Abstract
The mechanisms involved in the apoptotic effect of saikosaponin-d, a triterpene saponin from Bupleurum falcatum L., were studied in human CEM lymphocytes and compared with those of dexamethasone (3×10−7 M).
Saikosaponin-d (10−8 to 10−5 M) inhibited the serum-stimulated [3H]-thymidine incorporation in a concentration-dependent manner. Dexamethasone also inhibited serum-stimulated [3H]-thymidine incorporation.
Cell viability was unaffected by saikosaponin-d until 10−5–10−4 M. Dexamethasone significantly reduced the number of viable cells.
Following saikosaponin-d (10−5–10−4 M) treatment, flow cytometry analysis of propidium iodide-stained cells showed a significant increase in the percentage of cells in the apoptotic region. Dexamethasone also significantly increased the percentage of apoptotic cells. The supravital exposure to propidium iodide and annexin V labelling demonstrated that saikosaponin-d (10−5–10−4 M) induced apoptosis as well as necrosis.
The apoptotic effect of saikosaponin-d (3×10−6–10−4 M) was also demonstrated by TUNEL analysis and DNA laddering. The percentage of apoptotic cells induced by saikosaponin-d (3×10−6–10−5 M) was unaffected by the presence of Z-VAD-FMK, indicating that saikosaponin-d-induced apoptosis may not be mediated by caspase activity. However, the percentage of apoptotic cells induced by dexamethasone was significantly reduced by the presence of Z-VAD-FMK.
Levels of c-myc, p53, and bcl-2 mRNA were analysed by the reverse transcription-polymerase chain reaction. Levels of c-myc and p53 mRNA were significantly increased, while the level of bcl-2 mRNA was decreased, by saikosaponin-d (10−5 M) treatment. Dexamethasone did not significantly change the expression of these genes.
It is suggested that the apoptotic effect of saikosaponin-d may be partly mediated by increases in c-myc and p53 mRNA levels accompanied by a decrease in bcl-2 mRNA level.
Keywords: Saikosaponin-d, apoptosis, CEM lymphocytes, c-myc, p53, bcl-2, caspase
Introduction
Saikosaponins, triterpene saponins from the root of Bupleurum falactum, have a common steroid-like structure and are expected to have some steroid-related pharmacological activities. Glucocorticoid-induced apoptosis (programmed cell death) is a well-recognized physiological regulator of murine T-cell number and function (Brunetti et al., 1995). Saikosaponin-d (Figure 1) has been reported to have a cell type-dependent immuno-modulatory action (Kato et al., 1995). Thus, the apoptotic effect of saikosaponin-d was investigated in CEM lymphocyte and compared with that of dexamethasone, a synthetic glucocorticoid, in this study. Saikosaponins are reported to have a variety of therapeutic effects in hyperlipidaemia, hepatic injury, chronic hepatitis and inflammation (Ohuchi et al., 1985), and are the major components of a Chinese herbal medicine, xiao-chai-hu-tang (Japanese name: sho-saiko-to), a famous haematopoietic remedy in Oriental medicine. It has been reported that the herbal medicine sho-saiko-to and/or saikoasponins can induce apoptosis in hepatocellular carcinoma cell lines, liver cells, a cholangiocarcinoma cell line, a pancreatic cancer cell line, and a melanoma cell line (Motoo & Sawabu, 1994; Yano et al., 1994; Kato et al., 1995; Qian et al., 1995; Zong et al., 1996). It has also been demonstrated that the ginsenoside Rh2, one of the ginseng saponins, also induces apoptosis of human hepatoma cells (Park et al., 1997). Apoptosis plays a critical role in both the normal development and the pathology of a wide variety of tissues and is characterized by cytoplasmic shrinkage, nuclear condensation, and DNA fragmentation (Jacobson et al., 1997; Nagata, 1997). Initiation of apoptosis is controlled by regulation of the balance between the death and life signals perceived by the cell (Musci et al., 1997). In recent years, the regulation of the cell cycle and apoptosis has received much attention as a possible means of eliminating excessively proliferating cells. The processes involved in immune defense and inflammation consist of several proliferative responses at the tissue and cell lineage levels. Thus, saikosaponin-d may be useful as a new template for the development of better immunosuppressive and anti-inflammatory agents. Atherosclerosis and post-angioplastic restenosis are characterized by abnormal accumulation of vascular smooth muscle cells, inflammatory cells, and extracellular matrix proteins (Lundergan et al., 1991; Ross, 1993). Saikosaponin-d induces cell apoptosis in both lymphocytes and vascular smooth muscle cells and thus may be useful for the study and treatment of the pathological changes associated with atherosclerosis and restenosis. Yamamoto et al. (1975) have also reported that saikosaponin-a and saikosaponin-d, but not saikosaponin-c, have anti-inflammatory and plasma cholesterol-lowering effects.
Figure 1.
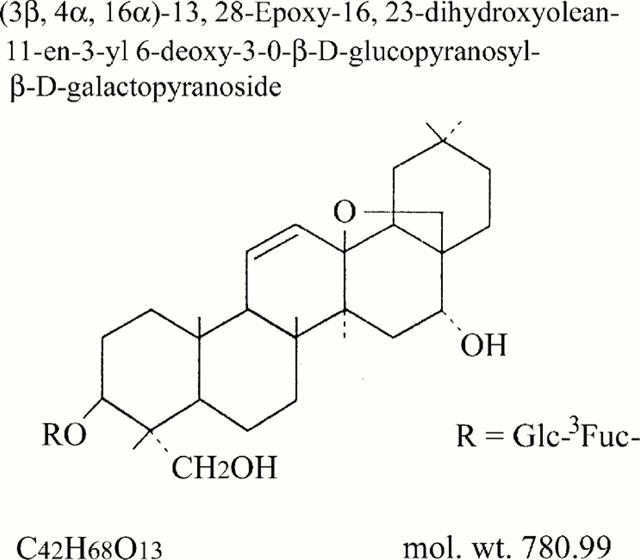
Structure of saikosaponin-d.
In the present study, the possible mechanisms involved in the apoptotic effect of saikosaponin-d were investigated in human CEM lymphocytes. The transmission of signals from the plasma membrane to the nucleus involves a number of different pathways. We have previously shown the apoptotic effect of curcumin to be mediated by inhibition of c-myc and bcl-2 mRNA expression (Chen & Huang, 1998) and it was therefore of interest to investigate the effects of saikosaponin-d on genes expression. Some proto-oncogenes, bcl-2 and c-myc, and a tumour suppressor gene, p53, were reported to regulate cell proliferation and/or apoptosis (Hale et al., 1996). In this study, the molecular mechanisms involved in the apoptotic effect of saikosaponin-d in CEM cells were investigated to determine whether the changes of gene expression were involved.
