Abstract
The present study investigated the central effects of the selective serotonin reuptake inhibitor (SSRI) fluoxetine and the role of 5-hydroxytryptamine3 (5-HT3) receptors in the core of the nucleus accumbens (NAc) on cocaine-induced behavioural changes in rats.
The 5-HT3 receptor antagonist ondansetron (1 or 10 ng) was microinjected bilaterally into the core of the NAc 60 min prior to peripheral cocaine (15 mg kg−1, i.p.) administration followed by the assessment of locomotor activity, rearing activity and head bobs. Both doses of ondansetron attenuated cocaine's stimulatory effect on behaviours.
Fluoxetine (0.05 or 5 μg) microinjected bilaterally into the core of the NAc 30 min before peripheral administration of cocaine produced dose-dependent biphasic effects on cocaine-induced behaviours. Intra-NAc administration of 0.05 μg fluoxetine resulted in a potentiation of cocaine-induced behaviours, while the higher dose of the SSRI (5 μg) attenuated the stimulant effect of cocaine on behaviours.
To investigate a possible involvement of 5-HT3 receptors in fluoxetine's facilitatory action, ondansetron (10 ng) was microinjected 30 min prior to fluoxetine (0.05 μg), which resulted in a significant attenuation of the facilitatory effect of fluoxetine on cocaine-induced behaviours.
Thus, 5-HT3 receptors in the core of the NAc appear to mediate stimulatory effects on cocaine-induced locomotor activity, rears and head bobs, whereas the attenuation of cocaine-induced behaviours by fluoxetine at the higher dose, suggests the involvement of a different 5-HT receptor subtype.
Keywords: Cocaine, locomotor activity, fluoxetine, ondansetron, 5-HT3, nucleus accumbens
Introduction
The behavioural characteristics of the psychomotor stimulant cocaine include an increase of locomotor activity and stereotypic behaviours at higher doses (Scheel-Krüger et al., 1977; for review see Johanson & Fischman, 1989). It is believed that the dopaminergic nerve terminals in the NAc with cell bodies located in the ventral tegmental area (VTA) are crucially involved in cocaine's stimulatory effect on locomotor activity in rats (Kelly & Iversen, 1976; Delfs et al., 1990; Kaddis et al., 1993; Neisewander et al., 1998). Besides cocaine's neurochemical effects on the dopaminergic mesoaccumbens circuit (Bradberry & Roth, 1989; Carboni et al., 1989; Chen & Reith, 1994), the psychomotor stimulant also interacts with the serotoninergic neurotransmitter system (for review see Cunningham et al., 1996), which may contribute to its overall behavioural profile. Indeed, an increasing number of behavioural studies suggest a modulatory role of 5-hydroxytryptamine (5-HT) on cocaine-induced locomotor activity in rodents. For example, pretreatment with the 5-HT biosynthesis inhibitor p-chlorophenylalanine enhanced the locomotor stimulatory properties of cocaine in rats, indicating an inhibitory role of 5-HT (Scheel-Krüger et al., 1977; Herges & Taylor, 1999a). It has been shown that this inhibitory effect of the serotoninergic neurotransmitter system may be mediated by somatodendritic 5-HT1A autoreceptors located in the dorsal raphe nucleus (Herges & Taylor, 1999b). In contrast to the proposed inhibitory role of 5-HT is our recent observation that peripheral administration of the selective serotonin reuptake inhibitor (SSRI) fluoxetine potentiated locomotor, rearing and head bobbing activities elicited by cocaine in rats (Herges & Taylor, 1998). In view of the observed inhibitory effect mediated by serotoninergic dorsal raphe nucleus (Herges & Taylor, 1999a,1999b), fluoxetine's facilitatory action on cocaine-induced motor behaviours may involve a different brain region.
Neurochemical evidence indicates that fluoxetine administered peripherally elicits an increase in extracellular 5-HT levels in the NAc (Guan & McBride, 1988). A proposed involvement of the serotoninergic nerve terminals in the NAc in fluoxetine's facilitatory effect on cocaine-induced behaviours poses the question of the 5-HT receptor subtype mediating its stimulatory action. Ample behavioural, electrophysiological and neurochemical data have accumulated suggesting a stimulatory effect of 5-HT3 receptors on the dopaminergic activity in the mesoaccumbens system (Costall et al., 1987; Hagan et al., 1987, 1990; Imperato & Angelucci, 1989; Sorensen et al., 1989; Jiang et al., 1990; Minabe et al., 1991a,1991b; Rasmussen et al., 1991; Prisco et al., 1992; Volonté et al., 1992; Pei et al., 1993; Mylecharane, 1996; Gillies et al., 1996). For example, the selective 5-HT3 receptor antagonist ondansetron (Brittain et al., 1987) administered into the NAc and VTA, respectively attenuated locomotor activity induced by intra-NAc injection of amphetamine (Costall et al., 1987) or peripheral administration of dexamphetamine in rats (Gillies et al., 1996). An involvement of 5-HT3 receptors in cocaine's behavioural profile is indicated by the reported attenuation of cocaine-induced locomotor activity after peripheral administration of the 5-HT3 receptor antagonists zacopride, ICS 205-930 and MDL 72222 in rodents (Reith, 1990; Svingos & Hitzemann, 1992), although preliminary results obtained in our laboratory failed to indicate a modulatory effect of ondansetron on the stimulant effect of cocaine on locomotor activity as well as fluoxetine's facilitatory effect in rats (Taylor & Megalogenis, 1994). Despite the lack of effect of peripherally administered ondansetron, a role of 5-HT3 receptors in cocaine's behavioural (Reith, 1990; Svingos & Hitzemann, 1992) and neurochemical actions (McNeish et al., 1993; Kankaanpää et al., 1996) is indicated by published reports, but the site of action mediating the facilitatory effect of 5-HT3 receptors on cocaine's behavioural responses still remains to be identified.
The rationale of the present study was to identify the site of action mediating the previously observed facilitatory effect of peripherally administered fluoxetine on cocaine-induced motor behaviours in rats. Due to the strong implication of the NAc in cocaine's locomotor stimulatory effect (Kelly & Iversen, 1976; Delfs et al., 1990; Kaddis et al., 1993; Neisewander et al., 1998), this dopaminergic brain region appeared to be a suitable target site to investigate fluoxetine's modulatory effect after microinjection into the NAc. In addition, the potential involvement of 5-HT3 receptors in cocaine's as well as fluoxetine's effects was investigated by intra-NAc administration of ondansetron. In view of the proposed regional differences between the core and the shell of the NAc (Alheid & Heimer, 1988; Pontieri et al., 1995; McMahon & Cunningham, 1999), the present study focussed on microinjections of 5-HT agents into the core of the NAc to assess its relative role in the behavioural effects of cocaine administered peripherally at a dose of 15 mg kg−1 (Herges & Taylor, 1998).
Methods
Animals
The experiments were conducted under the guidelines of the National Health and Medical Research Council of Australia and were approved by the Victorian College of Pharmacy, Monash University, Animal Ethics Committee.
Female Glaxo Wistar rats weighing 200–250 g were obtained from the animal house of the Victorian College of Pharmacy, Monash University. The animals were kept in a 12 h light/dark cycle (lights on 0600 h) in a temperature-regulated (22–23°C) room with free access to standard laboratory food (ARM pellets) and water, except during the behavioural sessions. Prior to the surgery, animals were housed in group cages of eight rats. Following implantation of the central cannulae, the rats were kept in individual cages.
