Abstract
Our previous studies have identified a role for annexin 1 as a mediator of glucocorticoid action in the neuroendocrine system. The present study centred on growth hormone (GH) and exploited antisense and immunoneutralization strategies to examine in vitro the potential role of annexin 1 in effecting the regulatory actions of glucocorticoids on the secretion of this pituitary hormone.
Rat anterior pituitary tissue responded in vitro to growth hormone releasing hormone, forskolin, 8-Bromo-cyclic adenosine 3′5′-monophosphate (8-Br-cyclic AMP) and an L-Ca2+ channel opener (BAY K8644) with concentration-dependent increases GH release which were readily inhibited by corticosterone and dexamethasone.
The inhibitory actions of the steroids on GH release elicited by the above secretagogues were effectively reversed by an annexin 1 antisense oligodeoxynucleotide (ODN), but not by control (sense or scrambled) ODNs, as also were the glucocorticoid-induced increases in annexin 1. Similarly, a specific anti-annexin 1 monoclonal antibody quenched the corticosterone-induced suppression of secretagogue-evoked GH release while an isotype matched control antibody was without effect.
Transmission electron micrographs showed that the integrity and ultrastructural morphology of the pituitary cells were well preserved at the end of the incubation and unaffected by exposure to the ODNs, antibodies, steroids or secretagogues.
The results provide novel evidence for a role for annexin 1 as a mediator of the inhibitory actions of glucocorticoids on the secretion of GH by the anterior pituitary gland and suggest that its actions are effected at a point distal to the formation of cyclic AMP and Ca2+ entry.
Keywords: Annexin 1 (lipocortin 1), growth hormone, glucocorticoids, antisense oligodeoxynucleotides, pituitary
Introduction
Although growth suppression is a common complication of systemic glucocorticoid (GC) therapy in children, the mechanisms which underlie this unwanted effect are not fully understood. Actions of the steroids within the periphery which, for example, oppose the growth promoting activity of growth hormone (GH) and of mediators such as epidermal growth factor (EGF) and insulin-like-growth factor-1 (IGF-1) are undoubtedly important in this regard (Tonshoff & Mehls, 1997; Allen et al., 1998; Jux et al., 1998). However, GCs also exert complex regulatory effects within the neuroendocrine system which modulate the secretion of growth hormone (GH) by the anterior pituitary gland (for review see Guistina & Veldhuis, 1998). These effects appear to be exerted largely at the hypothalamic level through increased expression and release of somatostatin (Papachristou et al., 1994; Fife et al., 1996; Lam & Srivastave, 1997) and decreased production of GH releasing hormone (GHRH, Fernandez-Vazquez et al., 1995; Fife et al., 1996; Lam & Srivastave, 1997). However, the steroids also exert complex regulatory actions on GH release at the pituitary level which vary with dose and time (Guistina & Veldhuis, 1998). Sustained exposure (>18 h) of pituitary tissue to GCs in vitro increases GHRH receptor (Tamaki et al., 1996; Miller & Mayo, 1997) and GH (Oosterom et al., 1983; Evans et al., 1992; Nogami et al., 1997) expression and thereby augments basal and GHRH-stimulated GH release (Vale et al., 1983). Such effects are not however apparent if the contact time is reduced; to the contrary, short-term exposure (<4 h) of pituitary tissue to GCs inhibits the neurochemically-evoked release of GH (Guistina & Veldhuis, 1998). Little is known of the mechanisms which effect this acute inhibitory action. Our earlier studies (Taylor et al., 1995a) showed that short-term exposure (2–3 h) of rat anterior pituitary tissue to GCs causes a marked increase in de novo protein synthesis (as indexed by protein content and by incorporation of 14C-labelled amino acids into protein), raising the possibility that the steroid-induced suppression of GH release requires a newly synthesized protein messenger(s). To the best of our knowledge this line of thought has not been pursued and the identity of any such protein(s) is thus obscure. One potential candidate is annexin 1, a GC-inducible protein (Buckingham & Flower, 1997) which is implicated in the processes of vesicle fusion and exocytosis (Gerke, 1996).
Annexin 1 (also known as lipocortin 1) is a well characterized member of the annexin family of Ca2+ and phospholipid binding proteins. It was first identified as a potential mediator of the therapeutically important anti-inflammatory actions of the GCs and has since been shown to contribute to the signalling mechanisms effecting the regulatory actions of the steroids in the neuroendocrine system (reviewed in Buckingham & Flower, 1997). Annexin 1 is found in abundance in the anterior pituitary gland, particularly in the S100-positive folliculo-stellate cells, and in specific loci in the hypothalamus where its expression and cellular disposition are regulated by GCs (Smith et al., 1993; Philip et al., 1997; Christian et al., 1999; Traverso et al., 1999). GCs thus induce de novo synthesis of annexin 1 in these tissues; they also promote the exportation of the newly synthesized protein from the cytoplasm to a pericellular site where it adheres to the cell membrane by a Ca2+-dependent mechanism (Taylor et al., 1993; 1997; Philip et al., 1997). Functional studies in which neutralizing antisera, antisense oligodeoxynucleotides (ODNs) and various annexin 1 related peptides have been used as probes, have identified a key role for annexin 1 in effecting the acute inhibitory actions of the GCs on the secretion of corticotrophin (ACTH) and its principal hypothalamic releasing hormone (CRH, Loxley et al., 1993; Taylor et al., 1993; 1995b; 1997). In addition, they have provided novel evidence of a role for annexin 1 at the pituitary level in the GC-regulation of prolactin release (Taylor et al., 1995a; 2000). In the present study we have used immunoneutralization and antisense strategies developed and validated in our laboratory to examine in vitro the potential role of this protein in the rat anterior pituitary gland as a mediator of the acute inhibitory actions of GCs on GH release.
Methods
Animals
Adult male Sprague Dawley (∼200 g) rats bred in-house from a closed colony were used. They were housed (5/cage) in a quiet room with controlled lighting (lights on 08.00–20.00 h), temperature (21–23°C) and humidity (∼50%). Food and water were available ad libitum. All experiments were started between 08.00–09.00 h to avoid any circadian influences.
