitively inhibited in the presence of 10 μM labetalol (Figure 5A), a less efficacious inverse agonist than timolol. The inverse agonistic pEC50-value of timolol was significantly reduced by labetalol, from 7.8±0.2 in the absence of the competing antagonist to 6.1±0.1 in the presence of 10 μM labetalol. From this shift in timolol sensitivity a pKB value of 6.5±0.2 could be calculated for labetalol, which is well below the pKB value for competition with (−)-isoprenaline at the β2-AR (7.4±0.2). The shift of the timolol curve by the competing inverse agonist labetalol appeared not to be due to the increased contraction in the presence of labetalol per se. In the initial presence of 10 nM timolol, which induces a similar contraction as 10 μM labetalol, the pEC50-value for timolol-induced contraction was not significantly changed (7.7±0.1 vs 8.0±0.1 in the paired controls without the initial presence of 10 nM timolol, P>0.05) (Figure 5B).
Figure 5.
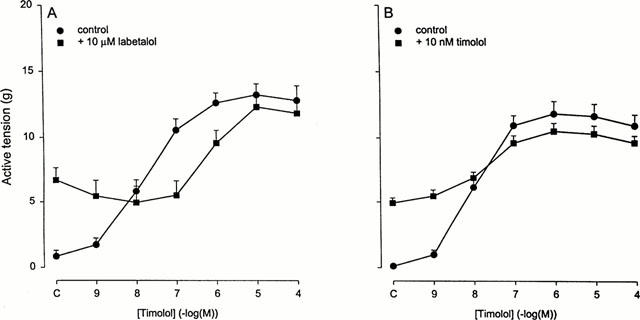
Inverse agonism by increasing concentrations of timolol in the absence or initial presence of 10 μM labetalol (A), or in the initial presence of 10 nM timolol (B). BTSM strips were preincubated with 10 μM fenoterol for 18 h, followed by thorough washout of the agonist. Contraction was measured in the presence of 25 mM KCl. Results are means±s.e.mean of 5–8 experiments.
Discussion
Incubation of bovine tracheal smooth muscle with the β2-agonist fenoterol resulted in a time- and concentration-dependent induction of constitutive β2-adrenergic receptor activity. This constitutive β2-AR activity was revealed by a decreased sensitivity towards the contractile agent KCl accompanied by a reduced maximal contraction towards this agent as compared to non-treated control preparations. Blockade of the constitutively active receptors of the fenoterol-treated preparations with the inverse agonist timolol significantly enhanced the sensitivity towards KCl, i.e. diminished the EC50, to values below those of non-treated controls, and augmented the maximal contraction as well. In addition to timolol, also the five other β-AR antagonists tested, applied at concentrations resulting in a 99.51 to 99.77% (sotalol: 98.44%) occupancy of the inactive receptor, displayed inverse activity on this tissue, with a rank order of inverse efficacy on a 40 mM KCl-induced tone of pindolol⩾timolol=propranolol>alprenolol⩾sotalol>labetalol.
With the first four compounds, the rank order of inverse efficacies on the constitutive active receptor corresponded with their receptor occupancies of the inactive receptor, except for alprenolol which displayed a lower inverse agonism. As shown in Figure 4B, sotalol clearly displayed a higher inverse efficacy than labetalol, despite the fact that the receptor occupancy of sotalol (98.44%) was less than that of labetalol (99.60%). Interestingly, a moderate inverse efficacy of labetalol was also found in Sf9 cells overexpressing the human β2-AR (Chidiac et al., 1994), with timolol and propranolol showing similar high inverse efficacies as in the present study. Pindolol, however, being a potent inverse agonist in fenoterol pretreated BTSM, was less efficacious in human β2-AR overexpressing Sf9 cells. Since isoprenaline-induced desensitization of overexpressed β2-AR has been found to potentiate the inverse efficacies of antagonists (Chidiac et al., 1996), it may be argued that the enhanced inverse efficacy of pindolol in BTSM was related to the fenoterol-pretreatment. However, desensitization does not change the rank order of inverse efficacies (Chidiac et al., 1996).
Despite the fact that fenoterol is known to be a short-acting β2-agonist in asthma, it could be envisaged that some retained fenoterol is causing the depression of the KCl-induced contraction. However, this is very unlikely for the following reasons. First, after incubation with fenoterol, the BTSM preparations were washed for at least 3 h with regular, repeated changes of buffer solution. Second, the rank order of the inverse efficacies of the six β-AR antagonists on the constitutive active receptor did not correspond with the receptor occupancies on the inactive receptor, as discussed above. Third, the enhanced sensitivity of fenoterol-treated BTSM contraction to KCl in the presence of timolol cannot be caused by displacement of residual β-agonist. Fourth (and most revealing), labetalol, the compound with the lowest inverse efficacy, was acting as an antagonist of the timolol-induced inverse agonism, as visualized by the rightward shift of the concentration-dependent increase of the contraction in the presence of 25 mM KCl (Figure 5A). If the effect of timolol was due to displacement of residually bound agonist, an additional β-blocker would not be expected to antagonize the effect. For the same reasons, the theoretical possibility that during the KCl-induced contraction, mediated by L-type calcium channels, some (facilitated) release of endogenous noradrenaline would contribute to the observed constitutive β2-AR activity, is not feasible. Furthermore, we recently found that muscarinic agonist- and histamine-induced contractions, mediated by phosphoinositide metabolism (Meurs et al., 1988; Van Amsterdam et al., 1989), are also susceptible to fenoterol-induced constitutive activity (unpublished observations).
