Abstract
A potent and highly selective DP prostanoid receptor antagonist radioligand, [3H]-cyclohexyl-N-BWA868C (3-benzyl-5-(6-carboxyhexyl)-1-(2-cyclohexyl-2-hydroxyethyl-amino) hydantoin, ([3H]-BWA868C)), has been generated for receptor binding and autoradiographic studies.
Specific [3H]-BWA868C binding to human platelet membranes achieved equilibrium within 60 min at 23°C and constituted up to 95% of the total binding. The association (K+1) and dissociation (K−1) rate constants of binding were 0.758±0.064 min−1, mmol and 0.0042±0.0002 min−1, respectively, yielding dissociation constants (KDs) of 5.66±0.44 nM (n=4).
Specific [3H]-BWA868C bound to DP receptors with a high affinity (KD=1.45±0.01 nM, n=3) and to a finite, saturable number of binding sites (Bmax=21.1±0.6 nmol g−1 wet weight).
DP receptor class prostanoids (e.g. ZK118182, BW245C, BWA868C, PGD2) exhibited high (nanomolar) affinities for [3H]-BWA868C binding, while prostanoids selective for EP, FP, IP and TP receptors showed a low (micromolar) affinity.
Specific DP receptor binding sites were autoradiographically localized on the ciliary epithelium/process, longitudinal and circular ciliary muscles, retinal choroid and iris in human eye sections using [3H]-BWA868C. While [3H]-PGD2 yielded similar quantitative distribution of DP receptors as [3H]-BWA868C, the level of non-specific binding observed with [3H]-PGD2 was significantly greater than that observed with [3H]-BWA868C.
It is concluded that [3H]-BWA868C is a high-affinity and very specific DP receptor radioligand capable of selectively labelling the DP receptor. [3H]-BWA868C may prove useful for future homogenate-based and autoradiographic studies on the DP receptor.
Keywords: [3H]-BWA868C, DP receptor, prostaglandins, PGD2, DP antagonist, human platelets, receptor autoradiography
Introduction
Prostaglandins (PGs) such as PGD2, PGE2, PGF2α, PGI2 represent the major endogenously generated prostanoids from arachidonic acid which have many different functions in the mammalian body (Bergstrom & Sjovall, 1957; Anderson & Ramwell, 1974). PGD2 is generated in the skin, lung, brain, mast cells and other tissues and has been implicated in the mediation/regulation of sleep, hormone secretion, body temperature, pain and intraocular pressure (IOP) amongst other functions (Giles & Leff, 1988; Coleman et al., 1990; 1994 for reviews). Thus, PGD2 can cause bronchoconstriction and allergic rhinitis (Anderson & Ramwell, 1974; Narumiya, 1994; Coleman et al., 1994) and has been shown to cause the inhibition of platelet aggregation (Narumiya, 1994; Coleman et al., 1990; 1994). Furthermore, DP receptor stimulation has shown potential therapeutic utility in lowering IOP as a treatment for glaucoma and ocular hypertension (Matsugi et al., 1995).
PGD2 interacts with receptors which are coupled, via a Gs-protein, to adenylyl cyclase and initiates the production of cyclic AMP (Trist et al., 1989; Crider et al., 1998; 1999). Based on the current nomenclature and receptor classification scheme for the prostanoid receptors (Coleman et al., 1994; Alexander & Peters, 1999), the PGD2-sensitive receptor selectively activated by prostanoids such as BW245C and ZK118041 and inhibited by BWA868C is known as the DP receptor. The molecular cloning of human and mouse DP receptors has recently been accomplished (Boie et al., 1995; Kiriyama et al., 1998) and these recombinant receptors shown to be members of the super-family of 7-transmembrane domain G-protein-coupled receptors (see Narumiya et al., 1993; Abramovitz et al., 1995 for review). The cloned receptors have been subsequently expressed in host cells and some pharmacological characterization described (Boie et al., 1995; Kiriyama et al., 1997; Wright et al., 1998).
Functionally coupled DP receptors in animal and human tissues/cells have been studied pharmacologically using selective and potent agonist prostanoids such as BW245C (Giles et al., 1989; Leff & Giles, 1992), ZK110841 and ZK118182 (Thierauch et al., 1988), SQ27986 (see Coleman et al., 1990; 1994), and a potent and a DP receptor-selective antagonist, BWA868C (Giles et al., 1989; Trist et al., 1989; Leff & Giles, 1992). However, there has been a paucity of suitable, stable, selective and high-affinity radioligands of sufficiently high specific activity for the characterization of the ligand binding site of the DP receptor. To the best of our knowledge, [3H]-PGD2 is the only commercially available radioligand available for conducting ligand binding studies on DP prostanoid receptors (Cooper & Ahern, 1979; Shimizu et al., 1982; Town et al., 1983). However, since PGD2 itself is a fairly non-selective agent possessing affinity and functional activity at other prostanoid receptors, notably at FP and TP receptors (see Coleman et al., 1994; Griffin et al., 1997; 1998; Sharif et al., 1998), and also since it is an agonist of rather low affinity (dissociation constant, KD=20–53 nM; Morii & Watanabe, 1991; Shimizu et al., 1982; Thierauch et al., 1988), yields low signal-to-noise and is metabolically unstable, [3H]-PGD2 is not an ideal radioligand for radioreceptor binding to tissue homogenates or for autoradiographic studies. In order to rectify this deficit in the prostanoid field, we decided to explore the possibility of generating a radioligand from BWA868C, (3-benzyl-5-(6-carboxyhexyl)-1-(2-cyclohexyl-2-hydroxyethyl-amino) hydantoin) (Figure 1), a functionally-defined DP-antagonist (Giles et al., 1989; Senior et al., 1993; Fernandes & Crankshaw, 1995; Crider et al., 1999). Therefore, the aims of our present studies were 4 fold: firstly, to determine the receptor binding affinity and selectivity of BWA868C using ligand binding experiments and thus verify its affinity and selectivity previously described in the literature using mostly isolated tissue functional assays; secondly, based on the DP-affinity/selectivity proceed to synthesize a suitable precursor to generate [3H]-BWA868C of acceptable specific activity; thirdly, to determine the biochemical and pharmacological characteristics of the DP receptor binding sites on human platelets using this novel radioligand; fourthly, to employ [3H]-BWA868C as a probe to autoradiographically localize and quantify DP receptors in human donor eye sections.
Figure 1.
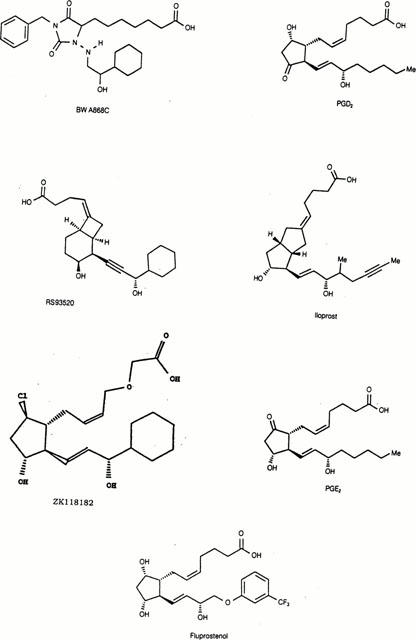
Structures of BWA868C and some other compounds used in the current studies.