Oligodeoxynucleotide preparations
In line with our previous studies (Taylor et al., 1997; 2000), the annexin 1 antisense ODN probe was targeted to bases 83–98 inclusive (3′-G GTC CTG GTG GAA ACA-5′) which code for amino acids 29–33 of the translated protein. This 16 base sequence, which comprises approximately 60% GC residues, is unique and specific to annexin 1. From this sequence the complementary antisense ODN (3′-TGT TTC CAC CAG GAC C-5′) and a scrambled ODN sequence (3′-TTC CTC TAC GAC CGA G-5′) were constructed together with the annexin 1 sense sequence (3′-G GTC CTG GTG GAA ACA-5′). The ODNs were protected from degradation by the addition of two phosphorothioate groups at both the 3′ and the 5′ ends (100% efficiency at 10 μM, Oswel, University of Southampton, U.K.).
Anti-annexin antisera
A well characterized neutralizing anti-annexin 1 monoclonal antibody (anti-annexin 1 mAb, Zymed, Cambridge Biosciences, U.K., clone Z013 raised against bovine lung annexin 1) of proven specificity and efficacy was used (Taylor et al., 1993; 1997). An isotype matched (IgG1) control mAb (anti-spectrin α and β mAb, Sigma Chemical Co., Poole, Dorset, U.K.) was also employed.
Antisense experiments
Preparation and incubation of dispersed anterior pituitary cells
Suspensions of dissociated anterior pituitary cells were prepared as described previously (Taylor et al., 1997). Briefly, anterior pituitary cells obtained post mortem from decapitated rats were dissociated by incubation (1 h, 37°C) with collagenase (0.2% w v−1. Boehringer Mannheim, Sussex, U.K.) and deoxyribonuclease (DNase, 0.05% w v−1, Sigma Chemical Co.) in Earle's balanced salt solution (EBSS, Sigma Chemical Co., pH 7.4, phenol red free) enriched with bovine serum albumin (BSA, 0.4%; Sigma Chemical Co.); the dispersion was aided by gentle trituration (30 s 10 min−1). The resulting cell suspension was centrifuged (300 ×g, 10 min), the pellet resuspended in 5 ml BSA-enriched EBSS and the suspension filtered through 20 μm nylon mesh to remove any large clumps of debris. The filtrate was then centrifuged (300 ×g, 10 min) and the pellet resuspended in 5 ml incubation medium [1% aprotinin v v−1 (Bayer Ltd., Saffron Waldon, Essex, U.K.), 1% penicillin/streptomycin v v−1 (Sigma Chemical Co.) in EBSS, pH 7.4]. The cells were examined at the light microscope level to verify the effectiveness of the dispersion and counted using a haemocytometer. Cell viability was assessed by the trypan blue exclusion test and always found to be >95%.
The cells were plated at a density of 2.5×105 cells ml−1 well−1 in 24-well cell culture plates (Costar, Cambridge, MA, U.S.A.) and incubated for 2.5 h at 37°C in a humidified atmosphere saturated with 95% O2/5% CO2 gas. They were then challenged for 1 h with GHRH (0.1 nM–1 μM), forskolin (0.1 nM–1 mM), 8-bromo-cyclic adenosine 3′5′ monophosphate (8-Br-cyclic AMP, 10 pM–10 μM) or an L-Ca+ channel opener (BAY K8644, 1–100 nM); controls were incubated in an equal volume of medium alone. After centrifugation (600×g, 4°C, 10 min), the supernatant fluid was harvested and either assayed immediately for ir-GH or stored in aliquots (300 μl) at −20°C for subsequent peptide measurement. In some experiments the pituitary cells were retained for electron microscopy. Where appropriate corticosterone (10 nM) or dexamethasone (100 nM) were included in the medium throughout the experiment. Annexin 1 antisense, sense or scrambled ODNs (50 nM) were also added to the medium at the beginning of the experiment as required and replenished at 1.5 h and 2.5 h.
Efficacy and specificity of the anti-sense probe
The antisense protocol used in this study was developed and validated in our laboratory and has been described in detail in two previous papers (Taylor et al., 1997; 2000). In line with the recommendations of Wahlstedt (1995), the antisense probe was directed against a 16 base sequence which is unique and specific to rat annexin 1 and which comprises approximately 55% guanine/cytosine residues; corresponding sense and scrambled sequences were used as controls. Our previous studies demonstrated that these ODN sequences are readily taken up in a time-dependent manner by enzymatically dispersed rat pituitary cells in vitro (Taylor et al., 1997) and that the antisense ODN specifically blocks the de novo synthesis of annexin 1 in this preparation (Taylor et al., 1997; 2000). The contact time (3.5 h) and concentration (50 nM) of ODN used in the present study (50 nM) were selected on the basis of earlier experiments (Taylor et al., 1997) which, in view of the potential toxicity of phosphorothioate derivatives (Wagner, 1995; Matteucci & Wagner, 1996), aimed to determine the concentration and contact time necessary to produce near maximal effects on annexin 1 expression in this preparation. Thus, as illustrated in Figure 1 (reproduced with permission from Taylor et al., 2000), the antisense ODN (50 nM, 3.5 h) effectively prevents the increase in annexin 1 synthesis induced by corticosterone (10 nM) or dexamethasone (100 nM). Its effects appear to be specific as the sense and scrambled ODN sequences are without effect. Furthermore, the synthesis of 35S-annexin 5, a closely related protein, is unaffected by the antisense, scrambled or sense ODNs and/or the steroids.
Figure 1.
