Abstract
Urotensin-II (U-II) and its G-protein-coupled receptor, GPR14, are expressed within mammalian cardiac and peripheral vascular tissue and, as such, may regulate mammalian cardiovascular function. The present study details the vasoconstrictor profile of this cyclic undecapeptide in different vascular tissues isolated from a diverse range of mammalian species (rats, mice, dogs, pigs, marmosets and cynomolgus monkeys).
The vasoconstrictor activity of human U-II was dependent upon the anatomical origin of the vessel studied and the species from which it was isolated. In the rat, constrictor responses were most pronounced in thoracic aortae and carotid arteries: −log[EC50]s 9.09±0.19 and 8.84±0.21, Rmaxs 143±21 and 67±26% 60 mM KCl, respectively (compared, for example, to −log[EC50] 7.90±0.11 and Rmax 142±12% 60 mM KCl for endothelin-1 [ET-1] in thoracic aortae). Responses were, however, absent in mice aortae (−log[EC50] <6.50). These findings were further contrasted by the observation that U-II was a ‘coronary-selective' spasmogen in the dog (−log[EC50] 9.46±0.11, Rmax 109±23% 60 mM KCl in LCX coronary artery), yet exhibited a broad spectrum of vasoconstrictor activity in arterial tissue from Old World monkeys (−log[EC50]s range from 8.96±0.15 to 9.92±0.13, Rmaxs from 43±16 to 527±135% 60 mM KCl). Interestingly, significant differences in reproducibility and vasoconstrictor efficacy were seen in tissue from pigs and New World primates (vessels which responded to noradrenaline, phenylephrine, KCl or ET-1 consistently).
Thus, human U-II is a potent, efficacious vasoconstrictor of a variety of mammalian vascular tissues. Although significant species/anatomical variations exist, the data support the hypothesis that U-II influences the physiological regulation of mammalian cardiovascular function.
Keywords: Urotensin-II, GPR14, SENR, endothelin-1, somatostatin, vascular reactivity, spasmogen, coronary artery, endothelium, vasoconstriction
Introduction
The integrated control of cardiovascular homeostasis is achieved through a combination of direct neuronal control and systemic activation of the neurohumoral axis. The principal mammalian vasoactive factors of this axis (angiotensin-II, endothelin [ET]-1, noradrenaline) exert their haemodynamic effects exclusively via interactions with specific seven transmembrane heterotrimeric G-protein-coupled receptors (GPCRs). Drugs which antagonize such interactions constitute one of the most successful classes of therapeutic agents identified to date (Stadel et al., 1997; Wilson et al., 1998). Nowhere is this more evident than within the vasculature where numerous agents have been developed successfully for the clinical management of diseases characterized by aberrant vasoconstriction and/or cardiac contractility. As such, the identification of novel agonist-GPCR interactions within the cardiovascular system offers great potential for the development of novel therapeutic modalities. The cloning of the vasoactive cyclic undecapeptide human Urotensin-II (U-II), a selective ligand for the novel serpentine seven transmembrane receptor, GPR14 (Ames et al., 1999), is one such pairing that has met with considerable interest recently (Hare et al., 1999; Davenport & Maguire, 2000; Newby & Jalan, 2000; Gray et al., 2000) since (a) human U-II and GPR14 are both expressed within the vasculature and (b) preliminary pharmacological evaluation indicates that human U-II possesses significant vasoactive properties (Ames et al., 1999; Bottrill et al., 2000; MacLean et al., 2000).
PreproU-II mRNA is expressed abundantly within the CNS of frogs, rats and humans (medulla oblongata, acetylcholinesterase-positive ventral horn motor neurones; Coulouarn et al., 1998; 1999; Ames et al., 1999). Indeed, rp-HPLC fractions of porcine spinal cord, bovine hypothalamic and squirrel monkey brain extracts selectively activate GPR14 (Mori et al., 1999; Nothacker et al., 1999). However, in addition to the CNS, U-II is clearly present within the mammalian periphery including the vasculature (cardiac myocytes, coronary atheroma; Ames et al., 1999) and several other peripheral tissues including kidney, adrenal gland, pancreas and liver (Coulouarn et al., 1998; Nothacker et al., 1999). Further, in addition to any putative paracrine action, U-II likely functions as an endocrine factor since U-II-like immunoreactivity has been detected within fish plasma (∼30 pM; Kobayashi et al., 1986; Winter et al., 1999). Irrespectively, however, the fact that the cognate receptor for U-II, GPR14, is expressed within the cardiovasculature (Ames et al., 1999) implies that the heart and peripheral vasculature represent ‘target organs' for this hormone.
The characterization of GPR14, a novel GPCR homologous to a rat ‘orphan' receptor originally designated SENR or GPR14 (Tal et al., 1995; Marchese et al., 1995), as a selective U-II receptor was originally made by Ames et al. (1999) and confirmed subsequently by several other groups using rat GPR14 (Liu et al., 1999; Mori et al., 1999; Nothacker et al., 1999). As with U-II, GPR14 expression is evident within the cardiovascular system. In addition to brain (e.g. cerebellum), spinal cord, skeletal muscle, pancreas and bladder, GPR14 mRNA is readily detectable within rat, mouse, bovine and human cardiomyocytes, arterial vascular smooth muscle (e.g. aortae, pulmonary artery) and vascular (arterial and venous) endothelium. Radioligand binding studies demonstrate the presence of [125I]-U-II binding sites in cardiac and arterial membrane preparations from the rat (Itoh et al., 1988; Ames et al., 1999). Thus, based on the expression profile of U-II/GPR14 in mammals, the initial aim of the present study was to characterize the vasoconstrictor properties of human U-II in a diverse range of native vascular tissue from several species including rat, mouse, dog, pig and New/Old World primates.
A preliminary account of some of the data described in this manuscript was presented to the 72nd American Heart Association Meeting and the VIth International Conference on Endothelin (Douglas et al., 1999a,1999b; Gray et al., 2000).
Methods
General preparation of isolated vascular tissue
All experiments were performed in accredited facilities in accordance with institutional guidelines, SmithKline Beecham (Animal Care and Use Committee) following the guidelines of the American Association of Laboratory Animal Care (DHSS #NIH 85-23) and the UK Animals (Scientific Procedures) Act of 1986. Vessels were isolated, cleaned of adherent tissue and denuded of endothelium using a pair of fine forceps (functional loss was confirmed using 10 μM carbachol). With the exception of non-rodent aortae (where helical strips were prepared), all vessels were suspended in 10 ml organ baths as rings bathed in Krebs of the following composition (mM): NaCl, 112.0; KCl, 4.7; KH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; NaHCO3, 25.0; dextrose, 11.0. Krebs was maintained at 37±1°C and gassed with 95%O2:5%CO2 (pH 7.4) and all experiments were performed in the presence of 10 μM indomethacin (0.1% ethanol). Agonist-induced variations in isometric tension were recorded using FT03 force-displacement transducers (Grass Instruments, Quincy, MA, U.S.A.) coupled to Model 7D polygraphs. Vessels were equilibrated for 90 min following which they were exposed to standard concentrations of KCl (60 mM) and noradrenaline (1 μM). All subsequent responses were normalized to these responses.
Contractile activity of human U-II in rat isolated tissue
Proximal descending thoracic aortae were isolated from adult male Sprague-Dawley rats (400 g; Charles River Breeding Labs, Wilmington, MA, U.S.A.) following sodium pentobarbitone overdose. No more than four 3 mm rings were isolated from any given rat and care was taken to isolate consistently a ∼12 mm length of descending aorta from the aortic arch at a point immediately distal to the origin of the left subclavian artery. Endothelium-denuded rings were randomly assigned to organ baths, placed under 1 g weight optimal resting tension and, once equilibrated, exposed to human U-II in a cumulative manner. The contractile potency and efficacy of this peptide was compared to that of a number of standard vasoactive peptide hormones, biogenic amines and eicosanoids.
