Abstract
In rats a single injection of the alkaloid monocrotaline (60 mg MCT kg−1 body weight, i.p.) caused right ventricular hypertrophy and heart failure. The aim of this study was to find out whether, in these MCT-treated rats, the cardiac neuronal noradrenaline uptake (uptake1) might undergo chamber-specific alterations.
For this purpose we assessed in right and left ventricular slices, uptake1 activity (by [3H]-noradrenaline accumulation), and in right and left ventricular membranes, uptake1 carrier protein density (by [3H]-nisoxetine binding).
Uptake1-inhibitors blocked [3H]-noradrenaline accumulation in ventricular slices and [3H]-nisoxetine binding in ventricular membranes with the order of potency: desipramine>nisoxetine>>cocaine⩾GBR 12909, indicating that with both approaches noradrenaline uptake1 was determined.
In right ventricular slices of MCT-treated rats uptake1 activity was significantly lower than in control rats (84.7±8.2 vs 145.1±6.2 pmol noradrenaline mg−1 tissue 15 min−1; P<0.05). This was accompanied by a significant decrease in the density of [3H]-nisoxetine binding sites (73.7±14.4 vs 125.9±9.1 fmol mg−1 protein; P<0.05).
In left ventricular slices of MCT-treated rats uptake1 activity was not significantly altered (131.2±10.5 vs 116.1±5.2 pmol noradrenaline mg−1 tissue 15 min−1); similarly, also the density of [3H]-nisoxetine binding sites was unchanged (108±9.7 vs 123±7.7 fmol mg−1 protein).
We conclude that in MCT-treated rats with right ventricular hypertrophy and heart failure uptake1 activity is chamber-specifically reduced possibly due to a decrease in carrier protein density.
Keywords: Monocrotaline, right ventricular hypertrophy, heart failure, noradrenaline, uptake1, [3H]-nisoxetine, uptake1 carrier sites
Introduction
During the last two decades growing evidence has accumulated that one typical feature of chronic heart failure, in humans as well as in experimental animal models, is a decrease in cardiac β-adrenoceptor function (for recent review see Brodde & Michel, 1999) that might be due to increased circulating noradrenaline, increased cardiac release of noradrenaline and/or a decreased cardiac neuronal noradrenaline uptake (uptake1) activity (for recent review see Esler et al., 1997). In fact, elevated circulating catecholamines (Thomas & Marks, 1978; Cohn et al., 1984), reduced myocardial catecholamine stores (Chidsey & Braunwald, 1966; Anderson et al., 1992), decreased neuronal uptake1 (Petch & Nayler, 1979) and a reduction in density of noradrenaline carrier sites (Böhm et al., 1995) have been described in the failing human heart. However, in animal models of right-sided and of left-sided heart failure a chamber-specific decrease in right or left ventricular neuronal uptake1 was observed (Liang et al., 1989; Himura et al., 1993; Yoshikawa et al., 1994) indicating that local rather than systemic effects might be responsible for these changes. In patients with primary pulmonary hypertension, who exhibit isolated right ventricular failure, catecholamine stores were depleted only in the failing right ventricle but not in the nonfailing left ventricle (Bristow et al., 1992). Similarly, chamber-specific depletion of myocardial stores were found in rabbits (Yoshikawa et al., 1994), dogs (Liang et al., 1989) and transgenic rats (Böhm et al., 1998).
Monocrotaline (MCT) is a pyrrolizidine alkaloid of the Crotalaria spectabilis. A single injection of MCT in the rat causes pulmonary hypertension and right ventricular hypertrophy; part of these animals develop cardiac failure (Kay et al., 1967; for recent review see Doggrell & Brown, 1998). In the present study we have used this MCT-rat model of pulmonary hypertension to further study whether local rather than systemic influences may affect the neuronal noradrenaline uptake in the heart. For this purpose we have assessed in right and left ventricles of MCT-treated rats [3H]-noradrenaline accumulation in tissue slices (as a measure of neuronal noradrenaline uptake1 activity). In addition we have used binding of [3H]-nisoxetine that has been recently shown to label the noradrenaline carrier sites in rat placenta (Shearman & Meyer, 1998) and rat heart (Böhm et al., 1998) in crude right and left ventricular membranes to determine the density of the uptake1 carrier sites.