Methods
Measurement of DNA synthesis and cell viability
DNA synthesis was measured by uptake of [3H]-thymidine (Watabe et al., 1984; Huang et al., 1992). Human CEM lymphocytes, a type of lymphoblastoid leukaemia cells, were grown in RPMI medium (supplemented with 10% v v−1 foetal calf serum (FCS), 100 ug ml−1 penicillin, and 1 μg ml−1 streptomycin). Prior to all experiments, confluent cells were rendered quiescent by culturing for 48 h (with one medium change at 24 h) in 0.5 % v v−1 FCS-containing medium. Then in quiescent CEM cells (3×103 cells well−1), 5% v v−1 FCS and the test compound were added to the medium and incubated for another 48 h. Twenty-four hours after FCS or test compound addition, [3H]-thymidine (1 μCi well−1) was included in the medium. After 24 h incubation, the cells were harvested and the [3H]-thymidine incorporated to cells was counted.
Each experiment was performed in triplicate and repeated five or six times. The inhibitory activity of test compound was expressed as a percentage of the serum-stimulated control value in the absence of test compound. The concentration evoking 50% inhibition (IC50) was calculated for each experiment.
Cell viability was determined with the trypan blue dye exclusion method. After addition of FCS or the test compound for 48 h, cells were harvested, treated with trypan blue and the viability was determined by cell counting using a haemocytometer.
Flow cytometric analysis
Cells, synchronized at the G0 phase by serum depletion for 48 h, were washed, then incubated in fresh medium containing 5% v v−1 FCS to allow the progression through the cell cycle. At various time periods after release from the quiescent state, cell cycle distribution was analysed by flow cytometry (Sherwood & Schimke, 1995; Chen & Huang, 1998). After various treatments, CEM cells (5×106 cells sample−1) were pelleted, washed twice with PBS (pH 7.4), and re-suspended in ice-cold 70% v v−1 ethanol at −20°C overnight. Cells were washed with 0.4 ml phosphate-citric acid buffer (pH 7.8) containing 50 mM Na2HPO4, 25 mM citric acid and 0.1% Triton X-100, for 5 min and stained with 1.5 ml propidium iodide (PI) staining buffer containing 0.1% Triton X-100, 10 mM PIPES, 100 mM NaCl, 2 mM MgCl2, 100 μg ml−1. RNase A, 50 μg ml−1 PI, for more than 30 min in the dark before cytometric analysis. Cells were filtered on a nylon mesh filter. The samples were analysed using FACScan and Cellquest program (Becton Dickinson). Each experiment was repeated five or six times.
Apoptotic cells were also detected by PI and/or annexin V labelling as described by Boersma et al. (1996) and Zamai et al. (1996). The double labelling was performed at 37°C by treating cells with PI (50 μg ml−1) and annexin V (2 μg ml−1) for 2 h. The staining was then immediately analysed on a FACScan. Annexin V is a protein that binds to phosphatidylserine residues, which are exposed on the cell surface of apoptotic, but not normal, cells. In healthy cells, the distribution of the phosphatidylserine groups in the plasma membrane is asymmetrical such that the groups are directed toward the inside of the cell. During apoptosis, this asymmetry is lost, and the phosphatidylserine groups are exposed to the exterior of the cell membrane. Annexin V staining is therefore an established biochemical marker of apoptosis. The partial loss of membrane integrity or functionality is a useful criterion for distinguishing apoptotic from necrotic and living cells.
The terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labelling (TUNEL) assay for apoptosis
Incorporation of modified nucleotides at the free 3′-OH ends of DNA in cultured cells was modified from the method described by Gavrieli et al. (1992). Following treatment with the test compound, CEM cells were collected and plated on glass slides by cytospin, then fixed with ice-cold 95% v v−1 ethanol. The slides were rehydrated in PBS and incubated in 0.3% v v−1 H2O2 in methanol for 30 min to block endogenous peroxidase. DNA nicks were determined using an in situ cell death detection kit (Boehringer Mannheim), then the specimens were covered for 1 h at 37°C with TUNEL reaction mixture containing enzyme solution (terminal deoxynucleotidyl transferase; TdT) and label solution (modified nucleotide mixture in reaction buffer). The reaction was terminated by washing with PBS and the slides covered with Converter-POD (Anti-fluorescein antibody conjugated with horse-radish peroxidase, POD) for 30 min and visualized by using 3,3′-diaminobenzidine for 10 min. Counterstaining was performed using 5% w v−1 methyl green in 0.1 M sodium acetate solution (pH 4.0) for 2 min, the stained cells being analysed by light microscopy. In each experiment, the negative control received only the label solution without the enzyme solution, and the positive control was exposed for 10 min at room temperature to DNaseI (1 μg ml−1 in 40 mM Tris-HCl, 6 mM MgCl2, pH 7.5) prior to the TUNEL reaction.
DNA laddering
Cleavage of DNA into oligonucleosomal fragments, recognizable as a DNA ladder when electrophoresed on an agarose gel, is usually considered as the biochemical hallmark of apoptosis. Following treatment with the test compound, cells (107 cells sample−1) were washed with PBS, and lysed in cell lysis buffer containing Tris, EDTA and sodium dodecyl sulphate. After the addition of RNase A (0.6 u ml−1), the mixture was incubated at 37°C for 30 min. Protein precipitation solution (ammonium acetate) was added to the samples to eliminate the contamination of proteins and centrifuged at 2000×g for 10 min. Cell lysates were treated with 100% isopropanol to precipitate DNA. The DNA pellet was washed with 70% v v−1 ethanol and dissolved in DNA hydration buffer containing Tris and EDTA. The DNA concentration was determined at 260 nm by spectrophotometry. Twenty μg DNA was electrophoresed on a 1% w v−1 agarose gel containing 0.5 μg ml−1 of ethidium bromide. DNA fragmentation bands were photographed under UV light and analysed using an image analyser (Winstar, Taiwan).
RT–PCR analysis of c-myc, p53 and bcl-2 mRNA expression
The expression of c-myc, p53, and bcl-2 mRNA was analysed by the reverse transcription-polymerase chain reaction (RT–PCR) technique (Wang et al., 1989; Huang et al., 1994). Prior to RNA extraction, confluent cells were rendered quiescent by culturing for 48 h in low serum (0.5% v v−1 FCS) medium, then the test compound together with 5% v v−1 FCS was added to the medium and incubated for the indicated time periods. Total cellular RNA was prepared by acid guanidinium thiocyanate extraction (Chomczynski & Sacchi, 1987) and reverse transcribed into cDNA. Two μg of oligo(dT) primer was added to 1 μg of total cellular RNA and the mixture heated in a 65°C water bath for 10 min to destroy the secondary structure. Twenty μl of reverse transcription reaction mixture containing 1 μg of total cellular RNA, 2 μg oligo(dT) primer, RT buffer (mM: Tris-HCl (pH 8.3) 100, KCl 40, MgCl2 10, spermidine 0.5), 1.25 mM deoxynucleoside triphosphates (dNTPs), 4 mM sodium pyrophosphate, 10 units of RNase inhibitor, and 5 units of AMV (avian myeloblastosis virus) reverse transcriptase was incubated at 42°C for 1 h, heated to 95°C for 5 min, and then quick-chilled at 4°C for 5 min. The amounts of reverse-transcriptase products taken for PCR amplification of the test transcripts were within the ranges of 0.5 to 5 μl. PCR was performed in a final volume of 50 μl in 1×PCR buffer containing 0.4 μM of each 3′ and 5′ primers, and 2.5 unit of Taq DNA polymerase (HT Biotech). The mixture was amplified for 30 cycles using a Perkin-Elmer thermal cycler (GeneAmp PCR System 9600), using an amplification profile of denaturation at 94°C for 45 s, annealing at 60°C for 45 s and extension at 72°C for 2 min. Primers for c-myc, p53, and bcl-2 were used to generate 479, 371, and 235 bp fragments, respectively. The GAPDH primer (452 bp) was used as the internal standard. In each experiment, a negative control without reverse transcriptase was performed. Amplification products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. The gel was photographed using Polaroid 667 film, and the digitized images analysed using an image analyser (Winstar, Taiwan). The signal intensity for the test gene was normalized to their respective GAPDH signal intensity and expressed in arbitrary units. The results were confirmed by Northern blotting using standard methods (Sambrook et al., 1989).
