Abstract
Local pressure-induced vasodilation (PIV) is a neural vasodilator response to non-nociceptive externally applied pressure in the skin, previously described in humans. We first determined whether PIV exists in rats and depends on capsaicin-sensitive fibres as it does in humans. We then examined the mediators involved in the efferent pathway of PIV.
Cutaneous blood flow was measured by laser Doppler flowmetry during 11.1 Pa s−1 increases in local applied pressure in anaesthetized rats. The involvement of capsaicin-sensitive fibres in PIV was tested in rats treated neonatally with capsaicin. To antagonize CGRP, neurokinin-1, -2, or -3 receptors, different groups of rats were treated with CGRP8–37, SR140333, SR48968 or SR142801, respectively. Prostaglandins involvement was tested with indomethacin treatment. To inhibit nitric oxide synthase (NOS) activity or specific neuronal NOS, rats were treated with NG-nitro-L-arginine or 7-nitroindazole, respectively.
PIV was found in rats, as in humans. PIV was abolished by neonatal treatment with capsaicin and by administration of CGRP8–37 but remained unchanged with SR140333, SR48968 and SR142801 treatments. Prostaglandin inhibition resulted in a significant decrease in PIV. Inhibition of NOS abolished PIV, whereas inhibition of neuronal NOS caused a diminution of PIV.
These data suggest that PIV depends on capsaicin-sensitive fibres in rats, as in humans. It appears that CGRP plays a major role in the PIV, whereas neurokinins have no role. Furthermore, PIV involves a contribution from prostaglandins and depends on endothelial NO, whereas neuronal NO has a smaller role.
Keywords: Capsaicin, CGRP, laser Doppler, neurokinins, NO, pressure, prostaglandins, skin blood flow
Introduction
Over the last two decades it has become clear that cutaneous vasodilation can be caused by the release of mediators from afferent nerve endings and from the endothelium. The cutaneous circulation participates in reflexes of nonthermoregulatory origin (Johnson, 1986; Rowell et al., 1973), such as external pressure stress (Fromy et al., 1998). Capsaicin-sensitive afferent neurones are connected to cutaneous receptors (Holzer, 1991) which enable them to detect noxious stimuli that are potentially or actually harmful to the tissue. Nevertheless, little is known about the response to non-noxious pressure application (Garell et al., 1996; Koltzenburg & Handwerker, 1994).
It is well known that ulcers are easily developed in paralysed patients (Schubert & Fagrell, 1991) and in elderly subjects (Schubert et al., 1994). Risk factors of decubitus ulcers are multiple, but the major ones are the intensity and the duration of applied pressure on the skin, particularly in subjects that remain immobile, and at bony prominences. The skin becomes ischaemic, and if the local pressure is maintained, necrosis occurs. Skin blood flow is regulated to respond to physical stimuli before such a noxious event occurs, in order to prevent ischaemia in the tissue. We recently reported a significant transient increase of cutaneous laser Doppler flow (LDF) during local non-noxious pressure applied progressively in the skin of humans (Fromy et al., 1998). A similar phenomenon was reported by Schubert & Fagrell (1989) in humans, during moderate step local pressure increases over the sacrum and in the gluteus region. In our previous study (Fromy et al., 1998), we found that capsaicin desensitization resulted in the total disappearance of this local pressure-induced vasodilation (PIV). These results demonstrate the existence of a vasodilatory reflex response to non-noxious pressure strain which is initiated by capsaicin-sensitive nerve terminals in human skin. This local reflex may have important implications for the cutaneous pathologies involved in various neurological diseases and in the pathophysiology of decubitus ulcers (Schubert & Fagrell, 1989).
In the present study, our first aim was to develop an animal model of the PIV to facilitate the study of mediators involved in this reflex. For this purpose, we verified the existence of a PIV in the skin of untreated anaesthetized rats. The involvement of capsaicin-sensitive fibres in this mechanism was then tested in rats treated neonatally with capsaicin. The properties peculiar to capsaicin and its characterization as a tool for sensory neurone research have been demonstrated (Jancso et al., 1967; 1968). Systemic treatment with capsaicin, as a denervating agent in neonatal rats, provokes the most extensive and consistent degeneration of capsaicin-sensitive fibres (Jancso et al., 1977; Saumet & Duclaux, 1982).
Our second aim was to verify whether similar neuropeptide receptors are involved in PIV as those which are involved in responses induced by nociceptive stimuli. Calcitonin gene-related peptide (CGRP) and neurokinins, among the main sensory neuropeptides, are released from small afferent nerve endings following their activation (Maggi & Meli, 1988; Otsuka & Yoshioka, 1993; Rossi & Johansson, 1998). It is known that neuropeptides (CGRP and neurokinins) are expressed, co-stored and co-released from capsaicin-sensitive primary afferent neurones. These concepts have received extensive confirmation over the past several years (Holzer, 1988; Maggi & Meli, 1988). Specifically, CGRP, a potent vasodilator (Brain et al., 1985; Kawasaki et al., 1988), has been co-localized with substance P (SP) in subepidermal and epidermal C-fibres (Gibbins et al., 1985). It can be selectively antagonized by CGRP8–37 (Chiba et al., 1989; Donoso et al., 1990; Hughes & Brain, 1991). Thus it is likely that CGRP and/or neurokinin receptors are involved in the mechanism of PIV. The neurokinin-1 (NK1) receptor is operationally defined as the mediator of the biological activities for which SP is a more potent agonist than neurokinin A (NKA) or neurokinin B (NKB) (Maggi, 1995; Scholzen et al., 1998). This definition does not imply that SP is the exclusive activator of NK1 receptor in physiological conditions (Otsuka & Yoshioka, 1993; Yanagisawa & Otsuka, 1990). SR140333 is a selective antagonist of NK1 receptors in various animal species (Emonds-Alt et al., 1993a; Herbert & Bernat, 1996; Jung et al., 1994). The neurokinin-2 (NK2) receptor presents the following rank order of potency of natural tachykinins: NKA>NKB>SP (Emonds-Alt et al., 1992; 1993b; Maggi et al., 1993). SR48968 is a selective non-peptide antagonist of the NK2 receptor (Advenier et al., 1992; Emonds-Alt et al., 1992; 1993b; Maggi et al., 1993) and is a useful tool for studying the distribution and function of NK2 receptors (Emonds-Alt et al., 1993b; Maggi et al., 1993). The neurokinin-3 (NK3) receptor is defined by the following rank order of potency of natural tachykinins: NKB>NKA>SP (Maggi et al., 1993). SR142801 is a selective non-peptide antagonist of the NK3 receptor (Emonds-Alt et al., 1995) and is a tool for investigation of the physiological and pathological role of the NK3 receptor.
The last aim of the present study was to examine whether the mechanism for the PIV response is endothelium dependent and to elucidate the roles of potential factors involved. Both prostaglandins and nitric oxide (NO) are possible mediators of an endothelial vasodilator mechanism (Shastry et al., 1998; Warren et al., 1994). The importance of prostaglandins in the microcirculation has been emphasized by cyclo-oxygenase inhibition, which attenuates the microvascular vasodilator response to inflammation (Williams & Peck, 1977). The role of prostaglandins was studied via administration of indomethacin (Friese et al., 1997; Whittle et al., 1980).
NO mediates most, if not all, the functions of endothelium derived relaxing factor (Furchgott & Zawadzki, 1980; Griendling & Alexander, 1996; Moncada et al., 1991; Palmer et al., 1987). NO was identified as a vasodilating substance that is synthesized from L-arginine (Moncada et al., 1989; Moncada & Higgs, 1993) by nitric oxide synthase (NOS) in the vascular endothelium. NO can also be released by autonomic nerves and may contribute to neurally mediated vasodilation in various tissues (Rajfer et al., 1992; Taylor & Bishop, 1993; Toda & Okamura, 1991). Indeed, two constitutive NO synthases have been described in vessels, an endothelial and a neuronal NOS. Both of these NOS isoforms can be blocked using NG-nitro-L-arginine (L-NNA) (Vargas et al., 1991). Recently 7-nitroindazole (7-NI), a specific inhibitor of neuronal NO synthase in vitro, has been identified (Babbedge et al., 1993; Moore et al., 1993), allowing the differentiation between neuronal and endothelial NOS activities.
Thus, in the present study, we sought to verify the presence of PIV in the skin of anaesthetized rats, and then to elucidate some of the mechanisms for this response. In all, eight related questions were addressed: (1) Does PIV exist in rats, as in humans? (2) Are capsaicin-sensitive fibres involved in PIV in rats? (3) Does CGRP play a major role in PIV? Are substance P (4), or other neurokinins (5) essential for the development of PIV? (6) Does the prostaglandin pathway play a major role in PIV? (7) Is the NO pathway essential for the development of PIV? (8) If so, is neuronal NO involved in this vasodilator response?
Methods
Animal instrumentation
The studies were performed in 218 Wistar rats (250–350 g). At least 2 days prior to the experiment (to prevent skin irritation during the experiment from confounding the results), the hair was removed from the heads of the animals with a depilator lotion, to present a hairless area for the LDF measurements and local pressure application. Animals were housed in a regulated environment with a constant ambient temperature of 24°C. Animals were deprived of food for 24 h prior to the experiments but allowed free access to tap water. Procedures for the maintenance and use of the experimental animals were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No. 85–23, revised 1985).