Stereotaxic surgical procedures
To induce anaesthesia, rats were injected i.p. with sodium amylobarbitone (27–33.3 mg kg−1) in combination with either sodium methohexitone (16.7 mg kg−1) or thiopentone sodium (62.5 mg kg−1). The head of the rat was shaved prior to being placed in a stereotaxic frame. An incision of approximately 4 cm was made to expose the skull above bregma. To enable bilateral microinjections into the core of the NAc of the right and left brain hemisphere, twin guide cannulae prepared from 23 gauge needles were aligned above the NAc, using the coordinates 1.6 mm anterior to bregma and±1.5 mm lateral to the midline according to the stereotaxic atlas of Paxinos & Watson (1986) and held in place by two stainless steel screws and dental acrylic cement.
The guide cannulae were sealed by two stainless steel stylets. The rats were injected with the penicillin ticarcillin (15 mg kg−1, i.p.) for 3 consecutive days to prevent postoperative infections. Following the surgery, the animals were housed in individual cages for a 5 day recovery period prior to the behavioural experiment.
Central and peripheral drug treatment
The central administration of ondansetron and fluoxetine was into the core of the NAc. Ondansetron (O) or saline (S) were microinjected into the NAc 30 min prior to fluoxetine (F) or saline (S) followed by the peripheral administration of cocaine (C) (15 mg kg−1, i.p.) or saline (S) (i.p.) 30 min later. The submaximal dose of 15 mg kg−1 cocaine is approximately in the middle of the dose-response curve (5–30 mg kg−1), allowing an attenuation or potentiation to be detected (Herges & Taylor, 1999b). For each treatment group, 5–12 rats were used. The animals were gently restrained, the stylets removed and replaced by two stainless steel needles (NAc: ventral, 6.6 mm), connected by polyethylene tubing to a CMA/100 microinjection pump (Carnegie Medicin, Stockholm, Sweden). The microinjection of fluoxetine (0.05 or 5 μg 0.5 μl−1 10 s−1) or saline (0.5 μl 10 s−1) was preceded 30 min earlier by intra-NAc administration of saline (0.5 μl 10 s−1). The intra-NAc administration of ondansetron (1 or 10 ng 0.5 μl−1 10 s−1) was followed 30 min later by microinjections of saline into the NAc (0.5 μl 10 s−1). Another group of animals received ondansetron (10 ng 0.5 μl−1 10 s−1) followed by fluoxetine (0.05 μg 0.5 μl−1 10 s−1). To allow the diffusion of the drugs, the injection needle remained in place in the NAc for 3 min after the microinjections. After removing the injection needles, the guide cannulae were sealed with stylets, and the animals returned to their home cage.
Behavioural testing
At the intermediate dose of 15 mg kg−1 cocaine elicits a complex behavioural pattern, consisting of increases in rearing activity and forward locomotion, which is associated with repetitive head bobbing activities (Herges & Taylor, 1998). The exploration of the environment appears to be the motivational input for the manifestation of this behavioural pattern and is clearly dissociated from stereotypic, oral hyperkinesia, which is apparently devoid of an environmental input (Le Moal & Simon, 1991). The manifestation of these exploratory behaviours were assessed in accordance with the previously employed methodology (Herges & Taylor, 1998).
The behavioural experiments were conducted between 0830 h and 1630 h in an isolated room under light conditions. Locomotor activity was quantified using one circular photobeam activity meter (diameter 67 cm, height 79 cm) equipped with six horizontal infrared photocell beams located 3 cm above the wire grid floor and spaced approximately 13 cm apart. All photobeam interruptions were recorded by two counters (one for each bank of three beams) and totalled for each behavioural session of 5 min duration.
All animals were habituated to the activity meter on the days before the behavioural testing. During the treatment and the time between the behavioural testing, the animals were housed in their familiar home cage in the main laboratory. The basal locomotor activity was quantified during each 5 min observation period 30 min (data not presented) and 10 min before cocaine administration, which allowed the previously habituated animals to familiarize with the environment and 10, 30 and 60 min after cocaine administration. Each animal was tested individually. To quantify the behaviours, the animal was placed in the centre of the wire grid floor of the activity meter. After 5 min testing period, the rat was replaced by the next one and the counters were re-zeroed after noting the scores. The animal was immediately returned to its home cage located in the adjacent laboratory. During the 5 min behavioural sessions 10, 30 and 60 min after cocaine administration, each rat was videotaped using a Panasonic WV-BL 200 video camera centred 1.6 m above the wire grid floor, connected to a Panasonic Super-VHS FS 90 video cassette recorder located in the adjacent laboratory. The behaviours rears (standing up on hind legs) and head bobs (lateral and upward head movements) were quantified from the videotape.
Histology
To determine the location of the injection needle, the animals were anaesthetized and microinjected with dye (0.5 μl 10 s−1) at the completion of the behavioural experiments, as previously described. The brains were removed and frozen at −15°C. The location of dye within the right and left NAc was determined by an observer unaware of the behavioural results obtained. For behavioural data to be included, both cannulae had to be placed within the core of the NAc. The approximate injection site was determined by comparison of the staining of the brain tissue slices to the appropriate plates of the rat brain atlas by Paxinos & Watson (1986). The location of the injection sites were between 6.5–7.5 ventral, 1.2–2.7 and 1.0–2.2 anterior to bregma.
Drugs
The following drugs were used: ondansetron HCl (Glaxo Wellcome, Melbourne, Australia), fluoxetine HCl (Eli Lilly, Indianapolis, IN, USA), cocaine HCl (Glaxo Wellcome, Melbourne, Australia), methohexitone sodium (Eli Lilly, West Ryde, Australia), amylobarbitone sodium (Eli Lilly, West Ryde, Australia), thiopentone sodium (May & Baker, Melbourne, Australia) and ticarcillin sodium (SmithKline Beecham, Melbourne, Australia). Cocaine HCl and fluoxetine HCl were dissolved in saline 0.9% w v−1. A solution of 0.2 mg ml−1 of ondansetron HCl in water for injection was prepared and the appropriate dilutions were made to volume with saline. Methohexitone sodium, amylobarbitone sodium, thiopentone sodium and ticarcillin sodium were prepared in water for injection.
Statistical analyses
The behavioural data were analysed at each time point measured after cocaine administration (10, 30 and 60 min). The effects of ondansetron on baseline behaviours were analysed by the parametric t-test or the non-parametric Mann–Whitney rank sum test (statistical data not presented). The effects of ondansetron and fluoxetine on cocaine-induced behaviours and the effects of fluoxetine on baseline behaviours were analysed by one-way analysis of variance (ANOVA), which is represented in the result section by the value of the calculated F-test and its degrees of freedom. Data, which failed the assumption of a Gaussian distribution was analysed by the non-parametric Kruskal–Wallis one-way analysis of variance (ANOVA on ranks) and is represented in the result section by the value of the calculated H-test and its degrees of freedom. Following data analyses by ANOVA and ANOVA on ranks, respectively pairwise multiple comparison was carried out by Student–Newman–Keuls test and Dunn's test. The probability level of the statistical analyses was 5%. The behavioural counts presented in the graphs represent the mean+s.e.mean (for clarity).