In a preliminary experiment, various aliquots taken from the RT reaction were subjected to PCR for analysis of the test transcripts. The amount of cDNA produced was proportional to the inputs of the RT–PCR products over the range of 0.5 to 5 μl and thus, in subsequent experiments, the amount of cDNA used for PCR amplification of the test transcripts was within this linear range, in order to ensure that the amounts of cDNA produced truly reflect the levels of mRNA in the original samples.
Materials
Saikosaponin-d (Figure 1) was purchased from Nacalai Tesque (Kyoto, Japan). Human CEM lymphocytes were purchased from the American Type Culture Collection (Rockville, MD, U.S.A.). FCS, penicillin, streptomycin, and RPMI 1640 medium were purchased from Gibco Lab. (Grand Island, NY, U.S.A.). Thymidine [methyl-3H] (5 Ci mmol−1) was purchased from Amersham Co. (Buckinghamshire, U.K.). Dexamethasone (water-soluble) and 3,3′-diaminobenzidine were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). The in situ cell death detection kit, Converter-POD (antifluorescein antibody, Fab fragment from sheep, conjugated with horse-radish peroxidase), and annexinV-FLUOS (fluorescence-conjugated anticoagulant) were purchased from Boehringer Mannheim Biochemicals (Indianapolis, IN, U.S.A.). The DNA isolation kit was purchased from Gentra systems, Inc. (Research Triangle Park, NC, U.S.A.). AMV reverse transcriptase and Taq DNA polymerase were purchased from HT Biotech. Ltd. (Cambridge, U.K.). Primer for bcl-2 (235 bp) was purchased from Maxim Biotech, Inc. (So. San Francisco, CA, U.S.A.). Primers for c-myc (479 bp), p53 (371 bp), and GAPDH (452 bp) were purchased from Clontech Lab., Inc. (Palo Alto, CA, U.S.A.). Z-VAD-FMK, the caspase inhibitor, was purchased from Calbiochem (San Diego, CA, U.S.A.).
Statistical analysis
The data are expressed as mean±s.e.mean. P values less than 0.05 were considered to be statistically significant (ANOVA and Student's t-test).
Results
Effect on DNA synthesis
DNA synthesis was studied by incorporation of [3H]-thymidine into cellular DNA. The control value for serum-stimulated [3H]-thymidine incorporation in CEM cells (3×103 cells well−1) in the absence of test compound was 9205±945 c.p.m. well−1. Exposure of CEM cells to saikosaponin-d (10−8–10−5 M) for 48 h significantly inhibited serum-stimulated [3H]-thymidine incorporation in a concentration-dependent manner (n=5) (Figure 2). The IC50 value of saikosaponin-d was 3.1±0.4×10−6 M and the maximal inhibition value was 100.0±0.0% at 10−5 M (n=5). In this study, dexamethasone (3×10−7 M) inhibited the serum-stimulated [3H]-thymidine incorporation by 93.0±3.3% (Figure 2) (n=5).
Figure 2.
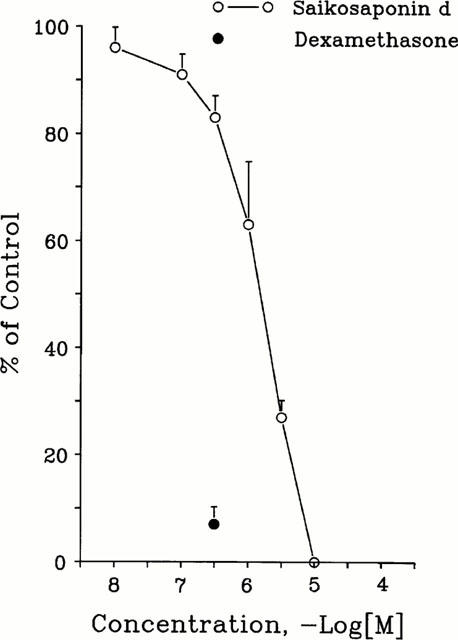
Effects of saikosaponin-d on DNA synthesis in CEM cells. DNA synthesis was measured by uptake of [3H]-thymidine. The control value for serum-induced [3H]-thymidine incorporation in the absence of test compound was 9205±945 c.p.m. well−1. The inhibitory activity of the test compound is expressed as a percentage of the control value (% of control). Each point with vertical line represents the mean and s.e. mean (n=5).
Cell viability
Cell viability, determined by trypan blue dye exclusion method, was not affected by 10−6 M saikosaponin-d (n=5) (Figure 3) and the number of viable cells was greater than the basal value (2×105 cells). However, in the concentration range of 10−6 to 10−4 M, saikosaponin-d significantly reduced cell viability (n=5). In this study, dexamethasone (3×10−7 M) also significantly reduced the numbers of viable cells (n=5).
Figure 3.
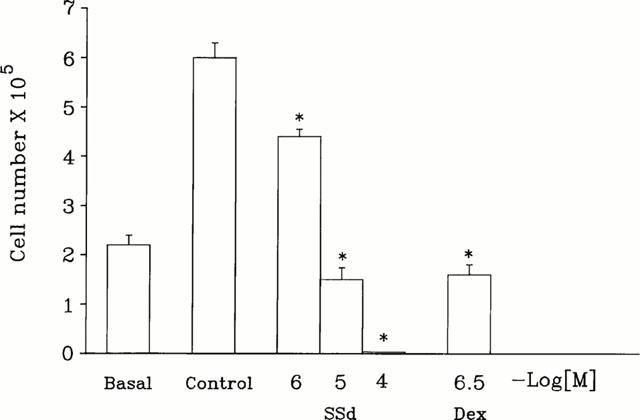
Effect of saikosaponin-d on CEM cell viability. Quiescent CEM cells (basal level, 2×105 cells) were stimulated with serum. After addition of serum for 48 h in the absence (control level) or presence of test compound, the cells were harvested and their viabilities were examined by trypan blue dye exclusion test. The number of viable cells was estimated using a haemocytometer. Each column represents the mean±s.e.mean (n=5). *P<0.05, compared to the cell number in control group without saikosaponin-d and dexamethasone treatment (control level).