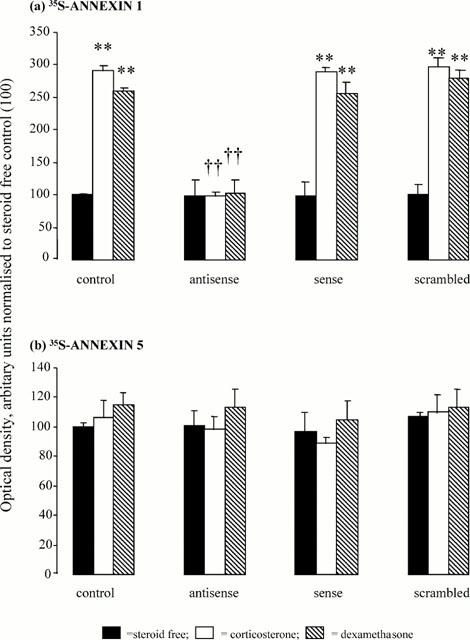
Effects of corticosterone (1 nM) and dexamethasone (100 nM) on the expression of newly synthesized (a) annexin 1 (as 35S-annexin 1) and (b) annexin 5 (as 35S-annexin 5) in anterior pituitary cells in vitro in the presence and absence of annexin 1 anti-sense, sense or scrambled oligodeoxynucleotides (ODNs, 50 nM, 3.5 h). Newly synthesized annexin 1 and 5 (labelled with 35S-tagged cysteine/methionine) were separated by immunoprecipitation and sodiumdodecyl sulphate polyacrilamide gel electrophoresis (SDS–PAGE). The radioactive bands on the gels were detected by exposure to X-ray film and band density on the resultant autoradiographs was measured with a Fujix-Bas 1500 imaging system with a low level light sensitive camera and TINA software (Raytek, Sheffield, U.K.). Responses to the steroids and the ODNs were calculated within autoradiographs as a percentage of the corresponding drug-free control. The data are expressed as the mean±s.e.mean of three autoradiographs. **P<0.001 vs control; ††P<0.001 vs steroid alone. This figure is reproduced with the permission of the Endocrine Society from Taylor et al. (2000).
Immunoneutralization experiments
The majority of these experiments were performed on segments of anterior pituitary tissue, according to the method of Taylor et al. (1995a). Briefly, anterior pituitary glands were removed from rats immediately after decapitation and divided into four pieces of approximately equal size. The segments were distributed randomly (one segment per well) in the wells of 24-well tissue culture plates (Costar) and incubated in 1 ml EBSS (pH 7.4, phenol red free) enriched with aprotinin (1%, Bayer Ltd.) for 2 h at 37°C in a humidified atmosphere saturated with 95% O2/5% CO2 gas; the medium was changed after 1 h and 1.5 h. The segments were then incubated for a further 1 h in medium containing GHRH (0.1 nM–1 μM), forskolin (0.1 nM–1 mM) or 8-Br-cyclic AMP (10 pM–10 μM); controls were exposed to an equal volume (1 ml) of medium alone. Where appropriate corticosterone was included in the medium throughout both the pre-incubation and final incubation periods. Anti-annexin 1 mAb or anti-spectrin α+β mAb (both diluted 1 : 15,000) were added to the final incubation medium as required. The medium from the final incubation was collected and either assayed immediately for ir-GH or stored in aliquots (300 μl) at −20°C for subsequent peptide measurement. The pituitary segments were weighed on a torsion balance and retained for electron microscopy. Additional immunoneutralization experiments were performed on enzymatically dispersed pituitary cells (Christian et al., 1997) which were prepared and incubated under the conditions described above. During the final incubation period (1 h) the cells were challenged with forskolin (100 μM) or incubated in medium alone (controls). When required corticosterone (1 nM) was included in the medium throughout the experiment; anti-annexin 1 mAb or anti-spectrin α+β mAb (both diluted 1 : 15,000) were added to the final incubation medium where appropriate.
Hormone assays
Growth hormone was measured by enzyme linked immunosorbent assay (ELISA) using a modification of the method of Farrington & Hymar (1987). The antiserum (monkey anti-rat GH coded anti-GH-S5) and standard preparation (rat GH, coded rGH-B13) were supplied by the National Hormone and Pituitary Programme (Ogden Bioservices, Rockville, U.S.A.). The sensitivity of the assay was 1.95 ng ml−1 with inter- and intra-assay coefficients of variation of 15.6 and 9.4% respectively (n=8). Dilution curves of test samples were parallel with those of the standard GH preparation. The antiserum showed negligible cross-reactivity with rat luteinizing hormone, thyrotrophin, prolactin, follicle stimulating hormone and adrenocorticotrophin; GHRH, forskolin, 8-Br-cyclic AMP, BAY K8644, anti-annexin 1 mAb, anti-spectrin α and β mAb, annexin ODNs (anti-sense, sense and scrambled sequences), corticosterone and dexamethasone, were also all inactive in the assay in concentrations likely to be present in the samples.
Electron microscopy
Anterior pituitary segments and dispersed pituitary cells were prepared for electron microscopy as described previously (Christian et al., 1999). Briefly, the tissues were post-fixed in 1% osmium tetroxide in 0.1 M phosphate buffer, stained in 2% uranyl acetate in distilled water, dehydrated through a graded series of increasing ethanol concentrations and embedded in Spurr's resin. Ultra-thin sections (50–80 nm) were cut using a Reichart-Jung Ultracut ultramicrotome and mounted onto formvar-coated 200-mesh nickel grids. Sections were double stained at room temperature, first in an aqueous solution of uranyl acetate (2% w v−1, 10 min) and subsequently with lead citrate (10 min) in a CO2-depleted environment (Hanaichi et al., 1986). Sections were viewed with a JOEL transmission microscope (JEM-100S).
Drugs
The following were used for in vitro studies: GHRH (Bachem U.K. Ltd., Saffron Walden, U.K.), forskolin, 8-Br-cyclic AMP (both from Sigma Chemical Co.), BAY K8644 (Semat, St. Albans, Herts., U.K.), dexamethasone sodium phosphate (David Bull Laboratories, Warwick, U.K.), corticosterone, (Sigma Chemical Co.). Forskolin and corticosterone were each dissolved initially in small amounts of ethanol and subsequently diluted in incubation medium; the final concentration of ethanol never exceeded 0.01% and appropriate controls were included in all experiments. The remaining drugs were dissolved directly and diluted in incubation medium immediately before use.