Results
Effects of ondansetron on cocaine-induced locomotor activity, rears and head bobs
The locomotor activity, the number of rears and head bobs of saline-treated animals were not altered by pretreatment with ondansetron into the core of the NAc (Figure 1a–c). A significant group effect for the locomotor activity (S-S-C, O 1 ng-S-C, O 10 ng-S-C) at 10 [F(2, 29)=4.16, P<0.05, n=8–12] and 30 min [F(2, 29)=7.51, P<0.01; n=8–12] was detected by ANOVA (Figure 1a). The stimulatory effect of cocaine on locomotor activity was attenuated by intra-NAc administration of ondansetron at both doses to a similar extent, which reached statistical significance at 10 and 30 min (Figure 1a).
Figure 1.
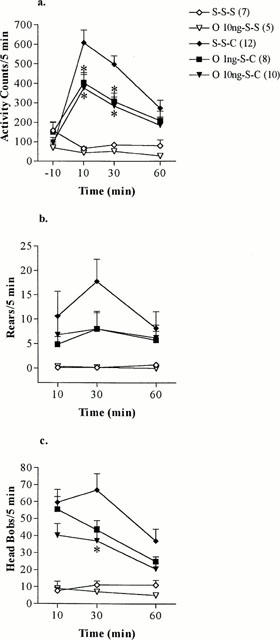
(a) Locomotor activity, (b) rears and (c) head bobs induced by 15 mg kg−1 cocaine (C) or saline (S) injected i.p. at time 0 in rats pretreated by intra-NAc administration of saline (S) 30 min earlier and ondansetron (O) 1 or 10 ng or saline (S) 60 min earlier. The values represent mean scores+s.e.mean (number of rats used for each treatment group are shown in parentheses). *P<0.05 (Student–Newman–Keuls test), O 1 ng-S-C and O 10 ng-S-C compared to S-S-C.
ANOVA on ranks did not detect a significant group effect (S-S-C, O 1 ng-S-C, O 10 ng-S-C) for the number of rears, although pretreatment with ondansetron tended to reduce the rearing activity of rats compared to cocaine-treated rats (Figure 1b). The head bobbing activity of cocaine treated rats was significantly altered by pretreatment with intra-NAc administration of ondansetron with a significant group effect (S-S-C, O 1 ng-S-C, O 10 ng-S-C) being detected by ANOVA at 30 min (F2, 29=3.69, P<0.05; n=8–12) (Figure 1c). Both doses of ondansetron reduced the head bobbing activity of cocaine treated rats, which reached statistical significance for 10 ng ondansetron at 30 min (Figure 1c).
Effects of fluoxetine on cocaine-induced locomotor activity, rears and head bobs
Following intra-NAc administration of fluoxetine, a significant group effect (S-S-S, S-F 0.05 μg-S, S-F 5 μg-S) for the basal locomotor activity was determined by ANOVA at 30 min [locomotor activity: F(2, 17)=5.23, P<0.05, n=5–7] (Figure 2a). In addition, a significant group effect (S-S-S, S-F 0.05 μg-S, S-F 5 μg-S) for the number of head bobs was revealed by ANOVA on ranks at 30 min [H(2)=6.36, P<0.05, n=5–7] (Figure 2c). The lower dose of fluoxetine (0.05 μg) did not alter baseline activities of saline treated animals (Figure 2a–c), while 5 μg fluoxetine tended to decrease the spontaneous locomotor and head bobbing activities of saline treated rats, which reached statistical significance at 30 min (Figure 2a,c).
Figure 2.
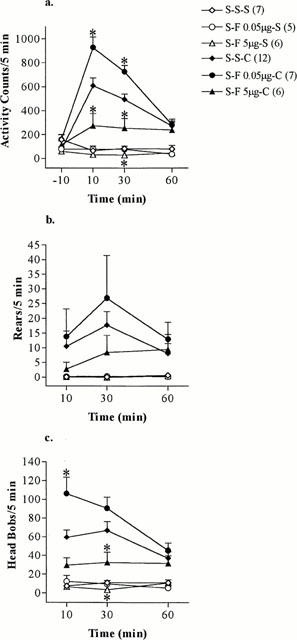
(a) Locomotor activity, (b) rears and (c) head bobs induced by 15 mg kg−1 cocaine (C) or saline (S) injected i.p. at time 0 in rats pretreated by intra-NAc administration of fluoxetine (F) 0.05 or 5 μg or saline (S) 30 min earlier and saline (S) 60 min earlier. The values represent mean scores+s.e.mean (number of rats used for each treatment are shown in parentheses). *P<0.05 (Student–Newman–Keuls test, Dunn's test), S-F 5 μg-S compared to S-S-S and S-F 0.05 μg-C and S-F 5 μg-C compared to S-S-C.
Fluoxetine had a biphasic dose-dependent effect on cocaine's stimulatory effect on behaviours. Following intra-NAc administration of fluoxetine a significant group effect (S-S-C, S-F 0.05 μg-C, S-F 5 μg-C) for the locomotor activity was detected by ANOVA at 10 [F(2, 24)=12.7, P<0.001, n=6–12] and 30 min [F(2, 24)=14.0, P<0.001, n=6–12] (Figure 2a). Pretreatment with 0.05 μg fluoxetine into the core of the NAc resulted in a significant potentiation of cocaine-induced locomotor activity at 10 and 30 min (Figure 2a). In contrast, intra-NAc administration of fluoxetine at the higher dose (5 μg) significantly attenuated the stimulant effect of cocaine on locomotor activity at 10 and 30 min (Figure 2a).
This modulatory effect of intra-NAc administration of fluoxetine on locomotor activity was similarly observed on the number of head bobs (Figure 2c). A significant group effect (S-S-C, S-F 0.05 μg-C, S-F 5 μg-C) for the number of head bobs was determined by ANOVA at 10 [F(2, 24)=9.92, P<0.001, n=6–12] and 30 min [F(2, 24)=5.60, P<0.05, n=6–12] (Figure 2c). An increase of cocaine-induced head bobs was observed following intra-NAc administration of fluoxetine at the lower dose (0.05 μg), which reached statistical significance at 10 min (Figure 2c). In contrast, 5 μg fluoxetine reduced the head bobbing activity following cocaine administration with a statistical significant difference being detected at 30 min (Figure 2c). However, intra-NAc administration of fluoxetine did not produce a statistically significant modulatory effect on cocaine-induced rearing activity (Figure 2b).
Effect of ondansetron and fluoxetine on cocaine-induced locomotor activity, rears and head bobs
The facilitatory effect of fluoxetine (0.05 μg) on cocaine-induced locomotor activity was attenuated by intra-NAc administration of ondansetron (Figure 3a). A significant group effect (O 10 ng-S-C, S-F 0.05 μg-C, O 10 ng-F 0.05 μg-C) for the locomotor activity was determined by ANOVA at 10 [F(2, 24)=12.4, P<0.001, n=7–10] and 30 min [F(2, 24)=13.2, P<0.001, n=7–10] (Figure 3a). Intra-NAc administration of ondansetron significantly attenuated the facilitatory effect of intra-NAc injection of fluoxetine (0.05 μg) on cocaine-induced locomotor activity at 10 and 30 min (Figure 3a). The activity level in ondansetron and fluoxetine pretreated rats was reduced to the locomotor activity counts observed in the ondansetron-cocaine control group (Figure 3a).
