Abstract
Whole-cell patch clamp recordings were made from rat striatal cholinergic interneurones in slices of brain tissue in vitro. Bath application of histamine (EC50 6.3 μM) was found to rapidly and reversibly depolarize these neurones through the induction of an inward current at −60 mV.
The effects of histamine were mimicked by the H1 receptor agonist 2-thiazolylethylamine (50 μM) and selectively inhibited by pre-incubation with the H1 receptor antagonist triprolidine (1 μM).
Ion substitution experiments under voltage clamp conditions revealed that the histamine activated current was comprised of two components. One component was sensitive to the concentration of extracellular Na+, whilst the other component was inhibited by intracellular Cs+ or extracellular Ba2+.
In situ hybridization experiments revealed that the majority of cholinergic interneurones in the rat striatum express the histamine H1 receptor but few neurones express H2 receptors. These findings were confirmed using single cell RT–PCR.
It is concluded that histamine depolarizes cholinergic interneurones in the rat striatum via a H1-receptor mediated mechanism.
Keywords: acetylcholine, interneurone, histamine, striatum
Introduction
The presence of histamine in the CNS has been known for over four decades (Kwiatowski, 1943) and its role as a central transmitter is now well established (Schwartz et al., 1991). Three subtypes of histamine receptor have been pharmacologically defined in the brain. H1 receptors are coupled to phospholipase C and stimulate the production of IP3/Ca2+ and diacylglycerol-PKC pathways (Claro et al., 1986). H2 receptors are linked to adenylyl cyclase (Traiffort et al., 1992) whilst the recently cloned H3 receptor is thought to be an autoreceptor that acts in an inhibitory manner at the presynaptic terminal (Leurs et al., 2000).
Histaminergic fibres are known to project to the striatum (Russell et al., 1997; Inagaki et al., 1988) and autoradiographic studies have demonstrated the presence of H1, H2 and H3 histamine receptors within the basal ganglia (Palacios et al., 1981; Arrang et al., 1987; Bouthenet et al., 1988; Ruat et al., 1990). Functionally, intrastriatal injection of H1 and H2 receptor agonists modulate the motor and cognitive behaviour associated with a learned helplessness model of depression (López-Garcia et al., 1993), whilst microinjections of histamine into the nucleus accumbens can induce a marked increase in locomotor activity (Bristow & Bennett, 1988). Striatal histamine may also play an important role in methamphetamine-induced stereotypic behaviours (Ito et al., 1996).
At the cellular level, both GABAergic medium spiny neurones and cholinergic interneurones are thought to be under the modulatory influence of histaminergic neurones. Dopamine D1 receptor-mediated release of GABA from the rat striatum is effectively inhibited by H3 antagonists (Garcia et al., 1997) whilst striatal superfusion with the histidine decarboxylase inhibitor (s)-α-fluoromethylhistidine can significantly reduce the release of acetylcholine (Prast et al., 1997). In addition to a role in normal behaviour and movement, histaminergic systems may be involved in disorders of the basal ganglia and striatum. The density of H2 histamine receptors in the putamen of patients with Huntington's disease is lower than in non-Huntington's patients whilst H2 antagonists are thought to aggravate the symptoms of Parkinson's disease and epilepsy (Martinez-Mir et al., 1993).
In view of the potential importance of this system and since the release of striatal acetylcholine has been shown to be modulated by histamine, we have examined the pharmacology of the histamine receptors expressed in identified cholinergic interneurones within the rat striatum using electrophysiology, single cell RT–PCR and in situ hybridization.
Methods
Preparation
14–28-day-old male Wistar rats were sacrificed by cervical dislocation, brains removed and 300 μm coronal slices containing the striatum were prepared in ice cold physiological saline using a vibratome. Cholinergic neurones were preferentially targeted using infra red videomicroscopy as previously described (e.g. Lee et al., 1998).
Recording and analysis
Recording electrodes were pulled from borosilicate glass capillaries and when filled with electrolyte had resistances of 2–5 MΩ. Electrophysiological signals were detected using an Axopatch-1D patch-clamp amplifier and were recorded onto digital audio tape for later figure production. Following formation of the whole-cell configuration, series resistance was partially compensated using the amplifier and cellular conductance was continually monitored via the injection of hyperpolarizing current (current-clamp mode; −100 pA in amplitude, 300 ms in duration at 0.1 Hz) or voltage (voltage-clamp mode; −10 mV in amplitude, 300 ms duration at 0.1 Hz). Membrane signals were filtered at 1 kHz and digitized at 5 kHz through a Digidata 1200B A/D converter using pCLAMP 8.0 software (Axon Instruments Inc., Foster City, CA, U.S.A.). In voltage-clamp recordings, neurones were clamped at −60 mV and current-voltage relationships were examined using a voltage ramp protocol between −140 mV and −60 mV (20 mV s−1).
Concentration-response data for histamine were fitted according to the relationship:
where I is the amplitude of the current in the presence of the test concentration of histamine, [histamine] is the test concentration of histamine, EC50 is the half maximal effective concentration of histamine and n is the Hill coefficient.
To assess whether a particular procedure led to a significant change in the magnitude of the current under study, data were subjected to a Student's t-test. All data in the text and figures are presented as mean values±s.e.mean unless otherwise stated.