Methods
[3H]-PGD2 binding assay
A DP receptor binding assay using [3H]-PGD2 and human platelets was first established for determining the affinity of some key reference prostanoids, including BWA868C. Briefly, assays were conducted in 25 mM Tris.HCl (pH 7.4) containing (in mM) NaCl 138, MgCl2 5, and EDTA 1. Frozen-thawed expired human blood platelets (40–60 mg ml−1 stock) were incubated with 2–10 nM [3H]-PGD2 in the absence and presence of 10 μM BWA868C or 10 μM unlabelled PGD2 to define total and non-specific binding, respectively, in a total volume of 500 μl. The incubations (20 min at 23°C; Cooper & Ahern, 1979; Shimizu et al., 1982) were terminated by rapid vacuum filtration (using Whatman GF/B glass fibre filters previously soaked in 1% polyethyleneimine (PEI) and 0.1% BSA), the receptor-bound radioactivity determined by scintillation spectrometry, and the data analysed by non-linear, iterative curve-fitting programs (Bowen & Jerman, 1995; Sharif et al., 1996; 1999).
[3H]-BWA868C binding assay
Tissue linearity studies, kinetic (associations over 5–180 min/dissociations over 10–360 min), saturation and competition (using 10 competitor concentrations) assays were performed with 5 nM [3H]-BWA868C in order to help determine the appropriate platelet concentration, equilibrium time, affinity and maximal receptor density on human platelets and the pharmacological specificity of the DP receptor on the latter cells. [3H]-BWA868C (5 nM final concentration) binding to human platelets was conducted in 25 mM Tris.HCl (pH 7.4) containing (in mM) NaCl 138, MgCl2 5, and EDTA 1. Frozen-thawed expired human blood platelets (20–80 mg ml−1 stock) were incubated with 1–38 nM [3H]-BWA868C in the absence and presence of 10 μM unlabelled PGD2 or 10 μM BWA868C to define total and non-specific binding, respectively, in a total volume of 500 μl. The incubations (various times at 23°C) were terminated by rapid vacuum filtration (using Whatman GF/B glass fibre filters previously soaked in 1% PEI and 0.1% bovine serum albumen [BSA]), and the receptor-bound radioactivity determined by beta-scintillation spectrometry.
EP1 and EP4 receptor binding assays
[3H]-PGE2 (0.3–3 nM) binding to HEK-293 cell membranes (4–8 μg protein well−1) expressing recombinant human EP1 and EP4 receptors was conducted in 10 mM 2-[N-Morpholino]ethanesulphonic acid (MES) buffer (pH 6.0) containing (in mM) EDTA 1, MnCl2 10 (pH 6.0) for 90 min at 23°C in a total volume of 500 μl. The assays were terminated and the resultant data analysed as described above and below.
EP3 receptor binding assay
The bovine corpus luteum has been shown to express high-affinity [3H]-PGE2 binding sites which appear to be of the EP3-subtype (Sharif et al., 1998). Total particulate bovine corpus luteum membranes (8–24 mg tube−1 final) were prepared by standard centrifugation (30,000× g/20 min at 4°C) procedures and incubated with [3H]-PGE2 (0.9–2 nM) in Krebs buffer (pH 7.4) for 1 h at 23°C. The non-specific binding was defined with 1 μM unlabelled PGE2. The assays were terminated by vacuum filtration (using Whatman GF/B glass fibre filters previously soaked in 0.3% PEI) and the data analysed as described above for DP assays.
FP receptor binding assay
The bovine corpus luteum has been shown to express high-affinity [3H]-PGF2α binding sites, in addition to [3H]-PGE2 binding, which appear to have pharmacological characteristics of FP receptors (Sharif et al., 1998). Total particulate bovine corpus luteum membranes (20 mg ml−1 final) were prepared as described above and were incubated with [3H]-PGF2α (0.9–1.5 nM) in Krebs buffer (pH 7.4) for 2 h at 23°C. The non-specific binding was defined with 1–10 μM unlabelled PGF2α or fluprostenol. The assays were terminated by vacuum filtration (using Whatman GF/B glass fibre filters previously soaked in 0.3% PEI) and the data analysed as described above for DP assays.
IP and TP receptor binding assays
Human platelets express specific IP (Armstrong et al., 1989) and TP (Ogletree & Allen, 1992) prostanoid receptors in addition to DP receptors (Cooper & Ahern, 1979; Shimizu et al., 1982). Frozen-thawed expired human platelet membranes (10 mg tube−1) dispersed in 50 mM Tris-HCl containing 10 mM MgSO4 (pH 7.4) were incubated with 1 nM [3H]-SQ29548 or 3 nM [3H]-Iloprost to label TP and IP receptors, respectively. The non-specific binding was defined with 10 μM unlabelled SQ29548 and with 10 μM iloprost for TP and IP receptors, respectively. The incubations (60 min at 23°C) were terminated by rapid vacuum filtration (using Whatman GF/B glass fibre filters previously soaked in 0.3% PEI) and the data analysed as described above for DP assays.
NovaScreen assays
Unlabelled BWA868C was evaluated over 1 nM–10 μM at NovaScreen (Hanover, MD, U.S.A.) for its ability to compete for ligand binding to 32 non-prostanoid receptor, uptake site, ion channels and enzyme binding sites in order to determine its specificity.
Autoradiographic studies
Human donor eyes received from various eye banks in ice-cold Dexol® or Optisol® corneal preservation buffer within 14–27 h of death were rapidly frozen onto microtome chucks in Tissue-Tek O.C.T plastic embedding material. The ages of the donors were 30, 59 and 75 years. Following equilibration of the frozen tissue, 20 μm sections from the eyes were cut at −17°C using a Brights freezing microtome and collected on gelatinized glass microscope slides as previously described (Sharif & Hughes, 1989; Davis & Sharif, 1999). The section-bearing slides were employed in the ligand binding experiments either on the day the sections were cut or kept frozen for up to 4 weeks at −80°C before being used. In order to conduct the receptor binding experiments on the eye sections, the slides were brought to 23°C and allowed to air-dry. The slides were laid flat on metal rods in a specially designed autoradiography acrylic box and then each section on the slide was covered with 1 ml of the different prepared solutions containing 5 nM [3H]-BWA868C in the assay buffer described for the test-tube assays above in the absence or presence of 100 μM unlabelled PGD2 to define total and non-specific binding, respectively. The same conditions were employed to study [3H]-PGD2 (5 nM) binding on human eye sections. The sections were allowed to incubate for 60 min at 23°C to achieve equilibrium after which time the solutions were poured off the slides and the slides loaded into metal carriers for washing. The sections were rinsed as follows. Glass slide trays were filled with 550 ml of ice-cold 50 mM Tris.HCl (pH 7.4) buffer and the slide-carriers placed in the trays. A series of three separate washes was then performed at 40 r.p.m. on a rotary mixer for 5 min each. Between washes, the carriers were removed from the trays while the buffer was replaced with fresh ice-cold buffer. After the last wash, the slides were drained by standing the carriers vertically on the bench. The slides were then rapidly dried in a stream of cool air. When dry by visual inspection, the carriers were placed in a vacuum dessicator overnight at room temperature. The slides were removed from the carriers and taped in a suitable arrangement, together with [3H]-Microscale® radiation standards, into X-ray film cassettes in order to image the receptor autoradiographs (Sharif, 1996). The slides were then overlaid with a sheet of radiation-sensitive film (Hyperfilm®) in total darkness and the cassettes closed. Films, were exposed to the film for up to 6 months at room temperature without intensifying screens. Individual sheets of film were then hand developed in Kodak D19® developer (3 min/17°C) and fixed in Kodafix® (3 min/17°C), washed in distilled water (7 min at 17°C) to generate the receptor images on the Hyperfilm® (Sharif, 1996). Film autoradiograms were scanned via an Agfa scanner into the Optiquant® software package of the Cyclone® phosphor-imager for quantitative image analysis. Individual tissue images were quantified and data generated as digital light units mm−2 as previously described (Davis & Sharif, 1999; Sharif et al., 1999).