Figure 3.
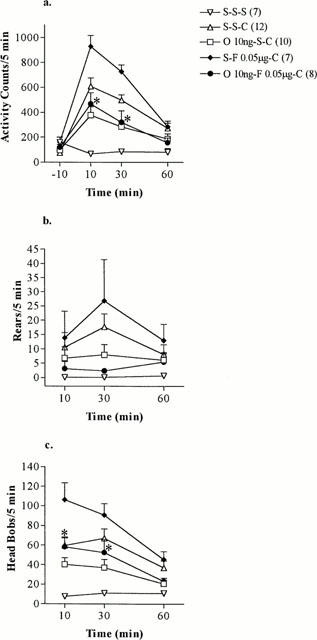
(a) Locomotor activity, (b) rears and (c) head bobs induced by 15 mg kg−1 cocaine (C) or saline (S) injected i.p. at time 0 in rats pretreated by intra-NAc administration of fluoxetine (F) 0.05 μg or saline (S) 30 min earlier and ondansetron (O) 10 ng or saline (S) 60 min earlier. The values represent mean scores+s.e.mean (number of rats used for each treatment are shown in parentheses). *P<0.05 (Student–Newman–Keuls test), O 10 ng-F 0.05 μg-C compared to S-F 0.05 μg-C.
Although the rearing activity in animals pretreated with ondansetron and fluoxetine into the core of the NAc followed by peripheral cocaine administration was reduced in comparison to the facilitatory effect of fluoxetine on cocaine-induced rearing activity, this did not reach statistical significance (Figure 3b). A significant group effect (O 10 ng-S-C, S-F 0.05 μg-C, O 10 ng-F 0.05 μg-C) for the number of head bobs was detected by ANOVA at 10 [F2, 24=8.98, P<0.01, n=7–10] and 30 min [F2, 24=7.67, P<0.01, n=7–10] and by ANOVA on ranks at 60 min [H2=6.06, P<0.05, n=7–10] (Figure 3c). Ondansetron microinjected into the core of the NAc produced a long-lasting reduction of the head bobbing activity induced by fluoxetine and cocaine treatment with statistical significant differences being detected at 10 and 30 min (Figure 3c). The number of head bobs observed in this group was not different to the head bobbing activity in cocaine treated rats pretreated with ondansetron (Figure 3c).
It should be noted, that the above described behavioural changes were observed following administration of the 5-HT agents into the core of the NAc and not adjacent brain regions outside the NAc.
Discussion
The psychomotor stimulant cocaine produced marked increases in exploratory behaviours consisting of forward locomotion associated with head bobbing activities and increases in rearing activities. This stimulatory effect of cocaine on locomotor and head bobbing activities appears to be facilitated by NAc 5-HT3 receptors located in the core of the NAc. In agreement with a facilitatory role of 5-HT3 receptors on locomotor activity induced by psychomotor stimulants is the reported observation, that the increased locomotion following intra-NAc administration of amphetamine was antagonized by ondansetron, however, the 5-HT3 receptor antagonist failed to reduce locomotor activity elicited by peripherally administered amphetamine (Costall et al., 1987). The reported failure of ondansetron on amphetamine's stimulatory properties on behaviours following peripheral administration was attributed to its additional interaction with the striatum (Costall et al., 1987), a dopaminergic brain region which is associated with stereotypic behaviours elicited by psychomotor stimulants (Kelly et al., 1975; Delfs et al., 1990). In the present study, the ability of ondansetron microinjected into the core of the NAc to reduce locomotor and head bobbing activities elicited by peripherally administered cocaine may suggest a preferential involvement of this brain region in these exploratory behaviours induced by cocaine. In agreement with this suggestion is neurochemical evidence, demonstrating a greater effect of peripherally administered cocaine on extracellular dopamine levels in the NAc compared to the striatum (Carboni et al., 1989; Kankaanpää et al., 1996).
Despite ondansetron's pronounced inhibitory effects on locomotor and head bobbing responses elicited by cocaine, the 5-HT3 receptor antagonist produced only a moderate attenuation of the cocaine-induced rearing activity, an exploratory behaviour that can be elicited by intra-NAc administration of dopamine (Makanjuolo et al., 1980). However, the definite role of the NAc in being the primary site of action in mediating rearing activity remains equivocal. Following local administration of dopamine into the nerve terminals of the mesoaccumbens system, Jackson et al. (1975) did not report an increase in rearing activity in rats, but observed continuous rearing in some animals treated with intra-striatum injection of dopamine. An additional involvement of the striatum in the mediation of this exploratory behaviour would explain the only modest attenuation by ondansetron as well as the limited modulatory effect of intra-NAc injection of fluoxetine. Besides the potential involvement of the striatum in cocaine's rearing activity, stimulation of 5-HT3 receptors located in the cell body region of the mesoaccumbens system has been implicated in mediating an increase in rearing activity in rats, a stimulatory effect that could be blocked by ondansetron (Gillies et al., 1996). In view of cocaine' ability to increase extracellular 5-HT levels in the VTA following peripheral administration (Chen & Reith, 1994), the potential involvement of 5-HT3 receptors located in the cell body region of the mesoaccumbens system may account for the modest modulatory effect of intra-NAc injection of ondansetron and fluoxetine on cocaine-induced rearing activity.
The underlying mechanism of the attenuated cocaine-induced locomotor and head bobbing activities after ondansetron pretreatment in the present study may be a reduced release of dopamine in the NAc. As indicated in the introduction, activation of 5-HT3 receptors increases dopaminergic activity in the mesoaccumbens system. For example, intraventricular administration of 2-methyl-5-HT increased dopamine release in the NAc, a facilitatory effect which was blocked by the 5-HT3 receptor antagonist granisetron (Jiang et al., 1990). Furthermore, an involvement of 5-HT3 receptors in cocaine's neurochemical action is indicated by the ability of the 5-HT3 receptor antagonists MDL 72222 (Kankaanpää et al., 1996) and zacopride (McNeish et al., 1993) to reduce the increased extracellular dopamine levels in the NAc.
Contrary to the observed inhibitory effect of 5-HT3 receptor antagonists on cocaine-induced locomotor activity (Reith, 1990; Svingos & Hitzemann, 1992; present study), is an earlier study from our laboratory, showing no modulatory effect of peripherally administered ondansetron on cocaine-induced locomotor activity in rats (Taylor & Megalogenis, 1994). The differential results of the latter study and the study by Reith (1990) may partly be explained by species differences (rats versus mice). In addition, Reith (1990) used 25 mg kg−1 cocaine, which may increase the sensitivity of the behavioural experiment. However, the present study used the same dose of cocaine and the same behavioural model, as had been employed previously in our laboratory by Taylor & Megalogenis (1994). The major methodological difference between the two studies is the routes of administration employed for ondansetron. In the previous study ondansetron was administered peripherally (Taylor & Megalogenis, 1994) compared with central microinjection in the present study. The reasons for the failure of peripherally administered ondansetron to modulate cocaine-induced locomotor activity are not clear. Ondansetron's lack of effect is unlikely to be attributable to an inefficacy of the doses employed (0.03–1.0 mg kg−1) (Taylor & Megalogenis, 1994), since similar doses of ondansetron (0.01–1.0 mg kg−1) have been shown to attenuate locomotor activity elicited by intra-NAc administration of amphetamine (Costall et al., 1987). However, it has been reported that peripheral administration of ondansetron (0.1 mg kg−1) failed to attenuate cocaine-induced elevation of extracellular dopamine in the NAc (Cervo et al., 1996). A possible explanation for the lack of behavioural and neurochemical effects following peripheral administration of ondansetron might be the recently proposed involvement of 5-HT3 receptors in the negative serotoninergic feedback mechanism by the dorsal raphe nucleus (Bagdy et al., 1998), although this speculation awaits further experimental research.