Apoptosis
Cell apoptosis in CEM cells was induced by saikosaponin-d (3×10−6–10−4 M) treatment for 24 and 48 h. Some shrunken cells, condensed chromatin and blebbed apoptotic bodies were observed. Some surviving cells also showed a certain degree of morphological change, such as having an elongated and bipolar appearance. The induced apoptosis was then characterized by flow cytometry, TUNEL analysis, and DNA laddering.
Flow cytometry analysis
The percentage of apoptotic CEM cells following test compound treatment was further analysed by flow cytometry (Figure 4). Prior to experiments, cells were synchronized at the G0 phase in the basal condition after 48 h of serum depletion, then the synchronized cells were stimulated with serum to allow progression through the cell cycle. Cells were stained with PI and analysed by flow cytometry. Figure 4 shows the concentration- and time-dependent effects of saikosaponin-d in inducing apoptosis. The percentage of cells in the apoptotic region (Ap, sub-G0/G1 peak, subdiploid peak) was significantly increased following 3×10−6 M saikosaponin-d treatment (48 h) from 1.1±0.32 to 5.1±0.6% (Figure 4) (n=3). At 10−5 M (48 h), saikosaponin-d increased the cells in the apoptotic region to 22.2±1.9% (n=3). In this study, 3×10−7 M dexamethasone (48 h) also significantly increased the percentage of cells in the apoptotic region to 11.5±0.8% (n=6) (Figure 4).
Figure 4.
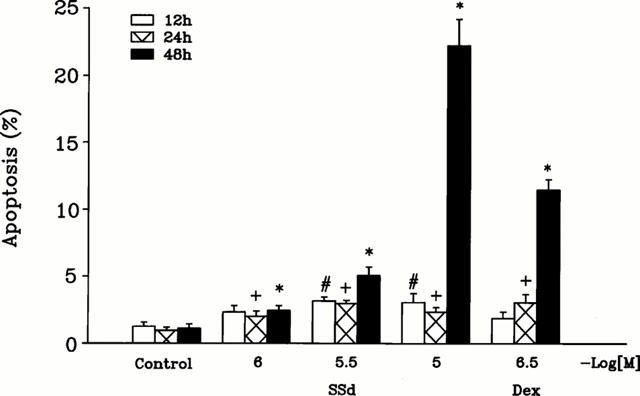
Effect of saikosaponin-d on the percentage of apoptotic CEM cells. Quiescent CEM cells were stimulated by serum in the absence or presence of test compound for the indicated time periods. The percentage of apoptotic cells untreated (control) and treated with saikosaponin-d for 12, 24 or 48 h was analysed by flow cytometric analysis of PI-stained cells as described in Methods. The effect of test compound was compared to that of 3×10−7 M dexamethasone. Each column represents the mean±s.e.mean (n=3 ∼ 6). #P<0.05, when compared to the control value (without test compound) for 12 h treatment. +P<0.05, when compared to the control value (without test compound) for 24 h treatment. *P<0.05, when compared to the control value (without test compound) for 48 h treatment.
We also used supravital exposure to PI and annexin V labelling for the detection of apoptosis. As compared to the untreated cells (Figure 5a), the cells treated with saikosaponin-d 3×10−6 M (Figure 5b) and 10−5 M (Figure 5c) showed a high proportion of annexin V+ and displayed two levels of labelling: the annexin V+ cells remained Pl− (lower right quadrant), corresponding to the early apoptotic cells, and the annexin V+PI+ cells (upper right quadrant), corresponding to the advanced apoptotic cells and/or necrotic cells. Thus, our results demonstrated that saikosaponin-d at 3×10−6 M induced cell apoptosis, while at 10−5 M it markedly induced cell necrosis. Dexamethasone at 3×10−7 M also induced cell apoptosis as evidenced by the increased number of annexin V+PI−-labelled cells (Figure 5d).
Figure 5.
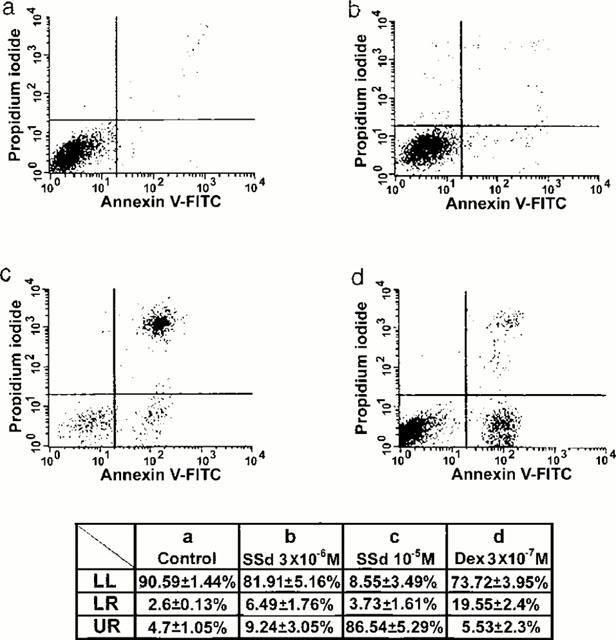
Contour diagram of annexin V/PI flow cytometry of CEM cells. CEM cells untreated (a), or treated with saikosaponin-d, 3×10−6 M (b) or 10−5 M (c), or 3×10−7 M dexamethasone (d) were performed PI and annexin V labelling as described in the Methods. The lower left quadrant of each panel (LL, annexin V−PI−) shows the viable cells, which exclude PI and are negative for annexin V binding. The lower right quadrant (LR, annexin V+PI−) represents the early apoptotic cells, annexin V positive and PI negative, demonstrating cytoplasmic membrane integrity. The upper right quadrant (UR, annexin V+PI+) contains advanced apoptotic cells and necrotic cells, which are positive for annexin V binding and for PI uptake. The percentages of cells in these three quadrants were calculated from four experiments and were summarized as shown in the table.
TUNEL assay
The TUNEL procedure stains nuclei that contain nicked DNA, a characteristic exhibited by cells in the early stages of apoptotic cell death (Figure 6). Untreated (Figure 6d) or cells treated with saikosaponin-d 10−6 M (Figure 6a) were almost negative for TUNEL staining, with only 1.6±0.2 and 3±0.1% of TUNEL-positive cells respectively. These cells with nuclei not stained with TUNEL (TUNEL-negative, green-coloured) were visualized by counterstaining with methyl green. In contrast, following 48 h treatment with 10−5 (Figure 6b) or 10−4 M (Figure 6c) saikosaponin-d, a large number of TUNEL-positive (brown) cells (53±1.01 and 100%, n=3, for 10−5 and 10−4 M, respectively) were seen, the stain being localized in the nuclei. TUNEL-positive cells (21±2.2%) were also seen following 3×10−7 M dexamethasone treatment (n=3) (Figure 6e).