Methods
Induction of acute right ventricular hypertrophy and heart failure in rats by monocrotaline (MCT)
Six-week-old male Wistar rats (obtained from Moellegard, Schönewald, Germany), housed in groups of three per cage in a controlled environment (12 h light/dark cycle at 22°C), were randomly divided into two experimental groups: one group received an intraperitoneal (i.p.) injection of monocrotaline (MCT, 60 mg kg−1 body weight), and the other group an equal volume of 0.9% saline (1 ml kg−1 body weight). MCT was dissolved in 1 N HCl, neutralized with 1 N NaOH, diluted with 0.9% saline and then injected at a concentration of 24 mg ml−1. The dosage of 60 mg MCT kg−1 i.p. was determined in pilot experiments and was selected with regard to survival and induction of cardiopulmonary injury.
MCT-treated rats had free access to food (25 g dry food day−1 per animal), whereas control rats were given only the quantity of food consumed by the MCT-treated rats the previous day (reduced to a minimum of 12.5 g dry food day−1 per animal). This diet was necessary because of toxic effects of MCT that elicit generalized illness and lack of appetite, mainly due to metabolic effects on the liver (Duecker et al., 1992).
Four to 6 weeks after MCT-injection all MCT-treated animals had developed hypertrophied right ventricles. About 40–50% of these animals showed signs of illness such as dyspnoea, cessation of eating, and loss of body weight. Within this time range the animals were anaesthetized with ether, killed by cervical dislocation and the hearts were rapidly excised. Meantime of sacrifice of the rats was 5.2 weeks (4–6 weeks). Right ventricular failure was confirmed by the presence of ascites, pericardial or pleural effusions. An equal number of control animals were killed at the same time. The hearts were divided into right ventricle (RV) and left ventricle including intraventricular septum (LV) and the ratio of the weight of each ventricle to the body weight was calculated as an index for ventricular hypertrophy. Because MCT-treated rats had significantly lost body weight (Table 1) we calculated in addition the ratio of LV/RV-weight as a body weight independent indicator of right ventricular hypertrophy. Subsequently right and left ventricular tissue were either directly used for measuring uptake1 activity or quickly frozen in liquid nitrogen and stored at −80°C until use for crude membrane preparations. The remaining MCT-treated rats not developing signs of cardiac failure were killed 6 weeks after the MCT-injection and were used in another study (Seyfarth et al., submitted for publication).
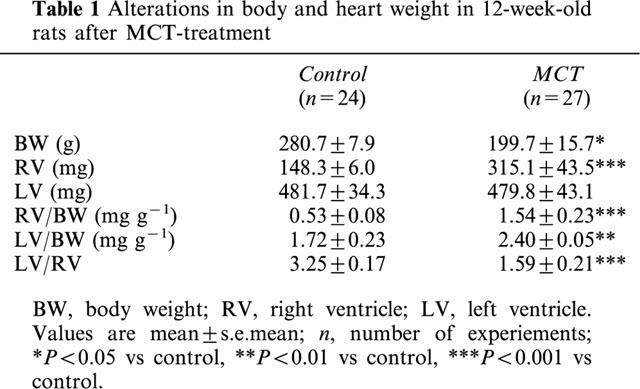
Table 1.
Alterations in body and heart weight in 12-week-old rats after MCT-treatment
All animal experiments were performed according to the German laws for animal welfare approved by the local committee for animal studies.