In a separate series of experiments, anatomical differences in vascular reactivity to human U-II were examined in 3 mm arterial and venous rings isolated from the proximal descending thoracic aorta, abdominal aorta (distal the iliolumbar vessels and immediately proximal to the iliac bifurcation), left common carotid artery and left jugular vein. With the exception of the carotid artery (0.5 g weight resting tension), all vessels were studied under predetermined 1 g weight optimal resting tension. In addition, epithelium-denuded rat tracheal rings were also isolated and exposed to human U-II or the airway smooth muscle spasmogen carbachol.
Isolated descending thoracic aortae were also exposed to a single EC80 concentration of human U-II (10 nM). The resultant contraction was allowed to plateau, at which point the organ bath was washed thoroughly a total of three times. The subsequent decay in agonist-induced tone was followed over 45 min. An identical procedure was performed using EC80 concentrations of [Arg8] vasopressin (10 nM), ET-1 (30 nM), angiotensin-II (30 nM), KCl (60 mM), noradrenaline (100 nM) and 5-HT (100 nM) for comparison.
Effects of human U-II in mouse isolated aortic rings
Following pentobarbitone overdose, the entire length of the descending aorta was isolated from adult male C57 Bl/6 mice (25 g; Charles River). Aortae were cut into three gross anatomical sections (proximal thoracic, medial and distal abdominal aortae, each approximately 3 mm in length) and suspended in organ baths under predetermined 0.5 g weight optimal resting tension. Changes in isometric tension were recorded following exposure to human U-II (either cumulative concentration-response curves or single bolus concentrations) or noradrenaline.
Regional differences in reactivity to human U-II in dog isolated arteries and veins
Blood vessels were isolated from adult (15 kg) male Beagle dogs (Marshall Farms, North Rose, NY, U.S.A) following sodium pentobarbitone overdose and, unless stated otherwise, studied under predetermined 5 g weight optimal resting tension: common carotid, renal, femoral, LAD and LCX coronary, internal mammary, pulmonary and mesenteric (tertiary branch; 2 g weight resting tension) and basilar (1 g weight resting tension) arteries, descending thoracic and abdominal aortae (2 g weight resting tension in the latter) and pulmonary, saphenous (1 g weight resting tension) and jugular veins. Vessels were exposed sequentially to cumulative concentrations of 5-HT, human U-II and, finally, noradrenaline. Once a cumulative curve had been constructed, tissues were washed repeatedly and allowed to recover over 90 min prior to the generation of the subsequent agonist-response curve (the exceptions being the basilar and coronary arteries which were exposed to ET-1 rather than noradrenaline since catecholamines relax these vessels).
Regional differences in reactivity to human U-II in porcine isolated arteries and veins
Blood vessels were isolated from (40 kg) male domestic pigs (Barton West End Farms, Oxford, NJ, U.S.A.) following sodium pentobarbitone overdose. In contrast to the left circumflex coronary and common carotid arteries (2 g weight resting tension), thoracic and distal abdominal aortae, renal, femoral, internal mammary, pulmonary and mesenteric arteries saphenous veins were suspended under predetermined 5 g weight optimal resting tension. Once equilibrated, cumulative concentration-response curves were generated to human U-II.
Regional differences in reactivity to human U-II in primate isolated arteries and veins
Blood vessels were isolated from adult (5 kg) cynomolgus monkeys (Macaca fascicularis) following sodium pentobarbitone overdose. Unless stated to the contrary, vessels were suspended under predetermined optimal resting tension of 2 g weight and included proximal descending/distal thoracic and distal abdominal aortae (1 g weight resting tension in the latter), basilar (1 g weight resting tension), common carotid (1 g weight resting tension), renal, femoral, LAD and LCX coronary, internal mammary, pulmonary and mesenteric (tertiary branch) arteries and pulmonary, jugular (both 1 g weight resting tension) and saphenous veins. Vessels were exposed sequentially to cumulative concentrations of 5-HT, human U-II and, finally, ET-1. Once a cumulative curve had been constructed, tissues were washed repeatedly and allowed to recover over 90 min prior to the generation of the subsequent agonist-response curve (the exceptions to this paradigm were the aortae and the carotid artery where human U-II-induced contractions were deemed ‘irreversible' i.e. U-II-induced tone took >6 h to return to basal values and, therefore, concentration-response curves could not be generated to ET-1).
In a separate series of experiments, blood vessels were isolated from adult (350 g) common marmosets (Callithrix jacchus) following sodium pentobarbitone overdose. Unless stated to the contrary, vessels were suspended under optimal resting tension of 2 g weight and included descending thoracic and distal abdominal aortae, common carotid, femoral (1 g weight resting tension) and mesenteric (secondary or tertiary branch, 1 g weight resting tension) arteries. Responses to human U-II were compared to those obtained with KCl (60 mM) or phenylephrine (1 μM).
Statistical and data analysis
All values are expressed as mean±s.e.mean and n represents the total number of animals from which vessels were isolated. Statistical comparisons were made by ANOVA (Fisher's protected least square difference), and differences considered significant where P<0.05. Concentration-response curves were fitted to a logistic equation (Douglas et al., 1995):
 |
where R is the contractile response, C the concentration of agonist, EC50 the concentration of agonist required to produce a half maximal response, nH the Hill coefficient and Rmax the maximal contractile response.
Drugs and reagents
Human U-II was prepared by Mr J. Martin (Protein Biochemistry, SmithKline Beecham). Goby U-I, somatostatin[1-14], angiotensin-II, MCH and porcine NPY (Bachem, King of Prussia, PA, U.S.A.), [Arg8] vasopressin (Sigma, St Louis, MO, U.S.A.), human adrenotensin (Phoenix Pharmaceuticals, Mountain View, CA, U.S.A.), and ET-l (American Peptide, Sunnyvale, CA, U.S.A.) were from commercial vendors (acetate salts stored as 1 mM stock solutions in distilled water at −20°C). Carbachol, indomethacin, (−)noradrenaline bitartrate (in ascorbate), phenylephrine, 5-HT creatinine sulphate and prostaglandin F2α were from Sigma. All other reagents used were of analytical grade. All drugs were made freshly on the day of experimentation and stored in a light-tight container on ice.
Results
Contractile activity of human U-II in rat isolated arteries and veins
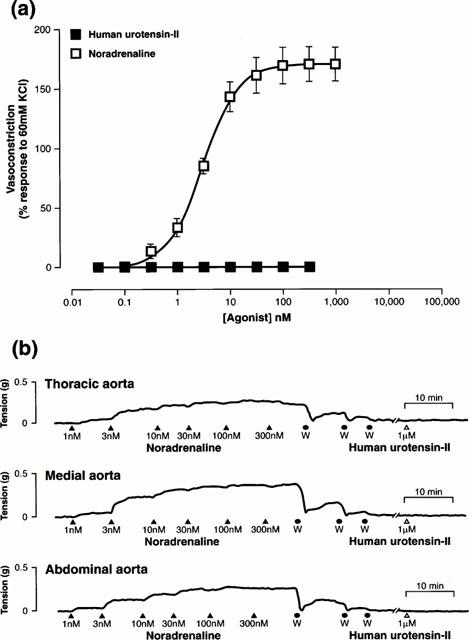
Exposure of the rat isolated descending thoracic aorta to human U-II (either as a single bolus 10 nM concentration or cumulative addition; Figure 1) resulted in a concentration-dependent contraction (Figure 2). Contraction was relatively slow in onset, sustained and occasionally associated with ‘cyclical' changes in tone as shown in Figure 1 (either upon exposure to the peptide or following removal of the peptide by washing or both). As a spasmogen, human U-II was both efficacious and potent (3 fold more potent and almost twice as efficacious as angiotensin-II and [Arg8]vasopressin in the rat isolated aorta; Table 1). Human U-II was 16, 32, 661 and 708 fold more potent than ET-1, noradrenaline, 5-HT and prostaglandin F2α in this tissue (Table 1, Figure 2). Goby U-I and cyclic somatostatin[1–14] were devoid of contractile activity in this tissue (⩽300 nM) as were NPY, MCH and adrenotensin. Contractile responses induced by 10 nM human U-II were characteristically sustained and resistant to washout in the rat aorta (Figure 1, Table 2). The time taken for human U-II-induced tone to fall by 75% upon removal of the agonist from the organ by washing (∼30 min) was an order of magnitude greater than that observed with EC80 concentrations of other pharmacologically distinct vasoactive factors including KCl, noradrenaline, [Arg8]vasopressin or angiotensin-II (Table 2). Human U-II-induced responses were, however, less resistant to washout than those induced by 30 nM ET-1 in this tissue.
