Abstract
To investigate the effects of the clove oil constituents β-caryophyllene oxide and eugenol on the heart muscle, experiments were performed on isolated papillary muscles and on ventricular myocytes of the guinea-pig. The results were compared with those obtained with the dihydropyridine, nifedipine.
All three substances exerted negative inotropic effects in heart muscle although with different potencies and different influences on the time course of the contraction curve.
They all reduced rested-state contractions (RSCs) in the presence of isoprenaline which are thought to be due to the Ca2+ current (ICa).
β-Caryophyllene oxide, eugenol and nifedipine inhibited the ICa in single cells from the guinea-pig ventricle in a concentration-dependent, reversible way, but with different potencies.
In addition to the ICa-inhibiting effect, β-caryophyllene oxide strongly inhibited and eugenol slightly inhibited the potassium current.
The action potential of papillary muscles at a 1 Hz contraction frequency was greatly shortened by nifedipine, slightly shortened by eugenol, but not changed by β-caryophyllene oxide.
The inhibition of the potassium current by β-caryophyllene oxide obviously prevents a shortening of the action potential due to the diminution of the ICa. Accordingly, the negative inotropic effect of β-caryophyllene oxide is closely related to the inhibition of ICa. In contrast, eugenol and nifedipine, which shorten the action potential, exert stronger negative inotropic effects than expected from their influence on ICa.
The results show that the negative inotropic effect of a calcium channel blocker can be attenuated by an additional inhibition of potassium channels.
Keywords: β-Caryophyllene oxide, eugenol, nifedipine, calcium inward current (ICa), potassium current, rested-state contraction (RSC), negative inotropic effect, heart muscle, ventricular myocardium, papillary muscle
Introduction
Calcium channel blockers inhibit the calcium influx into cells and thereby relax the vascular smooth muscle, which is a basis for the treatment of hypertension. In heart muscle, the reduced calcium inward current is important for the antiarrhythmic action of some of these substances. Although the reduced calcium influx into heart cells may be beneficial in certain diseases because of the diminution of the calcium load, it always leads to a negative inotropic effect. In the present investigation, we observed that constituents of clove oil (eugenol and β-caryophyllene oxide) which have relaxant effects in smooth muscle preparations (Reiter & Brandt, 1985) also block calcium channels of the heart. A comparison of the negative inotropic effects of these substances with that of the classic calcium channel blocker, nifedipine, showed that they differ with regard to their calcium channel blocking capacity. Nifedipine exerted the strongest effect on the force of contraction, which was much higher than expected from its calcium channel blocking effect. In contrast, the decrease in the force of contraction after the application of β-caryophyllene oxide was comparable to that which could be expected from its blocking effect on calcium channels.
The results show that there are calcium channel blocking substances which have a much lower negative inotropic effect on heart muscle than others, which may be explained by the additional influence on other ion channels.
Preliminary accounts of some of these data have been presented (Sensch et al., 1993a,1993b).
Methods
Isometric contraction recording
Guinea-pigs were stunned by a blow on the head (Sutherland & Festing, 1987) and the hearts rapidly excised. Right ventricular papillary muscles with a diameter of less than 1 mm were prepared and mounted vertically in a two-chambered organ bath with a volume of 50 ml containing a modified Krebs-Henseleit solution consisting of (in mmol l−1): NaCl 115, KCl 4.7, CaCl2 3.2, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, and glucose 10; pH 7.4. It was gassed and circulated by 95% O2 and 5% CO2 at 35°C. The muscles were stimulated at their base through punctate platinum electrodes with 1 ms square wave pulses of an intensity slightly above threshold. Contractions were recorded by an inductive force transducer connected to a storage oscilloscope and pen recorder. The resting force was kept at 4 mN during experiments performed at a constant contraction frequency of 1 Hz. To evaluate effects on the isometric contraction curve, the substances to be investigated were added cumulatively to the bath. The curves obtained at the various steady states were displayed on the oscilloscope and photographed and evaluated from fast speed records of the pen recorder. The peak force of contraction (FC) was determined from the peak height of the contraction curve and the time of contraction (tC) measured at 10% of the peak height.
Rested-state contraction (RSC)
After an equilibration period of 1 h at a contraction frequency of 1 Hz, the electrical stimulation of the papillary muscle was interrupted for 15 min to allow the accumulated activator calcium to leak completely from its intracellular stores. Five min prior to the next electrical stimulation, isoprenaline (0.06 μmol l−1, together with 40 μmol l−1 CaNa2EDTA) was added to the bath. It was removed again after the RSC by repeated washings with catecholamine-free solution, while the muscle contracted at 1 Hz frequency before a further rest period of 15 min and a new addition of isoprenaline allowed another RSC to be evoked. The release of endogenous catecholamines was avoided by pretreating the animals with reserpine (5 mg Kg−1 body wt. i.p., 24 h before the experiment). Substances to be tested were added to the bath at the beginning of the rest period. Up to four RSCs were performed with one papillary muscle kept at a constant length. Control experiments showed equal forces of the late peaks of repetitive RSCs in the absence of a test substance.
Recording of action potentials
Guinea-pig papillary muscles were horizontally mounted in a two-chambered organ bath with internal circulation. Action potentials were recorded with glass microelectrodes filled with a 3 mol l−1 KCl solution with DC resistances ranging from 20–25 MΩ. Potentials were measured with an amplifier (WPI, Berlin, Germany). An Ag-AgCl electrode connected to the bath solution via an agar bridge served as reference. The signals were photographed from an oscilloscope.