Single cell RT–PCR
The cytoplasm from presumed cholinergic interneurones was gently aspirated under visual control into the recording electrode until at least 40% of the somatic cytoplasm had been collected. The electrode was then withdrawn from the cell to form an outside-out patch which prevented contamination when subsequently withdrawn from the slice. The contents of the electrode were forced into a microtube and the RNA reverse transcribed using an anchored oligo dT primer and 200 Units of MMLV reverse transcriptase (BRL) according to the manufacturer's recommendations. After 60 min at 37°C the cDNA was stored frozen at −20°C prior to processing. After amplification of the cDNA using Taq polymerase (Dixon et al., 1998), the expression of specific genes was measured using primers designed to amplify products of between 150 and 250 base pairs in length, close to the 3′ ends of the mRNA transcripts.
The primers used were: for H1 receptor (Accession number D12800), forward primer (bases 3246–3266): CAAAGGAAAAGAGGTTCCTGG; reverse primer (bases 3438–3419): GTCACCCTCTGTGGACAGGT; H2 receptor (Accession number S57565), forward primer (bases 130–150): GGTTGAACAACTCTCTGCTGC; reverse primer (bases 376–357): CCTAAGAGAGCCAGCCATTG; choline acetyltransferase: forward primer (bases 2134–2155): TACTAAGCTCTGTTCCCATCCC; reverse primer (bases 2303–2285): ACCCAGGTTGCTTCCAAAC; preproenkephalin (PPE, Accession number S49491): forward primer (bases 1033–1050): AGTTGCCCTCGTGGTCTG; reverse primer (bases 1180–1159): TGTCAGAAGGGATGAGGTAACA; preprotachykinin (PPT, Accession number M34184): forward primer (bases 717–735): CAGAAAGGCTGCTGTGAGG; reverse primer (bases 877–850): AGTCACAACACAGGAAACATGC; preprosomatostatin (PPS, Accession number M25890): forward primer (bases 291–309): TGAGCAGGACGAGATGAGG; reverse primer (bases 441–421): AGAGGTCTGGCTGAGACAACA; α-tubulin (Accession number V01226): forward primer (bases 298–316): CACTGGTACGTGGGTGAGG; reverse primer (bases 469–448) TTTGACATGATACAGGGACTGC; synaptotagmin 1 (Accession number X52772): forward primer (bases 4019–4039): AGGGGCTTTCCTATCTAAGGG; reverse primer (bases 4220–4221): GTTGGCAGTGTTGCAAGAGA. These PCR reactions were run for 50 cycles of 92°C (denaturing, 2.5 min), 55°C (annealing, 1.5 min) and 72°C (extension, 1 min), followed by a final extension of 10 min at 72°C. The PCR products were separated on 2.5% agarose gels and the product sizes were as predicted from the sequences. In control experiments on diluted brain cDNA, the H1 and H2, primer pairs were able to detect positive products of the predicted size using 0.1 pg of cDNA but not 0.01 pg. Furthermore, in experiments where the electrode was positioned next to a cell without seal formation or harvesting of the cytoplasmic contents, no PCR products were detected (n=3).
Solution and drugs
The physiological saline contained (mM) NaCl 125; NaHCO3 25; glucose 10; KCl 2.5; NaH2PO4 1.25; CaCl2 2; MgCl2, 1, and was bubbled with a 95%, 5% O2/CO2 gas mixture. The intracellular (pipette) solution was adjusted to pH 7.4 using KOH in all experiments. For conventional whole cell recordings this solution comprised (mM) K-gluconate 120; NaCl 10; MgCl2 2; K2EGTA 0.5; HEPES 10; Na2ATP 4; Na2GTP, 0.3.
Throughout the course of an experiment, slices were continuously perfused at a rate of 3–4 ml min−1 with physiological saline by means of a gravity feed system. Histamine and other drugs used in this study were applied by changing the solution which superfused the slice to one which contained the drug. In voltage-clamp recordings, 1 μM tetrodotoxin (TTX), 50 μM D-2-amino-5-phosphovalerate (D-AP5) and 5 μM 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxalone (NBQX) were usually added to the physiological saline. All experiments were conducted at 32–35°C. All drugs were obtained from Sigma (Poole, U.K.) except NBQX and D-AP5 which were from Tocris Cookson (Bristol, U.K.) and 2-thiazolyethylamine dihydrochloride which was a gift from Dr J.M. Young, Cambridge University Department of Pharmacology, U.K.
In situ hybridization
Brains from 14–24-day-old Wistar rats were removed, rapidly frozen in and stored at −70°C until use. Coronal striatal sections were cut in a cryostat (5 μm) and thaw-mounted onto poly-L-lysine coated slides. Sections were then fixed in 4% paraformaldehyde (in phosphate buffered saline which comprised (mM) NaCl 120; KCl 2.7 and phosphate buffer salts 10, for 5 min and stored under 95% ethanol at 4°C. Sense and anti-sense cDNA oligonucleotide probes were designed complementary to the mRNA encoding the H1 receptor (Accession number D12800, bases 1117–1073): CCACTGTGACCAGGGAGATACTACTGAGAACCACCACCAGGGGCA; H2 receptor (Accession number S57565, bases 400–356): CATGGAGTCTGAGGCACTGCTGGATATGTCTTGAAGTGGCTTAGG. Probes were labelled with α-35S-dATP by terminal deoxynucleotidyl transferase at 37°C for 1 h. The labelled probes were added to hybridization buffer (50% deionized formamide, 4× saline-sodium phosphate-EDTA buffer, 10% dextran sulphate, 1× Denhardt's solution, 200 μg/ml denatured salmon sperm DNA, 100 μg/ml polyadenylic acid), to a final activity of 3000 c.p.m./ml. One hundred and fifty μl hybridization buffer containing labelled probe was applied to the appropriate sections, and incubated at 42°C overnight.