Figure 1.

Representative experimental traces illustrating the contractile responses obtained in rat proximal descending thoracic aortic rings isolated from three separate rats upon exposure to 10 nM human U-II. Contraction was sustained, and tone did not return to basal levels for >30 min following removal of peptide from the organ bath. Also evident are the cyclical changes in tone frequently observed upon (a) addition or (b) removal of human U-II to or from (or both, panel c) the organ bath. The vertical and horizontal scale bars represent tension (1 g) and time (10 min), respectively.
Figure 2.

Human U-II is a potent and efficacious spasmogen of rat isolated aortae. The figure shows log concentration-response curves to human U-II (n=15), [Arg8]vasopressin (n=9), angiotensin-II (n=12) and prostaglandin F2α (n=3) in rat isolated proximal descending thoracic aortae (responses are normalized to 60 mM KCl). All experiments were performed in endothelium-denuded aortae in the presence of 10 μM indomethacin. Values are mean and vertical bars represent the s.e.mean and n represents the number of vessels studied. Curves were derived by fitting experimental data to a logistic equation (Douglas et al., 1995).
Table 1.
Relative contractile potency and efficacy of human U-II in the rat isolated proximal descending thoracic aorta
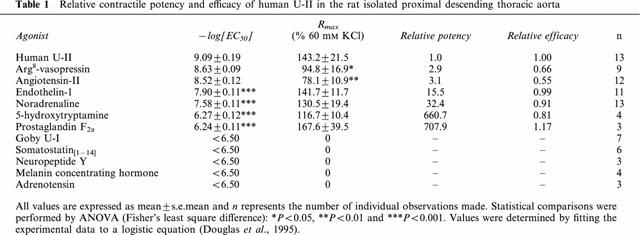
Table 2.
Resistance to washout of human U-II relative to equiefficacious (EC80) concentrations of standard spasmogens in the rat isolated proximal descending thoracic aorta

In contrast to the thoracic aorta, human U-II only induced a detectable contractile response in three out of four carotid arteries and two out of four distal abdominal aortae examined (although reproducible responses were obtained with KCl and noradrenaline). None of the jugular vein rings studied responded to this peptide, and contraction was not seen in isolated femoral and renal artery rings. As shown in Figure 3, the contractile potency of human U-II did not differ between the proximal descending thoracic aorta (−log[EC50] 9.09±0.19; 13/13 vessels responded), left common carotid artery (−log[EC50] 8.84±0.21; 3/4 vessels responded) and distal abdominal aorta (−log[EC50] between 8.54–9.00; 2/4 vessels responded). However, relative to the thoracic aorta (Rmax 143.2±21.5% 60 mM KCl), the contractile efficacy of human U-II was reduced in the carotid artery (Rmax 67.4±25.6% 60 mM KCl; P<0.05) and abdominal aorta (Rmax between 7.6–7.8% 60 mM KCl) by 53 and 95%, respectively.
Figure 3.

Differential anatomical reactivity to human U-II in rat isolated blood vessels. The figure illustrates log concentration-response curves to human U-II in the rat isolated proximal descending thoracic aortae (n=13), left common carotid artery (curve derived from the three out of four vessels that were responsive), distal abdominal aorta (curve derived from the two out of four vessels that were responsive) and left jugular vein (n=4). Responses in the proximal descending thoracic aorta are from Figure 2 and are shown for ease of comparison. All experiments were performed in endothelium-denuded aortae in the presence of 10 μM indomethacin. Values are mean and vertical bars represent the s.e.mean (with the exception of human U-II in the distal abdominal aorta where only two out of four vessels studied responded hence vertical bars represent the range) and n represents the number of vessels studied. Curves were derived by fitting experimental data to a logistic equation (Douglas et al., 1995). Responses are normalized to 60 mM KCl.
Contractile activity of human U-II in rat isolated airway smooth muscle
Human U-II was devoid of any detectable contractile activity in epithelium-denuded rat isolated tracheal rings (⩽1 μM; n=3), vessels which were clearly responsive to the airway smooth muscle spasmogen carbachol (−log[EC50] 7.03±0.13, Rmax 191.3±11.6% 60 mM KCl, n=3; Figure 4).
Figure 4.

Human U-II lacks contractile activity in rat isolated trachea. The figure illustrates log concentration-response curves to carbachol (n=3) and human U-II (n=3) in the rat isolated trachea. All experiments were performed in epithelium-denuded trachea in the presence of 10 μM indomethacin. Values are mean and vertical bars represent the s.e.mean (n represents the number of vessels studied). Curves were derived by fitting experimental data to a logistic equation (Douglas et al., 1995). Responses are normalized to 60 mM KCl.
Effects of human U-II in mouse isolated aortic rings
In contrast to the rat, neither cumulative addition of human U-II (⩽300 nM; Figure 5a) nor the addition of a single, bolus concentration of human U-II (1 μM; Figure 5b) elicited a contractile response in mouse isolated proximal thoracic, medial or abdominal aortae (n=8, 8 and 7, respectively). Isolated proximal thoracic, medial or abdominal aortae were viable, however, since exposure to noradrenaline resulted in robust, concentration-dependent vasoconstriction (−log[EC50]s 8.57±0.05, 8.46±0.05 and 8.53±0.07, Rmaxs 171.0±13.8%, 158.6±13.3% and 156.0±16.8% 60 mM KCl, n=8, 8 and 7, respectively; Figure 5).
Figure 5.
Human U-II lacks contractile activity in mouse isolated aortae. (a) Log concentration-response curves to human U-II (n=8) and noradrenaline (n=8) in the mouse isolated proximal descending thoracic aorta. Values are mean and vertical bars represent the s.e.mean (n represents the number of vessels studied). Curves were derived by fitting experimental data to a logistic equation (Douglas et al., 1995). Responses are normalized to 60 mM KCl. (b) Representative experimental traces illustrating the contractile responses obtained in mouse isolated proximal descending thoracic, medial and distal abdominal aortic rings upon cumulative exposure to noradrenaline. Tone returned to basal following removal of the catecholamine from the organ bath by washing (W) at which time tissues were exposed to 1 μM human U-II. The vertical and horizontal scale bars represents tension (0.5 g) and time (10 min), respectively. All experiments were performed in endothelium-denuded vessels in the presence of 10 μM indomethacin.
Regional differences in reactivity to human U-II in dog isolated arteries and veins
Human U-II exhibited a unique contractile profile in canine isolated vessels. All three canine LCX coronary arteries studied responded to human U-II (Figure 6a). U-II was 32 and 40 fold more potent than ET-1 and 5-HT (Table 3). Further, although the Rmax determined for human U-II was only half of that obtained with ET-1, it was double that achieved with 5-HT, an established coronary spasmogen of (patho)physiological relevance. Similar observations were made in the LAD coronary artery (Table 3) where human U-II was 10 fold more potent than ET-1 (of the three LAD vessels examined, one was refractory to 5-HT). Although the contractile potency of human U-II was consistent in the vessels studied from all three dogs, the contractile efficacy of U-II (and ET-1) varied considerably in the LAD (e.g. Rmax for human U-II ranged from 81 to 409% 60 mM KCl). In the three vessels removed from the arterial side of the pulmonary tree, no responses were observed for U-II (Figure 6b). In contrast, however, activity was detected in canine isolated pulmonary veins (Figure 6c). Responses were variable inasmuch as only two out of three vessels removed were reactive to human U-II. Human U-II (⩽100 nM) was devoid of detectable contractile activity in all canine isolated aortae (thoracic and abdominal segments), renal, internal mammary, femoral (Figure 6d), basilar, common carotid and mesenteric arteries and saphenous and jugular veins studied (vessels which responded to pharmacologically unrelated spasmogens such as noradrenaline and 5-HT).