For the experiments, animals were anaesthetized by intraperitoneal injection of thiopental at a dose of 50 mg kg−1 body weight. The level of anaesthesia was determined by testing eye reflexes. The left femoral vein was cannulated for drug or vehicle administration if necessary. In protocol 1, an arterial catheter was placed in the femoral artery to measure the mean arterial blood pressure of the rats. Rats were placed in an incubator (MMS, Chelles, France) warmed to 30°C to maintain stable body temperature, which was monitored with a rectal thermometer. The animals were placed in the prone position and the head was fixed on a frame. The rats were separated randomly into 16 groups, required for eight protocols.
Experimental procedure for assessment of PIV
A weighbridge was adapted to hold a laser Doppler probe (PF408, Periflux, Perimed, Sweden). The probe has a 12.6 mm2 circular contact surface and the measurement was performed in the centre of the hairless area. The probe is connected to a laser Doppler flowmeter (PF4001 Master, Periflux, Perimed, Sweden), which provides a good indicator of the response pattern of skin blood flow from the region of illuminated skin (Saumet et al., 1986). It has previously been shown that LDF measured from the skin surface is not influenced by blood flow to underlying skeletal muscle (Saumet et al., 1988).
Externally applied pressure was increased progressively at 11.1 Pa s−1 (5 mmHg min−1) through the LDF probe. The technical details of this method have been described (Fromy et al., 2000). Briefly, the weighbridge was carefully equilibrated and then positioned in the middle of the hairless skull of the rat. The LDF signal was digitized with a 5 Hz sample frequency using a computerized acquisition system (Biopac, Santa Barbara, California, U.S.A.). Data collection began with a 2-min control period prior to the onset of increasing pressure. The signals were continuously recorded for 30 min. The LDF signals were averaged every 30 s to reduce the instantaneous variability of the signals due to vasomotion. Rectal temperature was recorded throughout the experiment to verify the thermal stability of the rat. All animals were sacrificed at the end of the experiment by an overdose of the anaesthetic agent.
Protocol 1: PIV existence in rats
In protocol 1, the existence of PIV in untreated anaesthetized rats (n=12) was verified. We monitored systemic haemodynamics via the measurement of mean arterial blood pressure. Cutaneous vascular resistance was calculated as the ratio of mean arterial blood pressure to LDF, and thus had the units mmHg a.u.−1.
Protocol 2: Capsaicin-sensitive fibre involvement in PIV
To investigate the potential involvement of capsaicin-sensitive fibres in PIV the protocol was performed in rats treated neonatally with capsaicin (n=22). A separate group of untreated rats (n=17) was used as control.
Capsaicin was injected in neonatal rats intraperitoneally once a day for 5 days (50 mg kg−1). This treatment has been shown to destroy permanently the majority of small unmyelinated sensory nerve fibres (Jancso et al., 1977) including those implicated in nociception. We tested the nociceptive threshold in 8–10-week-old rats by a tail-flick test to verify the efficacy of the treatment. Capsaicin-treated neonatal rats showed a significant increase in the latency (from 15.6±0.8 s for untreated rats to 36.4±1.3 s for treated rats, P<0.001) of the response to the noxious thermal stimulus in the tail-flick test.
Protocol 3: CGRP receptor involvement in PIV
To examine the role of the CGRP receptor in this vasodilator response, we used the CGRP antagonist (CGRP8–37) which was injected intravenously (100 μg kg−1) 10 min prior to the start of the experiment (n=17). A control group (n=15) received the same volume of vehicle as the treated rats.
Protocol 4: NK1 receptor involvement in PIV
To test whether the NK1 receptor was involved in the vasodilator response, we used a NK1 receptor antagonist (SR140333), which was injected intravenously (200 μg kg−1) 5 min prior to the start of the experiment (n=20). A control group (n=17) received intravenously the same volume of vehicle as the treated rats.
Protocol 5: NK2 and NK3 receptors involvement in PIV
To test whether the NK2 and/or NK3 receptors were involved in the vasodilator response, we used NK2 and NK3 receptor antagonists. The NK2 receptor antagonist (SR48968) was injected into the femoral vein as a bolus (4 mg kg−1) 20 min prior to the start of the experiment (n=16). The NK3 receptor antagonist (SR142801) was injected intravenously (1 mg kg−1) 20 min prior to the start of the experiment (n=15). A control group (n=13) received intravenously the same volume of vehicle as treated rats and were studied 20 min after the injection.
Protocol 6: Prostaglandin involvement in PIV
Indomethacin was injected intraperitoneally (5 mg kg−1) 30 min prior to the start of the experiment (n=9). Inhibition of cyclo-oxygenase allowed us to test the role of prostaglandins in the PIV response. A control group (n=9) received the same volume of saline as the treated rats intraperitoneally.
Protocol 7: NO involvement in PIV
L-NNA was injected in the femoral vein as intravenous bolus (20 mg kg−1) 15 min prior to the start of the experiment (n=9). A control group (n=9) received the same volume of saline as the treated rats intravenously and were studied 15 min after the saline injection.
Protocol 8: Neuronal NO involvement in PIV
A single dose (50 mg kg−1) of 7-NI was injected intraperitoneally 45 min prior to starting the recordings (n=9). The control animals (n=9) were treated with the same volume of vehicle, and the recordings were started 45 min after its administration.
Drugs
Capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide) was purchased from Sigma company (St. Louis, Missouri, U.S.A.) and was dissolved in 10% ethanol, 10% Tween 80 and 80% distilled water. CGRP8–37, purchased from Sigma company (St. Louis, Missouri, U.S.A.), was dissolved in saline. SR140333 ((S)-1-{2-[3-(3,4-dichlorophenyl)-1-(3-isopropoxyphenylacetyl)piperidin-3-yl]ethyl}-4-phenyl-1-azoniabicyclo [2.2.2]octane chloride) (lot no. PG 13 2566), SR48968 ((S)-(N)-methyl-N[4-(acetylamino-4-phenylpiperidino)-2-(3,4-dichlorophenyl)butyl]benzamide) (lot noGX 4 677), SR142801 ((S)-(N)-{1-[3-(benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl)propyl]-4-phenylpiperidin-4-yl}-N-methylacetamide) (lot noVG 15 815) were kindly provided by Dr Emonds-Alt (Sanofi Recherche, Montpellier, France). SR140333 was dissolved in 4% ethanol and 96% saline. SR48968 and SR142801 were dissolved in 10% dimethyl sulphoxide (DMSO) and 90% saline. Indomethacin (1-[p-chlorobenzoyl]-5-methoxy-2-methylindole-3-acetic acid) and L-NNA were purchased from Sigma company (St. Louis, Missouri, U.S.A.) and were dissolved in saline. 7-NI, purchased from Sigma company (St. Louis, Missouri, U.S.A.), was dissolved in a mixture of 50% Tween 80 and 50% saline.
Analyses of results
Results are expressed as mean±s.e.mean. Resting values were calculated as the average over the 2 min of the control period prior to the local pressure application.
To determine the significance of our results between groups in every protocol, we performed a paired t-test or one-way ANOVA with repeated measures with a Bonferroni post test. In figures, following confirmation that two groups had overall responses that were significantly different, mean LDF for individual values of applied pressure were compared by t-test. One-way ANOVA with Dunnett's multiple comparison test (baseline as control) was performed to determine the differences due to the locally applied pressure within each group. A two-tailed P value less than 0.05 was regarded as statistically significant.
Results
Rectal temperature did not change significantly in any experiment. Further, for each protocol, baseline LDF values and the LDF values measured at the maximal applied pressure were not significantly different between the drug-treated rats and the vehicle treated rats.
Protocol 1: PIV existence in rats
Mean LDF, mean arterial blood pressure, mean vascular resistance and mean rectal temperature with increasing pressure for untreated rats are presented in Figure 1. As can be seen in this figure, mean arterial blood pressure and rectal temperature were stable throughout the experiment. Mean resting LDF was 98.9±4.8 arbitrary units (a.u.) in untreated rats. A significant increase in mean LDF was found from 0.7 kPa (117.7±7.7 a.u., P<0.01) and reached a maximal mean value of 124.0±8.5 a.u. at 1.3 kPa. Over the same range of pressures, mean vascular resistance decreased from 1.20±0.09 mmHg a.u.−1 to 0.98±0.09 mmHg a.u.−1 (P<0.0001). With further increases in pressure, mean LDF decreased slowly and reached 103.7±9.9 a.u. at 3.3 kPa. Mean values were significantly lower than mean resting LDF at 5.7 kPa (82.2±9.1 a.u., P<0.05). Mean LDF continued to decrease down to a plateau reached at the end of the experiment (46.1±4.0 a.u.).
Figure 1.