Figure 6.
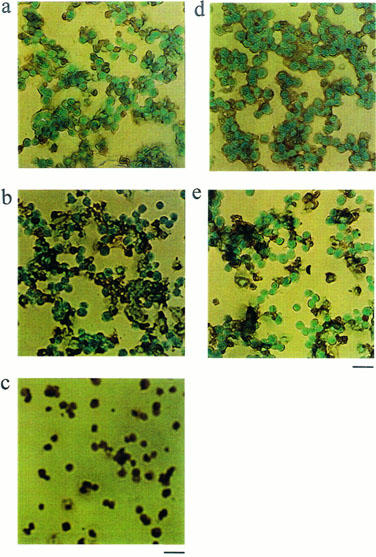
TUNEL analysis of CEM cells. CEM cells treated with (a) 10−6 M saikosaponin-d, (b) 10−5 M saikosaponin-d, (c) 10−4 M saikosaponin-d, (d) control, untreated with test compound, or (e) treated with 3×10−7 M dexamethasone were taken through the TUNEL procedure. TUNEL-positive cells were visualized using a peroxidase-substrate system as having brown nuclei. The green-blue nuclei as counter-stained with methyl green indicate TUNEL-negative. Few nuclei in untreated cells were positively labelled for DNA fragmentation, while a large proportion of the nuclei in 10−5 and 10−4 M saikosaponin-d-treated cells or 3×10−7 M dexamethasone-treated cells were TUNEL-positive. Bar=12 μm. Similar results were obtained in three independent experiments.
DNA laddering
Degradation of DNA into a specific fragmentation pattern is a characteristic feature of apoptosis. In contrast to the random fragmentation associated with necrosis, apoptosis-associated DNA fragmentation is characterized by cleavage of the DNA at regular intervals, visualized on agarose gel electrophoresis as a DNA ladder consisting of multimers of approximately 200 base pairs. After 48 h exposure to saikosaponin-d or dexamethasone, the genomic DNA from CEM cells was subjected to agarose gel electrophoresis (n=3) (Figure 7) and a clear DNA fragmentation ladder was seen in samples from the cells treated with 10−4 M saikosaponin-d (n=3) (Figure 7, lane 4); this effect was much less apparent in cells treated with 3×10−6, 10−5 M saikosaponin-d or 3×10−7 M dexamethasone (n=3) (Figure 7, lanes 3 and 5 for 10−5 M saikosaponin-d and 3×10−7 M dexamethasone, respectively). No fragmentation pattern was seen in untreated or 10−6 M saikosaponin-d-treated cells (n=3) (Figure 7, lanes 1 and 2).
Figure 7.
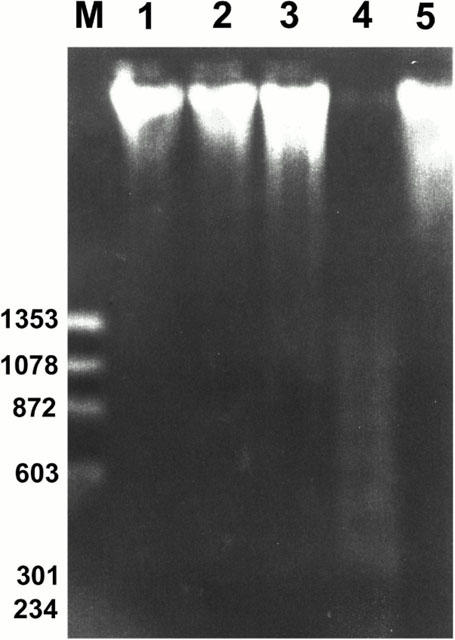
Electrophoresis of fragmented DNA in CEM cells. Genomic DNA was isolated from untreated (control) cells and cells treated with saikosaponin-d or dexamethasone for 48 h. DNA fragmentation was evaluated by electrophoresis on agarose gel containing ethidium bromide and photographed under UV light. The DNA ladder was detected in cells treated with 10−5 or 10−4 M saikosaponin-d. The effect of test compound was compared to that of 3×10−7 M dexamethasone. Lane M, ΦX174/HaeIII DNA size marker; lane 1, untreated control; lane 2, treated with 10−6 M saikosaponin-d; lane 3, treated with 10−5 M saikosaponin-d; lane 4, treated with 10−4 M saikosaponin-d; lane 5, treated with 3×10−7 M dexamethasone. Similar results were obtained in three independent experiments.
Effect of caspase inhibitor
Saikosaponin-d- or dexamethasone-induced apoptosis of CEM cells in the absence or presence of a caspase inhibitor were further analysed by flow cytometry with supravital exposure to PI and annexin V labelling. The percentage of apoptotic cells induced by saikosaponin-d (3×10−6–10−5 M) was unaffected by the presence of Z-VAD-FMK (10−4 M), a cell-permeable fluoromethylketone inhibitor of caspase (5.6±0.9 vs 5.1±0.9% for 3×10−6 M saikosaponin-d, n=6). However, the percentage of apoptotic cells induced by dexamethasone (3×10−7 M, 24 h) was significantly reduced by the presence of Z-VAD-FMK (10−4 M) (45.0±11.1% reduction, n=6).
Effects on c-myc, p53, and bcl-2 mRNA expression
The c-myc, p53, and bcl-2 mRNA levels in serum-stimulated CEM cells, either untreated or treated with test compound, were measured using the RT–PCR technique. The signal intensities for the test genes and GAPDH were quantified using an image analyser, and the changes in the signal intensity of the test genes relative to GAPDH are shown in Figure 8. All three genes were expressed in CEM cells in the presence of serum. In a preliminary experiment, the effect of saikosaponin-d on the expression of c-myc and p53 mRNA was found to be maximal at 12 h and changes in levels were therefore analysed at this time-point in subsequent studies. After addition of saikosaponin-d (10−5 M), the level of c-myc mRNA was significantly increased (3.5±0.8 fold increase, n=3) (lane 2), as was the level of p53 mRNA (6.1±1.1 fold increase, n=3) (lane 5), while the level of bcl-2 mRNA was significantly reduced (0.73±0.03 fold inhibition, n=3) (lane 8). Dexamethasone (3×10−7 M) increased the p53 mRNA level and reduced that for c-myc and for bcl-2 mRNA (lanes 3, 6 and 9); however, the changes did not achieve significance. The effects of saikosaponin-d on the mRNA levels were then confirmed by Northern blotting.
Figure 8.
