Abstract
The aim of this study was to investigate the effects of adenine nucleosides and nucleotides on contractility of the smooth muscle of rat prostate gland.
Nerve terminals within rat isolated prostatic tissues were electrically field stimulated (60 V, 0.5 ms, 10 Hz, 20 pulses every 60 s). Adenosine 5′-triphosphate (ATP), adenosine 5′-diphosphate (ADP), adenosine 5′-monophosphate (AMP) and adenosine had no effect on baseline smooth muscle tone but concentration-dependently inhibited electrically-evoked contractile responses. The relative order of potency was ATP ≅ AMP ≅ adenosine>ADP.
The inhibition by ATP and adenosine of field stimulation-induced contractions in the rat prostate was antagonized by 8-phenyltheophylline (10 μM), but not by suramin (100 μM) and only slightly by reactive blue 2 (5 μM).
The adenosine metabolizing enzyme adenosine deaminase (0.1 unit ml−1) inhibited the inhibitory effects of ATP and adenosine. The P2 purinoceptor agonist 2-methylthio ATP (10 nM–0.1 mM), had no effect on field stimulation-induced contractions of the rat prostate.
ATP and adenosine did not modify the contractile responses of the rat prostate to exogenously added noradrenaline (10 μM).
Inhibitory concentration-response curves to a number of adenosine analogues with differing stabilities and selectivities for the different adenosine receptors yielded a relative rank order of agonist potency of: N6-cyclopentyladenosine (CPA)>N6-cyclohexyladenosine (CHA) ≅ (−)-N6-(2-phenylisopropyl)-adenosine (R-PIA) ≅ 5′-(N-ethylcarboxamido)-adenosine (NECA)>(+)-N6-(2-phenylisopropyl)-adenosine (S-PIA)>2-p-[2-carboxyethyl]phenethyl-amino-5′-N-ethylcarboxamido-adenosine (CGS 21680).
These results indicate that adenine nucleoside and nucleotide induced inhibition of electrically-evoked contractions in the rat prostate occurs through activation of adenosine but not ATP receptors. The relative order of potency of adenosine analogues is consistent with activation of receptors of the A1-adenosine receptor subtype. These receptors appear to be prejunctional.
Keywords: Prostate, adenosine receptors, adenosine 5′-triphosphate, ATP, adenosine, neuromodulation, smooth muscle
Introduction
This study investigates the role of adenine nucleosides and nucleotides in the contractility of the smooth muscle of the rat prostate gland. Adenine nucleosides and nucleotides exhibit specific extracellular as well as intracellular signalling actions, so regulating a number of physiological systems. Cell surface purinoceptors are widely distributed throughout mammalian tissues (Windschief, 1996; Abbracchio & Burnstock, 1998; Rathbone et al., 1999). Most pertinent to this study is the ability of nucleosides and nucleotides to modulate autonomic neurotransmission (Abbracchio & Burnstock, 1998).
These receptors for ATP and adenosine can pharmacologically be classified into two primary groups. Adenosine receptors designated Ax and the P2 or ATP receptors which can be further divided into the P2X and P2Y families (Abbracchio & Burnstock, 1998; Rathbone et al., 1999).
ATP is colocalized with noradrenaline in postganglionic sympathetic nerve fibres (Abbracchio & Burnstock 1998). In this capacity, it acts as a co-transmitter in several genito-urinary tissues including the rat and guinea-pig vas deferens and the guinea-pig seminal vesicle, being co-released with noradrenaline upon nerve stimulation. In the rat vas deferens it has an excitatory effect on smooth muscle tone through interaction with P2X receptors (Khakh et al., 1995). ATP also contracts the rat cauda epididymis through stimulation of P2X receptors (Ventura & Pennefather, 1991). Recently ATP has been identified as an inhibitory co-transmitter with vasoactive intestinal polypeptide in the proximal hamster urethra where it acts at P2Y receptors (Pinna et al., 1998). There is similar evidence that ATP is a co-transmitter with acetylcholine in parasympathetic nerves, particularly in supplying the bladder, where it exhibits predominantly an excitatory effect (Burnstock et al., 1978) but also has some inhibitory effect (Boland et al., 1993).
Termination of ATP activation of purinoceptors is a result of the hydrolyses of the molecule by extracellular 5′-nucleotidases in the neuroeffector junction (Burnstock, 1972; Windschief, 1996). These hydrolyses systematically cleave a single phosphate moiety from the triphosphate tail to yield ADP, AMP and finally adenosine. The product of each hydrolysis consequently has a lower affinity for ATP or P2 receptors, and a greater affinity for adenosine or P1 receptors. This order of purinoceptor affinity, alternatively expressed as a rank order of potency, forms the basis for characterisation of purinoceptor subtypes (Burnstock, 1978; Windschief, 1996). In addition, the hydrolysis of ATP to adenosine provides a mechanism through which ATP can indirectly activate P1 purinoceptors (Windschief, 1996). Expression and enzymic activity of ecto 5′-nucleotidases have been demonstrated in male genital tissues including the prostate gland (Konrad et al., 1998).
P1 adenosine receptors have been subclassified into the A1, A2A, A2B and A3 subtypes (Fredholm et al., 1998), and there is evidence for the existence of a population of A1 receptors in various urogenital structures including the rat (Hourani et al., 1993; Kurz et al., 1993), guinea-pig (Driessen et al., 1994; Haynes et al., 1998) and mouse vas deferens (Kurz et al., 1993), and the rat (Ventura & Pennefather 1992) and guinea-pig cauda epididymis (Haynes et al., 1998). In all cases, these receptors are thought to mediate inhibition of noradrenaline release, consequently reducing electrical field stimulation-induced contractile responses or smooth muscle tone. Adenosine present in the neuroeffector junction of these tissues may result from enzymatic breakdown of ATP co-released from the sympathetic innervation of the tissue.