Preparation of single cells
Ventricular myocytes were isolated from hearts of adult guinea-pigs by enzymatic digestion in accordance with Bendukidze et al. (1985). After injection of heparin (5000 units Kg−1 body wt.), the animals (250–300 g) were stunned and the hearts rapidly excised and placed in a modified cold Krebs-Henseleit solution (see above). The hearts were mounted on a cannula and retrogradely perfused at 35°C with the same solution for 4 min followed by perfusion with nominal Ca2+-free Joklik solution (minimal essential medium; Gibco, Paisley, U.K.) for 4 min. Subsequently, Joklik solution containing (per litre) 250 μmol Ca2+ , 417 mg collagenase (Worthington 173 units mg−1, Biochrom, Berlin, Germany), 500 mg protease (0.41 units mg−1, Sigma, Deisenhofen, Germany), 200 mg trypsin (2.4 DMC units mg−1, Serva, Heidelberg, Germany), and 1 g albumin (bovine, Sigma, Deisenhofen, Germany) was recirculated for 10 min at 9 ml min−1. After mincing the ventricles with scissors, the tissue fragments were incubated in the same solution for 5 min at 35°C. Ventricular cells were then obtained by gentle filtering through a nylon mesh (pore radius 500 μm) and stored in Joklik solution containing 250 μmol Ca2+ l−1 and 10 g albumin l−1. The viable rod-shaped cells showed a clear cross-striation.
Voltage-clamp experiments
The cell suspension was placed in a perfusion chamber (volume 1.5 ml) mounted on an inverse microscope (IM 35; Zeiss, Oberkochen, Germany). The bath was continuously perfused with oxygenated (5% CO2 in O2) Krebs-Henseleit solution containing 1.2 mmol l−1 Ca2+ at a flow rate of 4 ml min−1. The temperature was 35±0.5°C. The experiments were carried out in the whole-cell patch-clamp configuration (Hamill et al., 1981). Micropipettes with DC resistances ranging from 1–2 MΩ were filled with a solution containing (in mmol l−1): KCl 140, NaCl 5, MgCl2 1, HEPES (Sigma, Deisenhofen, Germany) 5, EGTA (bis-(aminoethyl)-glycolether-N,N,N′,N′-tetraacetic acid; Merck, Darmstadt, Germany) 0.05, and Na2ATP 3, and adjusted to pH 7.2. They were connected to a patch-clamp amplifier (L/M EPC 7, List Electronic, Darmstadt, Germany); an Ag-AgCl electrode connected to the bath solution by an agar bridge served as reference. Fast and slow components of capacity and series resistance were adequately compensated. The current (after filtering with a 3 kHz 3-pole Bessel filter) and the voltage signals were digitized, stored, and evaluated by means of an AD converter (DAS-20, Keithley, München, Germany) using a program written in ASYST (Asyst, Rochester, N.Y. U.S.A.).
Calcium inward current (ICa)
To measure effects on the L-type calcium inward current, the holding potential of the cell was set to −80 mV, followed by a 600 ms depolarization step to −40 mV in order to inactivate the fast sodium current (Beeler & Reuter, 1970). The slow inward current was elicited by a subsequent 400 ms depolarization to 0 mV. This pattern of depolarization was repeated at a frequency of 0.2 Hz. To examine the voltage dependence of ICa, the potential of the second step was varied in steps of 10 mV from −40 to +50 mV. ICa was evaluated as the difference between the peak inward current and the current at the end of the 400 ms second clamp step.
Potassium current
While ICa was blocked by 0.1 mmol l−1 Cd2+ (see Lansman et al., 1986), the fast sodium current was inactivated by a conditioning depolarization from the resting potential of −80 mV to −40 mV. Subsequent 400 ms depolarizations in 10 mV increments from −80 mV to +80 mV elicited potassium current of the myocyte (see inset of Figure 6). Potassium current was assessed from the current at the end of the second (400 ms) clamp step.
Figure 6.
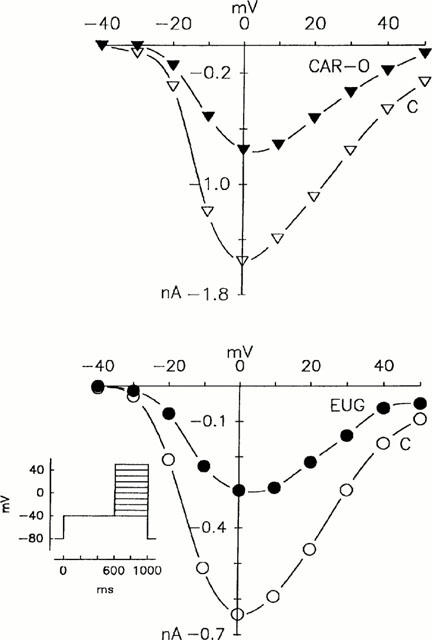
Current-voltage relationship of the ICa in cardiac myocytes. Protocol: 600 ms prepulses from −80 mV to −40 mV followed by 400 ms test pulses in steps of 10 mV from −40 to +50 mV. ICa in the absence (C) and presence of 30 μmol l−1 β-caryophyllene oxide (CAR-O) and 180 μmol l−1 eugenol (EUG). Abscissae: voltage in mV; ordinates: current in nA; representative experiments.