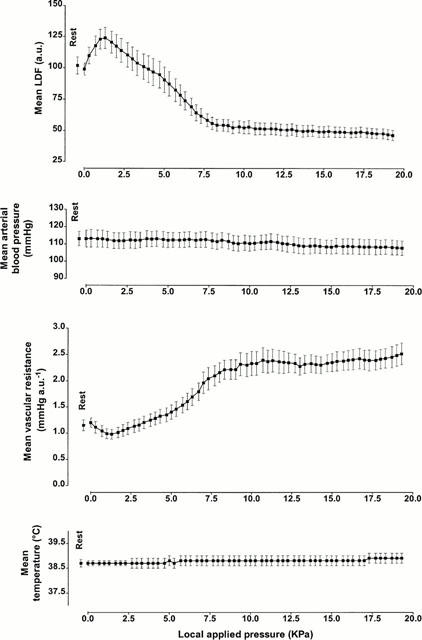
LDF, arterial blood pressure, vascular resistance and rectal temperature (mean±s.e.mean) obtained with 11.1 Pa s−1 local external pressure application in untreated anaesthetized rats. The first point, outside the scale of pressure, corresponds to the mean resting value before the start of local applied pressure.
Protocol 2: Capsaicin-sensitive fibre involvement in PIV
Mean LDF calculated with increasing pressure for untreated rats and rats treated with capsaicin are presented in Figure 2. Comparison between groups showed a significant difference (P<0.001).
Figure 2.
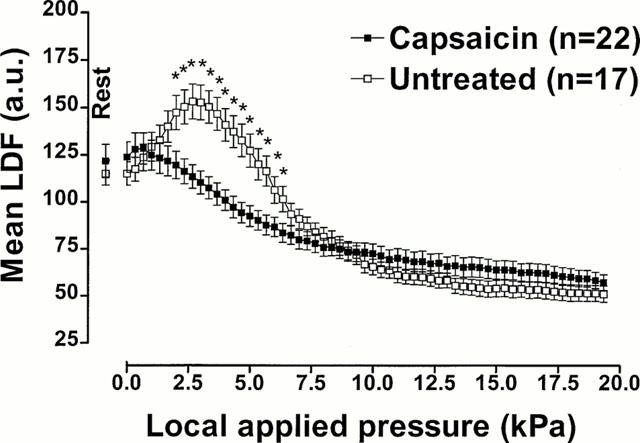
Capsaicin-sensitive fibre involvement in PIV. Mean±s.e.mean of LDF obtained with 11.1 Pa s−1 local external pressure application in rats treated with capsaicin as neonates and in untreated rats. The first point, outside the scale of pressure, corresponds to the mean resting value before the start of local applied pressure. Asterisks denote significant differences from control data (*P<0.05).
The rats treated with capsaicin had a mean resting LDF of 123.9±8.1 a.u. These rats did not show a significant increase in mean LDF at any time during the experiment. Mean LDF decreased to 119.6±7.3 at 1.7 kPa, and decreased significantly to 107.5±5.9 a.u. at 3.3 kPa. (P<0.05). With further pressure increases, mean LDF continued to decrease until it reached a plateau at the end of the experiment (57.3±4.2 a.u.).
Mean resting LDF was 115.1±6.2 a.u. in untreated rats. A significant increase in mean LDF was found from 0.3 kPa (117.4±6.3 a.u., P<0.01) and reached a maximal mean value of 153.3±9.2 a.u. at 2.7 kPa. With further increases in pressure, mean LDF decreased slowly, and was decreased to 150.4±8.6 a.u. at 3.3 kPa. Mean LDF reached 101.6±6.7 a.u., a value significantly lower than mean resting LDF, at 6.3 kPa (P<0.05) and continued to decrease down to a plateau reached at the end of the experiment (51.1±4.3 a.u.).
Protocol 3: CGRP receptor involvement in PIV
Mean LDF measured with increasing pressure in rats treated with CGRP8-37 or with its vehicle are presented in Figure 3. The comparison between groups showed a significant difference (P<0.001).
Figure 3.
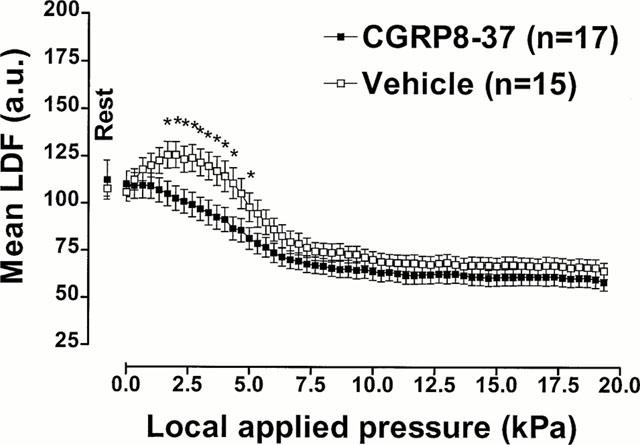
CGRP receptor involvement in PIV. Mean±s.e.mean of LDF obtained with 11.1 Pa s−1 local external pressure application in rats treated with CGRP8–37 (100 μg kg−1, i.v.) and with vehicle (saline, i.v.). The first point, outside the scale of pressure, corresponds to the mean resting value before the start of local applied pressure. Asterisks denote significant differences from control data (*P<0.05).
In this set of experiments, rats were treated with CGRP8-37 (100 μg kg−1, i.v.). The rats had a mean resting LDF of 110.1±6.8 a.u. and did not show significant increase in mean LDF at any time during the experiment. As soon as the applied pressure increased, a continuous decrease of cutaneous blood flow began, reaching 109.2±6.8 a.u. at 0.3 kPa. The observed decrease became significant at 1.7 kPa (105.0±6.6 a.u., P<0.05). With further pressure increases, mean LDF continued to decrease until it reached a plateau at the end of the experiment (60.1±4.4 a.u.).
The rats that received the vehicle of CGRP8-37 had a mean resting LDF of 105.7±4.8 a.u. and showed a significant increase of mean LDF at 0.3 kPa (112.4±5.6 a.u., P<0.01). The maximal increase in mean LDF was 125.6±6.9 a.u., occurring at 2.0 kPa. Further increases in pressure led to a progressive decrease in mean LDF, which reached significance for 85.9±6.1 a.u. at 6.0 kPa (P<0.05). The plateau, reached at the end of the experiment, was 65.8±4.5 a.u.
Protocol 4: NK1 receptor involvement in PIV
Comparison between both groups of rats treated with SR140333 or with its vehicle did not show any significant difference.
Mean resting LDF was 103.3±8.3 a.u. in the rats treated with SR140333 (200 μg kg−1, i.v.). In this group, mean LDF was significantly increased above the baseline starting at 0.3 kPa (106.6±9.0 a.u., P<0.05) and continued to increase progressively, reaching a maximal mean value of 116.7±8.8 a.u. at 1.7 kPa. Further increases in pressure resulted in a continuous decrease of mean LDF, which was decreased compared with resting values at 6.0 kPa (86.4±7.0 a.u., P<0.05). Mean LDF continued to decrease until it reached a plateau at the end of the experiment (57.5±4.3 a.u.).
The rats that received the vehicle of SR140333 had a mean resting LDF of 106.6±8.5 a.u. In these rats, mean LDF increased to 111.7±9.1 a.u. at 0.3 kPa (P<0.05) and reached a maximal mean value of 122.0±9.2 a.u. at 1.7 kPa. With further pressure increases, mean LDF decreased, reaching significance at 6.0 kPa (92.9±8.8 a.u., P<0.05) and continued to decrease down to a plateau at the end of the experiment (61.4±4.2 a.u.).
Protocol 5: NK2 and NK3 receptors involvement in PIV
Comparison between groups of rats treated with SR48968 or SR142801 or with their common vehicle did not show any significant differences. This indicates that as was true for the NK1 receptor, blockade of NK2/NK3 receptor did not affect the transient vasodilator response.
The rats treated with the NK2 receptor antagonist (SR48968) had a mean resting LDF of 95.0±2.0 a.u. A significant increase in mean LDF occurred starting at 0.3 kPa (97.7±2.1 a.u., P<0.01) which reached a maximal mean value of 105.1±2.2 a.u. at 1.7 kPa. As with previous protocols, further increases in applied pressure resulted in progressive decreases in mean LDF. The observed decrease became significantly different from baseline at 6.0 kPa (82.7±4.4 a.u., P<0.05). With further increases in pressure, mean LDF continued to decrease, until the plateau was attained at the end of the experiment (56.0±2.6 a.u.).
Mean resting LDF was 93.4±2.7 a.u. in the rats treated with the NK3 receptor antagonist (SR142801). A significant increase of mean LDF was found starting from 0.3 kPa (96.2±3.3 a.u., P<0.01) and reached a maximal mean value of 106.8±3.5 a.u. at 1.7 kPa. Further increases in pressure resulted in a continuous decrease of mean LDF. The plateau, reached at the end of the experiment, was 56.9±1.6 a.u.
The rats that received the vehicle of SR48968 and SR142801 had a mean resting LDF of 98.9±3.0 a.u. A significant increase in mean LDF was found starting from 1.0 kPa (100.7±4.2 a.u., P<0.01) and reached a maximal mean value of 105.3±4.5 a.u. at 2.7 kPa. With further increases in pressure, mean LDF decreased slowly. Mean LDF was significantly lower than mean resting LDF at 6.3 kPa (82.7±4.9 a.u., P<0.05) and continued to decrease until it reached a plateau at the end of the experiment (56.8±1.9 a.u.).