Interestingly, the formation of constitutive receptor activity by fenoterol was not only concentration-dependent but also a rapid process; after a 5 min treatment period, a marked depression of the KCl contraction curve already was found, whereas the effect of 30 min treatment was not further increased by prolonging the incubation period to 18 h. Since activation of βARK and subsequent β-AR phosphorylation are also known to occur rapidly (Roth et al., 1991), the findings would suggest that the fenoterol-induced constitutive activation of the receptor may involve receptor phosphorylation. This proposal would be in line with recent observations by Pei et al. (1994) with constitutively active mutant β2-ARs, reconstituted in phospholipid vesicles, showing that this receptor is phosphorylated by βARK to a similar extent as the agonist-stimulated wild type receptor. In addition, the phosphorylation of the mutant β2-AR was associated with constitutive desensitization of the receptor (Pei et al., 1994). Using BTSM preparations contracted with the muscarinic agonist methacholine, we similarly found that 10 μM fenoterol-induced constitutive β2-AR activity, as indicated by a reduced 1 μM methacholine-induced tone, was associated with desensitization of the receptor: both the pEC50 and the Emax of the isoprenaline-induced relaxation CRC were reduced as compared to control preparations (unpublished observations). Inverse agonists have been shown to diminish the basal phosphorylation of the constitutively active mutant receptor (Samama et al., 1994). Using overexpressed β2-ARs, agonist-induced desensitization was found to increase inverse efficacies (Chidiac et al., 1996), as mentioned above. Hence, an active and a non-active form of the desensitized receptor was proposed. Inverse agonists would stabilize the non-active form of the desensitized receptor with higher efficacy than of the non-desensitized receptor.
Thus, in line with these observations, the present findings would be congruent with the following scheme (Figure 6). In this scheme R represents the inactive receptor state with high affinity for inverse agonists, R* the active form with high affinity for agonists, Rd* the active desensitized state with relatively low affinities for agonists as well as inverse agonists and Rd the inactive desensitized state with very high affinity for inverse agonists. In native non-treated BTSM, basal isomerization of R to R* presumably is low in view of the inefficacy of timolol to enhance the KCl-induced contraction (Figure 2). During fenoterol treatment, rapid formation of AR* and, subsequently of ARd* will occur (heavy arrows) which will dissociate relatively rapidly after washing out. Thus, the resulting Rd* would be mainly responsible for the dose- and time-dependent depression of the KCl-induced contraction after the fenoterol treatment. During subsequent incubation with an inverse agonist B, forming BRd complexes, the decreased level of Rd* will enhance the KCl-induced contraction. The above described concept that Rd has a higher affinity for inverse agonists than the basal R state of the receptor (Chidiac et al., 1996) predicts the BRd complex to be tighter than the BR complex which is in line with the observation that in fenoterol-treated BTSM preparations, timolol induced a leftward shift of the KCl-induced CRCs (i.e. enhances the sensitivity for KCl) as compared to control preparations. In addition, the observation that the less efficacious inverse agonist labetalol is able to antagonize the effect of high efficacious timolol indicates that both ligands compete with the same receptor state (Figure 5A). In this context, it should be mentioned that a very similar antagonism of timolol by the low efficacious inverse agonist dichloroisoprenaline has been found in human β2-ARs overexpressed in Sf9 cells (Chidiac et al., 1994).
Figure 6.
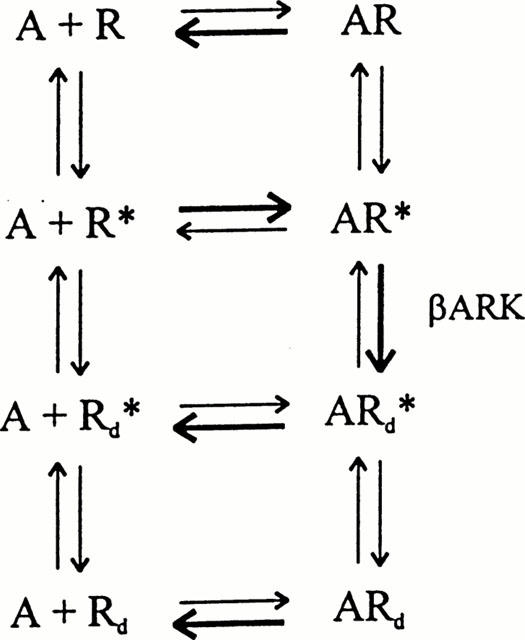
Scheme for the agonist-induced development of constitutive receptor activity. See text for further explanations.