Materials
[Cyclohexyl-3H (N)]BWA868C ([3H]-BWA868C; 51.5 Ci mmol−1; 1 mCi ml−1) was custom synthesized at NEN (Boston, MA, U.S.A.) by standard tritium-exchange procedures employing a suitable precursor provided by Alcon Research Ltd. [3H]-BWA868C was provided in ethanol. [3H]-PGD2 (115 Ci mmol−1), [3H]-PGE2 (171 Ci mmol−1); [3H]-PGF2α (170 Ci mmol−1), [3H]-SQ29548 (50.4 Ci mmol−1) were purchased from NEN (Boston, MA, U.S.A.); [3H]-iloprost (12.7 Ci mmol−1) was from Amersham Corp., (Arlington Heights, IL, U.S.A.). Tissue-Tek (Miles laboratories, Elkhert, CT, U.S.A.); [3H]-Microscales® and the radiation-sensitive Hyperfilm® for autoradiographic studies were purchased from Amersham Corp., (Arlington Heights, IL, U.S.A.). Cyclone® phosphor-imager and Optiquant® software package were purchased from Packard Instruments Co (Meriden, CT, U.S.A.). Kodak D19® and Kodafix® were purchased from a local photography shop. Frozen bovine corpus lutea were obtained from Pel-Freez (Rogers, AR, U.S.A.). Out-dated human platelet-enriched plasma was obtained from local blood banks. HEK-293 cell membranes expressing the human recombinant EP1 and EP4 prostanoid receptors were obtained from Receptor Biology Inc. (Beltsville, MD, U.S.A.). Standard reference prostanoids were purchased from Cayman Chemical Co. (Ann Arbor, MI, U.S.A.) or synthesized by our colleagues in the Medicinal Chemistry department. Other prostanoids were generous gifts from the following companies: ZK118182 (Schering, Berlin and Bergkamen, Germany), RS-93520 (Hoffman La-Roche, Basel, Switzerland), BWA868C (Glaxo-Wellcome, Stevenage, U.K.). All standard reagents, chemical and buffers were purchased from Sigma (St. Louis, MO, U.S.A.). Platelet enriched human plasma was kindly supplied by the Carter Blood Bank (Fort Worth, TX, U.S.A.) and was used to isolate the platelets. The chemical structures of some key prostanoids used in the current studies are as follows: ZK118182 ((5Z,13E)- (9R,11R,15S)0 -9-chloro- 15-cyclohexyl- 11,15-dihydroxy - 3 -oxa - 16, 17, 18, 19, 20 - pentanor - 5,13 - prostadienoic acid)); RS-93520 ((C3′S,1R,2R,3S,6R)-2-C3′-cyclohexyl-3′hydroxyprop-1-ynyl)-3-hydroxybicyclo[4.2.0]oct-7-ylidene) butyrate); SQ27986 ([1S-[1B,2B(5Z),3A(1E,3S),4B]]-7-[3-(3-cyclohexyl-3-hydroxy-1-propenyl)-7-oxabi-cyclo-[2.2.1]hept-2-yl]-5-heptenoic acid); BW245C (5-(6-carboxyhexyl)-1-(3-cyclohexyl-3-hydroxypropylhydantoin); cloprostenol, (16-m-chlorophenoxy tetranor prostaglandin F2α); fluprostenol, (5Z,13E)-(9S,11R,15S) - 9, 11, 15 - trihydroxy - 5, 13 - prostadienoic acid); U46619 (9,11 - di - deoxy -9α,11α-methanoepoxy prostaglandin F2α)) and BWA868C ((±) -3-benzyl-5 -(6-carboxyhexyl) -1-(2-cyclohexyl-2-hydroxyethylamino) hydantoin)).
Data analysis
The original data (d.p.m.) from saturation and competition experiments were analysed using non-linear, iterative curve-fitting computer programs (Bowen & Jerman, 1995; Sharif et al., 1996; 1998) which incorporated ‘LIGAND' (Munson & Rodbard, 1980). Kinetic data were analysed using ‘KINETIC' part of the set of computer programs as previously detailed (McPherson, 1983a,1983b). The inhibition constants (Kis) were calculated from IC50 values using the Cheng & Prusoff (1973) equation. The quantitative autoradiographic data were calculated as digital light units (mm2) (DLU mm−2) (Davis & Sharif, 1999; Sharif et al., 1999).
Results
Purity and stability of [3H]-BWA868C
[3H]-BWA868C (51.5 Ci mmol−1; 1 mCi ml−1; Lot # 2943-211) was separated by standard high pressure liquid chromatography methods and the purity of the radioligand (97.3%) was checked by thin-layer chromatography using toluene/dioxane/HOAc (20 : 10:1) on silica Gel GF at New England Nuclear (NEN) (Boston, MA, U.S.A.). The identity of the product was also confirmed by co-chromatography with a visualized authentic standard, and the structures of both the unlabelled and labelled BWA868C were confirmed by mass spectrometry at NEN (Boston, MA, U.S.A.).
Receptor binding characteristics of unlabelled BWA868C
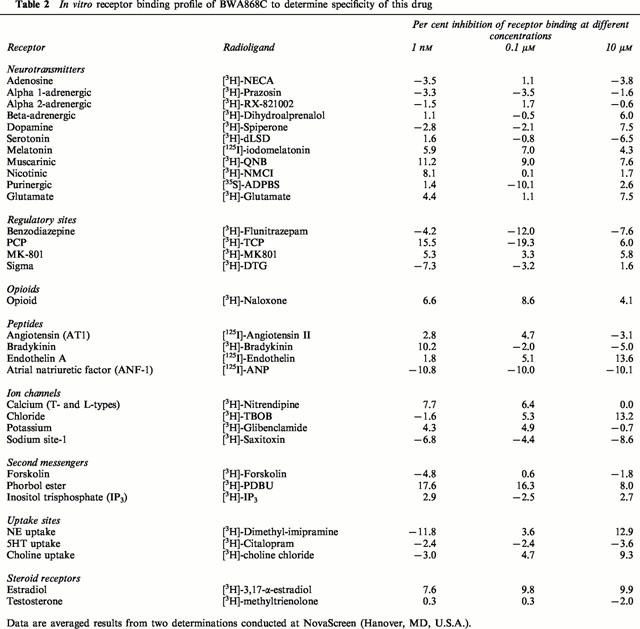
Unlabelled BWA868C was shown to have a high-affinity (Ki=22±3 nM, n=4) for [3H]-PGD2 binding to the DP receptor on human platelet membranes, but a very low affinity for the EP3, FP, IP and TP receptors (Table 1). In contrast, PGD2 exhibited a lower affinity and substantially lower selectivity than BWA868C at the prostanoid receptor subtypes (Table 1). In addition, when tested at NovaScreen (Hanover, MD, U.S.A.) at 1 nM–10 μM for its ability to interact with 32 non-prostanoid ligand binding sites (including adrenergic, serotonergic, muscarinic and dopaminergic receptors, various ion channels, uptake sites and enzyme binding sites, etc.), BWA868C showed minimal affinity for the afore-mentioned ligand binding sites (Table 2), indicating a high degree of DP-selectivity of the compound.
Table 1.
Estimated affinities of BWA868C and PGD2 for some of the major prostanoid receptors

Table 2.