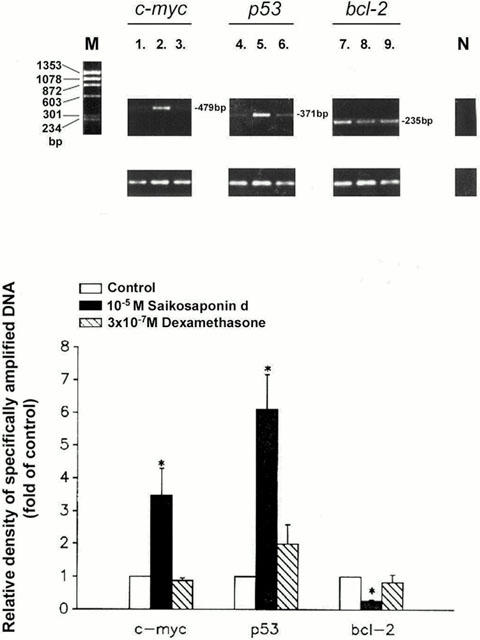
Effects of saikosaponin-d on c-myc, p53, and bcl-2 mRNA levels in CEM cells. Quiescent CEM cells were stimulated by serum in the absence or presence of test compound for 12 h. The serum-stimulated test gene mRNA levels in cells untreated or treated with either 10−5 M saikosaponin-d or 3×10−7 M dexamethasone were analysed by RT–PCR amplification as described in the Methods. Amplification products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. The signal intensities of test genes and GAPDH were quantified using image analyser, and the changes in the signal intensities of the test genes relative to GAPDH were calculated. Results are expressed as percentages of the control level without test compound (% of control) (bottom of figure) (n=3); the corresponding electrophoretic patterns of PCR products are shown at the top of each panel. Lane M, Φ X174/HaeIII DNA size marker; lane 1, 4, 7, control without test compound; lane 2, 5, 8, 10−5 M saikosaponin-d-treated; lane 3, 6, 9, 3×10−7 M dexamethasone-treated. c-myc mRNA level: lane 1, 2, 3; p53 mRNA level: lane 4, 5, 6; bcl-2 mRNA level: lane 7, 8, 9; lane N, negative control, no RT. Each column represents the mean±s.e.mean (n=3). *P<0.05, when compared to the control value without test compound.
Discussion
The present results demonstrate, for the first time, that saikosaponin-d inhibits DNA synthesis and induces cell apoptosis in human CEM lymphocytes. Saikosaponin-d is one of the active triterpenes in Bupleurum falcatum L. Saikosaponin-d has been reported to have a cell type-dependent immuno-modulatory action (Kato et al., 1995). Saikoasponin-d, which itself has no mitogenic activity, decreases the spleen cell proliferative response to T cell mitogens, but increases the response to B cell mitogens (Ushio & Abe, 1991). These authors also demonstrated that saikosaponin-d may stimulate, in vivo, lymphocyte functions, partly by activating certain macrophage functions (Ushio et al., 1991). Saikosaponin-a and -d are also reported to have both cholesterol-lowering and anti-inflammatory effects (Yamamoto et al., 1975). The increase in plasma levels of cholesterol and triglycerides by cholesterol feeding is reduced by saikosaponins (Yamamoto et al., 1975). Atherosclerosis and post-angioplastic restenosis are characterized by abnormal accumulation of vascular smooth muscle cells, inflammatory cells, and extracellular matrix proteins (Lundergan et al., 1991; Ross, 1993). The proliferative responses of both vascular smooth muscle cells and mononuclear cells are important in the pathogenesis of atherosclerosis and restenosis. Consistent with the results observed in CEM lymphocytes, we found that saikosaponin-d also inhibited DNA synthesis and induced cell apoptosis in both human peripheral blood mononuclear cells and A7r5 vascular smooth muscle cell line (data not shown). Thus, saikosaponin-d may also be useful in the study and treatment of atherosclerosis and restenosis. In addition to cell apoptosis, saikosaponin-d at the higher concentration (10−5 M) induced necrosis, as demonstrated by the positive annexin V and PI staining.
Apoptosis, a form of programmed cell death involved in tissue morphogenesis and homeostasis, is characterized by cytoplasmic shrinkage, nuclear condensation and DNA fragmentation. In addition to inhibiting the DNA synthesis, saikosaponin-d (3×10−6–10−4 M) induced cell apoptosis in CEM lymphocytes, as demonstrated by flow cytometry, TUNEL staining, and DNA laddering. The saikosaponin-d-induced apoptosis may, in part, contribute to the reduction of [3H]-thymidine incorporation at the higher concentration. Recent evidence suggests that the failure of cells to undergo apoptotic cell death may be involved in the pathogenesis of a variety of human diseases, including cancer, autoimmune diseases, and viral infections. Specific therapies designed to enhance or decrease the susceptibility of individual cell types to undergo apoptosis could form the basis for treatment of a variety of human diseases (Thompson, 1995).
The immunoregulatory action of saikosaponin-d has been demonstrated by Kato et al. (1994), who showed that saikosaponin-d uniquely modulates T lymphocyte function and that at least one target for the action of saikosaponin-d is located at, or before, the step of c-fos gene transcription, and following T-cell receptor/CD3-mediated protein tyrosine kinase activation. We have previously reported that the apoptotic effect of curcumin, a plant phenol, may partly be mediated by a reduction in c-myc and bcl-2 mRNA expression (Chen & Huang, 1998). Some oncogenes and oncosuppressor genes play a major role in cell cycle progression and cell apoptosis. The signals that induce apoptosis are varied, and the same signals can induce differentiation and proliferation in other situations. Some genes with established roles in the regulation of proliferation and differentiation are also important in controlling apoptosis. Several of these are proto-oncogene, e.g. c-myc, or tumour suppressor gene, e.g. p53. However, some pathways appear to be of particular significance in the control of cell death. One of these is the gene family related to the proto-oncogene, bcl-2 (Hale et al., 1996). We examined the effect of saikosaponin-d on c-myc, p53, and bcl-2 gene expression by measuring the mRNA levels using the RT–PCR technique. Levels of c-myc and p53 mRNA were significantly increased and the level of bcl-2 mRNA significantly decreased in the presence of saikosaponin-d (10−5 M). Thus, alteration in gene expression may partly contribute to the apoptotic effect of saikosaponin-d. However, the changes of the p53 and bcl-2 mRNA levels by dexamethasone treatment did not achieve significance. In mammalian cells, myc is a central regulator of cell proliferation and links external signals to the cell cycle machinery; it also induces cells to undergo apoptosis, unless specific signals provided either by cytokines or by oncogenes block the apoptotic pathway. Recent progress has shed light both on the factors regulating the function and expression of myc and on the downstream targets in the cell cycle. Together, these findings suggest the existence of a novel signal transduction pathway regulating both apoptosis and proliferation (Desbarats et al., 1996). The p53 tumour suppressor gene controls cellular growth after DNA damage through mechanisms involving growth arrest at the G1 phase of the cell cycle and apoptosis (Velculescu & El-Deiry, 1996; Yonish-Rouach, 1996). The outcome, either G1 arrest or apoptosis, will depend on a complex network of regulatory signals. On the other hand, evidence suggests that Bcl-2 can prevent the characteristic internucleosomal DNA fragmentation and cellular morphological changes. Apoptosis induced by saikosaponin-d, but not dexamethasone, in CEM lymphocytes may partly be mediated via c-myc, p53, and bcl-2 gene deregulation.