Very little attempt has been made to investigate purinoceptor mechanisms in the prostate gland. This is surprising when one considers that the presence or expression of purinoceptors (Fang et al., 1992; Janssens et al., 1996; Longhurst et al., 1996; Wasilenko et al., 1997) and of ecto 5′-nucleotidase (Konrad et al., 1998) has been reported in human prostate. ATP has also been shown to increase inositol phosphates in rat prostatic cultures (Guijarro et al., 1996).
The aims of this study were to investigate the role of purine nucleotides in neurotransmission to the smooth muscle of the rat prostate gland and to identify and characterize the receptor subtype responsible for mediation of any observed effect.
Methods
Animals and tissues
Male Sprague-Dawley rats (200–350 g) were housed at 22°C with a photoperiod of 12 h light/dark. Animals had free access to rodent chow and water ad libitum. Rats were killed by cervical dislocation or decapitation. An incision to the lower abdomen of the rat exposed the male urogenital tract. Both the ventral and dorsal lobes of the prostate were removed together, and placed in a petri dish containing Krebs-Henseleit solution (mM: NaCl 118.1, KCl 4.69, KH2PO4 1.2, NaHCO3 25.0, glucose 11.7, MgSO4 0.5, CaCl2 2.5). The prostatic capsule was carefully dissected away at this time. Ethical approval for these experiments was obtained from the Monash University Standing Committee of Ethics in Animal Experimentation.
Experimental procedures
The dissected prostates were separated into the left and right lobes, providing two preparations per animal. Tissues were mounted vertically in 10 ml water-jacketed glass organ baths, containing Krebs-Henseleit solution, maintained at 37°C and bubbled with 5% CO2 in O2. One end of the tissue was attached to the tissue holder, and the other to a Grass FTO3C-transducer for recording of isometric contractile activity of the smooth muscle. Developed force was recorded via a PowerLab data acquisition system (Chart 3.6) connected to a Macintosh 5500/225 computer. Preparations were equilibrated for 60 min under a resting force of 0.4–0.5 g.
Nerve terminals within the smooth muscle of the prostate stroma were electrically stimulated via two parallel platinum electrodes embedded in the tissue holder, connected to a Grass S88 stimulator. The electrical field stimulation applied to the tissues was with parameters: trains of 20 pulses of 0.5 ms pulse duration, at 10 Hz, a dial setting of 80 V, delivered every 60 s (Lau et al., 1998). Using these electrical field stimulation parameters, stimulation-induced contractile responses in the rat prostate have been shown to be tetrodotoxin-sensitive and are markedly attenuated in the presence of guanethidine and prazosin (Lau et al., 1998). This confirms that the responses are of neural origin, are sympathetically mediated and primarily noradrenergic in origin. Stimulation was first applied after the first 30 min of equilibration, and continued throughout the remainder of the 60 min equilibration period. Throughout the equilibration period, all tissues were washed every 10–15 min to eliminate the accumulation of prostatic secretions in the bath.
Agonist studies
Discrete log concentration-response curves to purinoceptor agonists were constructed using 0.5 log unit increments. Each concentration was added after five stable control electrical field stimulation induced contractions, and remained in contact with the tissue for 5 min before being washed out with fresh bathing medium. The next concentration was not applied until electrical field stimulation induced contractile responses returned to their control magnitude. Typically, this took 5–10 min depending on the agonist used. Only one agonist was used for each preparation, with paired tissues used for relevant vehicle control curves.
Effects of enzymes, inhibitors and antagonists
Discrete log concentration-response curves to the agonists ATP, adenosine and 2-methylthio ATP were constructed in the absence and presence of adenosine deaminase or the purinoceptor antagonists: 8-phenyltheophylline, reactive blue 2 and suramin. Only one curve was constructed on each preparation, with paired tissues from each animal used for time control curves. Most of these drugs were incubated with the tissues during the 1 h equilibration period, and were replaced in the bathing solution after each washout. The P2 receptor antagonist reactive blue 2 was incubated with the tissue for 5 min prior to each agonist addition. This particular protocol was adopted to avoid the non-selective desensitization that this agent causes after long periods of contact with isolated tissue preparations (Burnstock & Warland, 1987).
Postjunctional effects
A subset of experiments examined the effects of ATP and adenosine on the peak contractile response to exogenous application of a submaximal dose of noradrenaline (10 μM). In these experiments tissues were not field stimulated. Three control responses to exogenous noradrenaline (10 μM) were obtained at the beginning of each experiment. Tissues were then exposed to each agonist concentration for 3 min before a single application of noradrenaline (10 μM). Once the response to noradrenaline reached a plateau, the drugs were washed out with fresh bathing medium. The next dose of noradrenaline was not applied for 10 min after the previous wash. Discrete log concentration-response curves were constructed to each agonist using 0.5 log unit increments. To test for time related effects, three control responses to exogenous noradrenaline (10 μM) were also obtained at the end of each experiment.
Measurement and analysis of data
The mean peak force (g) of four electrical field stimulation-induced contractions was determined immediately before addition of each agonist concentration. After agonist addition, five responses were recorded before washout, and the mean peak force (g) of the last four electrical field stimulation induced contractions was calculated. Responses to inhibitory agonists were expressed as percentage inhibition of the initial mean response.
In the experiments examining postjunctional effects, the mean peak force (g) of three exogenously applied noradrenaline (10 μM)-induced contractions was determined before addition of the first agonist concentration. After each agonist addition, the response to noradrenaline (10 μM) was recorded before washout, and the mean peak force (g) of this contraction was determined. Responses to inhibitory agonists were expressed as percentage inhibition of the initial mean response.
All results were expressed as mean±s.e.mean. The n value represents the number of animals used.
Two-way repeated measure analysis of variance (ANOVA) were carried out to compare two or more treatment groups at all concentrations on the log concentration-response curve using Sigmastat (version 1.0). To evaluate statistical significance, P values representing a probability of a significant interaction between dose and treatment were used. Estimates of potency ratio between mean inhibitory log concentration-response curves were also calculated using GraphPad Prism (version 3.0). Two-tailed Student's paired t-tests were carried out to estimate the significance in basal electrical field stimulation induced contractile responses between control and treatment groups using GraphPad Prism (version 3.0). In all cases, values of P<0.05 were considered significant.