Substances used
β-Caryophyllene oxide (Sigma, Deisenhofen, Germany), β-caryophyllene, and eugenol acetate (Roth, Karlsruhe, Germany) were dissolved in Krebs-Henseleit solution by means of Arlatone 285 (castor oil, ethoxylated, CAS-No. 61791-12-6; ICI Surfactants, Essen, Germany), which did not influence contraction force in the final concentrations used. Eugenol (Fluka, Buchs, Switzerland) was dissolved in Krebs-Henseleit solution. Nifedipine (Bayer, Leverkusen, Germany) was dissolved in water containing 20% Cremophor EL (castor oil, ethoxylated, CAS-No. 61791-12-6; BASF; Ludwigshafen, Germany) and used only at sodium light. (−)-Isoprenaline hydrochloride (Sigma, Deisenhofen, Germany) was dissolved in water.
Statistics
The results are presented as single values or mean values±s.e.mean. IC50 values were obtained by fitting (Gauß-Newton method) to the experimental data using the relation:
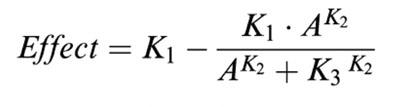 |
A=concentration, K1=control value, K2=steepness of the concentration-effect curve, and K3=IC50.
Octanol-water partition coefficients were calculated by automatic Log P estimation in accordance with Suzuki & Kudo (1990).
Results
Negative inotropic effects at 1 Hz contraction frequency
The negative inotropic effects of the dihydropyridine nifedipine, the sesquiterpene β-caryophyllene oxide, and the allylbenzene eugenol (chemical structures of the clove oil constituents, see Figure 1) are documented by the superimposed original tracings in Figure 2. Although all three substances reduced the force of contraction, they influenced the shape of the contraction curve in different ways. Whereas nifedipine and eugenol reduced contraction time with an increasingly negative inotropic effect leading to a shortening of the time to peak, β-caryophyllene oxide shortened the contraction time only at negative inotropic effects of more than 50%. There were also differences between nifedipine and eugenol: nifedipine reduced contraction time and contraction velocity (steepness of the ascending slope of the contraction curve) to a similar extent, whereas eugenol, at lower concentrations, reduced contraction time more than contraction velocity. Furthermore, the shortening of contraction time was more pronounced under the influence of eugenol.
Figure 1.
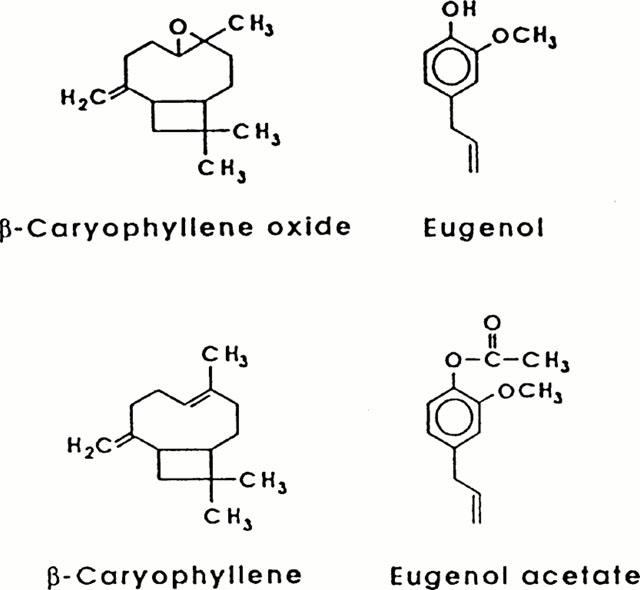
Chemical structures.
Figure 2.
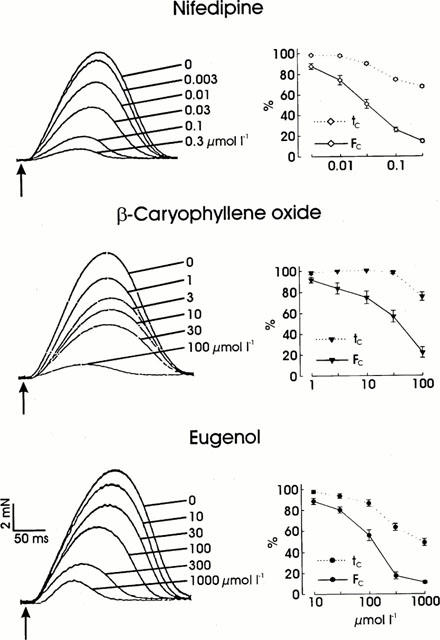
Effects of nifedipine, β-caryophyllene oxide, and eugenol on the force and duration of contraction of guinea-pig papillary muscles at a 1 Hz contraction frequency. Left side: superimposed isometric contraction curves of three different muscles without and under the influence of increasing concentrations of nifedipine, β-caryophyllene oxide, and eugenol. The electrical stimuli are marked by arrows. Right side: mean values±s.e.mean of the force (FC) and duration of contraction (tC) in per cent of the controls for the effects of nifedipine (n=6), β-caryophyllene oxide (n=5), and eugenol (n=6).