It has been reported that ginsenoside Rh2, a ginseng saponin, induces apoptosis in human hepatoma SK-HEP-1 cells by caspase-3 activation (Park et al., 1997). There are at least 10 caspases, cysteine proteases, and these form a cascade controlling death in most situations studied thus far (Xue et al., 1996; Alnemri, 1997). It has been demonstrated that dexamethasone-induced apoptosis involves caspase-3 in mouse lymphoma cells and caspase-1 in mouse thymocytes, and both are inhibited by Z-VAD-FMK, a cell-permeable fluoromethylketone inhibitor of caspases (McColl et al., 1998; Cifone et al., 1999). In our results, the apoptosis induced by saikosaponin-d was unaffected by treating cells with Z-VAD-FMK. This finding indicates that saikosaponin-d-induced apoptosis may be not mediated by caspase-1 or caspase-3 activity.
Since saikosaponins have a common steroid-like structure, they are expected to have some steroid-related pharmacological activities. One of the physiologically critical functions of glucocorticoids is to prevent the immune system from overreacting (Munck et al., 1984). Glucocorticoid-induced apoptosis is a well-recognized physiological regulator of murine T cell number and function (Brunetti et al., 1995). In this study, the apoptotic effect of saikosaponin-d was compared with that of dexamethasone, a synthetic glucocorticoid. Apoptosis induced by dexamethasone was also demonstrated by flow cytometry, TUNEL analysis and DNA laddering in our results. It has been reported that treatment of M1 myeloid leukaemia cells with dexamethasone down-regulated the expression of the apoptosis-suppressing gene bcl-2 and the apoptosis-promoting gene bax, but up-regulated expression of the apoptosis-suppressing gene bcl-xL (Lotem & Sachs, 1995). The proto-oncogene c-fos has also been reported to mediate dexamethasone-induced apoptosis in murine T lymphocytes (Pruschy et al., 1997). In the present study, dexamethasone increased p53 mRNA and decreased c-myc and bcl-2 mRNA levels, but these changes did not achieve significance, implying that dexamethasone may have a different mechanism of action.
In summary, we found that saikosaponin-d inhibits DNA synthesis and induces cell apoptosis in human CEM lymphocytes. Saikosaponin-d may be useful as a template for the development of better immunosuppressive and anti-inflammatory agents. Saikosaponin-d may also be useful for study and treatment of atherosclerosis and restenosis. Our results suggest that the apoptotic effect of saikosaponin-d may be partly mediated via an increase in c-myc and p53 mRNA levels and a decrease in bcl-2 mRNA level. Saikosaponin-d at the higher concentration (>10−5 M) also induced necrosis as demonstrated by annexin V staining. For treatment of atherosclerosis and restenosis, antiproliferative or apoptotic-inducing agents that can slow down the overdrive response of cell division, without deleterious effects on the stability of atherosclerotic plaques is needed.
Acknowledgments
This investigation was supported by grants from the National Science Council (NSC89-2320-B002-018), the Department of Health (CCMP89-RD-020) and the National Taiwan University Hospital, Taiwan.
Glossary
- PI
propidium iodide
- RT–PCR
the reverse transcription-polymerase chain reaction
- TUNEL
the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labelling assay
References
- ALNEMRI E.S. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J. Cell. Biochem. 1997;64:33–42. doi: 10.1002/(sici)1097-4644(199701)64:1<33::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- BOERSMA A.W.M., NOOTER K., OOSTRUM R.G., STOTER G. Quantification of apoptotic cells with fluorescein isothiocyanate-labeled Annexin V in Chinese hamster ovary cell cultures treated with cisplatin. Cytometry. 1996;24:123–130. doi: 10.1002/(SICI)1097-0320(19960601)24:2<123::AID-CYTO4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- BRUNETTI M., MARTELLI N., COLASANTE A., PIANTELLIi M., MUSIANI P., AIELLO F.B. Spontaneous and glucocorticoid-induced apoptosis in human mature T lymphocytes. Blood. 1995;86:4199–4205. [PubMed] [Google Scholar]
- CHEN H.W., HUANG H.C. Effects of curcumin on cell cycle progression and apoptosis in vascular smooth muscle cells. Br. J. Pharmacol. 1998;124:1029–1040. doi: 10.1038/sj.bjp.0701914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CIFONE M.G., MIGLIORATI G., PARRONI R., MARCHETTI C., MILLIMAGGI D., SANTONI A., RICCARDI C. Dexamethasone-induced thymocyte apoptosis: apoptotic signal involves the sequential activation of phosphoinositide-specific phospholiase C, acidic sphingomyelinase, and caspases. Blood. 1999;93:2282–2296. [PubMed] [Google Scholar]
- DESBARATS L., SCHNEIDER A., MULLER D., BURGIN A., EILERS M. Myc: a single gene controls both proliferation and apoptosis in mammalian cells. Experientia. 1996;52:1123–1129. doi: 10.1007/BF01952111. [DOI] [PubMed] [Google Scholar]
- GAVRIELI Y., SHERMAN Y., BEN-SASSON S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell. Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALE A.J., SMITH C.A., SUTHERLAND L.C., STONEMAN V.E., LONGTHORNE V.L., CULHANE A.C., WILLIAMS G.T. Apoptosis: molecular regulation of cell death. Eur. J. Biochem. 1996;236:1–26. doi: 10.1111/j.1432-1033.1996.00001.x. [DOI] [PubMed] [Google Scholar]
- HUANG H.C., HSIEH L.M., CHEN H.W., LIN Y.S., CHEN J.S. Effects of baicalein and esculetin on transduction signals and growth factors expression in T-lymphoid leukemia cells. Eur. J. Pharmacol. Mol. Pharmacol. 1994;268:73–78. doi: 10.1016/0922-4106(94)90121-x. [DOI] [PubMed] [Google Scholar]
- HUANG H.C., JAN T.R., YEH S.F. Inhibitory effect of curcumin, an anti-inflammatory agent, on vascular smooth muscle cell proliferation. Eur. J. Pharmacol. 1992;221:381–384. doi: 10.1016/0014-2999(92)90727-l. [DOI] [PubMed] [Google Scholar]