Protocol 6: Prostaglandin involvement in PIV
The mean LDF calculated with increasing pressure in rats treated with indomethacin or with its vehicle are presented in Figure 4. The comparison between groups showed a significant difference (P<0.001), but PIV was not entirely abolished by indomathacin treatment (see Figure 4).
Figure 4.

Prostaglandin involvement in PIV. Mean±s.e.mean of LDF obtained with 11.1 Pa s−1 local external pressure application in rats treated with indomethacin (5 mg kg−1, i.p.) and with vehicle (saline, i.p.). The first point, outside the scale of pressure, corresponds to the mean resting value before the start of locally applied pressure. Asterisks denote significant differences from control data (*P<0.05).
Mean resting LDF in the rats treated with indomethacin was 106.6±6.7 a.u. and increased to 117.1±12.9 a.u. at 1.7 kPa. Further increases in pressure led to a continuous decrease in mean LDF, and decreased to 78.1±7.2 a.u. at 6.3 kPa (P<0.05). The plateau, reached at the end of the experiment, was 54.2±5.6 a.u.
The rats that received the vehicle had a mean resting LDF of 127.0±4.9 a.u. and showed a significant increase starting at 0.7 kPa (155.2±8.4 a.u., P<0.05) and reached a maximal mean value of 167.8±14.2 a.u. at 1.7 kPa. With further pressure increases, LDF continued to decrease to reach significance at 5.0 kPa (98.4±9.0 a.u., P<0.05). The plateau reached at the end of the experiment was 56.9±6.7 a.u.
Protocol 7: NO involvement in PIV
Mean LDF values with increasing pressure in rats treated with L-NNA or with its vehicle are presented in Figure 5. The comparison between L-NNA and control groups showed a significant difference (P<0.001).
Figure 5.
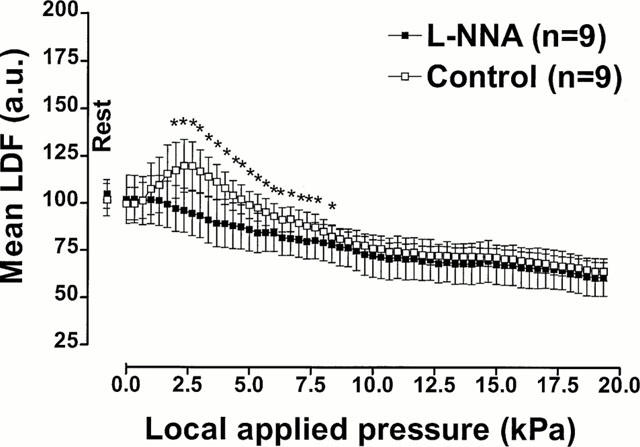
NO involvement in PIV. Mean±s.e.mean of LDF obtained with 11.1 Pa s−1 local external pressure application in rats treated with L-NNA (20 mg kg−1, i.v.) and with vehicle (saline, i.v.). The first point, outside the scale of pressure, corresponds to the mean resting value before the start of locally applied pressure. Asterisks denote significant differences from control data (*P<0.05).
No vasodilation was observed in L-NNA treated rats. The mean resting LDF was 102.0±12.9 a.u. and mean LDF began to decrease immediately with pressure increase. The decrease was significant at 2.0 kPa (97.2±12.2 a.u., P<0.05) and LDF further decreased with increasing pressure: 94.6±11.9 a.u. at 2.7 kPa and down to 60.9±10.0 a.u. at the end of the experiment.
In the group that received the vehicle, the mean resting LDF was 99.7±8.6 a.u. Mean LDF increased significantly at 2.0 kPa (117.4±14.8 a.u., P<0.01). The maximum increase of mean LDF was 119.8±12.8 a.u. (P<0.01) and occurred at a pressure of 2.7 kPa. With further increasing pressure, mean LDF decreased and became significantly different compared to the mean basal LDF at 9.3 kPa (78.0±4.7 a.u., P<0.05). The plateau, reached at the end of the experiment, was 64.0±4.9 a.u.
Protocol 8: Neuronal NO involvement in PIV
Mean LDF measured with increasing pressure in rats treated with 7-NI or with its vehicle are presented in Figure 6. The comparison between groups showed a significant difference (P<0.001).
Figure 6.
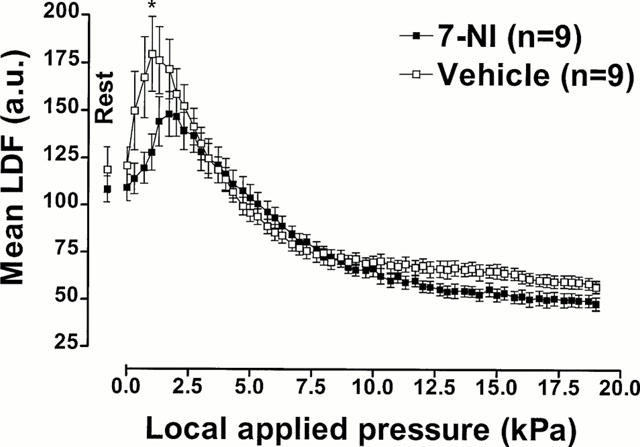
Neuronal NO involvement in PIV. Mean±s.e.mean of LDF obtained with 11.1 Pa s−1 local external pressure application in rats treated with 7-NI (50 mg kg−1, i.p.) and with vehicle (tween-saline, i.p). The first point, outside the scale of pressure, corresponds to the mean resting value before the start of locally applied pressure. Asterisk denotes significant differences from control data (*P<0.05).
In the last set of experiments, rats were treated with 7-NI. The rats had a mean resting LDF of 109.2±7.0 a.u. and showed a significant time averaged increase of LDF starting at 1.3 kPa (144.2±13.0 a.u., P<0.05) which increased to 148.1±11.7 a.u. at 1.7 kPa. Further increases in pressure led to progressive decreases in mean LDF. The decrease reached significance at 6.3 kPa (89.1±5.2 a.u., P<0.05) and continued down to a plateau of 48.0±3.4 a.u. at the end of the experiment.
The vehicle group had a mean resting LDF of 120.8±9.8 a.u. and increased to 179.6±19.6 a.u. at 1.0 kPa (P<0.05). Further increases in pressure led to progressive decreases in mean LDF, which reached significance at 5.0 kPa (96.0±4.6 a.u., P<0.05). The plateau reached at the end of the experiment was 56.9±3.2 a.u.
Discussion
Many investigations have been conducted to examine the thermal vascular responses elicited by cutaneous nociceptors (Handwerker & Kobal, 1993; Lynn, 1994), whereas there has been much less quantitative evaluation of the vascular response to mechanical stimuli. In this latter case, studies have been performed with intense stimuli, such as heavy pressure strains, but little is known about moderate or progressive pressure strains. In the rat, the majority of vasoactive C fibres belong to the cutaneous nociceptor class, some of which show relatively low threshold mechanical sensitivity (Gee et al., 1997; Hu & Sessle, 1988; Lynn et al., 1996).
We report here that progressive external pressure application resulted in a significant transient increase in mean LDF in rat skin, while mean arterial blood pressure remained stable during this increase. This increase in LDF indicates a vasodilator response of the skin, confirmed by the vascular resistance curve shown in Figure 1. Thus, our results indicate that cutaneous pressure-induced vasodilation (PIV) exists in rats, as in humans (Fromy et al., 1998). The results show that the cutaneous PIV is mediated by capsaicin-sensitive fibres and that CGRP is directly involved in this local vasodilator response. These conclusions are drawn from the following observations: PIV disappears in rats treated neonatally with capsaicin and in rats treated with CGRP8–37, whereas the response remains unchanged in rats treated with various neurokinin receptors antagonists. These latter results demonstrate that neurokinin receptors are not involved in the mechanism of PIV. Furthermore, we show here that PIV is impaired by indomethacin. Thus, this reflex involves a contribution from prostaglandins. Moreover, PIV is totally abolished with L-NNA treatment whereas 7-NI treatment provokes a diminution of PIV. Despite this diminution of PIV amplitude, a significant skin blood flow increase can be noted during 7-NI treatment. We conclude that PIV is dependent on NO and only partially dependent on neuronal NO.
Systemic administration of high doses of capsaicin has a definitive neurotoxic effect on a population of sensory neurones, the extent of damage depending on the dosage, route of administration, species and age of the animals. The most extensive and consistent lesions are produced by the systemic treatment of neonatal rats (Jancso et al., 1977; Saumet & Duclaux, 1982). To test whether PIV is dependent on capsaicin-sensitive fibres, as observed in humans on the dorsal aspect of the finger (Fromy et al., 1998), repeated administration of capsaicin in neonatal rats was performed in the present study in order to induce an irreversible destruction of these fibres (Jancso et al., 1977; Nagy et al., 1981). This was confirmed with the tail-flick test as previously demonstrated (Nagy & Van der Kooy, 1983). Since the use of capsaicin in neonates provokes a selective degeneration of small unmyelinated fibres, it is a useful tool for the elucidation of the participation of capsaicin-sensitive fibres in PIV. The marked reduction in this vasodilator response in the animals treated with capsaicin indicates that PIV is mediated by capsaicin-sensitive fibres, as in humans (Fromy et al., 1998). Data in both species support the view that PIV is mediated by the activation of unmyelinated cutaneous afferent nerve terminals.