In vitro receptor binding profile of BWA868C to determine specificity of this drug
Tissue linearity and kinetic characteristics of [3H]-BWA868C binding
Specific binding of [3H]-PGD2 and [3H]-BWA868C increased with increasing platelet membrane concentration (Figure 2a,b). The amount of specific [3H]-PGD2 binding was substantially less than that of [3H]-BWA868C binding (Figure 2a,b). [3H]-BWA868C binding to human platelet membranes achieved equilibrium within 60 min at 23°C; it remained stable and at a plateau for up to 3 h (Figure 3a,b), the specific binding constituting up to 95% of the total binding under these conditions. At equilibrium, [3H]-BWA868C bound to platelet membranes dissociated (t1/2 of 170 min at 23°C) from the DP receptor upon addition of 10 μM unlabelled PGD2 (Figure 4a,b). The association (K+1) and dissociation (K−1) rate constants for [3H]-BWA868C binding were 0.758±0.064 min−1, mmol (n=4) and 0.0042±0.0002 min−1 (n=4), respectively. Conversion of these data yielded dissociation constants (KDs) of 5.66±0.44 nM (n=4).
Figure 2.
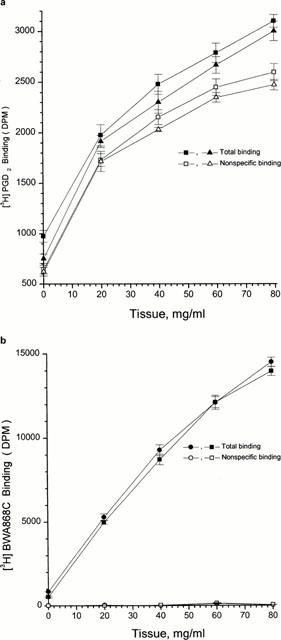
Binding of [3H]-PGD2 and [3H]-BWA868C to different concentrations of human platelet homogenates. (a) shows the total and non-specific binding of [3H]-PGD2 (10 nM) and (b) shows the total and non-specific binding of [3H]-BWA868C (5 nM). Membrane preparations from two separate platelet-enriched plasma donations were used. Experiments with both preparations and both radioligands were conducted in parallel for comparison purposes. Note the very high signal-to-noise observed for [3H]-BWA868C. The data shown are from two separate experiments. Vertical lines show s.e.mean within the same experiment.
Figure 3.
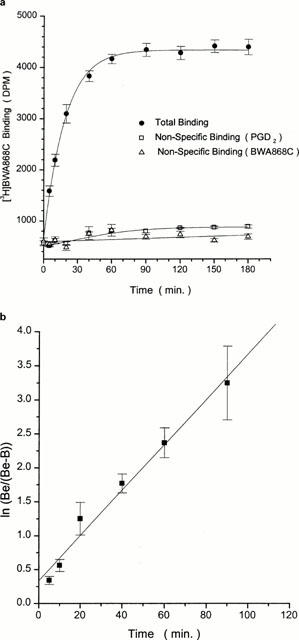
Time-course of [3H]-BWA868C binding to human platelet homogenates. The data shown in (a) represents total binding and non-specific binding as defined with 10 μM PGD2 or 10 μM BWA868C. (b) shows the linear transformation of the specific binding obtained from the initial binding points from (a). Plots shown are representative of ⩾ three experiments. Vertical lines show s.e.mean within the same experiment.
Figure 4.
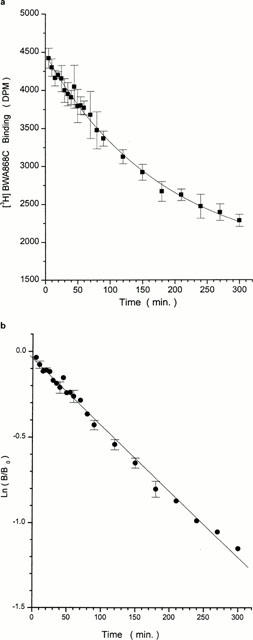
Time-course of dissociation of specific [3H]-BWA868C binding to human platelet homogenates. (a) [3H]-BWA868C (5 nM) binding was conducted up to equilibrium and the dissociation was then initiated by the addition of 100 μM unlabelled PGD2 and the residual specific binding monitored over time at room temperature. (b) shows the linear transformation of the data in (a). Plots shown are representative of ⩾ three experiments. Vertical lines show s.e.mean within the same experiment.
In a typical experiment, 207,127±817 d.p.m. of [3H]-BWA868C (approximately 5 nM final) were added to the human platelet membranes but less than 2.5% bound to the receptors. The level of non-specific binding (NSB) was very low and was similar if 100 μM PGD2 or 1–100 μM BWA868C was used, thus the actual representative d.p.m. bound were as follows: total binding=4438±115 d.p.m. (n=10), NSB with 10 μM BWA868C=630±24 d.p.m.; total binding=4470±109 d.p.m. (n=10), NSB with 10 μM PGD2=811±21 d.p.m. (n=10). The specific binding was therefore 82–86% of the total binding under these conditions. In contrast, [3H]-PGD2 (10 nM) binding yielded only 46% specific binding using the same assay conditions as above for [3H]-BWA868C. Based on these experiments all subsequent experiments with 5 nM [3H]-BWA868C were conducted at 23°C for 1 h using 10 mg ml−1 human platelet suspensions.
Saturation binding of [3H]-BWA868C
[3H]-BWA868C bound to human platelet membranes with a high affinity (dissociation constant; KD=1.45±0.012 nM, n=3) and to a finite number of binding sites (Bmax= 21.1±0.62 nmol g−1 wet weight) as determined from Scatchard analysis of saturation data (Figure 5a,b).
Figure 5.
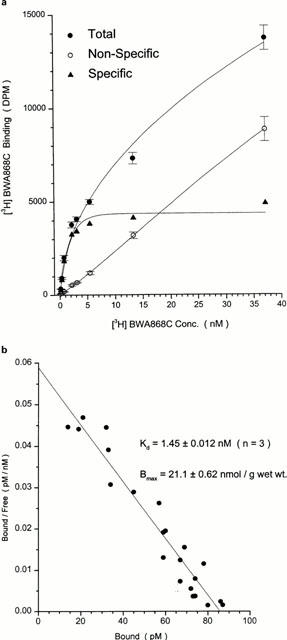
Saturation of [3H]-BWA868C binding to human platelet homogenates. Total, non-specific and specific binding is shown in (a) using 1–38 nM [3H]-BWA868C. Scatchard analysis of the specific binding component from (a) is shown in (b). Data are mean of three experiments. Vertical lines show s.e.mean.
Pharmacological characteristics of [3H]-BWA868C binding
The pharmacological specificity of [3H]-BWA868C (5 nM) binding was correlated very well with that of the unlabelled BWA868C (Tables 1 and 3) such that DP receptor prostanoid ligands (e.g. ZK118182, RS9320, BWA868C, PGD2) exhibited high (nanomolar) affinities for [3H]-BWA868C binding, while PGE2, cloprostenol, PGF2α, iloprost, U46691, etc. showed a low (micromolar) affinity for specific [3H]-BWA868C binding (Figure 6; Tables 2 and 3). The pharmacology of the displacement by various prostanoids of [3H]-BWA868C and [3H]-PGD2 binding to human platelet membranes was well correlated (Table 3; Figure 7). Moreover, there was a good correlation between the ability of a range of prostanoids to compete for [3H]-BWA868C binding and their ability to stimulate cyclic AMP production in embryonic bovine tracheal cells possessing a functional DP receptor (Crider et al., 1998; 1999) (Figure 8).
Table 3.