Following hybridization, the slides were washed in 1× saline-sodium citrate buffer (SSC; 20 min at 21°C followed by 30 min at 55°C), then sequentially rinsed in 1× SSC, 0.1× SSC, 70% ethanol, 95% ethanol and allowed to air dry for 2–3 h. The slides were dipped in photo-emulsion (LM-1, Amersham, Bucks, U.K.) and exposed for 8 weeks. After development, sections were lightly stained with cresyl violet, mounted with a coverslip and examined under a microscope. Sections were analysed using a Leica light microscope fitted with a ×40 objective lens using MCID software (MCID Version 4.0, Imaging Research Incorporated, Toronto, Canada). Cholinergic interneurones were preferentially targeted on the basis of somatic diameters. Thus only cells with a somatic diameter greater than 25 μm were regarded as presumptive cholinergic interneurones (e.g. DiFiglia & Carey, 1986; Kawaguchi, 1993) and included in subsequent analysis. For each oligonucleotide probe, the presence or absence of mRNA hybridization was examined in a sample of around 50 large presumptive cholinergic interneurones from at least three animals. For illustrative purposes, cells were photographed using a digital camera or MCID.
Results
Electrophysiology
This study was performed on a total of 84 visually identified cholinergic interneurones present in striatal slices taken from 51 animals. Following formation of the whole-cell configuration, these neurones displayed pharmacological and electrophysiological characteristics similar to those previously attributed to cholinergic interneurones (Lee et al., 1998). For example, in current-clamp recordings, these neurones had a resting membrane potential of −59.1±0.6 mV (n=73) and displayed action potentials which were associated with a large after-hyperpolarization (34.3±0.8 mV). Immediately after membrane breakthrough these neurones had an input resistance of 168.7±6.1 MΩ (n=73) and exhibited a characteristic reduction in apparent input resistance in response to hyperpolarizing current injection (Jiang & North, 1991).
To confirm the identity of these neurones, in 15 of these neurones we examined the effect of the NK1 receptor agonist [Sar9, Met(O2)11] substance P on the membrane properties of these neurones (Bell et al., 1998). In each case, 50 nM [Sar9, Met(O2)11] substance P produced 12.1±1.3 mV depolarization of the membrane potential (not shown). Furthermore, in 11 cells, following electrophysiological identification, cytoplasm was harvested and processed for reverse transcription and cDNA amplification. The resultant cDNA was subjected to PCR analysis with primers specific for the enzyme choline acetyltransferase (ChAT). All neurones identified electrophysiologically as cholinergic were positive for this enzyme.
Histamine-induced depolarization
In whole-cell current-clamp recordings, bath application of 10 μM histamine depolarized all striatal cholinergic interneurones by 11.9±0.8 mV (n=17). This depolarization was rapid in onset and was reversed slowly (5–10 min) following washout (Figure 1a). The depolarization was associated with an increase in apparent input resistance (of 100.3±10.2 MΩ; n=17) and was sufficient to stimulate an increase in firing frequency in all cells tested.
Figure 1.
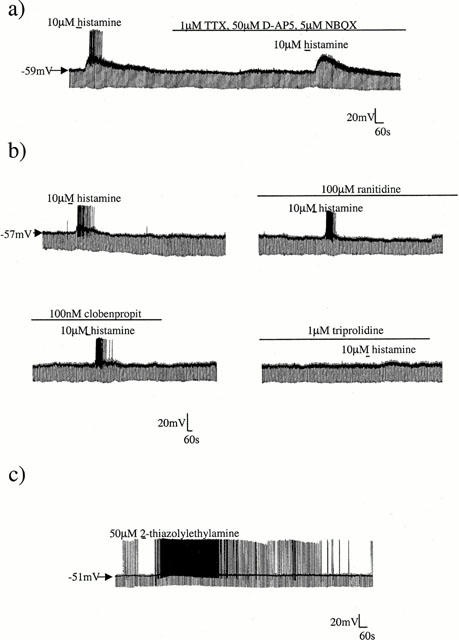
Histamine receptor agonists depolarize cholinergic interneurones. (a) Continuous whole-cell current-clamp recording illustrating the depolarizing effect of 10 μM histamine on resting membrane potential. This depolarization was maintained after addition of 1 μM TTX and ionotropic glutamate antagonists to the bath. (b) The selective H2 antagonist ranitidine (100 μM) and the potent H3 receptor antagonist clobenpropit (100 nM) did not affect the histamine evoked depolarization, however the H1 selective antagonist triprolidine (1 μM) was seen to block the response. (c) The effects of histamine could be mimicked by the H1 selective agonist 2-thiazolylethylamine.