The total disappearance of PIV in rats treated with CGRP8–37 indicates that CGRP participates in this vasodilator response. This antagonist was previously used (Holzer & Jocic, 1994; Kato et al., 1996) at a dose that was similar to that used in the present study (100 μg kg−1, i.v.). Our results are in accordance with the data of Merhi et al. (1998) who reported that the administration of CGRP8–37 before sciatic nerve stimulation resulted in significant inhibition (41%) of blood flux responses. They concluded that the peripheral release of sensory neuropeptides, such as SP and CGRP, initiates a cascade of events causing extravasation of plasma and vasodilation within the local microvasculature, where CGRP is implicated as the major mediator of neurogenic vasodilation.
In general, CGRP is a likely candidate to co-mediate the vasodilation (Brain et al., 1985; Escott & Brain, 1993), particularly in view of the finding that circulating CGRP is largely derived from perivascular capsaicin-sensitive nerve terminals (Zaidi et al., 1985). It appears that CGRP plays a major role in capsaicin-sensitive nerve mediated vasodilation (Holzer, 1988), as shown in the present study for non-nociceptive mechanical stimuli. Indeed CGRP8–37 was shown to inhibit completely the increase in cutaneous blood flow induced by capsaicin and also by its more potent analogue, olvanil (Hughes et al., 1992). Also in accordance with the present results, it has been shown that vasodilator responses to activation of sensory C-fibre afferents in the pig nasal mucosa and superficial skin are mainly dependent on CGRP, whereas NK1 receptor mechanisms seem to be of no or minor importance (Rinder & Lundberg, 1996). Delay-Goyet et al. (1992) concluded that CGRP rather than SP is likely to mediate the prolonged vasodilation seen in rat skin upon antidromic stimulation of sensory nerves.
The rats treated with SR140333 (200 μg kg−1, i.v.) demonstrated transient increases in LDF in response to locally applied pressure that were similar to those seen in control rats. This indicates that the NK1 receptor does not play a major role in PIV. Doses of SR140333 lower than 100 μg kg−1 show effective inhibition of various responses, such as neurogenic inflammation (Amann et al., 1995) or nociceptive transmission following trigeminal ganglion stimulation (Michaud et al., 1998). It has been shown that the inhibition is dose-dependent (Herbert & Bernat, 1996). A minimal administration of 100 μg kg−1, i.v., was needed to attain 100% inhibition of plasma extravasation in several studies (Emonds-Alt et al., 1993a; Herbert & Bernat, 1996), which was still effective 24 h after treatment (Amann et al., 1995; Emonds-Alt et al., 1993a). In the present study, a treatment regime with 200 μg kg−1, i.v., of SR140333 was used to ensure effective and selective blockade of NK1 receptors. The NK1 receptor is involved in nociceptive neurotransmission and SR140333 represents a potent drug for the relief of pain in humans (Li & Zhao, 1998; Michaud et al., 1998). In contrast, in conscious rats, systemic administration of SR140333 (100 μg kg−1, i.v.), at doses which cause inhibition of neurogenic inflammation, has no detectable effect on acute chemo- or thermonociception; although the decrease in thermal nociceptive threshold has been shown to be entirely dependent on capsaicin-sensitive afferents (Amann et al., 1995). Consistent with Amann et al. (1995), we found that SR140333 did not modify PIV, suggesting that the response to non-nociceptive mechanical stimulation does not involve NK1 receptor.
We further pursued the study of neurokinin involvement in PIV with rats treated with the NK2 and NK3 receptor antagonists, SR48968 and SR142801, respectively. The rats treated with SR48968 (4 mg kg−1, i.v.) or SR142801 (1 mg kg−1, i.v.) demonstrated a transient increase in skin blood flow in response to locally applied pressure, that was similar to the vasodilator response in control rats, showing that neither the NK2 nor the NK3 receptor play a major role in PIV. Santucci et al. (1993) studied the effects of SR48968 on the responses evoked by thermal or mechanical nociceptive cutaneous stimulation. Intravenous injection of SR48968 did not inhibit mechanically evoked responses in doses up to 2 mg kg−1, although these doses completely blocked the thermally evoked response. Thus we chose a dose superior at 2 mg kg−1 for use in the present study. Fleetwood-Walker et al. (1990) demonstrated that in cats, antagonists of NK2 receptors were found to attenuate cellular responses to thermal but not to mechanical nociceptive stimulation. Although our protocol did not involve nociceptive stimulation, our results are consistent with the report that the NK2 receptor was not involved in the vasodilator response to mechanical stimulation.
It is known that the NK3 receptor mediates potent endothelium-dependent vascular relaxation (Mizuta et al., 1995; Patacchini et al., 1995), but few studies have been conducted to investigate its role in the control of the peripheral vasculature. Mizuta et al. (1995) demonstrated that SR142801 inhibits NK3 receptor-induced relaxation in rat mesenteric artery and Patacchini et al. (1995) showed an insurmountable antagonism of the NK3 receptor in the portal vein of the rat. In contrast, we showed in the present study that the NK3 receptor was not involved in the vasodilator response to non-nociceptive mechanical stimulation.
Thus, the results of the present study show the involvement of CGRP but not of NK1, NK2 and NK3 receptors in cutaneous PIV. These results are consistent with those of Häbler et al. (1999), who concluded that in rat glabrous skin the vasodilatation evoked by a low level of activity in small diameter primary afferents resulted from the synergistic action of neurokinins (SP and/or NKA) and CGRP, while in hairy skin neurokinins are involved to a minor extent only. Similarly, multiple evidences arise from literature that neurokinins are unlikely to play a role in vasodilation whereas CGRP appears to be very important in this respect (Holzer, 1991; Lundberg, 1996; Rinder & Lundberg, 1996).
The last goal of the present study was to test whether the efferent pathway of PIV is dependent upon endothelial factors. Both a direct effect on smooth muscle relaxation and an endothelium dependent vasodilation can be hypothesized. In the present work, as in other studies on the neuronal control of microcirculation (Kajekar et al., 1995; Koller et al., 1998; Shastry et al., 1998), we tested whether prostaglandins and NO are involved in PIV, and specifically, whether this NO is of neuronal origin (Holzer et al., 1995).
Indomethacin is widely used at doses up to 5 mg kg−1 in order to test the role of prostaglandins in different processes and organs (Fabricio et al., 1998; Kato et al., 1997; Meadow et al., 1994; Tamori et al., 1998). In protocol 6 of the present study, cyclo-oxygenase inhibition via indomethacin (5 mg kg−1) altered PIV. Holzer et al. (1995) reported that indomethacin depressed sodium nitroprusside-induced hyperemia by 65% without altering baseline blood flow in rat skin. These authors suggested that the neurogenic hyperemia induced by administration of NO donors involves the formation of prostaglandins which in turn cause release of the vasodilator calcitonin gene-related peptide (CGRP) from perivascular afferent nerve fibres. Taken together with the results of the present study, this implies that prostaglandins may act as a co-mediator of PIV. Prostaglandins may also have an important role in mediating bradykinin-induced hyperalgesia to mechanical stimulation. Rueff & Dray (1993) showed that certain prostaglandins, as well as bradykinin and cyclic AMP enhance the responsiveness of peripheral sensory neurones to exogenous noxious and non-noxious stimuli.
On the basis of these observations, we also hypothesized that NO, implicated in the regulation of various vascular functions (Moncada et al., 1991), may be responsible for neurogenic vasodilation in skin during PIV in rats. NO is synthesized from L-arginine by NO-synthase (NOS). Two types of constitutive NOS have been identified, endothelial and neuronal (Bredt & Snyder, 1990). Non-specific inhibition of NOS with L-NNA allows for the study of involvement of NO in vasodilation (Preckel et al., 1996; Tabrizi-Fard & Fung, 1996; Taylor & Bishop, 1993). The intravenous administration of L-NNA at a dose of 20 mg kg−1 presents a mean residence time (biological average half-lives of 12 min) in plasma that suited in our experiment (Tabrizi-Fard & Fung, 1996). The total abolition of PIV following L-NNA administration in protocol 7 of the present study leads us to conclude that the NO pathway is essential for PIV. Protocol 8 was performed using 7-NI to determine whether NO release during PIV resulted from the neuronal NOS activation. Kajekar et al. (1995) showed a dose dependent inhibition with a significant effect on rat paw skin oedema being observed at a systemic dose of 10 mg kg−1. Although the dose we used (50 mg kg−1) was five times higher than theirs, PIV was diminished, but not totally abolished following 7-NI administration. This strongly suggests that neuronal NOS is partially involved in PIV in rats. Overall, our results are consistent with the idea that NO plays a major role in PIV.
These latter results are consistent with previous evidences which show that both prostaglandins and NO are co-released following stimulation by acetylcholine, ATP or bradykinins (Warren et al., 1994). Our data are also in accordance with Mehri et al. (1998) who concluded that NO (among other mediators) participates in neurogenic vasodilation in rat skin microvasculature.