Affinities of various prostanoids for specific [3H]-BWA868C and [3H]-PGD2 binding to human platelet membranes
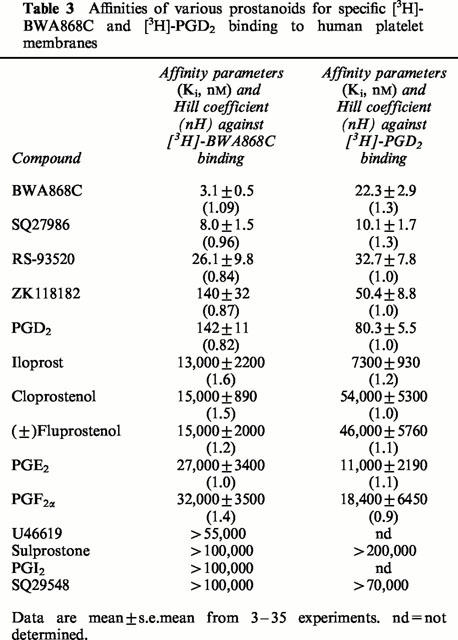
Figure 6.
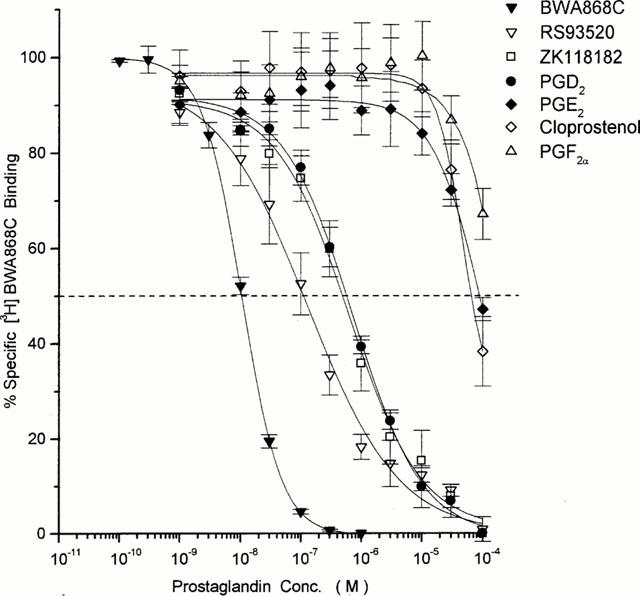
Competition by various prostanoids for specific [3H]-BWA868C binding to human platelet homogenates. Representative curves are shown for DP receptor-selective and non DP receptor-selective prostanoids. Data are mean from numerous experiments; the s.e.means are depicted as vertical lines. Numerical data obtained from 3–35 such experiments are shown in Table 4.
Figure 7.
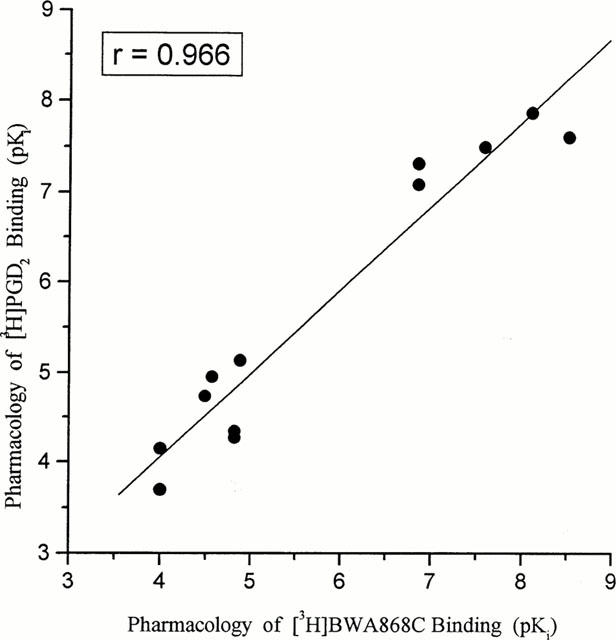
Correlation of the pharmacological specificity of displacement of [3H]-BWA868C and [3H]-PGD2 binding to human platelet homogenates. The mean pKi values from 3–35 experiments were used to construct this correlation plot.
Figure 8.
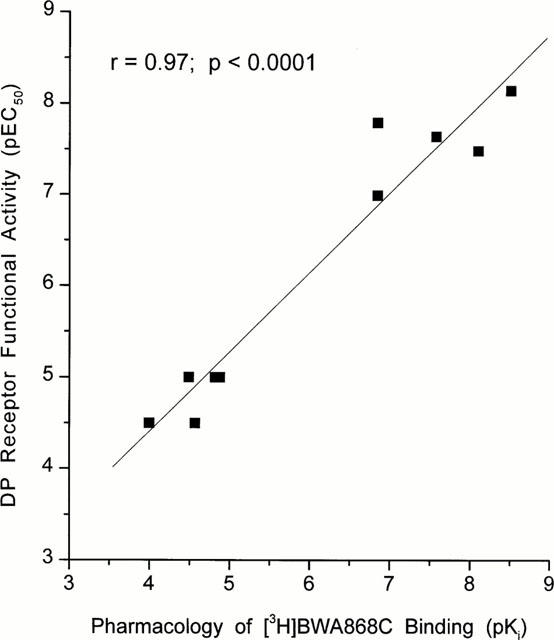
Correlation between the pharmacological specificity of displacement of [3H]-BWA868C binding to human platelet homogenates and the pharmacology of the DP receptor-mediated cyclic AMP production in embryonic bovine tracheal cells (Crider et al., 1998; 1999). Note the high degree of correlation between the two sets of parameters.
Quantitative autoradiographic studies
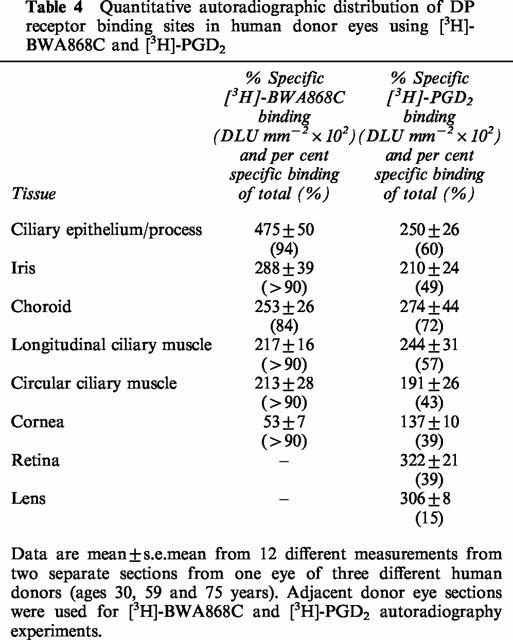
Using [3H]-BWA868C (5 nM) and quantitative autoradiographic techniques, specific DP receptor binding sites were densely localized on the ciliary epithelium/process, longitudinal and circular ciliary muscles, iris and retinal choroid in thin sections of human cadaver eyes (Figures 9 and 10; Table 4). [3H]-BWA868C yielded a high level of specific binding (over 84% of total) in the various ocular tissues studied (Table 4). The highest density of [3H]-BWA868C-labelled DP receptors was associated with the ciliary epithelial cells (Table 4). In comparison, the percentage of specific [3H]-PGD2 binding to human eye sections was less than that observed with [3H]-BWA868C (Figures 9 and 10; Table 4). However, the general distribution of sites labelled with [3H]-PGD2 appeared to be similar to that obtained with [3H]-BWA868C (Figures 9 and 10).
Figure 9.