Figure 6.

Human U-II constricts selected canine isolated blood vessels in a concentration-dependent manner. Log concentration-response curves to human U-II, endothelin-1, noradrenaline or 5-hydroxytryptamine in canine isolated (a) left circumflex (LCX) coronary arteries, (b) pulmonary arteries, (c) pulmonary veins and (d) femoral arteries. All experiments were performed in endothelium-denuded vessels in the presence of 10 μM indomethacin. Values are mean and vertical bars represent the s.e.mean. n=3 for all three agonists in all four vessels (with the exception of human U-II in the pulmonary vein where only two out of three vessels studied responded hence vertical bars represent the range). Curves were derived by fitting experimental data to a logistic equation (Douglas et al., 1995).
Table 3.
Regional differences in vascular reactivity to human U-II in dog isolated vascular tissue

Regional differences in reactivity to human U-II in porcine isolated arteries and veins
The vasoconstrictor profile determined for human U-II in the pig was variable: although vessels were viable (i.e. reactive to 60 mM KCl), no vasoconstriction was observed to human U-II (⩽300 nM) in porcine LCX coronary (n=4), renal (n=4), mammary (n=4) or carotid (n=3) artery or saphenous vein (n=4; Figure 7). Even in vessels deemed responsive, reactivity was only observed with an incidence of 50–75%. Responsive vessels could be sub-grouped based on the degree of contractile efficacy of human U-II, i.e. Rmax <10% or >10% 60 mM KCl. Human U-II was a potent spasmogen in two out of three thoracic aortae and two out of four pulmonary arteries studied (−log[EC50]s 8.82–8.86 and 8.34–8.56, respectively). Where responses were observed in thoracic aortae and pulmonary arteries, human U-II was clearly an efficacious spasmogen (Rmaxs 42.0–107.6% and 16.7–44.1% 60 mM KCl, respectively). Although human U-II was a potent spasmogen in three out of four femoral arteries and abdominal aortae studied (−log[EC50]s 9.37±0.25 and 8.69±0.03, respectively), this undecapeptide was not an efficacious spasmogen (Rmaxs 8.2±2.2 and 9.9±1.4% 60 mM KCl, respectively). Responses in isolated mesenteric arteries were also varied (two out of three vessels studied responded to human U-II with −log[EC50] 8.37–9.03, but the efficacy of this spasmogen ranged from 15.7–95.7% 60 mM KCl).
Figure 7.

Human U-II constricts selected porcine isolated blood vessels in a concentration-dependent manner. (a) Log concentration-response curves to human U-II in isolated thoracic aortae (responses detected in two out of three vessels studied) and pulmonary arteries (responses detected in two out of four vessels studied). (b) Log concentration-response curve responses to human U-II in isolated femoral arteries (responses detected in three out of four vessels studied), abdominal aortae (responses detected in three out of four vessels studied) and left circumflex (LCX) coronary arteries (no detectable response in any of the four vessels studied). All experiments were performed in endothelium-denuded vessels in the presence of 10 μM indomethacin. Values are mean and vertical bars represent the s.e.mean for femoral artery and abdominal aorta (vertical bars represent the range in thoracic aorta and pulmonary artery). Curves were derived by fitting experimental data to a logistic equation (Douglas et al., 1995).
Regional reactivity to human U-II in cynomolgus monkey isolated arteries and veins
Human U-II was a potent and efficacious spasmogen in all arterial blood vessels isolated from the cynomolgus monkey (Figure 8, Table 4). Contraction was observed in both LCX and LAD portions of the coronary arterial tree (Figure 8a, Table 4). Human U-II was equally as efficacious as a contractile agonist in both the LCX and LAD relative to ET-1, and was 10–14 fold more potent. Furthermore, not only was human U-II a more potent spasmogen than 5-HT (470 and 494 fold in the LAD and LCX, respectively), it was also clearly a more efficacious spasmogen than this biogenic amine.
Figure 8.
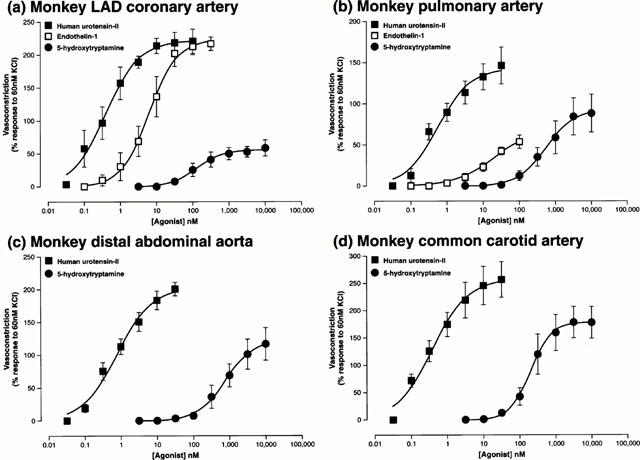
Human U-II constricts cynomolgus monkey arteries in vitro in a concentration-dependent manner. Log concentration-response curves in isolated (a) left anterior descending (LAD) coronary arteries, (b) pulmonary arteries, (c) distal abdominal aortae and (d) common carotid arteries following cumulative exposure to human U-II (n=4, 4, 5 and 5, respectively), 5-hydroxytryptamine (all n=3) or endothelin-1 (n=4 and 3 in coronary and pulmonary arteries, respectively). All experiments were performed in endothelium-denuded vessels in the presence of 10 μM indomethacin. Values are mean and vertical bars represent the s.e.mean. Curves were derived by fitting experimental data to a logistic equation (Douglas et al., 1995).
Table 4.
Regional differences in vascular reactivity to human U-II in vascular tissue isolated from cynomolgus monkeys

Human U-II induced potent and efficacious contraction of monkey isolated pulmonary arterial rings where it was 23 fold more potent and 2.5 fold more efficacious than ET-1 (Figure 8b, Table 4). Human U-II was three orders of magnitude more potent than 5-HT, and 60% more efficacious as a spasmogen in the isolated pulmonary artery. In contrast, exposure of isolated jugular, saphenous and portal veins to human U-II (⩽100 nM) failed to elicit a detectable contractile response (all venous tissue was viable, however, based on reactivity to both KCl, ET-1 and 5-HT). The only vessel of venous origin that responded to human U-II was isolated from the pulmonary vasculature.
Human U-II induced potent, efficacious and reproducible constriction in isolated renal, femoral and mesenteric arteries (Table 4). Although human U-II, ET-1 and 5-HT were all equally efficacious as spasmogens in all three vessels, human U-II was 19, 109 and 10 fold more potent than ET-1 and 152, 1,945 and 433 fold more potent than 5-HT, respectively. Further to this, both internal mammary and basilar arteries responded to human U-II. In terms of human U-II reactivity, the cynomolgus monkey basilar artery represents the most sensitive vessel identified to date. Clearly, human U-II was an efficacious spasmogen in the basilar and mammary arteries (10–30 fold greater Rmax than that determined for ET-1 and 5-HT in the latter).
Both the proximal/distal thoracic and distal abdominal (Figure 8c) sections of aortae responded to human U-II (Table 4). Interestingly, and in contrast to all other cynomolgus monkey arteries studied (where vascular tone was returned to basal conditions following washout of the organ bath within approximately 45–60 min), all three sections of aorta were characterized by the extremely sustained nature of the contractile responses to human U-II. Upon completion of a standard concentration-response curve to human U-II, some 6 to 8 h was required following the removal of human U-II from the organ bath by repeated washing before tone returned to basal levels (hence subsequent concentration-contraction curves could not be determined to ET-1 in the tissue available). In addition, this resistance to washout was also evident in the common carotid artery.