In summary, we report here that cutaneous pressure-induced vasodilation (PIV) is a phenomenon mediated by capsaicin-sensitive fibres in rats, as it is in humans. PIV was completely inhibited following CGRP antagonism, whereas the response remained unchanged following NK1, NK2 or NK3 receptor antagonism. Moreover, the total disappearance of cutaneous PIV was observed when the non specific NOS inhibitor was administered, whereas inhibition of prostaglandins or specific neuronal NOS only alters the normal response. Our conclusions regarding the mechanisms of cutaneous PIV are summarized schematically in Figure 7. Our data indicate that CGRP receptor plays a major role, whereas the neurokinin receptors do not have a role in the cutaneous vasodilator response to local externally applied pressure. PIV involves a contribution from prostaglandins and is dependent on endothelial NO, whereas neuronal release of NO is only partially involved.
Figure 7.
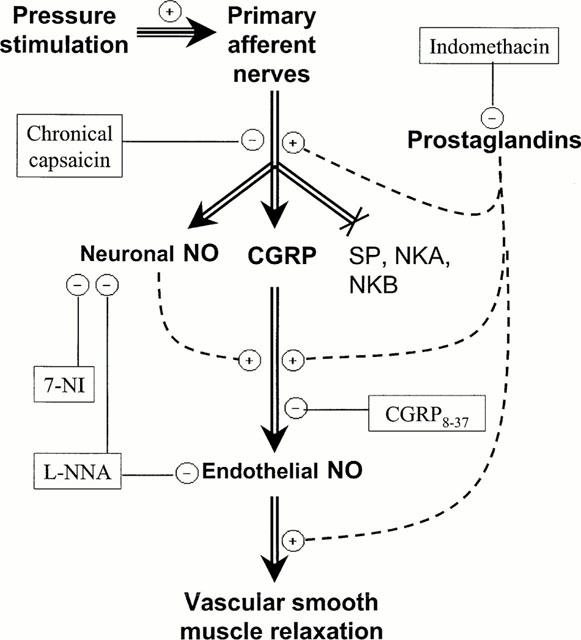
Schematic representation of the suggested mechanisms of vasodilation in response to local applied pressure. Our results show that the local pressure stimulus leads to a release of CGRP and neuronal NO by the primary afferent nerves. CGRP then induces the release of NO by the endothelium and subsequent relaxation of vascular smooth muscle. The results suggest that prostaglandins are involved in PIV. They may act at different levels. Neuronal NO participates to this reflex probably through the release of endothelial NO due to CGRP. Neurokinins do not appear to have any role in this reflex. The level at which each antagonist functions is indicated. Antagonists are represented in oval, negative signs represent inhibitory effects and positive signs represent stimulatory effects on the pathway.
Acknowledgments
The authors gratefully acknowledge Mr Michel Bedouet for technical assistance and Dr Nisha Charkoudian for reviewing the English of the manuscript. The authors also want to thank SANOFI (France) for the gracious gift of the NK1, NK2 and NK3 receptor antagonists.
Abbreviations
- a.u.
arbitrary units
- CGRP
calcitonin gene-related peptide
- DMSO
dimethyl sulphoxide
- LDF
laser Doppler flow
- L-NNA
NG-nitro-L-arginine
- 7-NI
7-nitroindazole
- NK1
neurokinin-1
- NK2
neurokinin-2
- NK3
neurokinin-3
- NKA
neurokinin A
- NKB
neurokinin B
- NO
nitric oxide
- NOS
nitric oxide synthase
- PIV
pressure-induced vasodilation
- SP
substance P
References
- ADVENIER C., ROUISSI N., NGUYEN Q.T., EMONDS-ALT X., BRELIERE J.C., NELIAT G., NALINE E, REGOLI D. Neurokinin A (NK2) receptor revisited with SR 48968, a potent non-peptide antagonist. Biochem. Biophys. Res. Commun. 1992;184:1418–1424. doi: 10.1016/s0006-291x(05)80041-5. [DOI] [PubMed] [Google Scholar]
- AMANN R., SCHULIGOI R., HOLZER P, DONNERER J. The non-peptide NK1 receptor antagonist SR140333 produces long-lasting inhibition of neurogenic inflammation, but does not influence acute chemo- or thermonociception in rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:201–205. doi: 10.1007/BF00176775. [DOI] [PubMed] [Google Scholar]
- BABBEDGE R.C., BLAND WARD P.A., HART S.L, MOORE P.K. Inhibition of rat cerebellar nitric oxide synthase by 7-nitro indazole and related substituted indazoles. Br. J. Pharmacol. 1993;110:225–228. doi: 10.1111/j.1476-5381.1993.tb13796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAIN S.D., WILLIAMS T.J., TIPPINS J.R., MORRIS H.R, MACINTYRE I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- BREDT D.S, SNYDER S.H. Isolation of nitric oxide synthase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. U.S.A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIBA T., YAMAGUCHI A., YAMATANI T., NAKAMURA A., MORISHITA T., INUI T., FUKASE M., NODA T, FUJITA T. Calcitonin gene-related peptide receptor antagonist human CGRP-(8-37) Am. J. Physiol. 1989;256:E331–E335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- DELAY-GOYET P., SATOH H, LUNDBERG J.M. Relative involvement of substance P and CGRP mechanisms in antidromic vasodilation in the rat skin. Acta Physiol. Scand. 1992;146:537–538. doi: 10.1111/j.1748-1716.1992.tb09460.x. [DOI] [PubMed] [Google Scholar]
- DONOSO M.V., FOURNIER A., ST PIERRE S, HUIDOBRO TORO J.P. Pharmacological characterization of CGRP1 receptor subtype in the vascular system of the rat: studies with hCGRP fragments and analogs. Peptides. 1990;11:885–889. doi: 10.1016/0196-9781(90)90003-n. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., BICHON D., DUCOUX J.P., HEAULME M., MILOUX B., PONCELET M., PROIETTO V., VAN BROECK D., VILAIN P., NELIAT G., SOUBRIE P., LE FUR G, BRELIERE J.C. SR 142801, the first potent non-peptide antagonist of the tachykinin NK3 receptor. Life Sci. 1995;56:PL27–PL32. doi: 10.1016/0024-3205(94)00413-m. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., DOUTREMEPUICH J.D., HEAULME M., NELIAT G., SANTUCCI V., STEINBERG R., VILAIN P., BICHON D., DUCOUX J.P., PROIETTO V., VAN BROECK D., SOUBRIÉ P., LE FUR G, BRELIÈRE J.C. In vitro and in vivo biological activities of SR140333, a novel potent non-peptide tachykinin NK1 receptor antagonist. Eur. J. Pharmacol. 1993a;250:403–413. doi: 10.1016/0014-2999(93)90027-f. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., GOLLIOT F., POINTEAU P., LE FUR G, BRELIERE J.C. Characterization of the binding sites of [3H]SR 48968, a potent nonpeptide radioligand antagonist of the neurokinin-2 receptor. Biochem. Biophys. Res. Commun. 1993b;191:1172–1177. doi: 10.1006/bbrc.1993.1340. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., VILAIN P., GOULAOUIC P., PROIETTO V., VAN BROECK D., ADVENIER C., NALINE E., NELIAT G., LE FUR G, BRELIÈRE J.C. A potent and selective non-peptide antagonist of the neurokinin A (NK2) receptor. Life Sci. 1992;50:PL101–PL106. doi: 10.1016/0024-3205(92)90352-p. [DOI] [PubMed] [Google Scholar]
- ESCOTT K.J, BRAIN S.D. Effect of a calcitonin gene-related peptide antagonist (CGRP8-37) on skin vasodilatation and oedema induced by stimulation of the rat saphenous nerve. Br. J. Pharmacol. 1993;110:772–776. doi: 10.1111/j.1476-5381.1993.tb13878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FABRICIO A.S., SILVA C.A., RAE G.A., D'ORLÉANS JUSTE P, SOUZA G.E. Essential role for endothelin ET(B) receptors in fever induced by LPS (E. coli) in rats. Br. J. Pharmacol. 1998;125:542–548. doi: 10.1038/sj.bjp.0702075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEETWOOD-WALKER S.M., MITCHELL R., HOPE P.J., EL YASSIR N., MOLONY V, BLADON C.M. The involvement of neurokinin receptor subtypes in somatosensory processing in the superficial dorsal horn of the cat [published erratum appears in Brain Res 1992 May 8;579(2):357] Brain Res. 1990;519:169–182. doi: 10.1016/0006-8993(90)90075-m. [DOI] [PubMed] [Google Scholar]
- FRIESE N., DIOP L., CHEVALIER E., ANGEL F., RIVIÈRE P, DAHL S.G. Involvement of prostaglandins and CGRP-dependent sensory afferents in peritoneal irritation-induced visceral pain. Regul. Pept. 1997;70:1–7. doi: 10.1016/s0167-0115(97)02141-1. [DOI] [PubMed] [Google Scholar]
- FROMY B., ABRAHAM P, SAUMET J.L. Non-nociceptive capsaicin-sensitive nerve terminal stimulation allows for an original vasodilatory reflex in the human skin. Brain Res. 1998;811:166–168. doi: 10.1016/s0006-8993(98)00973-1. [DOI] [PubMed] [Google Scholar]
- FROMY B., ABRAHAM P, SAUMET J.L. Progressive calibrated pressure device to measure cutaneous blood flow changes to external pressure strain. Brain Res. Protocols. 2000;5:198–203. doi: 10.1016/s1385-299x(00)00013-1. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F, ZAWADZKI J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GARELL P.C., MCGILLIS S.L, GREENSPAN J.D. Mechanical response properties of nociceptors innervating feline hairy skin. J. Neurophysiol. 1996;75:1177–1189. doi: 10.1152/jn.1996.75.3.1177. [DOI] [PubMed] [Google Scholar]