As an estimate of potency for each of the inhibitory adenosine receptor agonists, the concentration required to inhibit 25% of the initial mean response (IC25) was calculated using GraphPad Prism (version 3.0). Mean and 95% confidence limits of the IC25 value for each agonist were then determined.
Drugs
The following drugs were used: adenosine (Sigma), adenosine deaminase (Sigma), adenosine 5′-diphosphate (ADP; Sigma), adenosine 5′-monophosphate (AMP; Sigma), adenosine 5′-triphosphate (ATP; Sigma), 2-p-[2-carboxyethyl]phenethyl-amino-5′-N-ethylcarboxamido-adenosine (CGS 21680; Sigma), N6-cyclohexyladenosine (CHA; Sigma), N6-cyclopentyladenosine (CPA; Sigma), 2-methylthio ATP (Sigma), 5′-(N-ethylcarboxamido)-adenosine (NECA; Sigma), noradrenaline, (Sigma), 8-phenyltheophylline (Sigma), (−)-N6-(2-phenylisopropyl)-adenosine (R-PIA; Sigma), (+)-N6-(2-phenylisopropyl)-adenosine (S-PIA; Sigma), reactive blue 2 (Tocris) and suramin (Sigma).
CHA was dissolved in 50% ethanol (at 10 mM) then diluted to the required concentrations in distilled water. CPA was dissolved in 0.1 M NaOH (at 10 mM) then diluted to the required concentrations in distilled water. CGS 21680 was dissolved in 10% DMSO (at 10 mM) then diluted to the required concentrations in distilled water. Noradrenaline was dissolved and diluted in a catecholamine diluent comprising (mM: NaCl 154, NaH2PO4.2H2O 1.0, ascorbic acid 0.23). 8-Phenyltheophylline was dissolved in 80% methanol, 20% 0.2 M NaOH (at 10 mM) then diluted to the required concentrations in distilled water. All other drugs were dissolved and diluted to the required concentrations in distilled water. All drug solutions were made up fresh on the day of use.
pH determination
Since pH is known to affect responses to purinergic agonists (Wildman et al., 1998), the pH of the normal Krebs-Henseleit solution, bubbled with 5% CO2 in 95% O2, with no tissue present was tested in the presence and absence of both ATP (100 μM and 10 mM) and adenosine (100 μM and 10 mM).
Results
The contractile responses to electrical field stimulation remained stable throughout the experimental period (3–4 h), with contractions consistently returning to basal levels after washout. The mean magnitude of response to field stimulation in control tissues prior to addition of any drugs was 88±3 mg (n=145). During investigation of the following protocols, there were no noticeable differences in responses of prostates taken from within the same animal or between animals.
Responses to adenine nucleosides and nucleotides
The endogenous purines ATP, ADP, AMP and adenosine elicited concentration-dependent inhibition of electrical field stimulation induced contractile responses (Figure 1), but had no consistent effect on basal rat prostatic smooth muscle tone. The maximum inhibition of the contractile response was attained after approximately 2 min of tissue exposure to each agonist, and remained maximal until washout. The rank order of agonist potency was ATP ≅ AMP ≅ adenosine > ADP (Figure 2).
Figure 1.
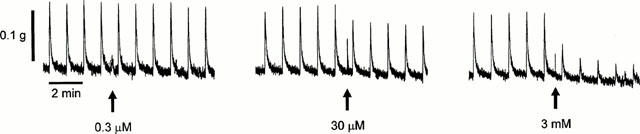
Representative trace showing the inhibitory effect of adenosine (0.3 μM–3 mM) on contractions evoked by trains of stimuli (0.5 ms pulse width, 60 V, 20 pulses at 10 Hz every 50 s) of the isolated rat prostate.
Figure 2.
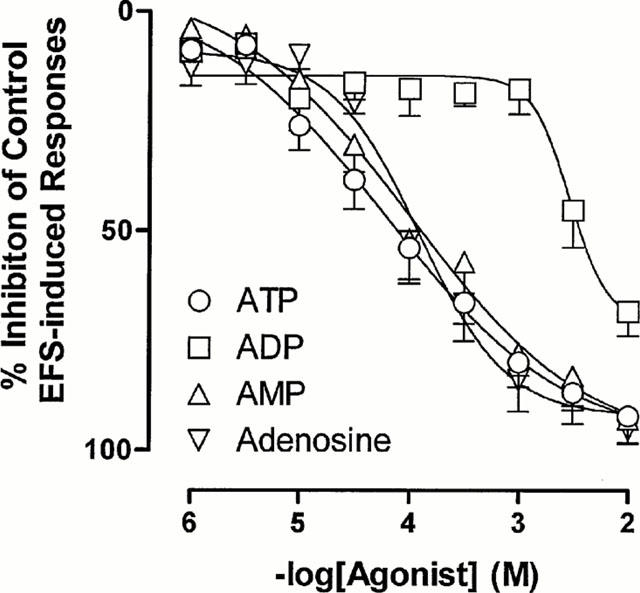
Mean log concentration-response curves for the inhibitory effects of ATP, ADP, AMP and adenosine on the field stimulation induced contractions in isolated rat prostatic preparations. Results are expressed as the percentage of basal control electrical field stimulation-induced responses. Each point represents the mean±s.e.mean from 6–7 experiments.
Effects of 8-phenyltheophylline
Preincubation of isolated rat prostates with the non-selective adenosine receptor antagonist 8-phenyltheophylline (10 μM) had no effect on responses to electrical field stimulation (P⩾0.542, Figure 3), but produced significant shifts to the right of the mean inhibitory log concentration response curves to both ATP (potency ratio=7.7, 95% confidence limits: 7.3–8.1) and adenosine (potency ratio=6.8, 95% confidence limits: 6.3–7.3) (Figure 3).
Figure 3.