Regional differences in reactivity to human U-II in marmoset isolated arteries and veins
The contractile profile of human U-II was assessed in vessels isolated from marmosets, New World primates. Vasoconstriction was recorded in four out of five thoracic aortae studied, but efficacy was modest (Rmax 18.9±5.2% 60 mM KCl, EC50 1 nM in all four reactive tissues). Responses in all other vessels studied were less consistent, including those observed in isolated abdominal aorta (responses observed in one out of three vessels where Rmax was 130% 60 mM KCl with an EC50 ∼0.5 nM) or mesenteric (two out of three vessels were refractory to human U-II, and in the one vessel which did appear to be contracted, high concentrations of human U-II were required and efficacy was low i.e. 10 nM threshold concentration, response to 100 nM human U-II was 11% 60 mM KCl) and carotid (only one of two vessels isolated responded where Rmax was 13% 60 mM KCl with an EC50 ∼2 nM) arteries. No contractile response to human U-II was observed in femoral arteries.
Discussion
U-II was originally purified from goby (Gillichthys mirabilis) urophysis extracts (Bern & Lederis, 1969; Pearson et al., 1980). Although this highly innervated and vascularized neurosecretory gland is believed to represent a vestigial organ, it is analogous in structure and function to the mammalian hypothalamo-neurohypophysial axis (Lederis, 1984; Bern et al., 1985). Although best known for their ability to elevate smooth muscle tone in fish/amphibian vascular, gastrointestinal and genitourinary tissues (Bern et al., 1985; Yano et al., 1994; Conlon et al., 1996; 1997), U-II isopeptides may also serve additional osmoregulatory and endocrine/metabolic functions (steroidogenesis, lipolysis, prolactin release; Bern et al., 1985; Loretz et al., 1985; Sheridan & Bern., 1986; Conlon et al., 1996; 1997; Kelsall & Balment, 1998). Since evolutionary pressure has acted to conserve this isopeptide family across a diverse range of species, it has been proposed that this peptide family exerts similar physiological actions in higher species including mammals. This hypothesis is supported by the recent identification of U-II isopeptides in the rat, mouse, pig and man (Ames et al., 1999; Coulouarn et al., 1998; 1999; Mori et al., 1999). The present study has evaluated the functional activity of human U-II in a range of native vascular tissue isolated from a variety of mammals.
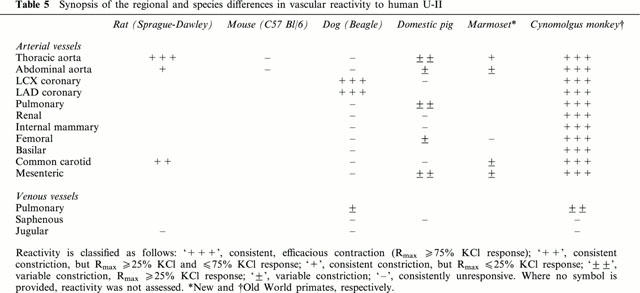
In accord with previous studies performed using the goby isoform of U-II in fish, amphibian and mammalian (rat and rabbit aortae) isolated vascular tissue (Muramatsu et al., 1979; Gibson, 1987; Gibson et al., 1988; Itoh et al., 1987; 1988; 1991; Conlon et al., 1996), potent, sustained and efficacious vasoconstriction was observed upon exposure of specific rat, dog, pig and primate isolated blood vessels to the human isoform of U-II (Table 5). Indeed, human U-II was between 8 and 109 fold more potent than ET-1. Generally, this endothelium-derived polypeptide is considered to be the most potent mammalian vasoconstrictor identified to date (Yanagisawa et al., 1988). Currently, therefore, human U-II represents the most potent mammalian spasmogen identified. Nevertheless, it should be acknowledged that, relative to U-II, ET-1 induces contractile responses in a more diverse range of conduit vessels. The EC50s generated in native mammalian vascular tissue were in accord with those affinities determined in stable/transient recombinant rat and human GPR14 cell lines (HEK293, CHO and COS-1) using both radioligand binding ([125I]-Tyr9-human U-II or [125I]-Ala1-frog U-II) and functional (Ca2+-mobilization [FLIPR and aequorin] or [3H]-arachidonate release) assays (Ames et al., 1999; Liu et al., 1999; Mori et al., 1999; Nothacker et al., 1999). Moreover, values were similar to affinities determined for [125I]-labelled goby (Itoh et al., 1988) and human (Ames et al., 1999) U-II in native cardiovascular tissue (rat cardiac and aortic membranes; Itoh et al., 1988, Ames et al., 1999).
Table 5.
Synopsis of the regional and species differences in vascular reactivity to human U-II
As with goby U-II (Gibson, 1987), human U-II-induced contraction was both slow in onset and sustained, consistent with the kinetic properties of [125I]-human U-II at recombinant GPR14 (<10% dissociation at 2 h; N.V. Aiyar, unpublished observation). Similarly, human U-II-induced contraction was extremely resistant to washout relative to other known spasmogens (with the exception of ET-1 which also has a very slow off rate from its receptor(s), frequently being described as a ‘pseudo-irreversible' ligand; Douglas & Ohlstein, 1997).
Human U-II elevates total peripheral resistance in the anaesthetized cynomolgus monkey (Ames et al., 1999). Paradoxically, however, although detailed regional haemodynamics have not been determined to date, neither goby nor human U-II isopeptides elicit systemic pressor responses in the anaesthetized or pithed rat (Gibson et al., 1986; Hasegawa et al., 1992; R.N. Willette, unpublished observation). It is unlikely that this reflects the use of non-rodent U-II isopeptides in the rat since human, rat and goby U-II isopeptides all exhibit identical affinities in both recombinant human and rat GPR14 radioligand binding/functional assays (Ames et al., 1999; Liu et al., 1999; Mori et al., 1999; Nothacker et al., 1999), and are equipotent spasmogens in the rat aorta (S.A. Douglas, unpublished observation). This apparent in vivo ‘paradox' may be related, however, to the observation that U-II isopeptides exhibit a unique ‘anatomically restricted' contractile profile in rat isolated vascular tissue. The relative contractile efficacy of human U-II is severely attenuated in vessels distal to the aortic arch, e.g. Rmax is reduced by 95% in the abdominal aorta (interestingly, expression of rat GPR14 mRNA is readily demonstrated in thoracic portions of rat aorta by RT–PCR, yet is undetectable in abdominal portions of the same vessel under identical conditions). This observation parallels an elegant study by Itoh et al. (1988) who correlated a loss in goby U-II contractile efficacy with a reduction in [125I]-goby U-II radioligand binding site density in rat isolated blood vessels. As such, there does not appear to be a significant ‘spare receptor reserve' for U-II in the rat vasculature. Even very modest changes in receptor expression may have profound effects on the contractile efficacy of this peptide. It is noteworthy that both chronic hypoxia and L-NAME administration augment vascular reactivity to human U-II in human and rat isolated pulmonary arteries (MacLean et al., 2000). Based on the ‘pseudo-irreversible' binding kinetics of the U-II isopeptides, rapid regulation of receptor density may afford greater physiological control of U-II-induced intracellular signalling (rather than uptake/metabolism of free ligand as it dissociates from the receptor complex).