The effects of 10 μM histamine on membrane potential and input resistance were not modified by the addition of 1 μM TTX to the bathing solution (control: 12.5±1.8 mV depolarization and 76.7±30.3 MΩ increase in input resistance; 1 μM TTX: 11.7±1.5 mV depolarization and 73.3±24.4 MΩ increase; n=4; Figure 1a). Similarly, the effects of histamine on membrane depolarization and apparent input resistance were not altered by the addition of ionotropic glutamate antagonists to the bathing solution (control: 13.5±1.1 mV depolarization; 5 μM NBQX and 50 μM D-AP5: 14.0±2.1 mV (n=3).
To examine the nature of the receptor responsible for the histamine-induced depolarization of these neurones, selective histamine receptor antagonists were employed. In all cells tested neither the potent H3 receptor antagonist clobenpropit (1 μM; n=3; Figure 1b) nor the H2 receptor antagonist ranitidine (up to 100 μM; n=4; Figure 1b) were able to modify the effect of 10 μM histamine. In contrast, preincubation of the slice with the H1 receptor antagonist triprolidine at a concentration of 1 μM virtually abolished the effects of histamine (n=4; Figure 1b). These findings suggest that the effects of histamine upon cholinergic interneurones are mediated predominantly via H1 receptors. In order to confirm this, the actions of the selective H1 receptor agonist 2-thiazolylethylamine were examined. As expected, 50 μM 2-thiazolylethylamine caused a rapid depolarization of cholinergic interneurones (of 7.1±0.7 mV; n=4; Figure 1c). This depolarization was associated with an increase in input resistance (of 42.5±9.7 MΩ; n=4) and was slowly reversible on washout.
Nature of the H1-activated current
When neurones were voltage-clamped at −60 mV, 10 μM histamine-induced an inward current 117.7±9.4 pA in magnitude which displayed a similar time course to the depolarization observed in current-clamp mode (n=43). To discount the possibility that histamine might be acting pre-synaptically via the release of other transmitters, Ca2+-free solutions were used containing 5 mM Mg2+, 5 μM NBQX and 50 μM D-AP5. Under these conditions, although the Ca2+-free solution reduced the level of baseline noise, the size of the histamine-induced current was unaffected (n=3, not shown).
When histamine was applied at short intervals or in large concentrations, the response to subsequent applications were reduced in magnitude suggesting that the histamine-activated response can exhibit desensitization under these conditions. When applied at 40 min intervals no desensitization was seen and the histamine-activated current was found to be concentration-dependent in nature (Figure 2a). Using this approach it was possible to construct a concentration-response relationship with a EC50 value of 6.3 μM and an associated Hill slope of 1.4 (Figure 2b).
Figure 2.
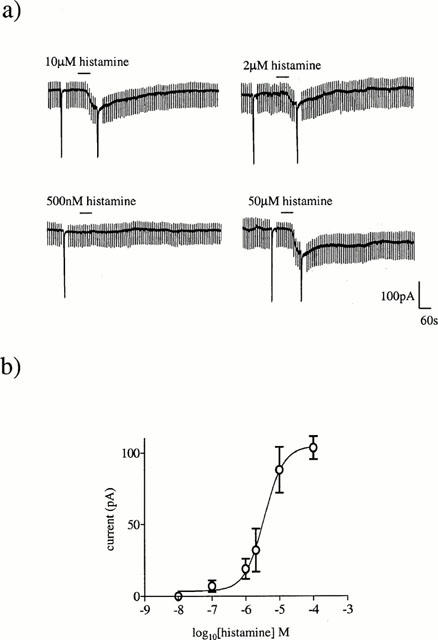
The histamine evoked current is concentration-dependent. (a) The effect of varying concentrations of histamine, applied at 40 min intervals, on the magnitude of the inward current at −60 mV. The large deflections are current responses to depolarizing voltage ramps. (b) Concentration-response curve for histamine. All points are means of three or four separate experiments. Vertical lines indicate s.e.mean. Where no lines are apparent, the error was within the size of the symbol.
To investigate the nature of this inward current, voltage ramps from −140 to −60 mV were applied in the absence and presence of the histamine-induced current. Under these conditions, it was not possible to obtain a clear reversal potential. To examine the nature of this current further, K+ channel blockers were added to the bathing medium. After incubation with 2 mM Ba2+ the response to histamine was reduced to 39.2±12.4% of its control value (n=5; P<0.05, not shown) indicating that a K+ conductance is involved in the generation of this current. To test whether a component of the histamine-activated current was due to Na+ influx, the external NaCl was replaced with Tris-HCl. Under NaCl-free conditions the amplitude of the histamine-induced current was reduced to 41.1±6.9% of its control value (n=5, P<0.05, Figure 3a). To confirm the mixed ionic nature of the response to histamine, in some cells the internal K-gluconate solution was replaced with CsCl. Under these conditions the magnitude of the current induced by 10 μM histamine was reduced (66.3±6.2 pA at −60 mV; n=4, P<0.05). The residual current observed in the presence of internal Cs+ was seen to be completely abolished after replacement of external Na+ with Tris-HCl (Figure 3b) but was unaffected by addition of 2 mM Ba2+ to the bathing medium (both n=3). This suggests that the current is carried by one component sensitive to external Na+ and a second component sensitive to blockade by external Ba2+ or internal Cs+. In agreement with this, when the extracellular Na+ was replaced with Tris-HCl the residual current had a reversal potential close to EK (−99.7±3.1 mV; n=5, Figure 3c). Conversely, after K+ channel blockade with Ba2+, the histamine-induced current was seen to reverse towards 0 mV (−2.7±10.9 mV; n=5, Figure 3d).