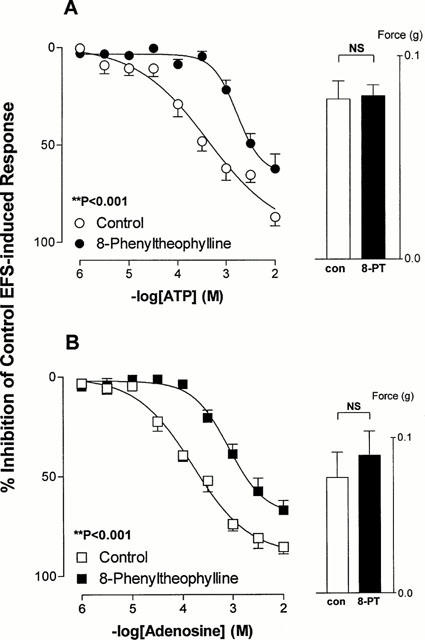
Mean log concentration-response curves for the inhibitory effects of ATP (A) and adenosine (B) on field stimulation induced contractions in rat prostatic preparations in the absence or presence of 8-phenyltheophylline (10 μM). Results are expressed as the percentage inhibition of basal control electrical field stimulation-induced responses. Each point represents the mean±s.e.mean of six experiments. P values are for the concentration×treatment interaction of a 2-way repeated measures ANOVA. The histogram columns represent the mean force developed by control tissues and in the presence of 8-phenyltheophylline prior to the addition of agonists. Vertical bars represent s.e.mean.
Effects of P2 purinoceptor antagonists
Preincubation of isolated rat prostates with the non-selective P2 purinoceptor antagonist suramin (0.1 mM) had no effect on responses to electrical field stimulation (P=0.645). Nor did this concentration of suramin affect the ATP-mediated inhibition (P=0.334; Figure 4). Preincubation of isolated rat prostates with the P2 receptor antagonist reactive blue 2 (5 μM) had no effect on basal electrical field stimulation induced contractile responses (P=0.398, Figure 4), but shifted the mean inhibitory log concentration-response curve to ATP to the right (potency ratio=3.6, 95% confidence limits: 3.2–4.0) (P=0.002; Figure 4).
Figure 4.
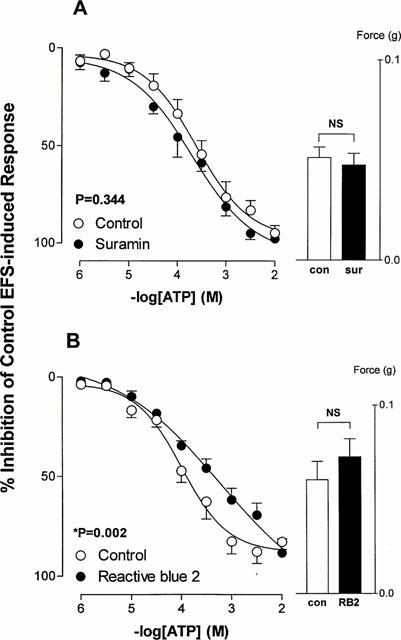
Mean log concentration-response curves for the inhibitory effects of ATP on field stimulation induced contractions in rat prostatic preparations in the absence or presence of (A) suramin (100 μM) and (B) reactive blue 2 (5 μM). Results are expressed as the percentage inhibition of basal control electrical field stimulation-induced responses. Each point represents the mean±s.e.mean of six experiments. P values are for the concentration×treatment interaction of a 2-way repeated measures ANOVA. The histogram columns represent the mean force developed by control tissues and in the presence of antagonist prior to the addition of agonists. Vertical bars represent s.e.mean.
Effects of adenosine deaminase
Preincubation of isolated rat prostates with adenosine deaminase (0.1 unit ml−1), the enzyme responsible for adenosine degradation to inosine, had no effect on basal electrical field stimulation induced contractile responses (P⩾0.271, Figure 5), but shifted the mean inhibitory log concentration-response curves to both ATP (potency ratio=2.8, 95% confidence limits: 2.3–3.3) and adenosine (potency ratio=6.0, 95% confidence limits: 5.4–6.6) to the right (Figure 5).
Figure 5.
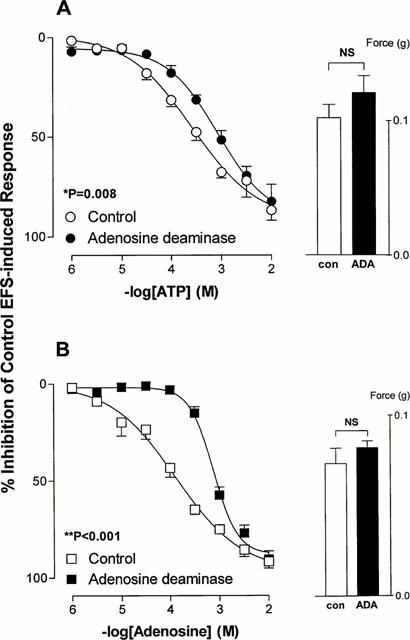
Mean log concentration-response curves for the inhibitory effects of ATP (A) and adenosine (B) on field stimulation induced contractions in rat prostatic preparations in the absence or presence of adenosine deaminase (0.1 unit ml−1). Results are expressed as the percentage inhibition of basal control electrical field stimulation-induced responses. Each point represents the mean±s.e.mean of six experiments. P values are for the concentration×treatment interaction of a 2-way repeated measures ANOVA. The histogram columns represent the mean force developed by control tissues and in the presence of adenosine deaminase prior to the addition of agonists. Vertical bars represent s.e.mean.
Effects of 2-methylthio ATP
The ATP analogue and P2 agonist, 2-methylthio ATP (10 nM–100 μM), had no effect on baseline smooth muscle tone or electrical field stimulation induced contractile responses (Figure 6).
Figure 6.