Data analysis
The data (expressed as mean±s.e.mean, n=6) were shown to be normally distributed (Shapiro and Wilks test) and analysed by standard parametric tests (ANOVA with post hoc comparisons by Duncan's multiple range test). Statistical comparisons were made within experiments only and differences were considered significant if P<0.05. Each of the studies was repeated several times (for specific details see legends) and in all instances the data profile was similar.
Results
Preliminary in vitro studies
Initial studies showed that both pituitary segments and enzymatically dispersed pituitary cells respond readily to GHRH (0.1–1000 nM), forskolin (100 nM–1 mM), 8-Br-cyclic AMP (10 pM–10 μM) and BAY K8644 (1–100 nM) with significant (P<0.01) concentration-dependent increases in immunoreactive- (ir-) GH release (data not shown). On the basis of these experiments, submaximal concentrations of these secretagogues were selected for further study, namely GHRH (10 nM), forskolin (100 μM), 8-Br-cyclic AMP (1 μM) and BAY K8644 (10 nM). Further experiments showed that the secretory responses evoked by these agents were prevented in a concentration-dependent manner by preincubation (2.5 h) of the segments/cells with corticosterone or dexamethasone (10 pM–100 nM, data not shown); concentrations of corticosterone (1 nM) or dexamethasone (100 nM) which reproducibly produced a 90–100% inhibition of secretagogue induced ir-GH release were used subsequently. Transmission electron micrographs showed that in both in vitro preparations the integrity and ultrastructural morphology of the pituitary cells were well preserved throughout the incubation (Figure 2a,c) and unaffected by exposure to the annexin 1 antisense ODN (Figure 1b) or anti-annexin 1 mAb (Figure 2d); similarly, within this time frame none of the other test substance tested (steroids, sense and scrambled ODNs or anti-spectrin α+βmAb) influenced the ultrastructure of the cells/tissue (data not shown).
Figure 2.
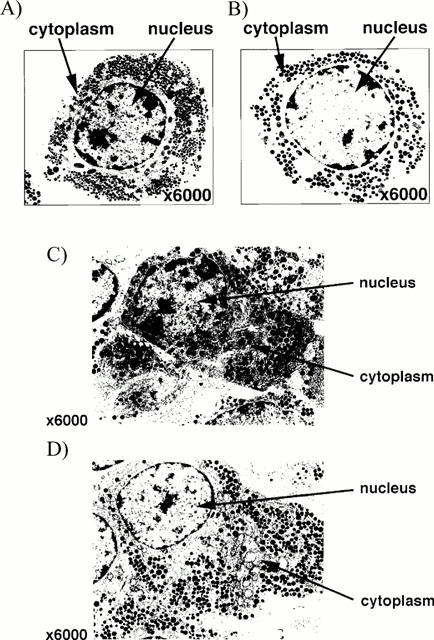
Electron micrographs (magnification ×6000) showing typical somatotrophs in (a,b) enzymatically dispersed pituitary cells and (c,d) the core region of a pituitary segment (diameter ∼1 mm) incubated for 3.5 h in the absence (a,c) or presence of annexin 1 ODN antisense (50 nM, b) or anti-annexin 1 mAb (diluted 1 : 15,000, d). Note (i) the electron-dense granules (diameter 200–350 nM) and extensive network of rough endoplasmic reticulum typical of somatotrophs, (ii) that in both preparations the cells appeared intact and well preserved at the end of the 3.5 h incubation and (iii) the appearance of the pituitary cells/tissue incubated with annexin 1 antisense ODN or anti-annexin mAb was indistinguishable from that of the controls; similarly, none of the other test substance tested (steroids, sense and scrambled ODNs or anti-spectrin α+β mAb) influenced the ultrastructure of the cells/tissue (data not shown).
Anti-sense studies
Figure 3 demonstrates the ability of the annexin 1 antisense ODN to reverse specifically the inhibitory actions of corticosterone on the release of ir-GH induced by submaximal concentrations of GHRH (10 nM, Figure 3a), forskolin (100 μM, Figure 3b) and 8-Br cyclic AMP (1 μM, Figure 3c). All three secretagogues produced clear (P<0.01) increases in ir-GH release which were significantly (P<0.01) reduced by pre-incubation of the cells with corticosterone (1 nM). In the absence of corticosterone, none of the ODNs tested (annexin 1 antisense, sense or scrambled sequences, 50 nM) influenced either the basal release of ir-GH (P>0.05) or secretory responses to GHRH, forskolin or 8-Br-cyclic AMP. In addition, all three ODNs failed to influence the resting ir-GH release in the presence of corticosterone (P>0.05). However, the inhibitory effects of the steroid on the release of ir-GH evoked by the three secretagogues were reversed substantially by the annexin antisense ODN (P<0.01). The annexin 1 sense and scrambled ODNs were inert in this respect and, thus, the pronounced (P<0.01) inhibitory effects of corticosterone on the release of ir-GH initiated by all three secretagogues persisted. Further experiments (Figure 4) revealed that the annexin 1 antisense ODN, but not the sense or scrambled sequences, also reversed the capacity of dexamethasone (100 nM) to suppress the significant increases (P<0.01) in ir-GH release induced by forskolin (Figure 4a, 100 μM) or BAY K8644 (Figure 4b, 10 nM).
Figure 3.

Blockade by the annexin 1 antisense oligodeoxynucleotide (ODN, 50 nM), but not by the scrambled or sense ODN sequences (50 nM), of the inhibitory effects of corticosterone (1 nM) on the release of ir-GH from freshly dispersed rat anterior pituitary cells induced in vitro by (a) growth hormone releasing hormone (GHRH, 10 nM), (b) forskolin (100 μM) and (c) 8-Br-cyclic AMP (1 μM). Each column represents the mean±s.e.mean (n=6); **P<0.01 vs corresponding secretagogue-free control; ††P<0.01 vs corresponding corticosterone-free control; NS=not significant; ANOVA+Duncan's multiple range test. Typical data from 3–4 replicate experiments.
Figure 4.