The complexity of the underlying interaction between 5-HT and dopamine is further confirmed by our interesting observation, that intra-NAc injection of the SSRI fluoxetine had dose-dependent biphasic effects on cocaine's behavioural responses. The potentiation of cocaine-induced locomotor and head bobbing activities following microinjection of the lower dose (0.05 μg) of fluoxetine into the core of the NAc, which was blocked by ondansetron suggests that the core of the NAc may mediate our previously reported facilitatory effect of peripherally administered fluoxetine (Herges & Taylor, 1998). One might speculate, that this facilitatory effect of the SSRI on cocaine-induced behaviours is the result of an increased release of dopamine in the NAc. Fluoxetine has been shown to increase extracellular 5-HT levels in the NAc (Guan & McBride, 1988), but failed to alter the dopamine levels (Guan & McBride, 1988; Tanda et al., 1994; Sakaue et al., 2000), although 5-HT applied into the NAc increased the extracellular dopamine levels (Parsons & Justice, 1993). This facilitatory effect of perfused 5-HT on extracellular dopamine levels in the NAc was attenuated by MDL 72222, a 5-HT3 receptor antagonist (Parsons & Justice, 1993), which is consistent with an increase of dopamine release following 5-HT3 receptor activation in rat forebrain regions (Jiang et al., 1990; Chen et al., 1991). Hence, stimulation of 5-HT3 receptors in the NAc, followed by an increased dopamine release may account for fluoxetine's facilitatory properties on cocaine-induced locomotor and head bobbing activities in rats, which is supported by the antagonism of its facilitatory effect by ondansetron in the present study. The lack of effect of fluoxetine on the extracellular dopamine levels in the NAc (Guan & McBride, 1988; Tanda et al., 1994; Sakaue et al., 2000) is in agreement with our behavioural observation, that the lower dose of the SSRI did not increase the basal activity of saline treated animals. The latter behavioural results may suggest, that fluoxetine's effect in the NAc depends on the dopaminergic tone, which is presumably low in saline-treated rats. By increasing the activity of dopaminergic neurones in the mesoaccumbens system following peripheral administration of cocaine, fluoxetine's indirect facilitatory effect by activation of 5-HT3 receptors becomes apparent, which is reflected in its potentiation of the stimulatory action of cocaine.
Contrary to the facilitatory effect of 0.05 μg fluoxetine, the higher dose (5 μg) of fluoxetine microinjected into the core of the NAc inhibited cocaine-induced locomotor and head bobbing activities. The effect of SSRIs on the extracellular 5-HT levels in forebrain regions has been suggested to reflect a balance between 5-HT reuptake inhibition and a reduced 5-HT release (Adell & Artigas, 1991; Rutter & Auerbach, 1993), resulting from 5-HT1A autoreceptor activation in the midbrain raphe nuclei (Rutter & Auerbach, 1993). Since fluoxetine was administered into the core of the NAc in the present study, an involvement of the midbrain raphe nuclei in a reduced 5-HT release may be excluded. However, an initial increase of the extracellular 5-HT levels in the synaptic cleft following intra-NAc administration of the higher dose of fluoxetine may be expected to result in a stimulation of presynaptic 5-HT1B autoreceptors, which has been associated with a decreased release of 5-HT (Cerrito & Raiteri, 1979; Göthert & Weinheimer, 1979; Baumann & Waldmeier, 1981; Mounsey et al., 1982; Göthert & Schlicker, 1983). Indeed, the greatest increase of extracellular 5-HT in the rat forebrain following peripheral administration of the SSRIs fluoxetine and paroxetine was reported after concurrent blockade of 5-HT1B and 5-HT1A autoreceptors by GR127935 and WAY100635, respectively (Gobert et al., 1997; Sharp et al., 1997). One might suggest, that the local administration of the higher dose of fluoxetine shifts the equilibrium between 5-HT reuptake inhibition and reduced 5-HT release (due to activation of presynaptic 5-HT1B autoreceptors) toward reduced extracellular 5-HT levels. Since 5-HT appears to facilitate cocaine's behavioural effects, as shown by the lower dose of fluoxetine, a reduction of the serotoninergic tone in the NAc may attenuate cocaine's stimulant action on locomotor activity.
Another possible explanation for the inhibitory effect of the SSRI at the higher dose is the involvement of a postsynaptic 5-HT receptor different from the 5-HT3 receptor. The 5-HT2 receptors have been suggested to mediate an inhibitory effect on dopamine-mediated locomotor activity and behaviour (Green et al., 1981; 1983). The non-selective 5-HT2 receptor antagonists metergoline and methysergide potentiated cocaine's stimulant effect on locomotor activity (Scheel-Krüger et al., 1977; Herges & Taylor, 1998), while the 5-HT2B/C receptor antagonist SB 206553 and the 5-HT2A receptor antagonist ketanserin had dose-dependent opposing effects on cocaine-induced hyperactivity (Herges & Taylor, 1998; McCreary & Cunningham, 1999). Although the non-selectivity of the 5-HT agents used complicates the interpretation of the behavioural studies, they are in agreement with the suggestion, that 5-HT2 receptors are involved in dopamine-mediated locomotor activity. In recent years, the 5-HT2C receptor subtype has been implicated in mediating an inhibitory role on the dopaminergic mesoaccumbens system (Lejeune et al., 1997; Spampinato et al., 1997; De Deurwaerdère & Spampinato, 1999), which may account for the potentiation of cocaine-induced locomotor activity by methysergide (Herges & Taylor, 1998) and SB 206553 (McCreary & Cunningham, 1999). Recent behavioural evidence presented by Filip & Cunningham (1998) demonstrated a slight increase of basal activity following microinjection of the selective 5-HT2C receptor antagonist RS 102221 into the shell of the NAc, which is in keeping with an increase of basal dopamine release in the NAc by the 5-HT2B/2C receptor antagonist SB 206553 (De Deurwaerdère & Spampinato, 1999). Thus, a stimulation of 5-HT2C receptors by intra-NAc administration of fluoxetine at the higher dose may be a plausible explanation for its inhibitory effect on cocaine-induced hyperactivity. Interestingly, the basal locomotor and head bobbing activities of rats treated with intra-NAc fluoxetine at the higher dose were decreased, as previously reported for spontaneous activity of rats by 5-HT locally administered into the NAc (Costall et al., 1976), which is in agreement with the well established 5-HT2C receptor-mediated hypolocomotion induced by 5-HT2C receptor agonists (Curzon & Kennett, 1990).