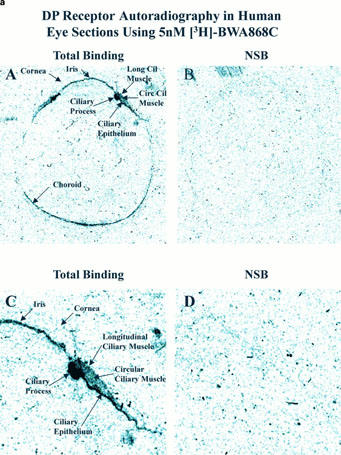
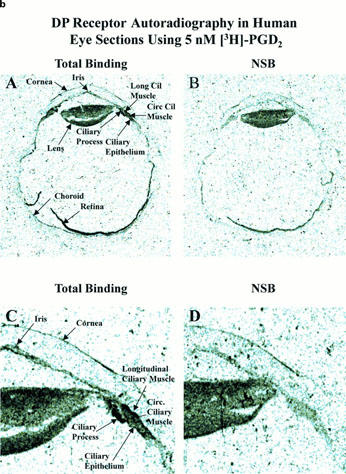
Autoradiographic localization of DP-receptors in human eye sections using [3H]-BWA868C (a) and [3H]-PGD2 (b). (a) and (b) show total binding and non-specific binding profile in the whole eye sections at low power (top two panels; A,B) and at high power (bottom two panels; C,D; anterior segments of eyes) using 5 nM [3H]-BWA868C (a) and using 5 nM (3H)-PGD2 (b). The images are in black and white. The magnifications of the images relative to originals were: (a,b) panels A and B=4×; panels C and D=14.7×.
Figure 10.
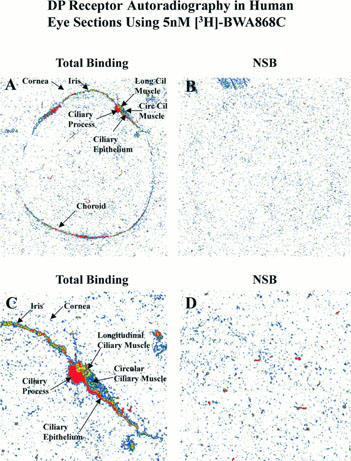
Pseudo colour-coded images of DP prostanoid receptor binding sites in human donor eye sections using 5 nM [3H]-BWA868C. The respective magnifications for the various images shown in the panels, relative to the original images, were as follows: A and B = 3.4×; C and D = 8.5×.
Table 4.
Quantitative autoradiographic distribution of DP receptor binding sites in human donor eyes using [3H]-BWA868C and [3H]-PGD2
Discussion
While [3H]-PGD2 of sufficiently high specific activity has been utilized to previously characterize DP receptor binding properties in the brain (Shimizu et al., 1982; Morii & Watanabe, 1991) and on human platelets (Cooper & Ahern, 1979; Thierauch et al., 1988), its general utility has been hampered by relatively low specific binding (see Results), low selectivity (binds to DP, FP and TP receptors; Coleman et al., 1994; Wright et al., 1998; Sharif et al., 1998), instability and a relatively low affinity (KD 20–53 nM) for the DP receptor (Shimizu et al., 1982; Morii & Watanabe, 1991). The present studies have demonstrated that [3H]-BWA868C is a stable, high-affinity (KD=1–2 nM) and selective antagonist radioligand of relatively high specific activity suitable for labelling the human DP receptor for studies on isolated cells and for autoradiographic visualization of the DP receptors in tissue sections. These are important attributes that are not currently afforded by any other DP receptor-selective radioligand and thus make [3H]-BWA868C a uniquely superior DP prostanoid receptor radioligand.
In the present studies, specific [3H]-BWA868C binding to human platelets comprised >95% of the total binding, was of high-affinity and attained equilibrium in an hour at 23°C. However, total and specific binding remained stable for up to 3 h, which is not the case for [3H]-PGD2 binding in many different tissues (Cooper & Ahern, 1979; Thierauch et al., 1988; Shimizu et al., 1982; Morii & Watanabe, 1991). Scatchard analysis of the saturation isotherms revealed that [3H]-BWA868C bound to a single population of high-affinity DP receptor binding sites in human platelets. There was a good correlation between the receptor dissociation constants derived from analyses of kinetic experiments and saturation binding data, indicating that [3H]-BWA868C bound to the DP receptor binding site within the laws of mass action. This was further reinforced by the monophasic displacement of specific [3H]-BWA868C binding from human platelets by unlabelled DP receptor ligands like BWA868C, ZK118182, RS93520 and PGD2 with Hill coefficients close to unity.
The pharmacological specificity of the displacement of [3H]-BWA868C binding to human platelets was notable. The only prostanoids which are known to exhibit high nanomolar potencies for DP receptors in functional assays, such as BW245C, ZK118182, SQ-27986, RS93520 and BWA868C (Giles & Leff, 1988; Giles et al., 1989; Trist et al., 1989; Leff and Giles, 1992; Coleman et al., 1994; Crider et al., 1999), displayed nanomolar affinities for [3H]-BWA868C binding to human platelets. Prostanoids selective for other prostanoid receptor subtypes, such as cloprostenol and fluprostenol (FP receptor-selective), SC-19220 (EP1-selective), sulprostone, enprostil, GR63799 (EP3-selective), iloprost (IP receptor-selective), SQ-29548, U46619 (TP receptor-selective) (Coleman et al., 1990; 1994; Figure 1) exhibited a very low affinity for [3H]-BWA868C binding. Although, [3H]-PGD2 yielded low specific binding and exhibited a lower affinity than [3H]-BWA868C binding to human platelets, we determined the pharmacological specificity of displacement of [3H]-PGD2 binding for comparative purposes using the same binding procedures as for [3H]-BWA868C. Interestingly, there was a relatively good correlation between the pharmacology of displacement by various prostanoids of [3H]-PGD2 and [3H]-BWA868C binding to human platelets. Moreover, even though the absolute affinity values of a range of prostanoids evaluated in the [3H]-BWA868C binding assay in human platelets (this study) were different to those previously obtained for brain and platelet membranes (Cooper & Ahern, 1979; Tokumoto et al., 1986; Thierauch et al., 1988; Shimizu et al., 1982; Morii & Watanabe, 1991) using [3H]-PGD2 as a probe, the rank orders of their affinities closely paralleled those determined for [3H]-BWA868C binding in the current studies. A similar good correlation existed between the rank orders of agonist and antagonist potency values derived from functional studies such as platelet aggregation (Giles & Leff, 1988; Giles et al., 1989), tissue contraction (Giles & Leff, 1988; Leff > Giles, 1992; Senior et al., 1993) and adenylyl cyclase (Trist et al., 1989; Sugama et al., 1989; Crider et al., 1999) assays and the rank order of affinities obtained for specific [3H]-BWA868C binding to human platelets in the current studies. We also obtained a good correlation between the prostanoid affinities in competition for [3H]-BWA868C binding and their agonist potencies in stimulating cyclic AMP in bovine embryonic tracheal fibroblast cells expressing functional endogenous DP receptors (see Results). These collective data support the identification of a single DP receptor in all these cells and tissues from different species, a conclusion further reinforced by the similarity of the amino acid sequence of the cloned human and mouse DP receptor (Narumiya et al, 1993; Narumiya, 1994; Boie et al., 1995; Abramovitz et al., 1995). However, one disparity observed for the recombinant mouse DP receptor expressed in host mammalian cells was the unexpectedly low affinities of BW245C and BWA868C (Kis>220 nM) competing for [3H]-PGD2 binding (Kiriyama et al., 1997). Likewise, it was rather curious that BWA868C acted as a potent partial agonist (EC50=7.5 nM) against the human DP receptor expressed in HEK-293 cells (Wright et al., 1998) whereas in the majority of other cells/tissues it behaves as a potent antagonist (Giles & Leff, 1988; Leff and Giles, 1992; Senior et al., 1993; Coleman et al., 1990; 1994; Fernandes & Crankshaw, 1995; Rangachari et al., 1995; Crider et al., 1999). Furthermore, the cloned and expressed human DP receptor was unable to discriminate between the affinities and potencies of BW245C, ZK118041 and PGD2 in both binding and functional assays (Wright et al., 1998), whereas against the endogenous DP receptors like in our studies and those of others (see above) such distinction was readily apparent. These types of observations and issues suggest caution in the interpretation of data from the use of recombinant receptors, and thus underscore the value of using naturally expressed receptors in cells and tissues of target organs. Whether these kinds of disparate observations in different biological systems provide further evidence for the potential multiplicity of DP receptors (e.g. Rangachari et al., 1995; Liu & Jackson, 1995) remains to be determined.