Blockade by the annexin 1 oligodeoxynucleotide (ODN, 50 nM), but not by the scrambled or sense ODN sequences (50 nM), of the inhibitory effects of dexamethasone (100 nM) on the release of ir-GH from freshly dispersed rat anterior pituitary cells induced in vitro by (a) forskolin (100 μM) and (b) BAY K8644 (10 nM). Each column represents the mean±s.e.mean (n=6); **P<0.01 vs corresponding secretagogue-free control; ††P<0.01 vs corresponding dexamethasone-free control; NS=not significant; ANOVA+Duncan's multiple range test. Typical data from 3–4 replicate experiments.
Immunoneutralisation studies
Figure 5 demonstrates the ability of an anti-annexin 1 mAb to reverse specifically the inhibitory actions of corticosterone on the release of ir-GH from rat anterior pituitary segments induced in vitro by submaximal concentrations of GHRH (Figure 5a), forskolin (Figure 5b) and 8-Br-cyclic AMP (Figure 5c). GHRH (10 nM), forskolin (100 μM) and 8-Br-cyclic AMP (1 μM) all caused significant (P<0.01) increases in ir-GH release which were inhibited by preincubation of the tissue with corticosterone (1 nM). Addition of either anti-annexin 1 mAb (diluted 1 : 15,000) or an equivalent dilution of an isotype-matched control antibody (anti-spectrin α+β mAb) to the medium had no significant effects on either basal or secretagogue-evoked ir-GH release. Both antibodies also failed to influence basal peptide release in the presence of corticosterone. However, anti-annexin 1 mAb quenched (P<0.01) the inhibitory actions of corticosterone on the release of ir-GH evoked by GHRH (Figure 5a), forskolin (Figure 5b) and 8-Br-cyclic AMP (Figure 5c). By contrast, anti-spectrin α+β mAb was ineffective in this regard. Further experiments (Figure 6) showed that anti-annexin 1 mAb also specifically reversed the inhibitory effects of corticosterone on forskolin-induced ir-GH release from enzymatically-dispersed pituitary cells.
Figure 5.
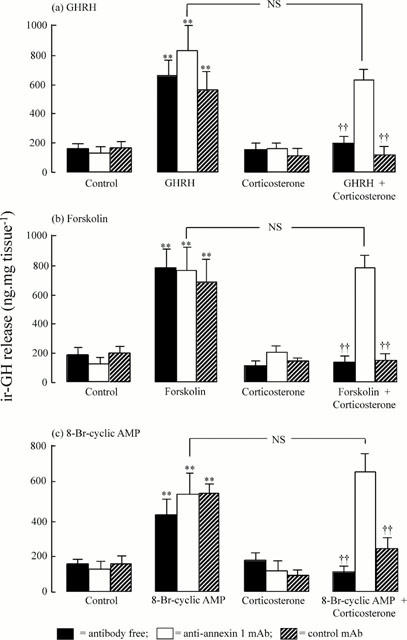
Neutralization by an anti-annexin 1 monoclonal antibody (anti-annexin 1 mAb, diluted 1 : 15,000) but not by an isotype-matched control mAb (anti spectrin α+β, diluted 1 : 15,000) of the inhibitory effects of corticosterone (1 nM) on the release of ir-GH from rat anterior pituitary segments induced in vitro by (a) growth hormone releasing hormone (GHRH, 10 nM), (b) forskolin (100 μM) and (c) 8-Br-cyclic AMP (1 μM). Each column represents the mean±s.e.mean (n=6); **P<0.01 vs corresponding secretagogue-free control; ††P<0.01 vs corresponding corticosterone-free control; NS=not significant; ANOVA+Duncan's multiple range test. Typical data from 3–4 replicate experiments.
Figure 6.

Neutralization by an anti-annexin 1 monoclonal antibody (anti-annexin 1 mAb, diluted 1 : 15,000) but not by an isotype-matched control mAb (anti spectrin α+β, diluted 1 : 15,000) of the inhibitory effects of corticosterone (1 nM) on the release in vitro of ir-GH from enzymatically dispersed rat anterior pituitary cells induced by forskolin (100 μM). Each column represents the mean±s.e.mean (n=6); **P<0.01 vs corresponding secretagogue-free control; ††P<0.01 vs corresponding corticosterone-free control; NS=not significant; ANOVA+Duncan's multiple range test. Typical data from two replicate experiments.
Discussion
Immunoneutralization and antisense techniques provide valuable means of examining the roles of specific proteins in the control of physiological processes. In the present study we have effectively exploited these techniques to explore the mechanisms underlying the acute inhibitory actions of GCs on GH release from the rat pituitary gland and thereby provided novel evidence for an obligatory role for the Ca2+ and phospholipid binding protein, annexin 1, in this process.
Our experiments were based on two well established in vitro preparations, namely static incubates of pituitary segments and enzymatically dispersed pituitary cells. The segment system, which we have used extensively for annexin 1 immunoneutralization studies (e.g., Taylor et al., 1993; 1995b), has the particular advantage that it retains the three dimensional structure of the heterogeneous cell population and thereby sustains the paracrine communication inherent to the tissue as a whole. Our electron microscopic studies showed that, irrespective of the addition of steroids and/or antibodies, the tissue was in good condition at the end of the incubation period with no signs of necrosis; this suggests that the flow of nutrients and metabolites to and from the cells was adequate and counters suggestions (reviewed in Gillies & Buckingham, 1995) that tissue viability may be compromised when incubations are continued for 3–4 h. Concerns about the ability of ODNs to penetrate the tissue segments and gain access to their intracellular targets led us to perform the antisense studies in a dispersed cell preparation in which ODN probes have been shown to enter the cells and accumulate mainly in the nucleus but also in the cytoplasm (Taylor et al., 1997). Inevitably, this preparation has the disadvantage that normal cell–cell contacts are not maintained; moreover, there is some loss of the non-secretory cell population during the separation process (Christian et al., 1997). However, as demonstrated by the electron micrographs, the ultrastructure of the cells was well maintained throughout the incubation period; furthermore, although phosphorothioate-protected nucleotide probes have been shown to be cytotoxic in some systems (Wagner, 1995; Wahlstedt, 1995) we could see no ill-effects of our ODNs or the other drugs employed on cell ultrastructure. Moreover, although there is a risk that cell surface receptors and other proteins may be lost during the cell separation process (Gillies & Buckingham, 1995), our previous studies have shown that the dispersed cells retain high affinity, proteinaceous annexin 1 binding sites on the cell surface (Christian et al., 1997) and exhibit annexin 1-dependent changes in their secretory activity when challenged with glucocorticoids (Christian et al., 1997; Taylor et al., 1997).