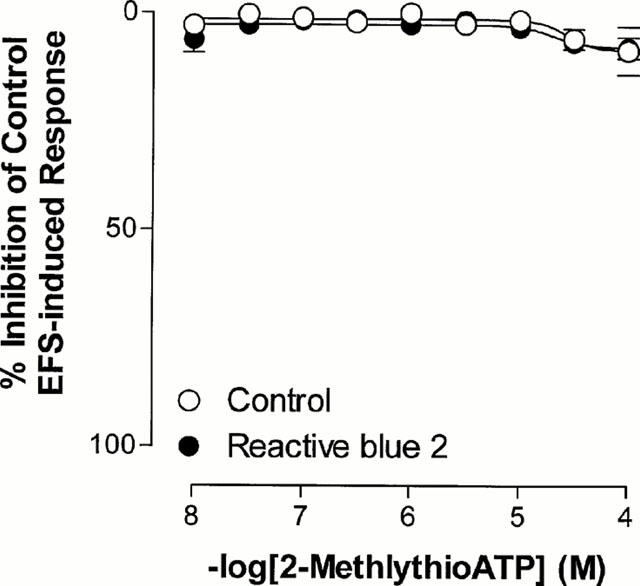
Mean log concentration-response curves for the inhibitory effects of 2-methylthio ATP on the field stimulation induced contractions in rat prostatic preparations in the absence and presence of reactive blue 2 (5 μM). Results are expressed as the percentage of basal control electrical field stimulation-induced responses. Each point represents the mean±s.e.mean.
Postjunctional effects
Noradrenaline (10 μM) elicited tonic contractions of unstimulated rat isolated prostates. Neither ATP nor adenosine (1 μM–1 mM) inhibited the contractile response to exogenous noradrenaline (10 μM) (Figure 7).
Figure 7.
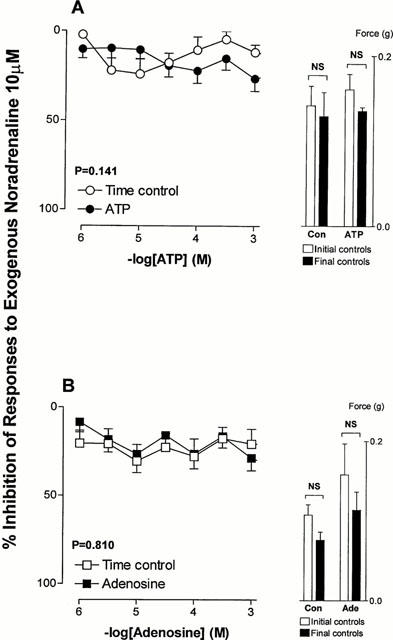
Mean log concentration-response curves for the inhibitory effects of ATP (A) and adenosine (B) on contractile responses to exogenously administered noradrenaline (10 μM) in rat prostatic preparations and mean contractile responses to noradrenaline as a function of time. Results are expressed as the percentage inhibition of basal control electrical field stimulation-induced responses. Each point represents the mean±s.e.mean of six experiments. P values are for the concentration×treatment interaction of a 2-way repeated measures ANOVA. The histogram columns represent the mean force developed by tissues prior to the addition of ATP or adenosine and prior to the commencement of time control studies and the mean force developed by tissues after washout of the final drug application. Vertical bars represent s.e.mean.
Effects of synthetic adenosine analogues
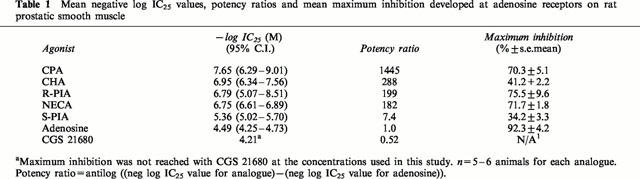
The synthetic adenosine analogues CPA, CHA, NECA, R-PIA, S-PIA and CGS 21680 elicited concentration-dependent inhibition of electrical field stimulation induced contractile responses. CPA, R-PIA and NECA caused a maximal inhibition of 70–75%, while CHA and S-PIA produced only 30–40% maximal inhibition. The selective A2A adenosine receptor agonist CGS 21680 was relatively inactive and its effect did not reach a plateau at the concentrations used in this study (up to 100 μM). The rank order of potency was CPA⩾CHA=R-PIA=NECA>S-PIA>CGS 21680 (Figure 8, Table 1).
Figure 8.
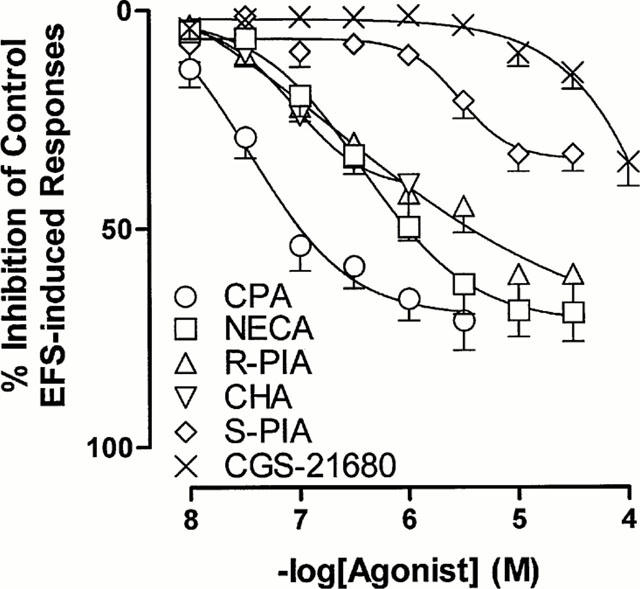
Mean log concentration-response curves for the inhibitory effects of CPA, CHA, R-PIA, NECA, S-PIA and CGS 21680 on the field stimulation induced contractions in control rat prostatic preparations. Results are expressed as the percentage of basal control electrical field stimulation-induced responses. Each point represents the mean±s.e.mean of 5–6 experiments.
Table 1.
Mean negative log IC25 values, potency ratios and mean maximum inhibition developed at adenosine receptors on rat prostatic smooth muscle
Effects of ATP and adenosine on bathing solution pH
The pH of the control Krebs-Henseleit solution was 7.37. In the presence of either ATP (100 μM) or adenosine (100 μM), this was still 7.34. In the presence of ATP (10 mM), this was reduced to 6.63, while in the presence of adenosine (10 mM) the pH of the bathing solution fell to 6.94.