An alternate explanation for the apparent disparity between the in vitro (vasoconstriction) and in vivo (systemic vasodepression) activities observed in the rat may be that the constrictor activity of U-II is ‘influenced' by the simultaneous release of modulatory factors, e.g. NO, PGI2. For example, goby U-II elicits transient endothelium-dependent relaxation in the rat aorta (although, in the rat aorta at least, responses are relatively refactory to endothelial denudation and indomethacin/NDGA; Gibson, 1987; Bottrill et al., 2000). However, regional and species differences in the extent of endothelium modulation cannot account for the differential effects recorded in the present study since all vessels were denuded of endothelium and studied in the presence of indomethacin. Interestingly, however, not only are significant anatomical differences observed for the vasoconstrictor activity of U-II, a similar phenomenon has recently been reported to exist with respect to the vasodilator capacity of U-II (Bottrill et al., 2000). In the rat, for example, a spectrum of vessel-dependent constrictor/dilator activity can be observed ranging from those preparations which (a) are refractory to U-II (where no constrictor or relaxant response is seen e.g. rat basilar artery), (b) exhibit mixed dilator/constrictor activity to U-II (rat coronary/mesenteric artery) or (c) only respond to U-II with a contractile response (e.g. absence of goby or human U-II-induced dilation in rat aorta; Bottrill et al., 2000). These regional differences are further complicated by the fact that the underlying mechanisms also differ between vascular beds (NO versus EDHF). Clearly, additional studies are warranted employing a range of species and vessels (both conduit and resistance) in order to appreciate fully the relative role(s) of U-II as a vasodilator/vasoconstrictor peptide. Based on published studies where systemic and regional haemodynamics have been examined in intact animals (Gibson et al., 1986; Hasegawa et al., 1992; Ames et al., 1999), the relaxant profile of the U-II is also likely to exhibit significant species differences.
Not only are anatomical differences observed between vessels, significant differences in reactivity are observed between species (Table 5). Such differences are important considerations when contemplating the utility of experimental animal models for the study of the physiology and pathophysiology of U-II, at least with respect to its vascular actions. As detailed above, one might argue that the use of a human isoform of U-II in non-human tissue underlies this lack of reactivity. However, as discussed below, such a hypothesis is not supported by radioligand binding or functional studies performed in both native tissue and recombinant rat/human GPR14 cell lines e.g. non-murine U-II isopeptides are active in mouse non-vascular tissue (goby U-II in the mouse anococcygeus; Gibson et al., 1984), mouse U-II contracts rat aortae and functions as a high affinity ligand at both rat and human GPR14 (Douglas & Aiyar, unpublished observations).
To illustrate further the differences in reactivity between species, U-II was found to exhibit a ‘coronary-selective' vasoconstrictor profile in the dog. This contrasted the vasoconstrictor profile of human U-II in the pig where, once again, the spasmogenic profile was unique to this species. Further, even in vessels deemed responsive to human U-II, reactivity was ‘inconsistent' in the pig. Similar intra-species variability has been reported in humans although, to date, only pulmonary arteries have been studied (MacLean et al., 2000). Where contractile responses were observed in porcine vessels, it was only with an incidence of 50–75%, and significant differences were observed between vessels with respect to contractile efficacy (Rmaxs ranging from 8 to 75% 60 mM KCl; thoracic aorta, pulmonary artery > mesenteric artery > femoral artery, abdominal aortae >4 saphenous vein, LCX, renal, mammary and carotid arteries).
In contrast to vessels isolated from the rat, mouse, dog and pig, human U-II was a ubiquitous, potent and efficacious spasmogen of cynomolgus monkey arterial blood vessels. In contrast, however, and with the one exception of the pulmonary vasculature, all isolated venous tissues studied (jugular, saphenous and portal veins) were refractory to human U-II (or, more accurately, responses were not detectable). Such an observation is consistent with RT–PCR analysis of human vascular tissue where GPR14 is ‘selectively' expressed in arterial (thoracic aorta) but not venous (vena cava) blood vessels and in human arterial (coronary artery and aortic) but not venous (renal vein) cultured smooth muscle cells (Ames et al., 1999). The detailed characterization of the contractile profile of U-II within the human vasculature (both arterial and venous) awaits elucidation. Clearly, based on the species-dependent variations described in the present study, it would be unwise to extrapolate from one species or another to man. Interestingly, however, human U-II is a more efficacious and consistent spasmogen in isolated vessels from Old World cynomolgus monkeys relative to those responses generated using tissues from marmosets, a New World monkey. New World and Old World primates evolved separately from ancestral primates. It is the Old World (or ‘true') monkey that is most closely related to man.
To date, the only human vessels that have been studied are from the pulmonary (arterial) vasculature where, of the 30% of vessels examined, contractile efficacy to human U-II was variable (ranging from 12 to 220% response to 50 mM KCl) and dependent on exposure to L-NAME (MacLean et al., 2000). This would appear to contrast the reactivity recorded in the corresponding pulmonary vessels from the cynomolgus monkey (all four vessels studied responded to human U-II in the present study). As such, the present study demonstrates that a complete contractile profile is warranted in man. Similarly, although rat tracheal rings are refractory to human U-II, a recent study reports significant bronchoconstrictor activity for U-II in primate tracheal/bronchial tissue (Hay et al., 2000). As such, similar studies aimed and investigating such differential intra-/inter-species effects are warranted using mammalian respiratory tissue.
In contrast to all other cynomolgus monkey arteries studied (where tone returned to basal conditions following washout of the organ bath within 1 h), human U-II-induced aortic and carotid artery contraction was characterized by sustained its nature (>6–8 h was required subsequent to removing human U-II from the organ bath by washing before tone returned to basal levels). Currently, this physical characteristic is little more than observational and has many potential explanations. However, it will be interesting to see if future studies establish if these contractile responses are mediated by the same receptor(s) as those which mediate constriction in vessels were responses are more readily reversed by washing.
Although the mechanism(s) which underlie the phenomenon of differential species/regional reactivity to U-II awaits elucidation, as stated earlier, the available data suggest that it is unlikely to reflect simply the use of a human U-II isoform in non-human tissues (i.e. cross-species isopeptide reactivity). Firstly, mammalian (rat and human) and non-mammalian (goby) U-II isopeptide isoforms are indistinguishable as spasmogens in the rat aorta (Ames et al., 1999; S.A. Douglas, unpublished observation). Similarly, to date, recombinant rat and human U-II receptors have been unable to differentiate between a range of U-II isopeptides (goby, rat, mouse, pig and human U-II isoforms all exhibit similar sub-nanomolar affinities for human and rat GPR14 as determined by both radioligand binding and [Ca2+]i-mobilization assays using recombinant HEK-293 cells; N.V. Aiyar, unpublished observation).
Such findings are in accord with independent studies where fish (goby), amphibian (frog) and mammalian (human U-II, porcine U-IIA- and U-IIB) U-II isoforms are reported to interact with rat GPR14 with comparable affinities (Liu et al., 1999; Mori et al., 1999; Nothacker et al., 1999). Further to this, the cyclic octapeptide core sequence of the U-II isopeptide family (D/E[CFWKYC]V/I), one which is absolutely conserved across all species identified, is reported to constitute the minimum sequence required for the retention of full biological activity (Itoh et al., 1987; 1988). This implies that the amino-tail of the U-II isopeptide family, a region divergent across species, does not grossly affect ligand binding/function. As such, to date, there is no precedence for the existence of a specific U-II receptor (from any tissue or species) which is capable of differentiating between U-II isoforms from different species. Consequently, a blood vessel such as the porcine isolated coronary artery that is refractory to human U-II, would be predicted to be unresponsive upon exposure to either of the known porcine U-II isoforms. Nevertheless, the definitive answer to such a hypothesis awaits experimental interrogation.
An alternative hypothesis for regional and inter-/intra-species variations in reactivity to U-II centres on the possibility that, in certain vascular beds, individuals or species, U-II receptors are expressed either (a) at levels below the threshold required to induce measurable contraction or (b) in an ‘uncoupled/refractory' state. This could, perhaps, result from full occupancy of the receptor population by U-II in situ (a ‘pseudo-irreversible' ligand with an extremely slow receptor dissociation rate) or might reflect differential species or anatomical regulation of the receptor and/or associated signal transduction (G-protein) mechanisms (e.g. by protein kinase-A/-C, putative receptor kinases, RGS proteins, arrestins, differences in post-translational modification etc.).