Our functional studies confirmed reports that short term exposure of pituitary tissue to GCs in vitro depresses secretagogue-driven GH release (Guistina & Veldhuis, 1998). They thus demonstrated a marked, concentration-dependent inhibition of GHRH-stimulated GH release following exposure of pituitary tissue to corticosterone or dexamethasone for 3.5 h. Forskolin-, 8-Br-cyclic AMP- and BAY K8644-stimulated GH secretion was also inhibited by the steroid treatments but basal GH secretion was unchanged, suggesting that the regulatory actions of the steroids on the somatotrophs are exerted at a point distal to the formation of cyclic AMP and the entry of Ca2+ into the cells. These studies also showed for the first time that the inhibitory effects of the steroids on the GH responses to each of the secretagogues tested were reduced markedly by inclusion in the medium of either the anti-annexin 1 ODN or the neutralizing anti-annexin 1 monoclonal antibody. In contrast, the isotype matched control antibody (anti-spectrin α+β) failed to modify the resting or evoked release of GH in the presence or absence of the steroids as also did control ODN sequences (sense and scrambled), indicating that the responses were specific. Further assurance of the specificity of the antisense action was provided by the fact that the ODN was directed against a sequence unique to rat annexin 1 and by our demonstration (Figure 1, Taylor et al., 1997; 2000) that the anti-sense (but not the sense or scrambled sequences) effectively blocked both the increase in de novo annexin 1 synthesis induced by both corticosterone and dexamethasone (Figure 1, Taylor et al., 1997; 2000). Taken together, these data suggest that annexin 1 plays an obligatory role in effecting the acute inhibitory effects of GCs on the secretion of GH.
The mechanism by which annexin 1 inhibits GH release remains to be determined. In addition to inducing de novo annexin 1 synthesis, GCs also promote the translocation of the protein from the cytoplasm to a pericellular site where it adheres to the cell membrane by a Ca2+-dependent mechanism. We have previously suggested that the latter process is critical to annexin 1 action as it provides a means whereby the protein may gain access to receptors on the outer surface of the cells and thereby initiate a biological response; annexin 1 may thus act as a paracrine or autocrine agent. This concept is supported by several lines of evidence. Firstly, while we have detected annexin 1 in both secretory and non-secretory adenohypophyseal cells by flow cytometry (Christian et al., 1999), data from our immunohistochemical studies at the light and electron microscope levels suggest the bulk of the protein is contained within the non-secretory S100-positive folliculostellate cells (Traverso et al., 1999); these cells are well positioned to exert paracrine influences on hormone secretion as their stellate projections lie in close apposition with the secretory cells (Traverso et al., 1999). Secondly, the antisense probe, which specifically reversed the inhibitory actions of glucocorticoids on GH release, inhibits de novo annexin 1 synthesis and thus also prevents the cellular exportation of the newly synthesized protein induced by GC (Taylor et al., 1997; 2000). Thirdly, the anti-annexin 1 antiserum, which, like the antisense ODN, readily quenched the antisecretory actions of the steroids, would not be expected to penetrate cell membranes readily but could effectively sequester annexin-1 at a pericellular site (Taylor et al., 1993; 1995a,1995b). Finally, we have demonstrated the presence of high affinity (Kd ∼13 nM), saturable, proteinaceous annexin 1 binding sites on the surface of several pituitary cell types, including somatotrophs (Christian et al., 1997); these sites resemble those on human peripheral leukocytes which have been deemed essential for annexin 1 activity (Goulding & Guyre, 1993). The signalling mechanisms employed by these ‘receptors' are unknown. However, there is evidence from other tissues that annexin 1 plays a role in the processes of vesicle fusion and exocytosis (Gerke, 1996). Our observation that the inhibitory actions of the glucocorticoids, and thus of annexin 1, on GH release are exerted at a point distal to the entry of Ca2+ into the cell together with evidence that annexin 1 does not influence Ca2+ influx the adenohypophyseal cells per se (Taylor, Davidson & Buckingham, unpublished) is consistent with an action late in the sequence of events which leads from cyclic AMP formation to exocytosis.
In conclusion, our results provide novel evidence that the acute inhibitory actions of glucocorticoids on GH secretion by the anterior pituitary gland are effected through induction of a protein, annexin 1, which suppresses cyclic AMP- and Ca2+-driven GH release. The significance of this mechanism in the control of GH release under physiological and pathophysiological conditions (e.g. stress) and following long-term glucocorticoid treatment remains to be elucidated and in vivo studies are now underway to address this point; in particular we are interested to determine the extent to which this mechanism counters the positive influence of the steroids on the expression of the GHRH receptors and GH itself. A further interesting and consistent finding which accords with other observations on the in vitro preparations used here (Taylor et al., 2000) is that, contrary to expectation, corticosterone was consistently more potent than dexamethasone. One possible explanation is that the effects of corticosterone are mediated via the high affinity mineralocorticoid receptor (MR) to which, unlike dexamethasone, it binds readily. However this seems unlikely as aldosterone, a potent, selective-MR agonist, does not influence the synthesis of cellular disposition of annexin 1 in our system (Taylor et al., 2000). Alternatively the differential potency of the two steroids may reflect the fact that dexamethasone was administered as the sodium phosphate salt and that its access to its receptors was thus limited by the rate at which the free base was liberated. In addition, the delivery of corticosterone to its receptors may have been enhanced by type 1 11β-hydroxysteroid dehydrogenase which is present in the anterior pituitary gland (Seckl, 1998) and which would reactivate any corticosterone converted to the inactive 11-dehydro species.
Acknowledgments
We are grateful to the Wellcome Trust for generous financial support (grant no 051887/B/97/Z) and to the National Hormone and Pituitary Programme for reagents for the GH assays.