Discussion
The present study has clearly demonstrated that adenine nucleosides and nucleotides elicit inhibition of electrical field stimulation-induced contractile responses in the rat prostate gland. This inhibition may be due to prejunctional inhibition of excitatory neurotransmitter release by stimulation of adenosine receptors possibly of the A1 subtype.
ATP and adenosine were equipotent in inhibiting electrical field stimulation induced contractile responses. This equipotency occurs in other sympathetically innervated tissues including the rat vas deferens (Clanachan et al., 1977), cauda epididymis (Ventura & Pennefather, 1992), heart (Khan & Malik, 1980) and the mouse vas deferens (von Kugelgen et al., 1989). At the neuroeffector junction, released ATP may be hydrolyzed to yield adenosine by extracellular ATPase and 5′-nucleotidase (Burnstock, 1972; Windschief, 1996), but the reverse is not possible. Adenosine receptors can therefore be activated directly by adenosine or indirectly by ATP, following hydrolysis to adenosine. This implies that inhibition of prostatic contractions by adenine nucleotides as well as by adenosine may be mediated by an adenosine receptor. In support of this proposal, the inhibition elicited by both ATP and adenosine was attenuated by the adenosine receptor antagonist 8-phenyltheophylline.
To evaluate the alternative or additional presence of an inhibitory P2-purinoceptor, the effects of the non-selective P2-purinoceptor antagonists suramin and reactive blue 2 on the inhibitory effects of ATP and adenosine were tested. Suramin was without effect on the inhibition caused by ATP and adenosine, while reactive blue 2 caused only a small attenuation of the ATP mediated inhibitory response. Reactive blue 2 has been shown to have nonspecific side effects at high concentrations (Reilly et al., 1987) and a general desensitizing effect when in contact with tissues for long periods (Burnstock & Warland, 1987), and although the concentration and contact period were reduced to avoid these effects, they may be confounding factors in the observed effect of reactive blue 2. Furthermore, it has been reported that reactive blue 2 can directly affect adenosine responses in certain vascular preparations (Burnstock & Warland, 1987; Hopwood & Burnstock, 1987).
Further investigation showed that 2-methylthio ATP had no effect on electrical field stimulation induced contractile responses, indicating the non-involvement of P2 purinoceptors. In addition, 2-methylthio ATP is less likely to undergo enzymatic hydrolysis to form adenosine and instead is degraded by ecto 5′-nucleotidases to form 2-methylthio adenosine (Welford et al., 1986), and as such is less likely to activate adenosine receptors. The inactivity of 2-methylthio ATP further supports the argument for indirect activation of an adenosine receptor by ATP.
Additional evidence suggesting that the inhibition of field stimulation-induced contractions was due to adenosine receptor stimulation came from the experiments using adenosine deaminase. This enzyme degrades adenosine to inosine (Windschief, 1996). The presence of this enzyme in the biophase causes increased breakdown of adenosine, so decreasing the amount of adenosine available for receptor activation, and thus attenuating the response. Inhibitory responses to both ATP and adenosine were shown to be sensitive to exogenously applied adenosine deaminase.
Synthetic adenosine analogues with differing selectivities for the adenosine receptor subtypes are very useful in the characterization of adenosine receptors (Fredholm et al., 1998). A1 adenosine receptors have an agonist potency ratio of CPA>CHA=R-PIA>NECA>CGS 21680. A2A adenosine receptors have an agonist potency order of NECA>CGS21680>CHA. A2B adenosine receptors have an agonist potency order of NECA>R-PIA>CPA>S-PIA>CGS 21680. A3 adenosine receptors have an agonist potency order of NECA>R-PIA=CPA>S-PIA.
In the present study, inhibitory log concentration-response curves to six adenosine analogues yielded the rank order of potency: CPA⩾CHA = R - PIA = NECAthinsp;> S - PIAthinsp;> CGS 21680, approximately correlating with the expected rank order of potency for an A1 adenosine receptor (Fredholm et al., 1998). This adenosine receptor subtype is known to be present presynaptically and its activation inhibits excitatory neurotransmission (Fredholm et al., 1998). Although the prejunctional location of the receptors at the neuroeffector junction was not directly examined in our study, it is implied by the findings that both ATP and adenosine were without effect on the contractions elicited by exogenous application of noradrenaline on unstimulated tissues. This observation also implies that a cross-talk mechanism by which postjunctional adenosine or ATP receptors could influence postjunctional α-adrenoceptors does not exist in the rat prostate.
In this study ATP, AMP and adenosine but not ADP all elicited close to 100% inhibition of electrical field stimulation induced responses, at their highest concentrations (10 mM). In contrast, the adenosine analogues, whilst clearly more potent, elicited a maximum inhibition of only 75%, with responses to some analogues plateauing at 30–40% inhibition. We found that at bath concentrations above 1 mM, ATP and adenosine both caused a decrease in the pH of the bathing solution. This pH change could alter the reactivity of ATP and adenosine by modifying enzymatic activity or decrease the tissue response to field stimulation by changing the ionic equilibrium of the smooth muscle cells and so accentuate the inhibition caused by very high concentrations of ATP and adenosine. Similar alterations in pH have previously been shown to modify the affinity of ATP for purinoceptors (Wildman et al., 1998), modifying the tissue response. Similarly, pilot studies in our laboratory have previously shown that addition of H+ to isolated organ baths containing rat prostate preparations causes a decrease in the magnitude of field stimulation induced contractions. The very low efficacy of CHA and S-PIA was also observed in our previous study of the A1-receptor of the rat cauda epididymis (Ventura & Pennefather, 1992). This suggests that these analogues may be partial agonists at urogenital A1 adenosine receptors.