- GEE M.D., LYNN B, COTSELL B. The relationship between cutaneous C fibre type and antidromic vasodilatation in the rabbit and the rat. J. Physiol. 1997;503:31–44. doi: 10.1111/j.1469-7793.1997.031bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBINS I.L., FURNESS J.B., COSTA M., MACINTYRE I., HILLYARD C.J, GIRGIS S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurones of guinea pigs. Neurosci. Lett. 1985;57:125–130. doi: 10.1016/0304-3940(85)90050-3. [DOI] [PubMed] [Google Scholar]
- GRIENDLING K.K, ALEXANDER R.W. Endothelial control of the cardiovascular system: recent advances. FASEB J. 1996;10:283–292. doi: 10.1096/fasebj.10.2.8641561. [DOI] [PubMed] [Google Scholar]
- HÄBLER H.J., TIMMERMANN L., STEGMANN J.U, JÄNIG W. Involvement of neurokinins in antidromic vasodilatation in hairy and hairless skin of the rat hindlimb. Neuroscience. 1999;89:1259–1268. doi: 10.1016/s0306-4522(98)00322-4. [DOI] [PubMed] [Google Scholar]
- HANDWERKER H.O, KOBAL G. Psychophysiology of experimentally induced pain. Physiol. Rev. 1993;73:639–671. doi: 10.1152/physrev.1993.73.3.639. [DOI] [PubMed] [Google Scholar]
- HERBERT J.M, BERNAT A. Effect of SR 140333, a selective NK1 antagonist, on antigen-induced oedema formation in rat skin. J. Lipid. Mediat. Cell Signal. 1996;13:223–232. doi: 10.1016/0929-7855(95)00052-6. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- HOLZER P, JOCIC M. Cutaneous vasodilatation induced by nitric oxide-evoked stimulation of afferent nerves in the rat. Br. J. Pharmacol. 1994;112:1181–1187. doi: 10.1111/j.1476-5381.1994.tb13208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER P., JOCIC M, PESKAR B.A. Mediation by prostaglandins of the nitric oxide-induced neurogenic vasodilation in rat skin. Br. J. Pharmacol. 1995;116:2365–2370. doi: 10.1111/j.1476-5381.1995.tb15081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER P., LIPPE I.I., RAYBOULD H.E., PABST M.A., LIVINGSTON E.H., AMANN R., PESKAR B.M., PESKAR B.A., TACHÉ Y, GUTH P.H. Role of peptidergic sensory neurons in gastric mucosal blood flow and protection. Ann. N.Y. Acad. Sci. 1991;632:272–282. doi: 10.1111/j.1749-6632.1991.tb33115.x. [DOI] [PubMed] [Google Scholar]
- HU J.W, SESSLE B.J. Properties of functionally identified nociceptive and nonnociceptive facial primary afferents and presynaptic excitability changes induced in their brain stem endings by raphe and orofacial stimuli in cats. Exp. Neurol. 1988;101:385–399. doi: 10.1016/0014-4886(88)90050-7. [DOI] [PubMed] [Google Scholar]
- HUGHES S.R, BRAIN S.D. A calcitonin gene-related peptide (CGRP) antagonist (CGRP8-37) inhibits microvascular responses induced by CGRP and capsaicin in skin. Br. J. Pharmacol. 1991;104:738–742. doi: 10.1111/j.1476-5381.1991.tb12497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES S.R., BUCKLEY T.L, BRAIN S.D. Olvanil: more potent than capsaicin at stimulating the efferent function of sensory nerves. Eur. J. Pharmacol. 1992;219:481–484. doi: 10.1016/0014-2999(92)90494-o. [DOI] [PubMed] [Google Scholar]
- JANCSO G., KIRALY E, JANCSO-GABOR A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- JANCSO N., JANCSO-GABOR A, SZOLCSANYI J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br. J. Pharmacol. 1967;31:138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANCSO N., JANCSO-GABOR A, SZOLCSANYI J. The role of sensory nerve endings in neurogenic inflammation induced in human skin and in the eye and paw of the rat. Br. J. Pharmacol. 1968;32:32–41. doi: 10.1111/j.1476-5381.1968.tb00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON J.M. Nonthermoregulatory control of human skin blood flow. J. Appl. Physiol. 1986;61:1613–1622. doi: 10.1152/jappl.1986.61.5.1613. [DOI] [PubMed] [Google Scholar]
- JUNG M., CALASSI R., MARUANI J., BARNOUIN M.C., SOUILHAC J., PONCELET M., GUEUDET C., EMONDS-ALT X., SOUBRIÉ P., BRELIÈRE J.C, LE FUR G. Neuropharmacological characterization of SR 140333, a non peptide antagonist of NK1 receptors. Neuropharmacology. 1994;33:167–179. doi: 10.1016/0028-3908(94)90004-3. [DOI] [PubMed] [Google Scholar]
- KAJEKAR R., MOORE P.K, BRAIN S.D. Essential role for nitric oxide in neurogenic inflammation in rat cutaneous microcirculation: evidence for an endothelium-independent mechanism. Circ. Res. 1995;76:441–447. doi: 10.1161/01.res.76.3.441. [DOI] [PubMed] [Google Scholar]
- KATO K., YANG H, TACHÉ Y. Role of prostaglandins and calcitonin gene-related peptide in central vagal cholinergic-dependent protection against gastric injury in urethane-anesthetized rats. Digestion. 1996;57:322–327. doi: 10.1159/000201352. [DOI] [PubMed] [Google Scholar]
- KATO S., HIRATA T, TAKEUCHI K. Nitric oxide, prostaglandin, and sensory neurons in gastric mucosal blood flow response during acid secretion in rats. Gen. Pharmacol. 1997;28:513–519. doi: 10.1016/s0306-3623(96)00350-3. [DOI] [PubMed] [Google Scholar]
- KAWASAKI H., TAKASAKI K., SAITO A, GOTO K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- KOLLER A., DÖRNYEI G, KALEY G. Flow-induced responses in skeletal muscle venules: modulation by nitric oxide and prostaglandins. Am. J. Physiol. 1998;275:H831–H836. doi: 10.1152/ajpheart.1998.275.3.H831. [DOI] [PubMed] [Google Scholar]
- KOLTZENBURG M, HANDWERKER H.O. Differential ability of human cutaneous nociceptors to signal mechanical pain and to produce vasodilatation. J. Neurosci. 1994;14:1756–1765. doi: 10.1523/JNEUROSCI.14-03-01756.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI H.S, ZHAO Z.Q. Small sensory neurones in the rat dorsal root ganglia express functional NK-1 tachykinin receptor. Eur. J. Neurosci. 1998;10:1292–1299. doi: 10.1046/j.1460-9568.1998.00140.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol. Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- LYNN B. The fibre composition of cutaneous nerves and the classification and response properties of cutaneous afferents, with particular reference to nociception. Pain Rev. 1994;1:172–183. [Google Scholar]
- LYNN B., SCHUTTERLE S, PIERAU F.K. The vasodilator component of neurogenic inflammation is caused by a special subclass of heat-sensitive nociceptors in the skin of the pig. J. Physiol. 1996;494:587–593. doi: 10.1113/jphysiol.1996.sp021516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGI C.A. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog. Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A, MELI A. The sensory-efferent function of capsaicin-sensitive sensory neurones. Gen. Pharmacol. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., PATACCHINI R., GIULIANI S, GIACHETTI A. In vivo and in vitro pharmacology of SR 48,968, a non-peptide tachykinin NK2 receptor antagonist. Eur. J. Pharmacol. 1993;234:83–90. doi: 10.1016/0014-2999(93)90709-q. [DOI] [PubMed] [Google Scholar]
- MEADOW W., RUDINSKY B., BELL A., LOZON M., RANDLE C, HIPPS R. The role of prostaglandins and endothelium-derived relaxation factor in the regulation of cerebral blood flow and cerebral oxygen utilization in the piglet: operationalizing the concept of an essential circulation. Pediatr. Res. 1994;35:649–656. doi: 10.1203/00006450-199406000-00006. [DOI] [PubMed] [Google Scholar]
- MEHRI M., DUSTING G.J, KHALIL Z. CGRP and nitric oxide of neuronal origin and their involvement in neurogenic vasodilatation in rat skin microvasculature. Br. J. Pharmacol. 1998;123:863–868. doi: 10.1038/sj.bjp.0701696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHAUD J.C., ALONSO R., GUEUDET C., FOURNIER M., CALASSI R., BRELIÈRE J.C., LE FUR G, SOUBRIÉ P. Effects of SR140333, a selective non-peptide NK1 receptor antagonist, on trigemino-thalamic nociceptive pathways in the rat. Fundam. Clin. Pharmacol. 1998;12:88–94. doi: 10.1111/j.1472-8206.1998.tb00929.x. [DOI] [PubMed] [Google Scholar]