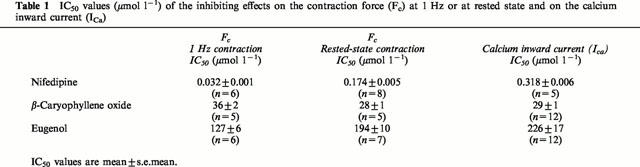
The IC50 value for the negative inotropic effect of nifedipine, 0.032 μmol l−1, is considerably lower than the values for β-caryophyllene oxide (36 μmol l−1) and eugenol (127 μmol l−1; see Table 1).
Table 1.
IC50 values (μmol l−1) of the inhibiting effects on the contraction force (Fc) at 1 Hz or at rested state and on the calcium inward current (ICa)
Influence on the rested-state contraction (RSC)
According to the frequency-force relationship of the guinea-pig papillary muscle, a rested-state contraction, which is defined as a contraction preceded by an interval of rest long enough for the strength of contraction to be independent of previous beats (Blinks & Koch-Weser, 1961), develops practically no force at all. However, a late contraction, developing about 100 ms after stimulation, can be elicited after adding a catecholamine to increase the ICa during the voltage activation of sarcolemmal L-type calcium channels (Seibel et al., 1978). It was suggested that the delayed contraction is activated only by the Ca2+ influx during depolarization (Reiter et al., 1984; Reiter, 1988). Since the force of the RSC then depends directly on the depolarization-induced Ca2+ influx, the degree of its diminution can be taken as a functional measure of the ICa inhibition.
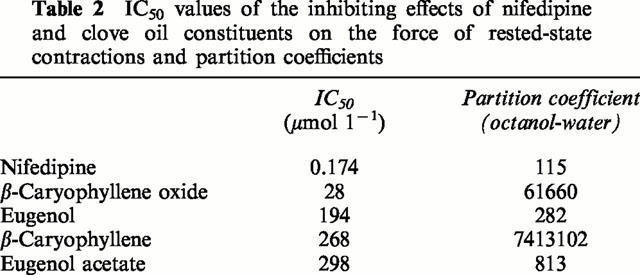
Figure 3 shows RSCs under the influence of isoprenaline without and with increasing concentrations of nifedipine, β-caryophyllene oxide, and eugenol. All substances reduced RSCs in a concentration-dependent way. In contrast to β-caryophyllene oxide, both nifedipine and eugenol evidently exerted a less potent effect on the RSCs than at a 1 Hz contraction frequency (Table 1), especially nifedipine for which a similar IC50 on RSCs (0.1 μmol l−1) was reported by Lynch (1991). The negative inotropic effect of β-caryophyllene oxide was combined with a marked prolongation, and that of nifedipine with a marked shortening of the RSC, whereas eugenol had a biphasic influence on the contraction time of the RSC (slight prolongation at 60 and 180 μmol l−1, shortening at 600 μmol l−1; Figure 3). A reduction of the outside [Ca2+] to one half (1.6 mmol l−1) did not significantly change the IC50 of eugenol (183±5 μmol l−1, n=4; experiments not shown). Eugenol acetate and β-caryophyllene, other constituents of clove oil (chemical structures see Figure 1), are relatively weak inhibitors of RSCs with IC50 values of 298±11 μmol l−1 (n=3) and 268±16 μmol l−1 (n=5) respectively (Table 2).
Figure 3.
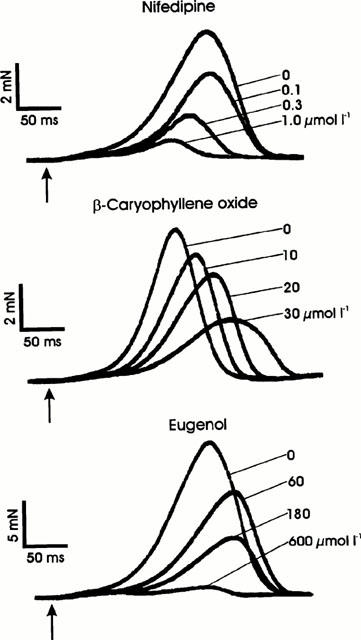
Rested-state contractions (RSCs) of guinea-pig papillary muscles (elicited in the presence of isoprenaline, 0.06 μmol l−1) under the influence of nifedipine, β-caryophyllene oxide, and eugenol. Superimposed contraction curves of three different muscles. The electrical stimuli are marked by arrows.
Table 2.
IC50 values of the inhibiting effects of nifedipine and clove oil constituents on the force of rested-state contractions and partition coefficients
Inhibition of ICa
The inhibitory effects of nifedipine, β-caryophyllene oxide, and eugenol on the ICa as observed in voltage clamp experiments on isolated cardiac ventricular cells are shown in Figure 4. It depicts single current tracings under control conditions and under the influence of nifedipine, eugenol, and β-caryophyllene oxide. It also shows the time-effect curves covering the concentration-effect relationships of the three substances in comparison to spontaneous decreases in the ICa. The respective IC50 values of nifedipine, β-caryophyllene oxide, and eugenol are 0.318, 29, and 226 μmol l−1 (see Table 1). The value of nifedipine corresponds to the values obtained by Bayer & Ehara (1978) in cat papillary muscles (0.3 μmol l−1) and by Uehara & Hume (1985) and Charnet et al. (1987) in the frog heart muscle (0.3 and 0.2 μmol l−1). If the influence of concentrations of the substances which reduced ICa by about 50% on the time course of the activation and inactivation of the ICa is considered, the time to peak was prolonged by about 20%, 10%, or was unchanged under the influence of nifedipine, β-caryophyllene oxide, and eugenol respectively. The decay of the current curve can be described by a bi-exponential function. None of the substances influenced the time constant of the fast component but all three reduced the time constant of the slow component by about 15%. The main difference between the three substances was that eugenol had a relatively stronger suppressive effect on the amplitude of the slow component than the other substances. Figure 5 shows the complete reversibility of the effects of β-caryophyllene oxide and eugenol. After application of the substances, the ICa decreased but then increased again after removal and reached values comparable to those before application if a small time-dependent run down of the current is taken into account.