The possibility that U-II lacks a significant (spare) receptor reserve in specific species or tissues is consistent with the observations of Itoh et al. (1987; 1988) where determination of radioligand binding Bmaxs and contractile Rmaxs for goby U-II in rat isolated arteries revealed that modest changes in receptor density correlated with profound changes in contractile efficacy. This may constitute a potential physiological mechanism whereby the ability of U-II to regulate systemic vascular resistance can be modified. Such a mechanism would be useful for regulating the chronic actions of a peptide that binds to its receptor within a ‘pseudo-irreversible' manner (it is noteworthy that in the cynomolgus aorta and carotid artery, some 6 to 8 h was required following the removal of human U-II from the organ bath for repeated washing to return tone to basal levels). The possibility also exists that the extent of U-II receptor ‘desensitization' differs between species and/or tissue. Interestingly, the sequence homology between rat and human GPR14 is divergent at both intracellular loop 3 (i3) and the cytoplasmic carboxyl-tail (i4), regions well documented to play critical roles in regulating the receptor activation/binding state (differences include several [Ser/Thr]- or [Tyr]-residues, potential sites for receptor phosphorylation). Such possibilities warrant further detailed investigation.
In summary, the present report is the first to detail the vasoconstrictor profile of human U-II in an array of blood vessels isolated from several different experimental animal species including rodents (mice, rats), dogs, pigs and primates. In those tissues where contraction is observed, human U-II is a universally potent and sustained spasmogen. However, significant anatomical and species differences exist with respect to vascular reactivity to this peptide family (differences which must be accounted for when such experimental species are employed to investigate the physiological and pathophysiological actions of U-II). Clearly, however, attempts must be made to understand how these data relate to the reactivity of the human vasculature and how vascular reactivity is influenced by pathophysiological conditions. Nevertheless, the data presented support a role for this isopeptide family in the regulation of vascular smooth muscle function in mammals.
Acknowledgments
The authors would like to express their gratitude to Susan Tirri, Daryl Ashton, Charles Sauermelch and Robert Coatney (Smith-Kline Beecham) for their assistance during the preparation of this manuscript.
Abbreviations
- FLIPR
fluorescent imaging plate reader
- GPCR
guanosine triphosphate-binding protein [G-protein]-coupled receptor
- LAD coronary artery
left anterior descending coronary artery
- LCX
left circumflex coronary artery
- SENR
sensory epithelial neuropeptide-like receptor
- U-II
Urotensin-II
References
- AMES R.S., SARAU H.M., CHAMBERS J.K., WILLETTE R.N., AIYAR N.V., ROMANIC A.M., LOUDEN C.S., FOLEY J.J., SAUERMELCH C.F., COATNEY R.W., AO Z., DISA J., HOLMES S.D., STADEL J.M., MARTIN J.D., LIU W.-S., GLOVER G.I., WILSON S., MCNULTY D.E., ELLIS C.E., ELSHOURBAGY N.A., SHABON U., TRILL J.J., HAY D.W.P., OHLSTEIN E.H., BERGSMA D.J., DOUGLAS S.A. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- BERN H.A., LEDERIS K. A reference preparation for the study of active substances in the caudal neurosecretory system of teleosts. J. Endocrinol. 1969;45:11–12. [PubMed] [Google Scholar]
- BERN H.A., PEARSON D., LARSON B.A., NISHIOKA R.S. Neurohormones from fish tails: the caudal neurosecretory system. I. “Urophysiology” and the caudal neurosecretory system of fishes. Recent Prog. Horm. Res. 1985;41:533–552. doi: 10.1016/b978-0-12-571141-8.50016-0. [DOI] [PubMed] [Google Scholar]
- BOTTRILL F.E., DOUGLAS S.A., HILEY C.R., WHITE R. Human urotensin-II is an endothelium-dependent vasodilator in rat small arteries. Br. J. Pharmacol. 2000;130:1865–1870. doi: 10.1038/sj.bjp.0703513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONLON M.J., TOSTIVINT H., VAUDRY H. Somatostatin- and urotensin II-related peptides: molecular diversity and evolutionary perspectives. Reg. Peptides. 1997;69:95–103. doi: 10.1016/s0167-0115(97)02135-6. [DOI] [PubMed] [Google Scholar]
- CONLON J.M., YANO K., WAUGH D., HAZON N. Distribution and molecular forms of urotensin II and its role in cardiovascular regulation in vertebrates. J. Exp. Zool. 1996;275:226–238. [PubMed] [Google Scholar]
- COULOUARN Y., JEGOU S., TOSTIVINT H., VAUDRY H., LIHRMANN I. Cloning, sequence analysis and tissue distribution of the mouse and rat urotensin II precursors. FEBS Lett. 1999;457:28–32. doi: 10.1016/s0014-5793(99)01003-0. [DOI] [PubMed] [Google Scholar]
- COULOUARN Y., LIHRMANN I., JEGOU S., ANOUAR Y., TOSTIVINT H., BEAUVILLAIN J.C., CONLON J.M., BERN H.A., VAUDRY H. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the U II gene in motoneurons of the spinal cord. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15803–15808. doi: 10.1073/pnas.95.26.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVENPORT A.P., MAGUIRE J.J. Urotensin II: fish neuropeptide catches orphan receptor. Trends Pharmacol. Sci. 2000;21:80–82. doi: 10.1016/s0165-6147(00)01449-8. [DOI] [PubMed] [Google Scholar]
- DOUGLAS S.A., OHLSTEIN E.H. Signal transduction mechanisms mediating the vascular actions of endothelin. J. Vasc. Res. 1997;34:152–164. doi: 10.1159/000159219. [DOI] [PubMed] [Google Scholar]
- DOUGLAS S.A., BECK G.R., JR, ELLIOTT J.D., OHLSTEIN E.H. Pharmacological evidence for the presence of three distinct functional endothelin receptor subtypes in the rabbit lateral saphenous vein. Br. J. Pharmacol. 1995;114:1529–1540. doi: 10.1111/j.1476-5381.1995.tb14936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS S.A., DISA J., NASELSKY D., ROMANIC A., LOUDEN C., AO Z., ELSHOURBAGY N.A., SARAU H.M., AMES R.S., OHLSTEIN E.H., WILLETTE R.N., AIYAR N.V. Pharmacological characterisation of a novel human G-protein-coupled receptor with specificity for the vasoactive human peptide urotensin-II. Circulation. 1999a;100:I6. [Google Scholar]
- DOUGLAS S.A., SAUERMELCH C.F., COATNEY R., ELSHOURBAGY N.A., AIYAR N.V., OHLSTEIN E.H., WILLETTE R.N. Characterisation of human urotensin-II as the most potent vasoconstrictor identified. Circulation. 1999b;100:I567–I568. [Google Scholar]
- GIBSON A. Complex effects of Gillichthys urotensin II on rat aortic strips. Br. J. Pharmacol. 1987;91:205–212. doi: 10.1111/j.1476-5381.1987.tb09000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., BERN H.A., GINSBURG M., BOTTING J.H. Neuropeptide-induced contraction and relaxation of the mouse anococcygeus muscle. Proc. Natl. Acad. Sci. U.S.A. 1984;81:625–629. doi: 10.1073/pnas.81.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., CONYERS S., BERN H.A. The influence of urotensin II on calcium flux in rat aorta. J. Pharm. Pharmacol. 1988;40:893–895. doi: 10.1111/j.2042-7158.1988.tb06298.x. [DOI] [PubMed] [Google Scholar]