In conclusion, the present study has shown that treatment of BTSM with the β2-agonist fenoterol causes a time- and concentration-dependent development of constitutive β2-AR activity, which can be reversed by various inverse agonists. The fenoterol-induced changes could represent a novel regulation mechanism of β2-AR activity by β-agonists.
Acknowledgments
The authors wish to thank Ellen H.A. Duerink, Jolanda M. Kooistra and Reinoud Gosens for expert technical assistance. The study was financially supported by the Netherlands Asthma Foundation (NAF 95.63).
Abbreviations
- β2-AR
β2-adrenergic receptor
- β-ARK
β-adrenergic receptor kinase
- BTSM
bovine tracheal smooth muscle
- CRC
concentration response curve
- DMEM
Dulbecco's modified Eagle's medium
- DR
dose ratio
- EC50
concentration causing 50% effect
- KH
Krebs-Henseleit
- pEC50
−log10 of the concentration causing 50% of effect
- pKB
−log10 of the dissociation equilibrium constant
- R
inactive receptor state
- R*
active receptor state
- Rd
inactive desensitized receptor state
- Rd*
active desensitized receptor state
References
- CHIDIAC P., HEBERT T.E., VALIQUETTE M., DENNIS M., BOUVIER M. Inverse agonist activity of β-adrenergic antagonists. Mol. Pharmacol. 1994;45:490–499. [PubMed] [Google Scholar]
- CHIDIAC P., NOUET S., BOUVIER M. Agonist-induced modulation of inverse agonist efficacy at the β2-adrenergic receptor. Mol. Pharmacol. 1996;50:662–669. [PubMed] [Google Scholar]
- CRUZ S.L., VILLARREAL J.E., VOLKOW N.D. Further evidence that naloxone acts as an inverse opiate agonist: Implications for drug dependence and withdrawal. Life Sci. 1996;58:PL381–PL389. doi: 10.1016/0024-3205(96)00250-0. [DOI] [PubMed] [Google Scholar]
- DE LEAN A., STADEL J.M., LEFKOWITZ R.J. A ternary complex model explains the agonist-specific binding of the adenylate cyclase-coupled beta-adrenergic receptor. J. Biol. Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- MACKAY D. How should values of pA2 and affinity constants for pharmacological competitive antagonists be estimated. J. Pharm. Pharmacol. 1978;30:312–313. doi: 10.1111/j.2042-7158.1978.tb13237.x. [DOI] [PubMed] [Google Scholar]
- MEURS H., ROFFEL A.F., POSTEMA J.B., TIMMERMANS A., ELZINGA C.R.S., KAUFFMAN H.F., ZAAGSMA J. Evidence for a direct relationship between phosphoinositide metabolism and airway smooth muscle contraction induced by muscarinic agonists. Eur. J. Pharmacol. 1988;156:271–274. doi: 10.1016/0014-2999(88)90331-7. [DOI] [PubMed] [Google Scholar]
- MILANO C.A., ALLEN L.F., ROCKMAN H.A., DOLBER P.C., MCMINN T.R., CHIEN K.R., JOHNSON T.D., BOND R.A., LEFKOWITZ R.J. Enhanced myocardial function in transgenic mice overexpressing the β2-adrenergic receptor. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- PEI G., SAMAMA P., LOHSE M., WANG M., CODINA J., LEFKOWITZ R.J. A constitutively active mutant β2-adrenergic receptor is constitutively desensitized and phosphorylated. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2699–2702. doi: 10.1073/pnas.91.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH N.S., CAMPBELL P.T., CARON M.G., LEFKOWITZ R.J., LOHSE M.J. Comparative rates of desensitization of β-adrenergic receptors by the β-adrenergic receptor kinase and the cyclic AMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A. 1991;88:6201–6204. doi: 10.1073/pnas.88.14.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMAMA P., COTECCHIA S., COSTA T., LEFKOWITZ R.J. A mutation-induced activated state of the β2-adrenergic receptor. J. Biol. Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- SAMAMA P., PEI G., COSTA T., COTECCHIA S., LEFKOWITZ R.J. Negative antagonists promote an inactive conformation of the β2-adrenergic receptor. Mol. Pharmacol. 1994;45:390–394. [PubMed] [Google Scholar]
- VAN AMSTERDAM R.G.M., MEURS H., BROUWER F., POSTEMA J.B., TIMMERMANS A., ZAAGSMA J. Role of phosphoinositide metabolism in functional antagonism of airway smooth muscle contraction by β-adrenoceptor agonists. Eur. J. Pharmacol. 1989;172:175–183. doi: 10.1016/0922-4106(89)90008-4. [DOI] [PubMed] [Google Scholar]
- WANG Z., BILSKY E.J., PORRECA F., SADEE W. Constitutive μ opioid receptor activation as a regulatory mechanism underlying narcotic tolerance and dependence. Life Sci. 1994;54:PL339–PL350. doi: 10.1016/0024-3205(94)90022-1. [DOI] [PubMed] [Google Scholar]