The low potency and efficacy of ADP compared to ATP, AMP and adenosine was unexpected given that extracellular ecto-nucleotidases which sequentially phosphorylate ATP to ADP, AMP and adenosine are present in many tissues. Furthermore, the human prostate is known to secrete soluble nucleotidases from its secretory cells (Kennedy et al., 1997). The decreased effect of ADP may be due to the presence of an unusual extracellular enzymatic metabolism system in the rat prostate whereby ADP is bypassed. If such an enzyme system was present in the rat prostate this may mean that the majority of ATP is metabolized directly to AMP and that ecto-ADPase is not present. This would result in a decreased rate of breakdown of ADP to AMP and then adenosine. Although this is mere speculation, such a phenomenon would explain the decreased effect of ADP.
The major findings of our study are that (1) adenine nucleosides and nucleotides cause inhibition of electrical field stimulation induced contractile responses in the rat prostate gland and (2) the observed data using agonists and antagonists plus the presynaptic inhibitory nature of the phenomenon points to the involvement of A1 adenosine receptors. To our knowledge, this is the first study to implicate purine nucleotides as modulators of neurotransmission to the smooth muscle of the prostate of any animal.
It remains to be established whether these findings have functional significance in the context of benign prostatic hyperplasia. Current pharmacological treatment with α1-adrenoceptor antagonists, such as terazosin, is directed at relieving the smooth muscle contraction believed to be responsible for many of the symptoms of benign prostatic hyperplasia. It is conceivable that the lower urinary tract symptoms associated with benign prostatic hyperplasia result from increased release of excitatory transmitter from the nerve terminal. The α1-adrenoceptor antagonists are thought to act by competitively antagonising the post-junctional receptor for noradrenaline on prostatic smooth muscle. As demonstrated in this study, adenosine receptor agonists may act proximal to this step in neurotransmission by stimulating prejunctional receptors that inhibit noradrenaline release from the nerve terminal. Successful utilization and manipulation of this inhibitory mechanism may subsequently provide an additional therapeutic target for the relaxation of prostate smooth muscle in those suffering from benign prostatic hyperplasia.
Acknowledgments
This research was supported by grants to Dr S. Ventura from the Appel Family Bequest, Arthur and Mary Osborn Estate and NH & MRC of Australia.
Abbreviations
- ADP
adenosine 5′-diphosphate
- AMP
adenosine 5′-monophosphate
- ATP
adenosine 5′-triphosphate
- CGS 21680
2-p-[2-carboxyethyl]phenethyl-amino-5′-N-ethylcarboxamido-adenosine
- CHA
N6-cyclohexyladenosine
- CPA
N6-cyclopentyladenosine
- NECA
5′-(N-ethylcarboxamido)-adenosine
- R-PIA
(−)-N6-(2-phenylisopropyl)-adenosine
- S-PIA
(+)-N6-(2-phenylisopropyl)-adenosine
References
- ABBRACCHIO M.P., BURNSTOCK G. Purinergic signalling: pathophysiological roles. Jpn. J. Pharmacol. 1998;78:113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- BOLAND B., HIMPENS B., PAQUES C., CASTEELS R., GILLIS J.M. ATP induced-relaxation in the mouse bladder smooth muscle. Br. J. Pharmacol. 1993;108:749–753. doi: 10.1111/j.1476-5381.1993.tb12872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. Purinergic nerves. Pharmacol. Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- BURNSTOCK G.A basis for distinguishing two types of purinergic receptor Cell Membrane Receptors for Drugs and Hormones: A Multidiscipinary Approach 1978New York: Raven Press; 107–118.eds. Bolis, L. & Staub, L.W. pp [Google Scholar]
- BURNSTOCK G., COCKS T., CROWE R., KASAKOVE L. Purinergic innervation of the guinea-pig urinary bladder. Br. J. Pharmacol. 1978;63:125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., WARLAND J.J.I. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2y- but not the P2x- purinoceptor. Br. J. Pharmacol. 1987;90:383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLANACHAN A.S., JOHNS A., PATON D.M. Presynaptic actions of adenine nucleotides and adenosine on neurotransmission in the rat vas deferens. Neuroscience. 1977;2:597–602. doi: 10.1016/0306-4522(77)90056-2. [DOI] [PubMed] [Google Scholar]
- DRIESSEN B., VON KUGELGEN I., STARKE K. P1-purinoceptor-mediated modulation of neural noradrenaline and ATP release in guinea-pig vas deferens. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;350:42–48. doi: 10.1007/BF00180009. [DOI] [PubMed] [Google Scholar]
- FANG W.-G., PIRNIA F., BANG Y.-J., MYERS C.E., TREPEL J.B. P2-purinergic receptor agonists inhibit the growth of androgen-independent prostate carcinoma cells. J. Clin. Invest. 1992;89:191–196. doi: 10.1172/JCI115562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDHOLM B.B., IJZERMAN A.P., JACOBSON K.A., LINDEN J., SCHWABE U., STILES G.L.Adenosine Receptors 199848–57.In the IUPHAR Receptor Compendium pp
- GUIJARRO L.G., JUARRANZ M.G., MARINERO M.J., PAJUELO L., CARMENA M.J., PRIETO J.C. Modulation of cyclic AMP and inositol phosphate production in rat prostatic cultures by VIP/PACAP, ATP, and carbachol: role in prostatic proliferation. Ann. New York Acad. Sci. 1996;72:723–728. doi: 10.1111/j.1749-6632.1996.tb17548.x. [DOI] [PubMed] [Google Scholar]