Abbreviations
- ACTH
corticotrophin
- ANOVA
analysis of variance
- 8-Br-cyclic AMP
8-bromo-cyclic adenosine 3′5′ monophosphate
- BSA
bovine serum albumin
- CRH
corticotrophin releasing hormone
- EBBS
Earle's balanced salt solution
- EGF
epidermal growth factor
- GC
glucocorticoid
- GH
growth hormone
- GHRH
growth hormone releasing hormone
- GR
glucocorticoid receptor
- IGF-1
insulin-like growth factor-1
- IR
immunoreactive
- mAb
monoclonal antibody
- MR
mineralocorticoid receptor
- ODN
oligodeoxynucleotide
- SDS–PAGE
sodium dodecyl sulphate polyacrilamide gel electrophoresis
References
- ALLEN D.B., JULIUS J.R., BREEN T.J., ATTIE K.M. Treatment of glucocorticoid-induced growth suppression with growth hormone. National Co-operative growth study. J. Clin. Endocrin. Metab. 1998;83:2824–2829. doi: 10.1210/jcem.83.8.5036. [DOI] [PubMed] [Google Scholar]
- BUCKINGHAM J.C., FLOWER R.J. Lipocortin 1: a second messenger of glucocorticoid action in the hypothalamo-pituitary axis. Mol. Medicine Today. 1997;3:296–302. doi: 10.1016/S1357-4310(97)88908-3. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN H.C., FLOWER R.J., MORRIS J.F., BUCKINGHAM J.C. Localisation and semi-quantitative measurement of lipocortin 1 in rat anterior pituitary cells by fluorescence-activated cell analysis/sorting and electron microscopy. J. Neuroendocrin. 1999;11:707–714. doi: 10.1046/j.1365-2826.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN H.C., TAYLOR A.D., MORRIS J.F., GOULDING N.J., FLOWER R.J., BUCKINGHAM J.C. Characterisation and localisation of lipocortin 1 binding sites in the anterior pituitary gland by fluorescence activated cell analysis/sorting. Endocrinology. 1997;138:5341–5352. doi: 10.1210/endo.138.12.5593. [DOI] [PubMed] [Google Scholar]
- EVANS R.M., BIMBERG N.C., ROSENFELD M.G. Glucocorticoids and thyroid hormones transcriptionally regulate growth hormone gene expression. Proc. Nat. Acad. Sci. U.S.A. 1992;79:7659–7663. doi: 10.1073/pnas.79.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARRINGTON M.A., HYMAR W.C. An enzyme immunoassay for rat growth hormone: application to the study of growth hormone varients. Life Sciences. 1987;40:2479–2488. doi: 10.1016/0024-3205(87)90068-3. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ-VAZQUEZ G., CACICEDO L., LORENZO M.J., TOLON R., LOPEZ K. Corticosterone modulates growth hormone-releasing factor and somatostatin in fetal rat hypothalamic cultures. Neuroendocrinology. 1995;61:31–35. doi: 10.1159/000126824. [DOI] [PubMed] [Google Scholar]
- FIFE S.K., BROGAN R.S., GUISTINA A., WEHRENBERG W.B. Immunocytochemical and molecular analysis of the effects of glucocorticoid treatment on the hypothalamic-somatotropic axis in the rat. Neuroendocrinology. 1996;64:131–138. doi: 10.1159/000127109. [DOI] [PubMed] [Google Scholar]
- GERKE V.Annexins and membrane traffic Annexins: Molecular Structure to Cellular Function 1996Austin, U.S.A.: R.G. Landes; 67–79.ed. Seaton, B.A. pp [Google Scholar]
- GILLIES G.E., BUCKINGHAM J.C.The application of in vitro models of pituitary function for toxicity testing In Vitro Toxicity Testing Protocols 199543New Jersey, U.S.A.: Humana Press Inc; 81–93.eds Hare, S.O. & Atterwill, C. vol [DOI] [PubMed] [Google Scholar]
- GOULDING N.J., GUYRE P.M. Annexin 1 binding to human leukocytes correlates with its ability to inhibit IgG interactions with FCγ receptors. Biochem. Biophys. Res. Commun. 1993;192:351–358. doi: 10.1006/bbrc.1993.1422. [DOI] [PubMed] [Google Scholar]
- GUISTINA A., VELDHUIS J.D. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and in the human. Endocrine Reviews. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- HANAICHI T., SATO T., IWAMOTO T., MALAVASI-YAMASHIRO J., HOSHINO M., MIZUNO N. A stable lead by modification of Satos' method. J. Electron Microscopy (Tokyo) 1986;35:304–306. [PubMed] [Google Scholar]
- JUX C., LEIBER K., HUGEL U., BLUM W., OHLSSON C., KLAUS G., MEHLS O. Dexamethasone impairs growth hormone (GH)-stimulated growth by suppression of local insulin-like growth factor (IGF)-1 production and expression of GH- and IGF-1-receptor in culture rat chondrocytes. Endocrinology. 1998;139:3296–3305. doi: 10.1210/endo.139.7.6099. [DOI] [PubMed] [Google Scholar]
- LAM K., SRIVASTAVE G. Gene expression of hypothalamic somatostatin and growth hormone releasing hormone in dexamethasone-treated rats. Neuroendocrinology. 1997;66:2–8. doi: 10.1159/000127212. [DOI] [PubMed] [Google Scholar]
- LOXLEY H.D., COWELL A-M., FLOWER R.J., BUCKINGHAM J.C. Modulation of the hypothalamo-pituitary-adrenocortical responses to cytokines in the rat by annexin 1 and glucocorticoids: A role for annexin 1 in the feedback inhibition of CRF-41 release. Neuroendocrinology. 1993;57:801–814. doi: 10.1159/000126439. [DOI] [PubMed] [Google Scholar]