In summary, the previously observed potentiation of cocaine's stimulatory effect on locomotor and head bobbing activities by peripherally administered fluoxetine appears to be mediated by 5-HT3 receptors located in the core of the NAc, while the limited modulation of cocaine-induced rearing activity may be indicative of an involvement of an additional brain region. Furthermore, the ability of ondansetron and fluoxetine to modulate the stimulatory properties of peripherally administered cocaine on locomotor and head bobbing activities may suggest a preferential involvement of this dopaminergic brain region in these behaviours. The striking observation that the higher dose of fluoxetine attenuated cocaine's stimulatory effect on locomotor, head bobbing and to some extent rearing activities complicates the proposal of a facilitatory role of 5-HT mediated by the core of the NAc and is the possible result of the involvement of either a post- or presynaptic 5-HT receptor subtype different from the 5-HT3 receptor.
Acknowledgments
The authors wish to thank Eli Lilly and Glaxo Wellcome for the generous supply of fluoxetine and ondansetron, respectively. Sonja Herges is a recipient of the Monash Graduate Scholarship.
Abbreviations
- F
fluoxetine
- 5-HT
5-hydroxytryptamine
- NAc
nucleus accumbens
- O
ondansetron
- S
saline
- SSRI
selective serotonin reuptake inhibitor
- VTA
ventral tegmental area
References
- ADELL A., ARTIGAS F. Differential effects of clomipramine given locally or systemically on extracellular 5-hydroxytryptamine in raphe nuclei and frontal cortex. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;343:237–244. doi: 10.1007/BF00251121. [DOI] [PubMed] [Google Scholar]
- ALHEID G.F., HEIMER L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- BAGDY E., SOLYOM S., HARSING L.G. Feedback stimulation of somatodendritic serotonin release: A 5-HT3 receptor-mediated effect in the raphe nuclei of the rat. Brain Res. Bull. 1998;45:203–208. doi: 10.1016/s0361-9230(97)00340-7. [DOI] [PubMed] [Google Scholar]
- BAUMANN P.A., WALDMEIER P.C. Further evidence for negative feedback control of serotonin release in the central nervous system. Naunyn-Schmiedebergs Arch. Pharmacol. 1981;317:36–43. doi: 10.1007/BF00506254. [DOI] [PubMed] [Google Scholar]
- BRADBERRY C.W., ROTH R.H. Cocaine increases extracellular dopamine in rat nucleus accumbens and ventral tegmental area as shown by in vivo microdialysis. Neurosci. Lett. 1989;103:97–102. doi: 10.1016/0304-3940(89)90492-8. [DOI] [PubMed] [Google Scholar]
- BRITTAIN R.T., BUTLER A., COATES I.H., FORTUNE D.H., HAGAN R., HILL J.M., HUMBER D.C., HUMPHREY P.P.A., IRELAND S.J., JACK D., JORDAN C.C., OXFORD A., STRAUGHAN D.W., TYERS M.B. GR38032F, a novel selective 5HT3 receptor antagonist. Br. J. Pharmacol. 1987;90 Suppl.:87P. [Google Scholar]
- CARBONI E., IMPERATO A., PEREZZANI L., DI CHIARA G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neurscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- CERRITO F., RAITERI M. Serotonin release is modulated by presynaptic autoreceptors. Eur. J. Pharmacol. 1979;57:427–430. doi: 10.1016/0014-2999(79)90506-5. [DOI] [PubMed] [Google Scholar]
- CERVO L., POZZI L., SAMANIN R. 5-HT3 Receptor antagonists do not modify cocaine place conditioning or the rise in extracellular dopamine in the nucleus accumbens of rats. Pharmacol. Biochem. Behav. 1996;55:33–37. doi: 10.1016/0091-3057(96)00046-9. [DOI] [PubMed] [Google Scholar]
- CHEN N.-H., REITH M.E.A. Autoregulation and monoamine interactions in the ventral tegmental area in the absence and presence of cocaine: a microdialysis study in freely moving rats. J. Pharmacol. Exp. Ther. 1994;271:1597–1610. [PubMed] [Google Scholar]
- CHEN J., VAN PRAAG H.M., GARDNER E.L. Activation of 5-HT3 receptor by 1-phenylbiguanide increases dopamine release in the rat nucleus accumbens. Brain Res. 1991;543:354–357. doi: 10.1016/0006-8993(91)90050-6. [DOI] [PubMed] [Google Scholar]
- COSTALL B., DOMENEY A.M., NAYLOR R.J., TYERS M.B. Effects of the 5-HT3 receptor antagonist, GR38032F, on raised dopaminergic activity in the mesolimbic system of the rat and marmoset brain. Br. J. Pharmacol. 1987;92:881–894. doi: 10.1111/j.1476-5381.1987.tb11394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTALL B., NAYLOR R.J., MARSDEN C.D., PYCOCK C.J. Serotoninergic modulation of the dopamine response from the nucleus accumbens. J. Pharm. Pharmacol. 1976;28:523–526. doi: 10.1111/j.2042-7158.1976.tb02783.x. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM K.A., BRADBERRY C.W., CHANG A.S., REITH M.E.A. The role of serotonin in the actions of psychostimulants: molecular and pharmacological analyses. Behav. Brain. Res. 1996;73:93–102. doi: 10.1016/0166-4328(96)00077-0. [DOI] [PubMed] [Google Scholar]
- CURZON G., KENNETT G.A. m-Cpp: a tool for studying behavioural responses associated with 5-HT1C receptors. Trends Pharmacol. Sci. 1990;11:181–182. doi: 10.1016/0165-6147(90)90109-l. [DOI] [PubMed] [Google Scholar]
- DE DEURWAERDÈRE P., SPAMPINATO U. Role of serotonin2A and serotonin2B/2C receptor subtypes in the control of accumbal and striatal dopamine release elicited in vivo by dorsal raphe nucleus electrical stimulation. J. Neurochem. 1999;73:1033–1042. doi: 10.1046/j.1471-4159.1999.0731033.x. [DOI] [PubMed] [Google Scholar]
- DELFS J.M., SCHREIBER L., KELLY A.E. Microinjection of cocaine into the nucleus accumbens elicits locomotor activity in the rat. J. Neurosci. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILIP M., CUNNINGHAM K.A. Serotonin 5-HT2C receptors in the shell of the nucleus accumbens modulate cocaine-induced hyperactivity. Soc. Neurosci. Abstr. 1998;24:2171. [Google Scholar]
- GILLIES D.M., MYLECHARANE E.J., JACKSON D.M. Effects of 5-HT3 receptor-selective agents on locomotor activity in rats following injection into the nucleus accumbens and the ventral tegmental area. Eur. J. Pharmacol. 1996;303:1–12. doi: 10.1016/0014-2999(96)00028-3. [DOI] [PubMed] [Google Scholar]