Figure 4.
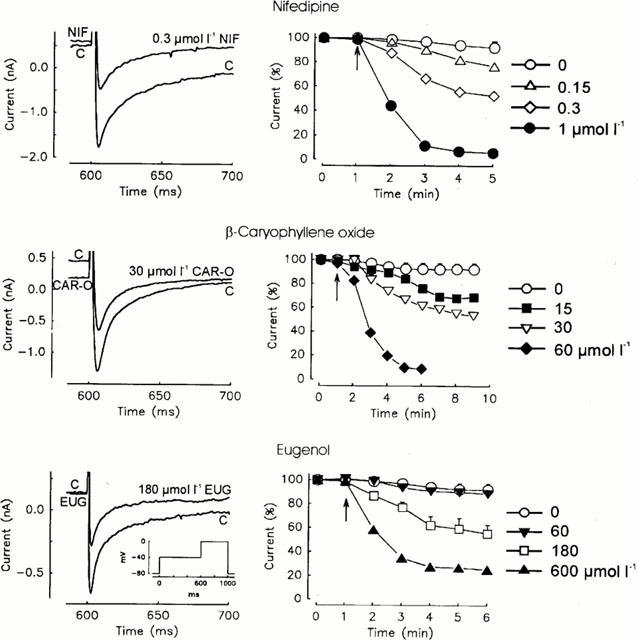
Effects of nifedipine, β-caryophyllene oxide, and eugenol on the ICa of cardiac ventricular myocytes. Left side: superimposed current tracing under control conditions (C) and in the presence of 0.3 μmol l−1 nifedipine (NIF), 30 μmol l−1 β-caryophyllene oxide (CAR-O), and 180 μmol l−1 eugenol (EUG). Depolarization protocol: 600 ms prepulses from the holding potential of −80 mV to −40 mV followed by 400 ms pulses to 0 mV to elicit ICa. Right side: time course of ICa under control conditions (always n=4) and in presence of 0.15 (n=1), 0.3 (n=3), and 1.0 (n=1) μmol l−1 nifedipine, 15 (n=3), 30 (n=6) and 60 (n=3) μmol l−1 β-caryophyllene oxide, and 60 (n=3), 180 (n=6), and 600 (n=3) μmol l−1 eugenol. Arrows indicate the time of addition. Abscissae: time in min; ordinates: ICa in per cent.
Figure 5.
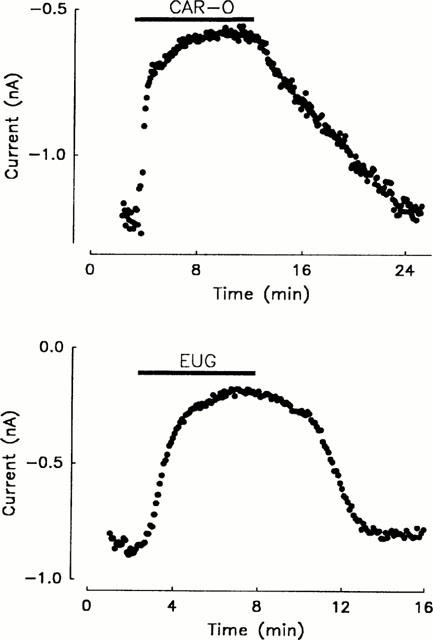
Development and reversibility of the suppression of the peak ICa after the addition and removal (as indicated by the heavy lines) of 30 μmol l−1 β-caryophyllene oxide (CAR-O) and 600 μmol l−1 eugenol (EUG); representative experiments.
To investigate a possible influence of β-caryophyllene oxide and eugenol on the current-voltage relationship of the ICa, their effects on the ICa were measured over the depolarization range between −40 and +50 mV. As shown in Figure 6, both substances decreased the current amplitude in the entire potential range tested without shifting the current-voltage relationship.
Effects on potassium current
The great prolongation of the contraction curve under rested-state conditions produced by β-caryophyllene oxide suggests that β-caryophyllene oxide is not a pure calcium channel blocker but rather has an additional influence on the electrical events of the myocardial cell, probably by additionally inhibiting potassium currents. An indication for such an effect is the suppression of the outward current in the experiments shown in Figure 4 at a holding potential of −40 mV before eliciting ICa. In the experiments depicted in Figure 7, the influence of β-caryophyllene oxide and eugenol on the outward potassium current was investigated in the potential range between −80 mV and +80 mV. The controls showed the usual N-shaped current-voltage relationship. 30 μmol l−1 β-caryophyllene oxide clearly inhibited the potassium current in the ranges from −60 mV to −30 mV and from 0 mV up to +80 mV. This contrasts with the slight effect of 180 μmol l−1 eugenol on the potassium current. The effects on different potassium channels were not differentiated further because the primary aim of the study was to investigate whether the different influences of the substances on contraction can be explained by a blockade of potassium channels leading to a change in action potentials. With regard to nifedipine, Bayer et al. (1977) reported that it does not alter the late potassium outward current. Another dihydropyridine derivative, nisoldipine, was found to inhibit the potassium current only in concentrations three orders of magnitude higher than that inhibiting the ICa (Hume, 1985).