- MIZUTA A., TAKANO Y., HONDA K., SAITO R., MATSUMOTO T, KAMIYA H. Nitric oxide is a mediator of tachykinin NK3 receptor-induced relaxation in rat mesenteric artery. Br. J. Pharmacol. 1995;116:2919–2922. doi: 10.1111/j.1476-5381.1995.tb15945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONCADA S, HIGGS A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J, HIGGS E.A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem. Pharmacol. 1989;38:1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J, HIGGS E.A. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MOORE P.K., BABBEDGE R.C., WALLACE P., GAFFEN Z.A, HART S.L. 7-Nitro indazole, an inhibitor of nitric oxide synthase, exhibits anti-nociceptive activity in the mouse without increasing blood pressure. Br. J. Pharmacol. 1993;108:296–297. doi: 10.1111/j.1476-5381.1993.tb12798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGY J.I, VAN DER KOOY D. Effects of neonatal capsaicin treatment on nociceptive thresholds in the rat. J. Neurosci. 1983;3:1145–1150. doi: 10.1523/JNEUROSCI.03-06-01145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGY J.I., HUNT S.P., IVERSEN L.L, EMSON P.C. Biochemical and anatomical observations on the degeneration of peptide-containing primary afferent neurones after neonatal capsaicin. Neuroscience. 1981;6:1923–1934. doi: 10.1016/0306-4522(81)90032-4. [DOI] [PubMed] [Google Scholar]
- OTSUKA M, YOSHIOKA K. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- PALMER R.M.J., FERRIGE A.G, MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PATACCHINI R., BARTHÒ L., HOLZER P, MAGGI C.A. Activity of SR142801 at peripheral tachykinin receptors. Eur. J. Pharmacol. 1995;278:17–25. doi: 10.1016/0014-2999(95)00090-8. [DOI] [PubMed] [Google Scholar]
- PRECKEL M.P., LEFTHERIOTIS G., FERBER C., DEGOUTE C.S., BANSSILLON V, SAUMET J.L. Effect of nitric oxide blockade on the lower limit of the cortical cerebral autoregulation in pentobarbital-anaesthetized rats. Int. J. Microcirc. Clin. Exp. 1996;16:277–283. doi: 10.1159/000179186. [DOI] [PubMed] [Google Scholar]
- RAJFER J., ARONSON W.J., BUSH P.A., DOREY F.J, IGNARRO L.J. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N. Engl. J. Med. 1992;326:90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- RINDER J, LUNDBERG J.M. Effects of hCGRP 8-37 and the NK1-receptor antagonist SR 140.333 on capsaicin-evoked vasodilation in the pig nasal mucosa in vivo. Acta Physiol. Scand. 1996;156:115–122. doi: 10.1046/j.1365-201X.1996.431164000.x. [DOI] [PubMed] [Google Scholar]
- ROSSI R, JOHANSSON O. Cutaneous innervation and the role of neuronal peptides in cutaneous inflammation: a minireview. Eur. J. Dermatol. 1998;8:299–306. [PubMed] [Google Scholar]
- ROWELL L.B., WYSS C.R, BRENGELMAN G.L. Sustained human skin and muscle vasoconstriction with reduced baroreceptor activity. J. Appl. Physiol. 1973;34:639–643. doi: 10.1152/jappl.1973.34.5.639. [DOI] [PubMed] [Google Scholar]
- RUEFF A, DRAY A. Sensitization of peripheral afferent fibres in the in vitro neonatal rat spinal cord-tail by bradykinin and prostaglandins. Neuroscience. 1993;54:527–535. doi: 10.1016/0306-4522(93)90272-h. [DOI] [PubMed] [Google Scholar]
- SANTUCCI V., GUEUDET C., EDMONDS-ALT X., BRELIÈRE J.C., SOUBRIÉ P, LE FUR G. The NK2 receptor antagonist SR48968 inhibits thalamic responses evoked by thermal but not mechanical nociception. Eur. J. Pharmacol. 1993;237:143–146. doi: 10.1016/0014-2999(93)90104-p. [DOI] [PubMed] [Google Scholar]
- SAUMET J.L, DUCLAUX R. Analgesia induced by neonatal capsaicin treatment in rats. Pharmacol. Biochem. Behav. 1982;16:241–243. doi: 10.1016/0091-3057(82)90155-1. [DOI] [PubMed] [Google Scholar]
- SAUMET J.L., DITTMAR A, LEFTHERIOTIS G. Non-invasive measurement of skin blood flow: comparison between plethysmography, laser-Doppler flowmeter and heat thermal clearance method. Int. J. Microcirc. Clin. Exp. 1986;5:73–83. [PubMed] [Google Scholar]
- SAUMET J.L., KELLOGG D.L., TAYLOR W.F, JOHNSON J.M. Cutaneous laser-Doppler flowmetry: influence of underlying muscle blood flow. J. Appl. Physiol. 1988;65:478–481. doi: 10.1152/jappl.1988.65.1.478. [DOI] [PubMed] [Google Scholar]
- SCHOLZEN T., ARMSTRONG C.A., BUNNETT N.W., LUGER T.A., OLERUD J.E, ANSEL J.C. Neuropeptides in the skin: interactions between the neuroendocrine and the skin immune systems. Exp. Dermatol. 1998;7:81–96. doi: 10.1111/j.1600-0625.1998.tb00307.x. [DOI] [PubMed] [Google Scholar]
- SCHUBERT V, FAGRELL B. Local skin pressure and its effects on skin microcirculation as evaluated by laser Doppler fluxmetry. Clin. Physiol. 1989;9:535–545. doi: 10.1111/j.1475-097x.1989.tb01007.x. [DOI] [PubMed] [Google Scholar]
- SCHUBERT V, FAGRELL B. Postocclusive reactive hyperemia and thermal response in the skin microcirculation of subjects with spinal cord injury. Scand. J. Rehabil. Med. 1991;23:33–40. [PubMed] [Google Scholar]
- SCHUBERT V. , PERBECK L, SCHUBERT P.A. Skin microcirculatory and thermal changes in elderly subjects with early stage of pressure sores. Clin. Physiol. 1994;14:1–13. doi: 10.1111/j.1475-097x.1994.tb00484.x. [DOI] [PubMed] [Google Scholar]
- SHASTRY S., DIETZ N.M., HALLIWILL J.R., REED A.S, JOYNER M.J. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J. Appl. Physiol. 1998;84:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- TABRIZI-FARD M.A, FUNG H.L. Pharmacokinetics and steady-state tissue distribution of L- and D-isomers of nitroarginine in rats. Drug Metab. Dispos. 1996;24:1241–1246. [PubMed] [Google Scholar]
- TAMORI K., YONEDA M., NAKAMURA K, MAKINO I. Effect of intracisternal thyrotropin-releasing hormone on hepatic blood flow in rats. Am. J. Physiol. 1998;274:G277–G282. doi: 10.1152/ajpgi.1998.274.2.G277. [DOI] [PubMed] [Google Scholar]
- TAYLOR W.F, BISHOP V.S. A role for nitric oxide in active thermoregulatory vasodilation. Am. J. Physiol. 1993;264:H1355–H1359. doi: 10.1152/ajpheart.1993.264.5.H1355. [DOI] [PubMed] [Google Scholar]
- TODA N, OKAMURA T. Role of nitric oxide in neurally induced cerebroarterial relaxation. J. Pharmacol. Exp. Ther. 1991;258:1027–1032. [PubMed] [Google Scholar]
- VARGAS H.M., CUEVAS J.M., IGNARRO L.J, CHAUDHURI G. Comparison of the inhibitory potencies of N(G)-methyl-, N(G)-nitro-, N(G)-amino-L-arginine on EDRF function in rats: Evidence for continuous basal EDRF release. J. Pharmacol. Exp. Ther. 1991;257:1208–1215. [PubMed] [Google Scholar]
- WARREN J.B., LOI R.K, WILSON A.J. PGD2 is an intermediate in agonist-stimulated nitric oxide release in rabbit skin microcirculation. Am. J. Physiol. 1994;266:H1846–H1853. doi: 10.1152/ajpheart.1994.266.5.H1846. [DOI] [PubMed] [Google Scholar]
- WHITTLE B.J., HIGGS G.A., EAKIN K.E., MONCADA S, VANE J.R. Selective inhibition of prostaglandin production in inflammatory exudates and gastric mucosa. Nature. 1980;284:271–273. doi: 10.1038/284271a0. [DOI] [PubMed] [Google Scholar]
- WILLIAMS T.J, PECK M.J. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977;270:530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA M, OTSUKA M. Pharmacological profile of a tachykinin antagonist, spantide, as examined on rat spinal motoneurones. Br. J. Pharmacol. 1990;100:711–716. doi: 10.1111/j.1476-5381.1990.tb14080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAIDI M., BEVIS P.J., GIRGIS S.I., LYNCH C., STEVENSON J.C, MACINTYRE I. Circulating CGRP comes from the perivascular nerves. Eur. J. Pharmacol. 1985;117:283–284. doi: 10.1016/0014-2999(85)90616-8. [DOI] [PubMed] [Google Scholar]