- OOSTEROM R., VERLEUN T., LAMBERTS S.W.J. Human growth hormone-secreting pituitary adenoma cells in long-term culture: effects of dexamethasone and growth hormone releasing factor. J. Endocrinol. 1983;100:353–360. doi: 10.1677/joe.0.1000353. [DOI] [PubMed] [Google Scholar]
- PAPACHRISTOU D.N., LIU J., PATEL Y.C. Glucocorticoids regulate somatostatin peptide and steady state messenger ribonucleic acid levels in normal rat tissues and in a somatostatin-producing islet tumour cell line (1027B2) Endocrinology. 1994;134:2259–2266. doi: 10.1210/endo.134.5.7908873. [DOI] [PubMed] [Google Scholar]
- PHILIP J.G., FLOWER R.J., BUCKINGHAM J.C. Glucocorticoids modulate the cellular disposition of lipocortin in the rat brain in vivo and in vitro. NeuroReport. 1997;8:1871–1876. doi: 10.1097/00001756-199705260-00016. [DOI] [PubMed] [Google Scholar]
- MATTEUCCI M.D., WAGNER R.W. In pursuit of antisense. Nature. 1996;384:20–22. [PubMed] [Google Scholar]
- MILLER T.L., MAYO K.E. Glucocorticoids regulate pituitary growth hormone-releasing hormone receptor messenger ribonucleic acid expression. Endocrinology. 1997;138:2458–2465. doi: 10.1210/endo.138.6.5184. [DOI] [PubMed] [Google Scholar]
- NOGAMI H., INOUE K., KAWAMURA K. Involvement of glucocorticoid-induced factor(s) in the stimulation of growth hormone expression in the fetal rat pituitary gland in vitro. Endocrinology. 1997;138:1810–1815. doi: 10.1210/endo.138.5.5124. [DOI] [PubMed] [Google Scholar]
- SECKL J. 11ß-hydroxysteroid dehydrogenase in the brain: a novel regulator of glucocorticoid action. Frontiers in Neuroendocrinology. 1998;18:49–99. doi: 10.1006/frne.1996.0143. [DOI] [PubMed] [Google Scholar]
- SMITH T., FLOWER R.J., BUCKINGHAM J.C. Lipocortins 1, 2 and 5 in the central nervous system and pituitary gland of the rat: selective induction by dexamethasone of annexin 1 in the anterior pituitary gland. Mol. Neuropharmacol. 1993;3:45–55. [Google Scholar]
- TAMAKI M., SATO M., MATSUBARA S., WADA Y., TAKAHARA J. Dexamethasone increases growth hormone (GH)-releasing hormone (GHR) receptor mRNA levels in cultured rat anterior pituitary cells. J. Neuroendocrinol. 1996;8:475–480. doi: 10.1046/j.1365-2826.1996.04779.x. [DOI] [PubMed] [Google Scholar]
- TAYLOR A.D, , CHRISTIAN H.C., MORRIS J.F., FLOWER R.J., BUCKINGHAM J.C. An antisense oligodeoxynucleotide to lipocortin 1 reverses the inhibitory actions of dexamethasone on the release of ACTH from rat pituitary tissue in vitro. Endocrinology. 1997;138:2909–2918. doi: 10.1210/endo.138.7.5260. [DOI] [PubMed] [Google Scholar]
- TAYLOR A.D., COWELL A-M., FLOWER R.J., BUCKINGHAM J.C. Lipocortin 1 mediates an early inhibitory action of glucocorticoids on the secretion of ACTH by the rat anterior pituitary gland in vitro. Neuroendocrinology. 1993;58:430–439. doi: 10.1159/000126572. [DOI] [PubMed] [Google Scholar]
- TAYLOR A.D., COWELL A-M., FLOWER R.J., BUCKINGHAM J.C. Dexamethasone suppresses the release of prolactin from the rat anterior pituitary gland by lipocortin 1 dependent and independent mechanisms. Neuroendocrinology. 1995a;62:530–542. doi: 10.1159/000127044. [DOI] [PubMed] [Google Scholar]
- TAYLOR A.D., LOXLEY H.D., FLOWER R.J., BUCKINGHAM J.C. Immunoneutralization of lipocortin 1 reverses the acute inhibitory effects of dexamethasone on the hypothalamo-pituitary-adrenocortical responses to cytokines in the rat in vitro and in vivo. Neuroendocrinology. 1995b;62:19–31. doi: 10.1159/000126984. [DOI] [PubMed] [Google Scholar]
- TAYLOR A.D., PHILIP J.G., JOHN C.D., COVER P.O., MORRIS J.F., FLOWER R.J., BUCKINGHAM J.C. Annexin 1 (lipocortin 1) mediates the inhibitory action of glucocorticoids on 3′,5′-cyclic adenosine phosphate stimulated release of prolactin. Endocrinology. 2000;141:2209–2219. doi: 10.1210/endo.141.6.7512. [DOI] [PubMed] [Google Scholar]
- TONSHOFF B., MEHLS O. Interactions between glucocorticoids and the growth hormone-insulin-like growth factor axis. Pediatric Transplantation. 1997;1:183–189. [PubMed] [Google Scholar]
- TRAVERSO V., CHRISTIAN H.C, , MORRIS J.F., BUCKINGHAM J.C. Lipocortin 1 (annexin 1): a candidate paracrine agent localised in pituitary folliculo-stellate cells. Endocrinology. 1999;140:4311–4319. doi: 10.1210/endo.140.9.7008. [DOI] [PubMed] [Google Scholar]
- VALE W., VAUGHAN J., YAMAMOTO G., SPIESS J., RIVIER J. Effects of synthetic human pancreatic (tumor) GH releasing factor and somatostatin, tri-iodothyronine and dexamethasone on GH secretion in vitro. Endocrinology. 1983;112:1553–1555. doi: 10.1210/endo-112-4-1553. [DOI] [PubMed] [Google Scholar]
- WAGNER R.W. The state of art of antisense research. Nature Medicine. 1995;1:1116–1118. doi: 10.1038/nm1195-1116. [DOI] [PubMed] [Google Scholar]
- WAHLSTEDT C. Antisense oligonucleotide strategies in neuropharmacology. Trends Pharmacol. Sci. 1995;15:42–46. doi: 10.1016/0165-6147(94)90107-4. [DOI] [PubMed] [Google Scholar]