Figure 7.
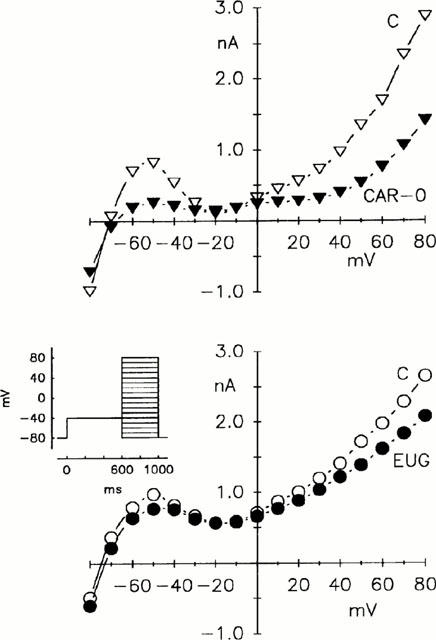
Current-voltage relationship of the potassium current in cardiac myocytes. Protocol: prepulses of 600 ms to −40 mV followed by test pulses in steps of 10 mV from −80 to +80 mV. ICa was blocked by the addition of 0.1 mmol l−1 Cd2+. Potassium current in the absence (C) and presence of 30 μmol l−1 β-caryophyllene oxide (CAR-O) and 180 μmol l−1 eugenol (EUG). Abscissae: voltage in mV; ordinates: current in nA; representative experiments.
Effects on action potential duration
The influence of nifedipine, β-caryophyllene oxide, and eugenol on the action potential duration at a 1 Hz contraction frequency is shown in Figure 8. Nifedipine strongly reduced the action potential duration (by about 50% at 30% repolarization). This reduction agrees with earlier observations in which a shortening of the action potential duration under the influence of nifedipine was shown at 0.25 and 1.5 Hz (Bayer et al., 1977) and under rested-state conditions (Reiter et al., 1984). β-Caryophyllene oxide did not change the action potential duration, while eugenol shortened it somewhat less than nifedipine (by about 25% at 30% repolarization).
Figure 8.
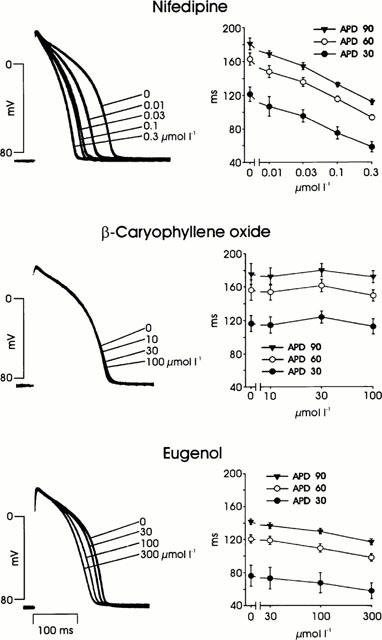
Action potentials of guinea-pig papillary muscles under the influence of nifedipine, β-caryophyllene oxide, and eugenol. Left side: superimposed action potentials (three different muscles). Right side: action potential duration at 30, 60 and 90% repolarization (APD 30, APD 60, APD 90); mean values±s.e.mean of six (nifedipine and eugenol) and four (β-caryophyllene oxide) muscles.
Discussion
Both clove oil constituents, β-caryophyllene oxide and eugenol, exerted negative inotropic effects in heart muscle which were comparable to that of the calcium channel blocker nifedipine. These negative inotropic effects can be related to the blocking of calcium channels: β-caryophyllene oxide, eugenol, and nifedipine inhibited rested-state contractions and reduced the ICa.
It has been suggested the rested-state contractions are determined exclusively by calcium flowing into the cell during excitation (Reiter et al., 1984; Reiter, 1988). The contractile proteins receive their activator calcium from the ICa after emptying intracellular calcium stores during the rest period (Wendt-Gallitelli, 1985). Therefore, a reduction of these contractions can be indicative of a calcium channel blocking effect of a substance.
Since the potencies of the clove oil constituents for suppressing the RSCs are three orders of magnitude less than that of nifedipine, the question arises as to whether the effects of the volatile oil constituents can be regarded as specific or non-specific effects on the sarcolemmal membrane depending on their lipophilic nature. Such an influence on the membrane is unlikely since the IC50 values of β-caryophyllene oxide, eugenol, and their derivatives caryophyllene and eugenol acetate (Table 2) do not correlate with their octanol-water partition coefficients as calculated according to Suzuki & Kudo (1990).
The calcium channel blocking effect of β-caryophyllene oxide and eugenol was demonstrated in voltage clamp experiments by measuring the ICa in isolated cardiac myocytes: β-caryophyllene oxide and eugenol reduced the ICa without changing the current voltage relationship. Therefore, ICa inhibition must be regarded as the common mechanism of the negative inotropic effects of these clove oil constituents.
In spite of the similarities between the effects of β-caryophyllene oxide, eugenol, and nifedipine, there are profound differences regarding their influence on the time course of the contraction and the relation between calcium channel blocking and negative inotropic effects. At a 1 Hz contraction frequency, β-caryophyllene oxide reduced the peak contraction without shortening the contraction time over most of the concentration range. In contrast, eugenol and nifedipine shortened the contraction time. Under rested-state conditions, β-caryophyllene oxide strongly prolonged and nifedipine shortened the contraction while eugenol had only slight effects on the time course of contraction.