- GOBERT A., RIVET J.-M., CISTARELLI L., MILLAN M.J. Potentiation of the fluoxetine-induced increase in dialysate levels of serotonin (5-HT) in the frontal cortex of freely moving rats by combined blockade of 5-HT1A and 5-HT1B receptors with WAY 100,635 and GR 127,935. J. Neurochem. 1997;68:1159–1163. doi: 10.1046/j.1471-4159.1997.68031159.x. [DOI] [PubMed] [Google Scholar]
- GÖTHERT M., SCHLICKER E. Autoreceptor-mediated inhibition of 3H-5-hydroxytryptamine release from rat brain cortex slices by analogues of 5-hydroxytryptamine. Life Sci. 1983;32:1183–1191. doi: 10.1016/0024-3205(83)90186-8. [DOI] [PubMed] [Google Scholar]
- GÖTHERT M., WEINHEIMER G. Extracellular 5-hydroxytryptamine inhibits 5-hydroxytryptamine release from rat brain cortex slices. Naunyn Schmiedebergs Arch. Pharmacol. 1979;310:93–96. doi: 10.1007/BF00499879. [DOI] [PubMed] [Google Scholar]
- GREEN A.R., HALL J.E., REES A.R. A behavioural and biochemical study in rats of 5-hydroxytryptamine receptor agonists and antagonists, with observations on structure-activity requirements for the agonists. Br. J. Pharmacol. 1981;73:703–719. doi: 10.1111/j.1476-5381.1981.tb16806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN A.R., O'SHAUGHNESSY K., HAMMOND M., SCHÄCHTER M., GRAHAME-SMITH D.G. Inhibition of 5-hydroxytryptamine-mediated behaviour by the putative 5-HT2 antagonist pirenperone. Neuropharmacol. 1983;22:573–578. doi: 10.1016/0028-3908(83)90147-8. [DOI] [PubMed] [Google Scholar]
- GUAN X.-M., MCBRIDE W.J. Fluoxetine increases the extracellular levels of serotonin in the nucleus accumbens. Brain Res. Bull. 1988;21:43–46. doi: 10.1016/0361-9230(88)90118-9. [DOI] [PubMed] [Google Scholar]
- HAGAN R.M., BUTLER A., HILL J.M., JORDAN C.C., IRELAND S.J., TYERS M.B. Effect of the 5-HT3 receptor antagonist, GR38032F, on responses to injection of a neurokinin agonist into the ventral tegmental area of the rat brain. Eur. J. Pharmacol. 1987;138:303–305. doi: 10.1016/0014-2999(87)90450-x. [DOI] [PubMed] [Google Scholar]
- HAGAN R.M., JONES B.J., JORDAN C.C., TYERS M.B. Effect of 5-HT3 receptor antagonists on responses to selective activation of mesolimbic dopaminergic pathways in the rat. Br. J. Pharmacol. 1990;99:227–232. doi: 10.1111/j.1476-5381.1990.tb14685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERGES S., TAYLOR D.A. Involvement of serotonin in the modulation of cocaine-induced locomotor activity in the rat. Pharmacol. Biochem. Behav. 1998;59:595–611. doi: 10.1016/s0091-3057(97)00473-5. [DOI] [PubMed] [Google Scholar]
- HERGES S., TAYLOR D.A. Modulatory effect of p-chlorophenylalanine microinjected into the dorsal and median raphe nuclei on cocaine-induced behaviour in the rat. Eur. J. Pharmacol. 1999a;374:329–340. doi: 10.1016/s0014-2999(99)00333-7. [DOI] [PubMed] [Google Scholar]
- HERGES S., TAYLOR D.A. Modulation of cocaine-induced locomotor activity, rears and head bobs by application of WAY100635 into the dorsal and median raphe nuclei of the rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999b;360:129–134. doi: 10.1007/s002109900058. [DOI] [PubMed] [Google Scholar]
- IMPERATO A., ANGELUCCI L. 5-HT3 receptors control dopamine release in the nucleus accumbens of freely moving rats. Neurosci. Lett. 1989;101:214–217. doi: 10.1016/0304-3940(89)90533-8. [DOI] [PubMed] [Google Scholar]
- JACKSON D.M., ANDÉN N.-E., DAHLSTRÖM A. A functional effect of dopamine in the nucleus accumbens and in some other dopamine-rich parts of the rat brain. Psychopharmacol. 1975;45:139–149. doi: 10.1007/BF00429052. [DOI] [PubMed] [Google Scholar]
- JIANG L.H., ASHBY C.R., JR, KASSER R.J., WANG R.Y. The effect of intraventricular administration of the 5-HT3 receptor agonist 2-methylserotonin on the release of dopamine in the nucleus accumbens: an in vivo chronocoulometric study. Brain Res. 1990;513:156–160. doi: 10.1016/0006-8993(90)91103-n. [DOI] [PubMed] [Google Scholar]
- JOHANSON C.-E., FISCHMAN M.W. The pharmacology of cocaine related to its abuse. Pharmacol. Reviews. 1989;41:3–52. [PubMed] [Google Scholar]
- KADDIS F.G., WALLACE L.J., URETSKY N.J. AMPA/Kainate antagonists in the nucleus accumbens inhibit locomotor stimulatory response to cocaine and dopamine agonists. Pharmacol. Biochem. Behav. 1993;46:703–708. doi: 10.1016/0091-3057(93)90565-b. [DOI] [PubMed] [Google Scholar]
- KANKAANPÄÄ A., LILLSUNDE P., RUOTSALAINEN M., AHTEE L., SEPPÄLÄ T. 5-HT3 Receptor antagonist MDL 72222 dose-dependently attenuates cocaine- and amphetamine-induced elevations of extracellular dopamine in the nucleus accumbens and the dorsal striatum. Pharmacol. Toxicol. 1996;78:317–321. doi: 10.1111/j.1600-0773.1996.tb01382.x. [DOI] [PubMed] [Google Scholar]
- KELLY P.H., IVERSEN S.D. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: Abolition of psychostimulant-induced locomotor activity in rats. Eur. J. Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- KELLY P.H., SEVIOUR P.W., IVERSEN S.D. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- LEJEUNE F., GOBERT A., RIVERT J.-M., MILLAN M.J. Serotonin 5-HT2C receptors inhibit the activity of mesocortical and mesolimbic dopaminergic pathways: A combined dialysis and electrophysiological analysis. Soc. Neurosci. Abstr. 1997;23:975. [Google Scholar]
- LE MOAL M., SIMON H. Mesocorticolimbic dopaminergic network: Functional and regulatory roles. Pharmacol. Reviews. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- MAKANJUOLA P.O.A., DOW R.C., ASHCROFT G.W. Behavioural responses to stereotactically controlled injections of monoamine neurotransmitters into the accumbens and caudate-putamen nuclei. Psychopharmacol. 1980;71:227–235. doi: 10.1007/BF00433056. [DOI] [PubMed] [Google Scholar]
- MCCREARY A.C., CUNNINGHAM K.A. Effects of the 5-HT2C/2B antagonist SB 206553 on hyperactivity induced by cocaine. Neuropsychopharmacol. 1999;20:556–564. doi: 10.1016/S0893-133X(98)00087-6. [DOI] [PubMed] [Google Scholar]
- MCMAHON L.R., CUNNINGHAM K.A. Antagonism of 5-hydroxytryptamine4 receptors attenuates hyperactivity induced by cocaine: Putative role for 5-hydroxytryptamine4 receptors in the nucleus accumbens shell. J. Pharmacol. Exp. Ther. 1999;291:300–307. [PubMed] [Google Scholar]