Figure 3.
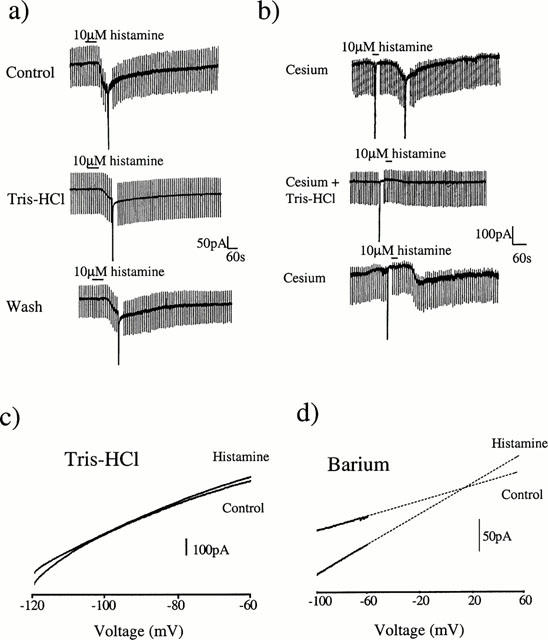
The histamine-activated current is sensitive to extracellular [Na+]. (a) Ten minutes after replacing all the extracellular NaCl with Tris-HCl the response to histamine at −60 mV was diminished. Thirty minutes after returning to normal physiological saline, the response to histamine had returned. (b) Control response to 10 μM histamine after blockade of potassium channels by addition of 120 mM Cs+ to the electrode solution. Ten minutes after replacing all the extracellular NaCl with Tris-HCl the response to histamine was abolished in a reversible manner. (c) After complete replacement of external NaCl with Tris-HCl, voltage ramps were recorded before and after administration of 10 μM histamine. Under these conditions the histamine-activated current was seen to reverse at approximately −95 mV which is close to EK. (d) After addition of 2 mM Ba2+ to normal physiological saline, voltage ramps were recorded before and after administration of 10 μM histamine. In this example extrapolation of the current traces using linear regression revealed that the histamine-activated current reversed towards 0 mV.
Gene expression studies
To investigate the expression of histamine receptors in striatal neuronal populations, in situ hybridization was performed on 5 μm coronal striatal sections using oligonucleotide probes specific to H1 and H2 receptors. Cholinergic interneurones were visually identified on the basis of relative size and distribution in the striatum (DiFiglia & Carey, 1986; Kawaguchi, 1993).
The expression of H1 histamine receptors was relatively widespread amongst cholinergic interneurones (Figure 4a). Thus, H1 receptor mRNA was detected in 68.9±8.9% of cells (n=63, three animals). In contrast, H2 receptor mRNA expression was relatively scarce in these cells (4 of 50 neurones, three animals, Figure 4a).
Figure 4.
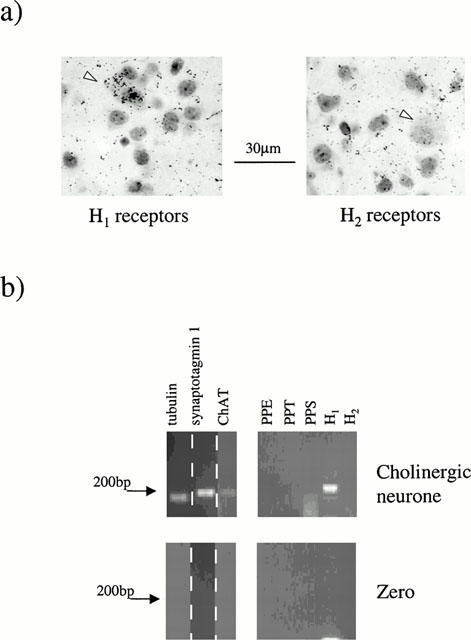
Expression of histamine receptor mRNA in striatal cholinergic interneurones. (a) The expression of H1 histamine receptors was relatively widespread amongst cholinergic interneurones (white arrowhead) and is visible as black grains colocalized with large interneurones. In contrast, hybridization against H2 receptor mRNAs was relatively scarce in cholinergic interneurones (white arrowheads). (b) Single cell RT–PCR analysis of histamine receptor expression in a cholinergic interneurone using primers specific for ChAT, H1 and H2 receptors. Lower panel shows a negative control in which the electrode was placed near the neurone but no cytosol was harvested.
To confirm these results single cell RT–PCR was performed on visually identified cholinergic interneurones. The cytosolic contents were aspirated from 11 cells in total (Figure 4b). Under these conditions H1 receptor mRNA expression was detected in 6 of 11 cells expressing ChAT mRNA whilst detectable levels of H2 receptor mRNA was absent from all cells tested (n=11).
Discussion
Identification of cholinergic interneurones
Within the striatum, cholinergic interneurones account for 1–2% of the total neuronal population. Despite their small number, these neurones possess extensive dendritic and axonal fields and are believed to exert a profound modulatory influence upon the medium spiny neurone and hence basal ganglia activity (Di Chiara et al., 1994).
In the present study, we have exploited several characteristics exhibited by these cells in order to distinguish them from other striatal subtypes. It is well documented that these neurones have the largest somatic size of all striatal neurones (typically in excess of 30 μm in the longest axis; Kawaguchi et al., 1995). Consequently, we have used the ability to visually identify neurones within brain slices in order to selectively record from these morphologically distinct cells.