- JACOBSON M.D., WEIL M., RAFF M.C. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- KATO M., PU M.Y., ISOBE K., HATTORI T., YANAGITA N., NAKASHIMA I. Cell type-oriented differential modulatory actions of saikosaponin-d on growth responses and DNA fragmentation of lymphocytes triggered by receptor-mediated and receptor-bypassed pathways. Immunopharmacology. 1995;29:207–213. doi: 10.1016/0162-3109(95)00059-3. [DOI] [PubMed] [Google Scholar]
- KATO M., PU M.Y., ISOBE K., IWAMOTO T., NAGASE F., LWIN T., ZHANG Y.H., HATTORI T., YANAGITA N., NAKASHIMA I. Characterization of the immunoregulatory action of saikosaponin-d. Cell. Immunol. 1994;159:15–25. doi: 10.1006/cimm.1994.1291. [DOI] [PubMed] [Google Scholar]
- LOTEM J., SACHS L. Regulation of bcl-2, bcl-XL and bax in the control of apoptosis by hematopoietic cytokines and dexamethasone. Cell. Growth Differ. 1995;6:647–653. [PubMed] [Google Scholar]
- LUNDERGAN C.F., FOEGH M.L., RAMWELL P.W. Peptide inhibition of myointimal proliferation by angiopeptin, a somatostatin analogue. J. Am. Coll. Cardiol. 1991;17:132B–136B. doi: 10.1016/0735-1097(91)90949-a. [DOI] [PubMed] [Google Scholar]
- MCCOLL K.S., HE H., ZHONG H., WHITACRE C.M., BERGER N.A., DISTELHORST C.W. Apoptosis induction by the glucocorticoid hormone dexamethasone and the calcium-ATPase inhibitor thapsigargin involves Bcl-2 regulated caspase activation. Mol. Cell. Endocrinol. 1998;139:229–238. doi: 10.1016/s0303-7207(98)00051-3. [DOI] [PubMed] [Google Scholar]
- MOTOO Y., SAWABU N. Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma cell lines. Cancer Lett. 1994;86:91–95. doi: 10.1016/0304-3835(94)90184-8. [DOI] [PubMed] [Google Scholar]
- MUNCK A., GUYRE P.M., HOLBROOK N.J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrinol. Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- MUSCI M.A., LATINIS K.M., KORETZKY G.A. Signaling events in T lymphocytes leading to cellular activation or programmed cell death. Clin. Immunol. Immunop. 1997;83:205–222. doi: 10.1006/clin.1996.4315. [DOI] [PubMed] [Google Scholar]
- NAGATA S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- OHUCHI K., WATANABE M., OZEKI T., TSURUFUJI S. Pharmacological influence of saikosaponins on prostaglandin E2 production by peritoneal macrophages. Planta. Med. 1985;51:208–212. doi: 10.1055/s-2007-969458. [DOI] [PubMed] [Google Scholar]
- PARK J.A., LEE K.Y., OH Y.J., KIM K.W., LEE S.K. Activation of caspase-3 protease via a Bcl-2-insensitive pathway during the process of ginsenoside Rh2-induced apoptosis. Cancer Lett. 1997;121:73–81. doi: 10.1016/s0304-3835(97)00333-9. [DOI] [PubMed] [Google Scholar]
- PRUSCHY M., SHI Y.Q., CROMPTON N.E., STEINBACH J., AGUZZI A., GLANZMANU C., BODIS S. The proto-oncogene c-fos mediates apoptosis in murine T-lymphocytes induced by ionizing radiation and dexamethasone. Biochem. Biophys. Res. Common. 1997;241:519–524. doi: 10.1006/bbrc.1997.7846. [DOI] [PubMed] [Google Scholar]
- QIAN L., MURAKAMI T., KIMURA Y., TAKAHASHI M., OKITA K. Saikosaponin A-induced cell death of a human hepatoma cell line (HuH-7): the significance of the ‘sub-G1 peak' in a DNA histogram. Pathol. Int. 1995;45:207–214. doi: 10.1111/j.1440-1827.1995.tb03444.x. [DOI] [PubMed] [Google Scholar]
- ROSS R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- SAMBROOK J., FRITSH E.F., MANIATIS T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York; 1989. [Google Scholar]
- SHERWOOD S.W., SCHIMKE R.T. Cell cycle analysis of apoptosis using flow cytometry. Met. Cell. Biol. 1995;46:77–97. doi: 10.1016/s0091-679x(08)61925-1. [DOI] [PubMed] [Google Scholar]
- THOMPSON C.B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- USHIO Y., ABE H. The effects of saikosaponin on macrophage functions and lymphocyte proliferation. Planta. Med. 1991;57:511–514. doi: 10.1055/s-2006-960195. [DOI] [PubMed] [Google Scholar]
- USHIO Y., ODA Y., ABE H. Effect of saikosaponin on the immune responses in mice. Int. J. Immunopharmacol. 1991;13:501–508. doi: 10.1016/0192-0561(91)90069-j. [DOI] [PubMed] [Google Scholar]
- VELCULESCU V.E., EI-DEIRY W.S. Biological and clinical importance of the p53 tumor suppressor gene. Clin. Chem. 1996;42:858–868. [PubMed] [Google Scholar]
- WANG A.M., DOYLE M.V., MARK D.F. Quantiation of mRNA by the polymerase chain reaction. Proc. Natl. Acad. Sci. U.S.A. 1989;86:9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATABE S., SENDO F., KIMURA S., ARAI S. Activation of cytotoxic polymorphonuclear leukocytes by in vivo administration of a streptococcal preparation, OK432. J. Natl. Cancer. Inst. 1984;72:1365–1370. [PubMed] [Google Scholar]
- XUE D., SHAHAM S., HORVITZ H.R. The Caenorhabditis elegans cell-death protein CED-3 is a cysteine protease with substrate specificities similar to those of the human CPP32 protease. Genes. Dev. 1996;10:1073–1083. doi: 10.1101/gad.10.9.1073. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO M., KUMAGAI A., YAMAMURA Y. Structure and action of saikosaponins isolated from Bupleurum falcatum L. II. Metabolic actions of saikosaponins, especially a plasma cholesterol-lowering action. Arzneimittel-Forschung. 1975;25:1240–1243. [PubMed] [Google Scholar]
- YANO H., MIZOGUCHI A., FUKUDA K., HARAMAKI M., OGASAWARA S., MOMOSAKI S., KOJIRO M. The herbal medicine sho-saiko-to inhibits proliferation of cancer cell lines by inducing apoptosis and arrest at the G0/G1 phase. Cancer Res. 1994;54:448–454. [PubMed] [Google Scholar]
- YONISH-ROUACH E. The p53 tumor suppressor gene: a mediator of a G1 growth arrest and of apoptosis. Experientia. 1996;52:1001–1007. doi: 10.1007/BF01920109. [DOI] [PubMed] [Google Scholar]
- ZAMAI L., FALCIERI E., MARHEJKA G., VITALE M. Supravital exposure to propidium iodide identifies apoptotic cells in the absence of nucleosomal DNA fragmentation. Cytometry. 1996;23:303–311. doi: 10.1002/(SICI)1097-0320(19960401)23:4<303::AID-CYTO6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- ZONG Z-P., FUJIKAWA-YAMAMOTO K., TANINO M., TERAOKA K., YAMAGISHI H., ODASHIMA S. Saikoasponin b2-induced apoptosis of cultured B16 melanoma cell line through down-regulation of PKC activity. Biochem. Biophys. Res. Common. 1996;219:480–485. doi: 10.1006/bbrc.1996.0259. [DOI] [PubMed] [Google Scholar]