- HAYNES J.M., ALEXANDER S.P.H., HILL S.J. A1 adenosine receptor modulation of electrically-evoked contractions in the bisected vas deferens and cauda epididymis of the guinea-pig. Br. J. Pharmacol. 1998;124:964–970. doi: 10.1038/sj.bjp.0701909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPWOOD A.M., BURNSTOCK G. ATP mediates coronary vasoconstriction via P2x-purinoceptors and coronary vasodilatation via P2y-purinoceptors in the isolated perfused rat heart. Eur. J. Pharmacol. 1987;136:49–54. doi: 10.1016/0014-2999(87)90777-1. [DOI] [PubMed] [Google Scholar]
- HOURANI S.M.O., NICHOLLS J., LEE B.S.S., HALFHIDE E.J., KITCHEN I. Characterization and ontogeny of P1-purinoceptors on rat vas deferens. Br. J. Pharmacol. 1993;108:754–758. doi: 10.1111/j.1476-5381.1993.tb12873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSENS R., COMMUNI D., PIROTTON S., SAMSON M., PARMENTIER M., BOEYNAEMS J.-M. Cloning and tissue distribution of the human P2Y1 receptor. Biochem. Biophys. Res. Comm. 1996;221:588–593. doi: 10.1006/bbrc.1996.0640. [DOI] [PubMed] [Google Scholar]
- KENNEDY C., TODOROV L.D., MIHAYLOVA-TODOROVA S., SNEDDON P. Release of soluble nucleotidases: a novel mechanism for neurotransmitter inactivation. Trends Pharmacol. Sci. 1997;18:263–266. doi: 10.1016/s0165-6147(97)01088-2. [DOI] [PubMed] [Google Scholar]
- KHAKH B.S., SURPRENANT A., HUMPHREY P.P. A study on P2X purinoceptors mediating the electrophysiological and contractile effects of purine nucleotides in rat vas deferens. Br. J. Pharmacol. 1995;115:177–185. doi: 10.1111/j.1476-5381.1995.tb16336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAN M.T., MALIK K.U. Inhibitory effect of adenosine and adenine nucleotides on potassium-evoked efflux of [3H]noradrenaline from the rat isolated heart: Lack of relationship to prostaglandins. J. Pharmacol. Exp. Ther. 1980;68:551–561. doi: 10.1111/j.1476-5381.1980.tb14571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONRAD L., SCHIEMANN P., RENNEBERG H., WENNEMUTH G., FINI C., AUMÜLLER G. Expression and enzymatic activity of ecto 5′-nucleotidase in the human male genital tract. Biol. Reprod. 1998;59:190–196. doi: 10.1095/biolreprod59.1.190. [DOI] [PubMed] [Google Scholar]
- KURZ K., VON KUGELGEN I., STARKE K. Prejunctional modulation of noradrenaline release in mouse and rat vas deferens: contribution of P1- and P2-purinoceptors. Br. J. Pharmacol. 1993;110:1465–1472. doi: 10.1111/j.1476-5381.1993.tb13986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAU W.A.K., VENTURA S., PENNEFATHER J.N. Pharmacology of neurotransmission to the smooth muscle of the rat and guinea-pig prostate glands. J. Auton. Pharmacol. 1998;18:349–356. doi: 10.1046/j.1365-2680.1998.1860349.x. [DOI] [PubMed] [Google Scholar]
- LONGHURST P.A., SCHWEGEL T., FOLANDER K., SWANSON R. The human P2X1 receptor: molecular cloning, tissue distribution, and localization to chromosome 17. Biochim. Biophys. Acta. 1996;1308:185–188. doi: 10.1016/0167-4781(96)00112-1. [DOI] [PubMed] [Google Scholar]
- PINNA C., PUGLISI L., BURNSTOCK G. ATP and vasoactive intestinal polypeptide relaxant responses in hamster isolated proximal urethra. Br. J. Pharmacol. 1998;124:1069–1074. doi: 10.1038/sj.bjp.0701908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATHBONE M.P., MIDDLEMISS P.J., GYSBERS J.W., ANDREW C., HERMAN M.A.R., REED J.K., CICCARELLI R., DI IORIO P., CACIAGLI F. Trophic effects of purines in neurons and glial cells. Prog. Neurobiol. 1999;59:663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- REILLY W.M., SAVILLE V.L., BURNSTOCK G. An assessment of the antagonistic activity of reactive blue 2 at P1- and P2-purinoceptors: supporting evidence for purinergic innervation of the rabbit portal vein. Eur. J. Pharmacol. 1987;140:47–53. doi: 10.1016/0014-2999(87)90632-7. [DOI] [PubMed] [Google Scholar]
- VENTURA S., PENNEFATHER J.N. Sympathetic co-transmission to the cauda epididymis of the rat: characterization of postjunctional adrenoceptors and purinoceptors. Br. J. Pharmacol. 1991;102:540–544. doi: 10.1111/j.1476-5381.1991.tb12207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENTURA S., PENNEFATHER J.N. Inhibition of field stimulation-induced contractions of rat cauda epididymis by purinoceptor agonists but not by adrenoceptor agonists. J. Auton. Pharmacol. 1992;12:299–309. doi: 10.1111/j.1474-8673.1992.tb00379.x. [DOI] [PubMed] [Google Scholar]
- VON KUGELGEN I., SCHOFFEL E., STARKE K. Inhibition by nucleotides acting at presynaptic P2-receptors of sympathetic neuroeffector transmission in the mouse isolated vas deferens. Naunyn-Schmiedeberg's Arch. Pharmacol. 1989;340:522–561. doi: 10.1007/BF00260607. [DOI] [PubMed] [Google Scholar]
- WASILENKO W.J., COOPER J., PALAD A.J., SOMERS K.D., BLACKMORE P.F., RHIM J.S., WRIGHT G.L. , Jr, SCHELLHAMMER P.F. Calcium signalling in prostate cancer cells: evidence for multiple receptors and enhanced sensitivity to bombesin/GRP. Prostate. 1997;30:167–173. doi: 10.1002/(sici)1097-0045(19970215)30:3<167::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- WELFORD L.A., CUSACK N.J., HOURANI S.M. ATP analogues and the guinea-pig taenia coli: a comparison of the structure-activity relationships of ectonucleotidases with those of the P2-purinoceptor. Eur. J. Pharmacol. 1986;129:217–224. doi: 10.1016/0014-2999(86)90431-0. [DOI] [PubMed] [Google Scholar]
- WILDMAN S.S., KING B.F., BURNSTOCK G. Zn+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. Br. J. Pharmacol. 1998;123:1214–1220. doi: 10.1038/sj.bjp.0701717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDSCHIEF U. Purinoceptors: from history to recent progress. A review. J. Pharm. Pharmacol. 1996;48:993–1011. doi: 10.1111/j.2042-7158.1996.tb05891.x. [DOI] [PubMed] [Google Scholar]