These neurones also demonstrate a number of electrophysiological and pharmacological characteristics which distinguish them from other striatal subtypes. For example, these neurones demonstrate a relatively depolarized resting membrane potential and are often seen to fire spontaneous action potentials at rest which are followed by a relatively large and prolonged after-hyperpolarization (Kawaguchi, 1992; 1993). Under resting conditions, these neurones exhibit a large input resistance and display a prominent sag in the voltage response to hyperpolarizing current injection due to the presence of an IH like conductance (Jiang & North, 1991). Pharmacologically, cholinergic interneurones are readily depolarized by the NK1 receptor stimulation (Aosaki & Kawaguchi, 1996; Bell et al., 1998) and together with the somatostatin-containing interneurones are known to exclusively express tachykinin NK1 receptors within the striatum (Kaneko et al., 1993). Thus we have been able to use these electrophysiological and pharmacological criteria to confirm the nature of these visually identified neurones. Finally, to confirm the cholinergic nature of these cells, 11 were examined for the expression of choline acetyltransferase (ChAT), the marker enzyme for cholinergic cells, using the technique of single cell RT–PCR. Using this technique, all cells identified as cholinergic on the basis of the above criteria were found to be positive for ChAT and thus, by definition, were cholinergic.
Nature of the histamine receptor
In the present study, histamine is shown to depolarize cholinergic interneurones within the rat striatum via the activation of an inward current. The effects of histamine were mimicked by the highly selective H1 receptor agonist 2-thiazolylethylamine and blocked by the potent and selective H1 antagonist triprolidine suggesting that these effects are mediated via the H1 histamine receptor subtype. This finding is in agreement with reports that 2-thiazolylethylamine can increase acetylcholine release from the rat striatum in vivo in a manner that is blocked by H1 antagonists (Prast et al., 1997). The nature of the histamine receptor responsible for these effects was confirmed in the current study using single cell RT–PCR and in situ hybridization. This finding represents the first demonstration of histamine receptor expression in these cells. A proportion of cholinergic interneurones did not express detectable levels of H1 receptor and this finding suggests that cholinergic interneurones may not be a homogeneous population of neurones or may modulate their expression of H1 receptor mRNA in a temporal or spatial fashion.
In large neurones acutely dissociated from the rat striatum, histamine has been reported to evoke a depolarization that is mediated predominantly by H1 receptors at concentrations up to 1 μM and by H2 receptors at higher concentrations (Munakata & Akaike, 1994). In the present study we were unable to detect any H2 histamine receptor mRNA by single cell RT–PCR in any of the cholinergic neurones tested. Similarly using in situ hybridization, H2 receptors were only identified in a very small number of cells. In light of these findings, since the neurones investigated in the former study were not confirmed as cholinergic interneurones, it is possible that other striatal interneurones may express the H2 receptor.
Mechanism of action
Some studies have reported that histamine can modulate the release of dopamine from striatal nerve terminals (Nowak, 1985) and recent studies have shown that dopamine can depolarize cholinergic interneurones through a direct D1 receptor-mediated activation (Aosaki et al., 1998). However, the persistence of a histamine-induced depolarization in the presence of TTX and low Ca2+-containing solutions in the present study indicates that histamine acts directly on cholinergic interneurones and is not mediating its effect by stimulating the release of dopamine or other depolarizing substance from surrounding neurones. This is consistent with previous reports of the direct postsynaptic actions of histamine in other areas of the brain such as the suprachiasmatic nucleus (Stehle, 1991), supraoptic nucleus (Li & Hatton, 1996), nucleus basalis (Khateb et al., 1995) and the medial septum (Gorelova & Reiner, 1996).
In normal physiological saline the histamine-induced inward current was not associated with a consistent change in membrane conductance or reversal potential. To examine the exact nature of this current, the ionic content of the bathing solution was manipulated and modulators of ion channels were included. Thus the histamine-activated current was seen to be sensitive to the replacement of external Na+ with Tris-HCl and the blockade of K+ channels with external Ba2+ or intracellular Cs+. These observations are consistent with the histamine-activated current being carried in part by a Na+-dependent cation channel, and in part by the inhibition of a K+ conductance. Similar findings have been reported in cholinergic neurones of the medial septum and diagonal band of Broca (Gorelova & Reiner, 1996), whilst in neurones from rat supraoptic nucleus, cortex and thalamus histamine has been shown to suppress a K+ leak conductance (McCormick & Williamson, 1991; Reiner & Kamondi, 1994; Li & Hatton, 1996).
Functional significance
The data presented provides an explanation for the observation that histamine enhances striatal acetylcholine release in vivo (Prast et al., 1997). The rapid depolarization induced by histamine acting at H1 receptors readily brings cholinergic interneurones to threshold for firing action potentials and suggests that histamine may be a significant neuromodulator in these cells. In behavioural studies, intrastriatal injection of histamine has been shown to have a marked effect on behaviour and locomotion possibly through modulation of cholinergic cell activity (Bristow & Bennett, 1988; López-Garcia et al., 1993; Ito et al., 1996). Furthermore, since it has been demonstrated that histaminergic transmission may be altered in diseases of the basal ganglia such as Huntington's disease (Martinez-Mir et al., 1993), it is conceivable that modulation of histamine action in these neurones may offer considerable scope for therapeutic intervention (Graybriel, 1990).