The influence of β-caryophyllene oxide on the time course of contraction at 1 Hz differs from the effect of nifedipine and, therefore, is not compatible with a pure calcium channel blocking effect. Accordingly, we found that in addition to blocking calcium channels, β-caryophyllene oxide also blocks potassium channels. At a 1 Hz contraction frequency, the influence on potassium channels obviously prevents the shortening of the action potential and contraction by reducing the ICa. Indeed, the action potential at 1 Hz was not changed by β-caryophyllene oxide. In contrast, eugenol slightly shortened and nifedipine greatly shortened the action potential. This shortening of the action potential was not only observed under the influence of nifedipine, but also under the influence of other dihydropyridines like elgodipine or oxodipine (Tamargo et al., 1991) as well as lacidipine (Cerbai et al., 1997) and structurally unrelated compounds like mefloquine (Coker et al., 2000) and clotrimazole (Thomas et al., 1999), which exert calcium channel blocking effects in addition to their main effects.
The fact that β-caryophyllene oxide did not change the time course of the action potential and contraction at 1 Hz means that the negative inotropic effect of β-caryophyllene oxide is solely produced by the reduced influx of calcium due to the blockade of calcium channels. Accordingly, the IC50 value of β-caryophyllene oxide for inhibiting the ICa was similar to that for inhibiting the force of contraction at 1 Hz (see Table 1). In contrast, the IC50 of nifedipine for the inhibition of the 1 Hz contraction was only 10% of that of the ICa. At physiological contraction frequencies, a slight inhibition of the ICa led to a strong diminution of the contraction. Eugenol occupies an intermediate position between nifedipine and β-caryophyllene oxide: at a 1 Hz contraction frequency, the action potential was shortened but to a lesser extent than under the influence of nifedipine. The IC50 for contraction was 55% of that of the ICa. The difference in comparison to nifedipine can be explained by a weak potassium channel blocking effect.
The different influences of the investigated compounds on the relationship between ICa and FC and on the duration of contraction and action potential at 1 Hz show that the negative inotropic effect of a calcium channel blocker is due to two effects: (1) the reduction of the calcium influx into the cell and (2) the shortening of the action potential duration as a consequence of the fact that the ICa contributes to the plateau of the action potential. The shortened action potential then probably again influences the contraction in two ways: it additionally reduces the calcium influx during excitation and ‘curtails' the contraction at an earlier time. The discrepancy between the IC50 values with regard to the ICa and force of contraction does not exclude other reasons: for instance, special kinetics of receptor binding due to the different frequencies used, as has been discussed for nifedipine (Uehara & Hume, 1985; Tsuchida et al., 1991; Okuyama et al., 1994). Additionally, the fact that eugenol had a weaker effect than nifedipine on action potential duration but a stronger effect on contraction time probably indicates that there is a complex interaction between the magnitude and duration of calcium influx and contraction.
In summary, the results show that the additional inhibition of potassium currents by the naturally occurring sesquiterpene β-caryophyllene oxide reduces the negative inotropic effect due to the blockade of calcium channels. The modification of the inotropic effect of a calcium channel blocker may be interesting if the diminution of ICa is used, for example, in certain arrhythmias where the negative inotropic effect should be avoided as far as possible.
Acknowledgments
We would like to thank Prof. F. Ebner, München, for statistical help, Dr A. Pauli, Würzburg, for calculating the octanol-water partition coefficients, Christa Baumgärtl and Beate Probst for technical assistance, and Ms S. Klein and Dr H.-J. Daleiden, Bayer Research Center Wuppertal, for a search of the literature. The study was supported by Deutsche Herzhilfe e.V., München.