- MCNEISH C.S., SVINGOS A.L., HITZEMANN R., STRECKER R.E. The 5-HT3 antagonist zacopride attenuates cocaine-induced increases in extracellular dopamine in rat nucleus accumbens. Pharmacol. Biochem. Behav. 1993;45:759–763. doi: 10.1016/0091-3057(93)90118-d. [DOI] [PubMed] [Google Scholar]
- MINABE Y., ASHBY C.R., JR, SCHWARTZ J.E., WANG R.Y. The 5-HT3 receptor antagonists LY 277359 and granisetron potentiate the suppressant action of apomorphine on the basal firing rate of ventral tegmental dopamine cells. Eur. J. Pharmacol. 1991a;209:143–150. doi: 10.1016/0014-2999(91)90162-j. [DOI] [PubMed] [Google Scholar]
- MINABE Y., ASHBY C.R., JR, WANG R.Y. The effect of acute and chronic LY 277359, a selective 5-HT3 receptor antagonist, on the number of spontaneously active midbrain dopamine neurons. Eur. J. Pharmacol. 1991b;209:151–156. doi: 10.1016/0014-2999(91)90163-k. [DOI] [PubMed] [Google Scholar]
- MOUNSEY I., BRADY K.A., CARROLL J., FISHER R., MIDDLEMISS D.N. K+-evoked [3H]-5-HT release from rat frontal cortex slices: The effect of 5-HT agonists and antagonists. Biochem. Pharmacol. 1982;31:49–53. doi: 10.1016/0006-2952(82)90234-9. [DOI] [PubMed] [Google Scholar]
- MYLECHARANE E.J. Ventral tegmental area 5-HT receptors: mesolimbic dopamine release and behavioural studies. Behav. Brain. Res. 1996;73:1–5. doi: 10.1016/0166-4328(96)00061-7. [DOI] [PubMed] [Google Scholar]
- NEISEWANDER J.L., FUCHS R.A., O'DELL L.E., KHROYAN T.V. Effects of SCH-23390 on dopamine D1 receptor occupancy and locomotion produced by intraaccumbens cocaine infusion. Synapse. 1998;30:194–204. doi: 10.1002/(SICI)1098-2396(199810)30:2<194::AID-SYN9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- PARSONS L.H., JUSTICE J.B., JR Perfusate serotonin increases extracellular dopamine in the nucleus accumbens as measured by in vivo microdialysis. Brain Res. 1993;606:195–199. doi: 10.1016/0006-8993(93)90984-u. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The rat brain in stereotaxic co-ordinates 1986Sydney Australia: Academic Press; 2nd edn [Google Scholar]
- PEI Q., ZETTERSTRÖM T., LESLIE R.A., GRAHAME-SMITH D.G. 5-HT3 receptor antagonists inhibit morphine-induced stimulation of mesolimbic dopamine release and function in the rat. Eur. J. Pharmacol. 1993;230:63–68. doi: 10.1016/0014-2999(93)90410-j. [DOI] [PubMed] [Google Scholar]
- PONTIERI F.E., TANDA G., DI CHIARA G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the ‘shell' as compared with the ‘core' of the rat nucleus accumbens. Proc. Natl. Acad. Sci. U.S.A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRISCO S., PESSIA M., CECI A., BORSINI F., ESPOSITO E. Chronic treatment with DAU 6215, a new 5-HT3 receptor antagonist, causes a selective decrease in the number of spontaneously active dopaminergic neurons in the rat ventral tegmental area. Eur. J. Pharmacol. 1992;214:13–19. doi: 10.1016/0014-2999(92)90089-m. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN K., STOCKTON M.E., CZACHURA J.F. The 5-HT3 receptor antagonist zatosetron decreases the number of spontaneously active A10 dopamine neurons. Eur. J. Pharmacol. 1991;205:113–116. doi: 10.1016/0014-2999(91)90781-k. [DOI] [PubMed] [Google Scholar]
- REITH M.E.A. 5-HT3 receptor antagonists attenuate cocaine-induced locomotion in mice. Eur. J. Pharmacol. 1990;186:327–330. doi: 10.1016/0014-2999(90)90454-e. [DOI] [PubMed] [Google Scholar]
- RUTTER J.J., AUERBACH S.B. Acute uptake inhibition increases extracellular serotonin in the rat forebrain. J. Pharmacol. Exp. Ther. 1993;265:1319–1324. [PubMed] [Google Scholar]
- SAKAUE M., SOMBOONTHUM P., NISHIHARA B., KOYAMA Y., HASHIMOTO H., BABA A., MATSUDA T. Postsynaptic 5-hydroxytryptamine1A receptor activation increases in vivo dopamine release in rat prefrontal cortex. Br. J. Pharmacol. 2000;129:1028–1034. doi: 10.1038/sj.bjp.0703139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEEL-KRÜGER J., BRAESTRUP C., NIELSON M., GOLEMBIOWSKA K., MOGILNICKA E.Cocaine: Discussion on the role of dopamine in the biochemical mechanism of action Cocaine and other stimulants 1977New York: Plenum Press; 373–407.ed. Ellinwood, Jr. E.H. & Kilbey, M.M., pp [Google Scholar]
- SHARP T., UMBERS V., GARTSIDE S.E. Effect of a selective 5-HT reuptake inhibitor in combination with 5-HT1A and 5-HT1B receptor antagonists on extracellular 5-HT in rat frontal cortex in vivo. Br. J. Pharmacol. 1997;121:941–946. doi: 10.1038/sj.bjp.0701235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SORENSEN S.M., HUMPHREYS T.M., PALFREYMAN M.G. Effect of acute and chronic MDL 73,147EF, a 5-HT3 receptor antagonist, on A9 and A10 dopamine neurons. Eur. J. Pharmacol. 1989;163:115–118. doi: 10.1016/0014-2999(89)90402-0. [DOI] [PubMed] [Google Scholar]
- SPAMPINATO U., STINUS L., DE DEURWAERDÈRE P. Effect of dorsal raphe electrical stimulation on striatal and accumbal dopamine release in the rat: Role of 5-HT2C/2B and 5-HT3 receptors. Soc. Neurosci. Abstr. 1997;23:2326. doi: 10.1523/JNEUROSCI.18-16-06528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVINGOS A.L., HITZEMANN R. 5-HT3 receptor antagonists block cocaine-induced locomotion via a PCPA-sensitive mechanism. Pharmacol. Biochem. Behav. 1992;43:871–879. doi: 10.1016/0091-3057(92)90420-k. [DOI] [PubMed] [Google Scholar]
- TANDA G., CARBONI E., FRAU R., DI CHIARA G. Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential. Psychopharmacology. 1994;115:285–288. doi: 10.1007/BF02244785. [DOI] [PubMed] [Google Scholar]
- TAYLOR D.A., MEGALOGENIS A. Fluoxetine and clomipramine augment cocaine's motor stimulant activity but not by activation of 5-HT3 receptors. Canadian J. Physiol. Pharmacol. Abstr. 1994;72:364. [Google Scholar]
- VOLONTÉ M., CECI A., BORSINI F. Effect of haloperidol and clozapine on (+) SKF 10,047-induced dopamine release: role of 5-HT3 receptors. Eur. J. Pharmacol. 1992;213:163–164. doi: 10.1016/0014-2999(92)90250-8. [DOI] [PubMed] [Google Scholar]