Abbreviations
- cDNA
complementary deoxyribonucleic acid
- ChAT
choline acetyltransferase
- D-AP5
D-2-amino-5-phosphovalerate
- mRNA
messenger ribonucleic acid
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxalone
- RT–PCR
reverse transcriptase polymerase chain reaction
- SSC
saline-sodium citrate buffer
- TTX
tetrodotoxin
References
- AOSAKI T, KAWAGUCHI Y. Actions of substance P on rat striatal neurons in vitro. J. Neurosci. 1996;16:5141–5153. doi: 10.1523/JNEUROSCI.16-16-05141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOSAKI T., KITUCHI K, KAWAGUCHI Y. Dopamine D1-like receptor activation excites rat striatal large aspiny neurons in vitro. J. Neurosci. 1998;18:5180–5190. doi: 10.1523/JNEUROSCI.18-14-05180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARRANG J.M., GARBARG M., LANCELOT J.C., LECONTE J.M., POLLARD H., ROBBA M., SCHUNACK W, SCHWARTZ J.C. Highly potent and selective ligands for histamine H3-receptors. Nature. 1987;327:117–123. doi: 10.1038/327117a0. [DOI] [PubMed] [Google Scholar]
- BELL M.I., RICHARDSON P.J, LEE K. Characterization of the mechanism of action of tachykinins in rat striatal cholinergic interneurons. Neuroscience. 1998;87:649–658. doi: 10.1016/s0306-4522(98)00187-0. [DOI] [PubMed] [Google Scholar]
- BOUTHENET M.L., RUAT M., SALES N., GARBARG M, SCHWARTZ J.C. A detailed mapping of histamine H1-receptors in guinea-pig central nervous system established by autoradiography with [125I]iodobolypyramine. Neuroscience. 1988;26:553–600. doi: 10.1016/0306-4522(88)90167-4. [DOI] [PubMed] [Google Scholar]
- BRISTOW L.J, BENNETT G.W. Biphasic effects of intra-accumbens histamine administration on spontaneous motor activity in the rat: a role for central histamine receptors. Br. J. Pharm. 1988;95:1292–1302. doi: 10.1111/j.1476-5381.1988.tb11767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARO E., ARBONÉS L., GARCÍA A, PICOTOSTE F. Phosphoinositidase hydrolysis mediated by histamine H1-receptors in rat brain cortex. Eur. J. Pharm. 1986;123:187–196. doi: 10.1016/0014-2999(86)90659-x. [DOI] [PubMed] [Google Scholar]
- DI CHIARA G., MORELLI M, CONSOLO S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- DIFIGLIA M, CAREY J. Large neurons in the primate neostriatum examined with the combined Golgi-electron microscope method. J. Comp. Neurol. 1986;244:36–52. doi: 10.1002/cne.902440104. [DOI] [PubMed] [Google Scholar]
- DIXON A.K., RICHARDSON P.J., LEE K., CARTER N.P, FREEMAN T.C. Expression profiling of single cells using three prime end amplification (TPEA) PCR. Nucleic Acids Research. 1998;26:4426–4431. doi: 10.1093/nar/26.19.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA M., FLORAN B., ARIAS-MONTANO J.A., YOUNG J.M, ACEVES J. Histamine H3 receptor activation selectively inhibits dopamine D1 receptor-dependent [3H]GABA release from depolarization-stimulated slices of rat substantia nigra pars reticulata. Neuroscience. 1997;80:241–249. doi: 10.1016/s0306-4522(97)00100-0. [DOI] [PubMed] [Google Scholar]
- GORELOVA N, REINER P.B. Histamine depolarizes cholinergic septal neurons. J. Neurophysiol. 1996;75:707–714. doi: 10.1152/jn.1996.75.2.707. [DOI] [PubMed] [Google Scholar]
- GRAYBRIEL A.M. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- INAGAKI N., YAMATODANI A., ANDO-YAMAMOTO M., TOHYAMA M., WATANABE T, WADA H. Organisation of histaminergic fibres in rat brain. J. Comp. Neurol. 1988;273:283–300. doi: 10.1002/cne.902730302. [DOI] [PubMed] [Google Scholar]
- ITO C., ONODERA K., SAKURAI E., SATO M, WATANABE T. Effects of dopamine antagonists on neuronal histamine release in the striatum of rats subjected to acute and chronic treatments with methamphetamine. J. Pharm. Exp. Ther. 1996;279:271–276. [PubMed] [Google Scholar]
- JIANG Z.-G, NORTH R.A. Membrane properties and synaptic responses of rat striatal neurons in vitro. J. Physiol. 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANEKO T., SHIGEMOTO R., NAKANISHI S, MIZUNO N. Substance P receptor-immunoreactive neurons in the rat neostriatum are segregated into somatostinergic and cholinergic aspiny neurons. Brain Res. 1993;631:297–303. doi: 10.1016/0006-8993(93)91548-7. [DOI] [PubMed] [Google Scholar]
- KAWAGUCHI Y. Large aspiny cells in the matrix of the rat neostriatum in vitro: Physiological identification, relation to the compartments and excitatory postsynaptic currents. J. Neurophysiol. 1992;67:1669–1682. doi: 10.1152/jn.1992.67.6.1669. [DOI] [PubMed] [Google Scholar]