Abbreviations
- APD 30 (60, 90)
action potential duration at 30% (60%, 90%) repolarization
- C
control
- CAR-O
β-caryophyllene oxide
- EUG
eugenol
- Fc
peak force of contraction
- NIF
nifedipine
- RSC
rested-state contraction
- tc
time of contraction
References
- BAYER R., EHARA T. Comparative studies on calcium antagonists. Progr. Pharmacol. 1978;2:31–37. [Google Scholar]
- BAYER R., RODENKIRCHEN R., KAUFMANN R., LEE J.H., HENNEKES R. The effects of nifedipine on contraction and monophasic action potential of isolated cat myocardium. Naunyn-Schmiedeberg's Arch. Pharmacol. 1977;301:29–37. doi: 10.1007/BF00501261. [DOI] [PubMed] [Google Scholar]
- BEELER G.W., REUTER H. Voltage clamp experiments on ventricular myocardial fibres. J. Physiol. 1970;207:165–190. doi: 10.1113/jphysiol.1970.sp009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENDUKIDZE Z., ISENBERG G., KLÖCKNER U. Ca-tolerant guinea-pig ventricular myocytes as isolated by pronase in the presence of 250 μM free calcium. Basic Res. Cardiol. 1985;80 Suppl. 1:13–17. doi: 10.1007/978-3-662-11041-6_2. [DOI] [PubMed] [Google Scholar]
- BLINKS J.R., KOCH-WESER J. Analysis of the effects of changes in rate and rhythm upon myocardial contractility. J. Pharmacol. Exp. Ther. 1961;134:373–389. [PubMed] [Google Scholar]
- CERBAI E., GIOTTI A., MUGELLI A. Characteristics of L-type calcium channel blockade by lacidipine in guinea-pig ventricular myocytes. Br. J. Pharmacol. 1997;120:667–675. doi: 10.1038/sj.bjp.0700951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARNET P., OUADID H., RICHARD S., NARGEOT J. Electrophysiological analysis of the action of nifedipine and nicardipine on myocardial fibers. Fundam. Clin. Pharmacol. 1987;1:413–431. doi: 10.1111/j.1472-8206.1987.tb00575.x. [DOI] [PubMed] [Google Scholar]
- COKER S.J., BATEY A.J., LIGHTBOWN I.D., DIAZ M.E., EISNER D.A. Effects of mefloquine on cardiac contractility and electrical activity in vivo, in isolated cardiac preparations, and in single ventricular myocytes. Br. J. Pharmacol. 2000;129:323–330. doi: 10.1038/sj.bjp.0703060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HUME J.R. Comparative interactions of organic Ca++ channel antagonists with myocardial Ca++ and K+ channels. J. Pharmacol. Exp. Ther. 1985;234:134–140. [PubMed] [Google Scholar]
- LANSMAN J.B., HESS P., TSIEN R.W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+: Voltage and concentration dependence of calcium entry into the pore. J. Gen. Physiol. 1986;88:321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYNCH C., III Pharmacological evidence for two types of myocardial sarcoplasmic reticulum Ca2+ release. Am. J. Physiol. 1991;260:H785–H795. doi: 10.1152/ajpheart.1991.260.3.H785. [DOI] [PubMed] [Google Scholar]
- OKUYAMA R., ADACHI-AKAHANE S., NAGAO T. Differential potentiation by depolarization of the effects of calcium antagonists on contraction and Ca2+ current in guinea-pig heart. Br. J. Pharmacol. 1994;113:451–456. doi: 10.1111/j.1476-5381.1994.tb17010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REITER M. Calcium mobilization and cardiac inotropic mechanisms. Pharmacol. Rev. 1988;40:189–217. [PubMed] [Google Scholar]
- REITER M., BRANDT W. Relaxant effects on tracheal and ileal smooth muscles of the guinea pig. Drug. Res. 1985;35:408–414. [PubMed] [Google Scholar]
- REITER M., VIERLING W., SEIBEL K. Excitation-contraction coupling in rested-state contractions of guinea-pig ventricular myocardium. Naunyn-Schmiedeberg's Arch. Pharmacol. 1984;325:159–169. doi: 10.1007/BF00506196. [DOI] [PubMed] [Google Scholar]
- SEIBEL K., KAREMA E., TAKEYA K., REITER M. Effect of noradrenaline on an early and a late component of the myocardial contraction. Naunyn-Schmiedeberg's Arch. Pharmacol. 1978;305:65–74. doi: 10.1007/BF00497007. [DOI] [PubMed] [Google Scholar]
- SENSCH O., VIERLING W., BRANDT W., REITER M. Eugenol and caryophyllene oxide as calcium current inhibitors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993a;347:R78. [Google Scholar]
- SENSCH O., VIERLING W., BRANDT W., REITER M. Calcium-channel blocking effect of constituents of clove oil. Planta Med. 1993b;59:A687. [Google Scholar]
- SUTHERLAND S.D., FESTING M.F.W.The guinea pig The UFAW Handbook on the Care and Management of Laboratory Animals 1987Essex: Longman Scientific & Technical; 393–410.ed. Poole, T.B. pp [Google Scholar]
- SUZUKI T., KUDO Y. Automatic log P estimation based on combined additive modelling methods. J. Computer-Aided Molecular Design. 1990;4:155–198. doi: 10.1007/BF00125317. [DOI] [PubMed] [Google Scholar]
- TAMARGO J., LOPEZ-SENDON J., DELPON E., GONZALES-MORALES M., DE MIGUEL E. Cardiovascular effects of the new dihydropyridine derivative elgodipine. Drug Res. 1991;41:895–900. [PubMed] [Google Scholar]
- THOMAS G.P., KARMAZYN M., ZYGMUNT A.C., ANTZELEVITCH C., NARAYANAN N. The antifungal antibiotic clotrimazole potently inhibits L-type calcium current in guinea-pig ventricular myocytes. Br. J. Pharmacol. 1999;126:1531–1533. doi: 10.1038/sj.bjp.0702475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUCHIDA K., KANEKO K., AIHARA H. Electrophysiological effects of CD-349, a dihydropyridine-type calcium antagonist, on goat cardiac purkinje fibers. J. Cardiovasc. Pharmacol. 1991;18:769–776. doi: 10.1097/00005344-199111000-00016. [DOI] [PubMed] [Google Scholar]
- UEHARA A., HUME J.R. Interactions of organic calcium channel antagonists with calcium channels in single frog atrial cells. J. Gen. Physiol. 1985;85:621–647. doi: 10.1085/jgp.85.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WENDT-GALLITELLI M.F. Presystolic calcium-loading of the sarcoplasmic reticulum influences time to peak force of contraction. X-ray microanalysis on rapidly frozen guinea-pig ventricular muscle preparation. Basic Res. Cardiol. 1985;80:617–625. doi: 10.1007/BF01907860. [DOI] [PubMed] [Google Scholar]