With respect to the localization of DP receptors in human eye sections, our autoradiographic results compared well with those of Matsuo & Cynader (1993) who used [3H]-PGD2 as the radioligand. We found, however, that [3H]-BWA868C afforded much higher resolution and substantially lower non-specific binding than [3H]-PGD2. The rather high density of DP receptors in the ciliary body region, including ciliary epithelium (which produces the aqueous humor) and longitudinal ciliary muscle (which appears to be involved in the outflow mechanism), may correlate with the ability of BW245C to lower intraocular pressure (Matsugi et al., 1995) by inhibiting the production of aqueous humor and also promoting the outflow of the aqueous humor via the trabecular meshwork (Matsugi et al., 1995). Interestingly, the ocular distribution of DP receptors determined in the current studies using [3H]-BWA868C was distinctly different from that of the FP receptors determined using an IoP-lowering FP receptor-selective agonist, [3H]-AL-5848 (Sharif et al., 1999), or even using [3H]-PGF2α (Davis & Sharif, 1999).
It is concluded that [3H]-BWA868C is a novel DP receptor prostanoid antagonist radioligand of sufficiently high specific activity which exhibits superior receptor binding properties to [3H]-PGD2, including high levels of specific binding, high affinity, selectivity and stability. [3H]-BWA868C should prove a useful new radioligand for further characterization and autoradiographic localization of DP receptors in various mammalian tissues and cells and to better define the distribution and possible functions of the DP receptors in the mammalian body.
Acknowledgments
The authors thank our colleagues in the Medicinal Chemistry group at Alcon Research, Ltd. for synthesizing some of the PGs used in the current studies. The authors also acknowledge the helpful comments of Dr M.R. Hellberg and Dr T.R. Dean.
Abbreviations
- BSA
bovine serum albumen
- HEK
human embryonic kidney (cells)
- MES
2-[N-Morpholino]ethanesulphonic acid
- NEN
New England Nuclear
- NSB
non-specific binding
- PEI
polyethyleneimine
- PG
prostaglandin
References
- ABRAMOVITZ M., ADAM M., BOIE Y., GRYGORCZYK R., RUSHMORE T.H., FUNK C.D., BASTIEN L., SAWYER N., ROCHETTE C., SLIPTZ D.M, METTERS K.M. Human prostanoid receptors: cloning and characterization. Adv. Prost. Thromb. Leuk. Res. 1995;23:499–504. [PubMed] [Google Scholar]
- ALEXANDER S.P.H, PETERS J.A.Tips receptor and ion channel nomenclature supplement Trends Pharmacol. Sci. 1999Suppl.64–66.10th edn
- ANDERSEN N.H, RAMWELL P.W. Biological aspects of prostaglandins. Arch. Intern. Med. 1974;133:30–50. [PubMed] [Google Scholar]
- ARMSTRONG R.A., LAWRENCE R.A., JONES R.L., WILSON N.H, COLLIER A. Functional and ligand binding studies suggest heterogeneity of platelet prostacyclin receptors. Br. J. Pharmacol. 1989;97:657–668. doi: 10.1111/j.1476-5381.1989.tb12001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGSTROM S, SJOVALL J. The isolation of prostaglandins. Acta Chem. Scand. 1957;11:1086. [Google Scholar]
- BOIE Y., SAWYER N., SLIPTZ D.M., METTERS K.M, ABRAMOVITZ M. Molecular cloning and characterization of the human prostanoid DP receptor. J. Biol. Chem. 1995;270:18910–18916. doi: 10.1074/jbc.270.32.18910. [DOI] [PubMed] [Google Scholar]
- BOWEN W.P, JERMAN J. Nonlinear regression using spreadsheets. Trends Pharmacol. Sci. 1995;16:413–417. doi: 10.1016/s0165-6147(00)89091-4. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C, PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., KENNEDY I., HUMPHREY P.P.A., BUNCE K, LUMLEY P.Prostanoids and their receptors Comprehensive Medicinal Chemistry, Vol. 3: Membranes and Receptors 1990Pergamon Press: Oxford, UK; 643–714.ed. Emmett, J.C. pp [Google Scholar]
- COLEMAN R.A., SMITH W.L, NARUMIYA S. VIII International Union of Pharmacology classification of prostanoid receptors: Properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- COOPER B, AHERN D. Characterization of the platelet prostaglandin D2 receptor. J. Clin. Invest. 1979;64:586–590. doi: 10.1172/JCI109497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRIDER J.Y., GRIFFIN B.W, SHARIF N.A. Prostaglandin DP receptors positively coupled to adenylyl cyclase in embryonic bovine tracheal (EBTr) cells: pharmacological characterization using agonists and antagonists. Br. J. Pharmacol. 1999;127:204–210. doi: 10.1038/sj.bjp.0702490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRIDER J.Y., XU S.X., GRIFFIN B.W, SHARIF N.A. Use of a semi-automated robotic radioimmunoassay to measure cAMP generated by activation of DP-, EP2- and IP-prostaglandin receptors in human ocular and other cell-types. Prostagland. Leukotri. Essent. Fatty Acids. 1998;59:77–82. doi: 10.1016/s0952-3278(98)90055-2. [DOI] [PubMed] [Google Scholar]
- DAVIS T.L, SHARIF N.A. Quantitative autoradiographic visualization and pharmacology of FP-prostaglandin receptors in human eyes using the novel phosphor-imaging technology. J. Ocular Pharmacol. Ther. 1999;15:323–336. doi: 10.1089/jop.1999.15.323. [DOI] [PubMed] [Google Scholar]
- FERNANDES B, CRANKSHAW D. Functional characterization of the DP receptor in human myometrium. Eur. J. Pharmacol. 1995;283:73–81. doi: 10.1016/0014-2999(95)00288-v. [DOI] [PubMed] [Google Scholar]
- GILES H, LEFF P. The biology and pharmacology of prostaglandin D2. Prostaglandins. 1988;35:277–300. doi: 10.1016/0090-6980(88)90093-7. [DOI] [PubMed] [Google Scholar]
- GILES H., LEFF P., BOLOF M.L., KELLY M.G, ROBERTSON A.D. The classification of prostaglandin DP-receptors in platelets and vasculature using BWA868C, a novel, selective and potent competitive antagonist. Br. J. Pharmacol. 1989;96:291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN B.W., MAGNINO P.E., PANG I-H, SHARIF N.A. Pharmacological characterization of an FP prostaglandin receptor on rat vascular smooth muscle cells (A7r5) coupled to phosphoinositide turnover and intracellular calcium mobilization. J. Pharmacol. Exp. Ther. 1998;286:411–418. [PubMed] [Google Scholar]
- GRIFFIN B.W., WILLIAMS G.W., CRIDER J.Y, SHARIF N.A. FP prostaglandin receptors mediating inositol phosphate generation and calcium mobilization in Swiss 3T3 cells: a pharmacological study. J. Pharmacol. Exp. Ther. 1997;281:845–854. [PubMed] [Google Scholar]