- KAWAGUCHI Y. Physiological, morphological, and histochemical characterization of three classes of interneurones in rat neostriatum. J. Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAGUCHI Y., WILSON C.J., AUGOOD S.J, EMSON P.C. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- KHATEB A., FORT P., PEGNA A., JONES B.E, MÜHLETHALER M. Cholinergic nucleus basalis neurons are excited by histamine in vitro. Neuroscience. 1995;69:495–506. doi: 10.1016/0306-4522(95)00264-j. [DOI] [PubMed] [Google Scholar]
- KWIATOWSKI H. Histamine in nervous tissue. J. Physiol. 1943;102:32–41. doi: 10.1113/jphysiol.1943.sp004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE K., DIXON A.K., FREEMAN T.C, RICHARDSON P.J. Characterization of the KATP channel current present in striatal cholinergic interneurones. J. Physiol. 1998;510:441–453. doi: 10.1111/j.1469-7793.1998.441bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEURS R., HOFFMANN M., WIELAND K, TIMMERMAN H. H3 receptor is cloned at last. Trends Pharm. Sci. 2000;21:11–12. doi: 10.1016/s0165-6147(99)01411-x. [DOI] [PubMed] [Google Scholar]
- LI Z, HATTON G.I. Histamine-induced prolonged depolarization in rat supraoptic neurons: G protein-mediated, Ca2+ independent suppression of K+ leakage conductance. Neuroscience. 1996;70:145–158. doi: 10.1016/0306-4522(95)00373-q. [DOI] [PubMed] [Google Scholar]
- LÓPEZ-GARCIA J.A., RAMIS C., NICOLAU M.C., ALEMANY G., PLANAS B, RIAL R. Histaminergic drugs in the caudate nucleus: effects on learned helplessness. Pharm. Biochem. Behav. 1993;45:275–282. doi: 10.1016/0091-3057(93)90239-p. [DOI] [PubMed] [Google Scholar]
- MARTINEZ-MIR M.I., POLLARD H., MOREAU J., TRAIFFORT E., RUAT M., SCHWARTZ J.C, PALACIOS J.M. Loss of striatal histamine H2 receptors in Huntington's chorea but not Parkinson's disease: comparison with animal models. Synapse. 1993;15:209–220. doi: 10.1002/syn.890150306. [DOI] [PubMed] [Google Scholar]
- MCCORMICK D.A, WILLIAMSON A. Modulation of neuronal firing mode in cat and guinea pig LGNd by histamine: possible cellular mechanisms of histaminergic control of arousal. J. Neurosci. 1991;11:3188–3199. doi: 10.1523/JNEUROSCI.11-10-03188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNAKATA M, AKAIKE N. Regulation of K+ conductance by histamine H1 and H2 receptors dissociated from rat neostriatum. J. Physiol. 1994;480:233–245. doi: 10.1113/jphysiol.1994.sp020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOWAK J.Z. Depolarization-evoked release of dopamine and histamine from brain tissue and studies on presynaptic dopamine-histamine interaction. Pol. J. Pharmacol. Pharm. 1985;37:359–382. [PubMed] [Google Scholar]
- PALACIOS J.M., WAMSLEY J.K, KUHAR M.J. The distribution of histamine H1 receptors in the rat brain: an autoradiographic study. Neuroscience. 1981;6:15–37. doi: 10.1016/0306-4522(81)90240-2. [DOI] [PubMed] [Google Scholar]
- PRAST H., FISCHER H., TRAN M.H., GRASS K., LAMBERTI C, PHILIPP U. Modulation of acetylcholine release in the ventral striatum by histamine receptors. Inflamm. Res. 1997;46:S37–S38. [PubMed] [Google Scholar]
- REINER P.B, KAMONDI A. Mechanisms of antihistamine-induced sedation in the human brain: H1 receptor activation reduces a background leakage potassium current. Neuroscience. 1994;59:579–588. doi: 10.1016/0306-4522(94)90178-3. [DOI] [PubMed] [Google Scholar]
- RUAT M., TRAIFFORT E., BOUTHENET M.L., SCHWARTZ J.C., HIRSCHFELD J., BUSCHAUER A, SCHUNACK W. Reversible and irreversible labelling and autoradiographic localization of the cerebral histamine H2 receptor using [125I] iodinated probes. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1658–1662. doi: 10.1073/pnas.87.5.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL W.L., HENRY D.P., PHEBUS L.A, CLEMENS J.A. Release of histamine in rat hypothalamus and corpus striatum in vivo. Brain Res. 1997;512:95–101. doi: 10.1016/0006-8993(90)91175-g. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ J.-C., ARRANG J.-M., GARBARG M., POLLARD H, RUAT M. Histaminergic transmission in the mammalian brain. Physiol. Rev. 1991;71:1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- STEHLE J. Effects of histamine on spontaneous electrical activity of neurons in rat suprachiasmatic nucleus. Neurosci. Lett. 1991;130:217–220. doi: 10.1016/0304-3940(91)90400-n. [DOI] [PubMed] [Google Scholar]
- TRAIFFORT E., RUAT M., ARRANG J.M., LEURS R., PIOMELLI D, SCHWARTZ J.C. Expression of a cloned rat histamine H2 receptor mediating inhibition of arachidonate release and activation of cAMP accumulation. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2649–2653. doi: 10.1073/pnas.89.7.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]