- GIBSON A., WALLACE P., BERN H.A. Cardiovascular effects of urotensin II in anaesthetised and pithed rats. Gen. Comp. Endocrinol. 1986;64:435–439. doi: 10.1016/0016-6480(86)90080-8. [DOI] [PubMed] [Google Scholar]
- GRAY G.A., BATTISTINI B., WEBB D.J. Endothelins are potent vasoconstrictors, and much more besides. Trends Pharmacol. Sci. 2000;21:38–40. doi: 10.1016/s0165-6147(99)01431-5. [DOI] [PubMed] [Google Scholar]
- HARE J.M., KASS D.A., STAMLER J.S. The physiological response to cardiovascular ‘orphan' G protein-coupled receptor agonists. Nature Med. 1999;5:1241–1242. doi: 10.1038/15193. [DOI] [PubMed] [Google Scholar]
- HASEGAWA K., KOBAYASHI Y., KOBAYASHI H. Vasodepressor effects of urotensin II in rats. Neuroendocrinol. Lett. 1992;14:357–363. [Google Scholar]
- HAY D.W.P., LUTTMANN M.A., DOUGLAS S.A. Human urotensin-II is a potent spasmogen of primate airway smooth muscle. Br. J. Pharmacol. 2000;131:10–12. doi: 10.1038/sj.bjp.0703533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITOH H., HIGUCHI H., HIRAOKA N., ITO M., KONISHI T., NAKANO T., LEDERIS K. Contraction of rat thoracic aorta strips by endothelin-1 in the absence of extracellular Ca2+ Br. J. Pharmacol. 1991;104:847–852. doi: 10.1111/j.1476-5381.1991.tb12516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITOH H., ITOH Y., RIVIER J., LEDERIS K. Contraction of major artery segments of rat by fish neuropeptide urotensin II. Am. J. Physiol. 1987;252:R361–R366. doi: 10.1152/ajpregu.1987.252.2.R361. [DOI] [PubMed] [Google Scholar]
- ITOH H., MCMASTER D., LEDERIS K. Functional receptors for fish neuropeptide urotensin II in major rat arteries. Eur. J. Pharmacol. 1988;149:61–66. doi: 10.1016/0014-2999(88)90042-8. [DOI] [PubMed] [Google Scholar]
- KELSALL C.J., BALMENT R.J. Native urotensins influence cortisol secretion and plasma cortisol concentration in the euryhaline flounder. Platichthys flesus. Gen. Comp. Endocrinol. 1998;112:210–219. doi: 10.1006/gcen.1998.7166. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI Y., LEDERIS K., RIVIER J., KO D., MCMASTER D., POULIN P. Radioimmunoassay for fish tail neuropeptides. II: development of a specific and sensitive assay for and the occurrence of immunoreactive urotensin II in the central nervous system and blood of Catostomus commersoni. J. Pharmacol. Meth. 1986;15:321–333. doi: 10.1016/0160-5402(86)90011-2. [DOI] [PubMed] [Google Scholar]
- LEDERIS K.The fish urotensins: hypophyseal and peripheral actions in fishes and mammals Frontiers in neuroendocrinology 1984New York: Raven Press; 247–263.ed. Martin, L. & Ganong, W.F. pp [Google Scholar]
- LIU Q., PONG S.S., ZENG Z., ZHANG Q., HOWARD A.D., WILLIAMS D.L., JR, DAVIDOFF M., WANG R., AUSTIN C.P., MCDONALD T.P., BAI C., GEORGE S.R., EVANS J.F., CASKEY C.T. Identification of urotensin II as the endogenous ligand for the orphan G-protein-coupled receptor GPR14. Biochem. Biophys. Res. Commun. 1999;266:174–178. doi: 10.1006/bbrc.1999.1796. [DOI] [PubMed] [Google Scholar]
- LORETZ C.A., HOWARD M.E., SIEGEL A.J. Ion transport in goby intestine: cellular mechanism of urotensin II stimulation. Am. J. Physiol. 1985;249:G284–G293. doi: 10.1152/ajpgi.1985.249.2.G284. [DOI] [PubMed] [Google Scholar]
- MACLEAN M.R., ALEXANDER D., STIRRAT A., GALLAGHER M., DOUGLAS S.A., OHLSTEIN E.H., MORECROFT I., POLLARD K. Contractile responses to human urotensin-II in rat and human pulmonary arteries: effect of endothelial factors and chronic hypoxia in the rat. Br. J. Pharmacol. 2000;130:201–204. doi: 10.1038/sj.bjp.0703314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCHESE A., HEIBER M., NGUYEN T., HENG H.H., SALDIVIA V.R., CHENG R., MURPHY P.M., TSUI L.C., SHI X., GREGOR P., GEORGE S.R., O'DOWD B.F., DOCHERTY J.M. Cloning and chromosomal mapping of three novel genes, GPR9, GPR10, and GPR14, encoding receptors related to interleukin 8, neuropeptide Y, and somatostatin receptors. Genomics. 1995;29:335–344. doi: 10.1006/geno.1995.9996. [DOI] [PubMed] [Google Scholar]
- MORI M., SUGO T., ABE M., SHIMOMURA Y., KURIHARA M., KITADA C., KIKUCHI K., SHINTANI Y., KUROKAWA T., ONDA H., NISHIMURA O., FUJINO M. Urotensin II is the endogenous ligand of a G-protein-coupled orphan receptor, SENR (GPR14) Biochem. Biophys. Res. Commun. 1999;265:123–129. doi: 10.1006/bbrc.1999.1640. [DOI] [PubMed] [Google Scholar]
- MURAMATSU I., FUJIWARA M., HIDAKA H., AKUTAGAWA H. Pharmacological analysis of urotensin-induced contraction and relaxation in isolated rabbit aortas. Gunma Symp. Endocrinol. 1979;16:39–47. [Google Scholar]
- NEWBY D.E., JALAN R. Urotensin II: better than somatostatin for portal hypertension. Hepatology. 2000;31:1201–1202. doi: 10.1002/hep.510310524. [DOI] [PubMed] [Google Scholar]
- NOTHACKER H.P., WANG Z., MCNEILL A.M., SAITO Y., MERTEN S., O'DOWD B., DUCKLES S.P., CIVELLI O. Identification of the natural ligand of an orphan G-protein-coupled receptor involved in the regulation of vasoconstriction. Nature Cell Biol. 1999;1:383–385. doi: 10.1038/14081. [DOI] [PubMed] [Google Scholar]
- PEARSON D., SHIVELY J.E., CLARK B.R., GESCHWIND I.I., BARKLEY M., NISHIOKA R.S., BERN H.A. Urotensin-II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc. Natl Acad. Sci. U.S.A. 1980;77:5021–5024. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERIDAN M.A., BERN H.A. Both somatostatin and the caudal neuropeptide, urotensin II, stimulate lipid mobilisation from coho salmon liver incubated in vitro. Reg. Peptides. 1986;14:333–344. doi: 10.1016/0167-0115(86)90175-8. [DOI] [PubMed] [Google Scholar]
- STADEL J.M., WILSON S., BERGSMA D.J. Orphan G protein-coupled receptors: a neglected opportunity for pioneer drug discovery. Trends Pharmacol. Sci. 1997;18:430–437. doi: 10.1016/s0165-6147(97)01117-6. [DOI] [PubMed] [Google Scholar]
- TAL M., AMMAR D.A., KARPUJ M., KRIZHANOVSKY V., NAIM M., THOMPSON D.A. A novel putative neuropeptide receptor expressed in neural tissue, including sensory epithelia. Biochem. Biophys. Res. Commun. 1995;209:752–759. doi: 10.1006/bbrc.1995.1563. [DOI] [PubMed] [Google Scholar]
- WILSON S., BERGSMA D.J., CHAMBERS J.K., MUIR A.I., FANTOM K.G., ELLIS C., MURDOCK P.R., HERRITY N.C., STADEL J.M. Orphan G-protein-coupled receptors: the next generation of drug targets. Br. J. Pharmacol. 1998;125:1387–1392. doi: 10.1038/sj.bjp.0702238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTER M.J., HUBBARD P.C., MCCROHAN C.R., BALMENT R.J. A homologous radioimmunoassay for the measurement of urotensin II in the euryhaline flounde. Platichthys flesus. Gen. Compar. Endocrinol. 1999;114:249–256. doi: 10.1006/gcen.1998.7245. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA M., KURIHARA H., KIMURA S., TOMOBE Y., KOBAYASHI M., MITSUI Y., YAZAKI Y., GOTO K., MASAKI T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- YANO K., VAUDRY H., CONLON J.M. Spasmogenic actions of frog urotensin II on the bladder and ileum of the frog, Rana catesbeiana. Gen. Comp. Endocrinol. 1994;96:412–419. doi: 10.1006/gcen.1994.1197. [DOI] [PubMed] [Google Scholar]