- KIRIYAMA M., USHIKUBI F., KOBAYASHI T., HIRATA M., SUGIMOTO Y, NARUMIYA S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFF P, GILES H. Classification of platelet and vascular prostaglandin D2 (DP) receptors: estimation of affinities and relative efficacies for a series of novel bicyclic ligands with an appendix on goodness-of-fit analyses. Br. J. Pharmacol. 1992;106:996–1003. doi: 10.1111/j.1476-5381.1992.tb14447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y.-J, JACKSON D.M. Are there subtypes of prostaglandin DP receptors. Br. J. Pharmacol. 1995;115 Suppl.:134. [Google Scholar]
- MATSUGI T., KAGEYAMA M., NISHIMURA K., GILES H, SHIRASAWA E. Selective prostaglandin D2 receptor stimulation elicits ocular hypotensive effects in rabbits and cats. Eur. J. Pharmacol. 1995;275:245–250. doi: 10.1016/0014-2999(94)00788-9. [DOI] [PubMed] [Google Scholar]
- MATSUO T, CYNADER M.S. The EP2 receptor is the predominant prostanoid receptor in the human ciliary muscle. Br. J. Ophthalmol. 1993;77:110–114. doi: 10.1136/bjo.77.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCPHERSON G.A. A practical computer based approach to the analysis of radioligand binding experiments. Computer Prog. Biomed. 1983a;17:107–114. doi: 10.1016/0010-468x(83)90031-4. [DOI] [PubMed] [Google Scholar]
- MCPHERSON G.A. Analysis of radioligand binding experiments on a microcomputing system. Trends Pharmacol. Sci. 1983b;4:369–370. [Google Scholar]
- MORII H, WATANABE Y. Solubilization of prostaglandin D2 binding protein from porcine temporal cortex. Biochem. Biophys. Res. Commun. 1991;174:364–371. doi: 10.1016/0006-291x(91)90529-g. [DOI] [PubMed] [Google Scholar]
- MUNSON P.J, RODBARD D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- NARUMIYA S.Prostanoid receptors: Structure, function, and distribution Cellular Generation, Transport, and Effects of Eicosanoids: Biological Roles and Pharmacological Intervention 1994The New York Academy of Sciences: New York, NY; 126–138.ed. Goetzl E.J., Lewis R.A. & Rola-Pleszczynski M. pp [DOI] [PubMed] [Google Scholar]
- NARUMIYA S., HIRATA M., NAMBA T., HAYAISHI Y., USHIKUBA F., SUGIMOTO Y., NEGISHI M, ICHIKAWA A. Structure and function of prostanoid receptors. J. Lipid Mediators. 1993;6:155–161. [PubMed] [Google Scholar]
- OGLETREE M.L, ALLEN G.T. Interspecies differences in thromboxane receptors: studies with thromboxane receptor antagonists in rat and guinea pig smooth muscles. J. Pharmacol. Expt. Ther. 1992;260:789–794. [PubMed] [Google Scholar]
- RANGACHARI P.K., BETTI P.-A., PRIOR E.T, ROBERTS L.J. Effects of a selective DP receptor agonist (BW245C) and antagonist (BWA868C) on the canine colonic epithelium: an argument for a different DP receptor. J. Pharmacol. Exp. Ther. 1995;275:611–617. [PubMed] [Google Scholar]
- SENIOR J., MARSHALL K., SANGHA R, CLAYTON J.K. In vitro characterization of prostanoid receptors on human myometrium at term pregnancy. Br. J. Pharmacol. 1993;108:501–506. doi: 10.1111/j.1476-5381.1993.tb12832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARIF N.A.Quantitative autoradiographic methods Brain mapping: the methods 1996Academic Press Inc: New York; 115–144.ed. Toga A.W. & Mazziota, J.C. pp [Google Scholar]
- SHARIF N.A., DAVIS T.L, WILLIAMS G.W. [3H]AL-5848 ([3H]9-β-[+]fluprostenol): carboxylic acid of travoprost (AL-6221), a novel FP prostaglandin to study the pharmacology and autoradiographic localization of the FP receptor. J. Pharmac. Pharmacol. 1999;51:685–694. doi: 10.1211/0022357991772989. [DOI] [PubMed] [Google Scholar]
- SHARIF N.A, HUGHES J. Discrete mapping of brain mu and delta opioid receptors using selective peptides: quantitative autoradiography, species differences and comparison with kappa receptors. Peptides. 1989;10:499–522. doi: 10.1016/0196-9781(89)90135-6. [DOI] [PubMed] [Google Scholar]
- SHARIF N.A., XU S.X., MILLER S.T., GAMACHE D.A, YANNI J.M. Characterization of the ocular anti-allergic and anti-histaminic effects of olopatadine (AL-4943A), a novel drug for treating ocular allergic diseases. J. Pharmacol. Exp. Ther. 1996;278:1252–1261. [PubMed] [Google Scholar]
- SHARIF N.A., XU S.X., WILLIAMS G.W., GRIFFIN B.W., CRIDER J.Y, DAVIS T.L. Pharmacology of [3H]Prostaglandin E1/[3H]Prostaglandin E2 and [3H]Prostaglandin F2α binding to EP3 and FP receptor binding sites in bovine corpus luteum: characterization and correlation with functional data. J. Pharmacol. Exp. Ther. 1998;286:1094–1102. [PubMed] [Google Scholar]
- SHIMIZU T., YAMASHITA A, HAYAISHI O. Specific binding of prostaglandin D2 to rat brain synaptic membrane. Occurrence, properties and distribution. J. Biol. Chem. 1982;257:13570–13575. [PubMed] [Google Scholar]
- SUGAMA K., TANAKA T., YOKOHAM H., NEGISHI M., HAYAISHI H., ITO S, HAYAISHI O. Stimulation of cAMP formation by prostaglandin D2 in primary cultures of bovine adrenal medullary cells: identification of the responsive population as fibroblasts. Biochem. Biophys. Acta. 1989;1011:75–80. doi: 10.1016/0167-4889(89)90081-5. [DOI] [PubMed] [Google Scholar]
- THIERAUCH K.-H., STURZEBECHER C.-ST, SCHILLINGER E. Stable 9α- or 11α-halogen-15-cyclohexyl-prostaglandins with high affinity to the PGD2-receptor. Prostaglandins. 1988;35:855–868. doi: 10.1016/0090-6980(88)90112-8. [DOI] [PubMed] [Google Scholar]
- TOKUMOTO H., WATANABE Y., YAMASHITA A., ARAI Y, HAYAISHI O. Specificity of prostaglandin D2 binding to synaptic membrane fraction of rat brain. Brain Res. 1986;362:114–121. doi: 10.1016/0006-8993(86)91404-6. [DOI] [PubMed] [Google Scholar]
- TOWN M.-H., CASALS-STENZEL J, SCHILLINGER E. Pharmacological and cardiovascular properties of a hydantoin derivative, BW245C, with high affinity and selectivity for PGD2 receptors. Prostaglandins. 1983;25:13–28. doi: 10.1016/0090-6980(83)90131-4. [DOI] [PubMed] [Google Scholar]
- TRIST D.G., COLLINS B.A., WOOD J., KELLY M.G, ROBERTSON A.D. The antagonism of BWA868C of PGD2 and BW245C activation of human platelet adenylate cyclase. Br. J. Pharmacol. 1989;96:301–306. doi: 10.1111/j.1476-5381.1989.tb11817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRIGHT H.D., METTERS K.M., ABRAMOVITZ M, FORD-HUTCHINSON A.W. Characterization of the recombinant human prostanoid DP receptor and identification of L-644,698, a novel selective DP agonist. Br. J. Pharmacol. 1998;123:1317–1324. doi: 10.1038/sj.bjp.0701708. [DOI] [PMC free article] [PubMed] [Google Scholar]