[3H]-noradrenaline uptake1 activity
[3H]-noradrenaline uptake1 activity was assessed as described by Liang et al. (1989) with minor modifications. Right and left ventricular tissue taken from saline- and MCT-treated rats was chopped in modified Krebs-Henseleit solution (mM: NaCl 118, KCl 4.7, CaCl2 2.5, MgCl2 0.54, NaHCO3 25, NaH2PO4 1, glucose 11, EDTA 0.094, ascorbic acid 1.14, nialamide 0.067) into 250×250 μm slices with a McIllwain tissue chopper (Bachhofer, Reutlingen, Germany). The slices were resuspended in fresh Krebs-Henseleit solution, and noradrenaline uptake1 activity was measured in duplicates by incubating 10 mg ventricular slices at 37°C for 15 min in oxygenated Krebs-Henseleit solution containing 1.56, 3.125, 6.25, 12.5 and 25 nM D,L-[7-3H(N)]-noradrenaline hydrochloride (13.5 Ci mmol−1) in a final volume of 500 μl. Nonspecific accumulation of radioactivity was determined by parallel incubation at 4°C. The incubation was terminated by rapid filtration of the entire reaction mixture through Whatman GF/C filters that had been presoaked for 15 min in ice-cold Krebs-Henseleit solution containing 0.05% polyethylenimine. Each filter was washed three times with 2 ml of ice-cold Krebs-Henseleit solution and the filters were transferred into scintillation vials containing 3% trichloracetic acid (TCA), followed by an incubation at 4°C overnight. The [3H]-radioactivity was counted after adding 4 ml scintillation cocktail (Lumasafe™ Plus, Lumec; Groningen, Netherlands) by liquid scintillation spectrometry (Packard TRI-Carb 2250CA) at an efficiency of 61%. Specific uptake was defined as total uptake (37°C) minus unspecific uptake (4°C). Six separate experiments were performed for control group and 12 for MCT-treated group.
Kinetic parameters of uptake1
To characterize the transport kinetics of noradrenaline in right and left ventricles of saline- and MCT-treated rats tissue slices were incubated as described above. For determination of the kinetic parameters, unlabelled (−)-noradrenaline was mixed in the incubation medium with [3H]-noradrenaline to give a final noradrenaline concentration of 1.56 to 200 nM. Specific uptake was defined as total uptake (37°C) minus unspecific uptake (4°C). Three separate experiments were performed for control group and six for MCT-treated group.
Drug competition experiments
Competition studies with desipramine, nisoxetine, cocaine and GBR 12909 were conducted as described above by incubating tissue slices taken from left ventricles of saline-treated rats with a fixed concentration of [3H]-noradrenaline (25 nM) in the presence of eight concentrations of each competitive drug and specific uptake was determined as described earlier. Five separate experiments were performed for each drug.
To further clarify which uptake system (uptake1 or uptake2) was active under the experimental conditions described above, tissue slices taken from right and left ventricles of saline- and MCT-treated rats were incubated with a fixed concentration of [3H]-noradrenaline (25 nM) in the presence or absence of 40 μM of the uptake2-inhibitor corticosterone or 1 μM of the uptake1-inhibitor nisoxetine. Nonspecific accumulation of radioactivity was determined by parallel incubation at 4°C. Specific uptake was defined as total uptake (37°C) minus unspecific uptake (4°C). Three separate experiments were performed for each drug.
[3H]-nisoxetine saturation analysis and drug competition experiments
The preparation of crude membranes isolated from right and left ventricles of saline- and MCT-treated rats for [3H]-nisoxetine binding studies was performed as described by Shearman & Meyer (1998), with minor modifications. 100–150 mg of frozen right and left ventricle were thawed on ice in 5 ml incubation buffer (mM: Na2HPO4 10, NaCl 120, KCl 5, pH 7.4) containing 0.25 M sucrose. The tissue was minced with scissors and gradually homogenized with an Ultra-Turrax (Ultra-Turrax T25, Jahne & Kunkel IKA® Labortechnik, Germany) at maximal setting (24 000 r.p.m.) for 10 s and twice at 17 500 r.p.m. for 20 s with 1 min intervals at 4°C. The homogenate was brought up to 20 ml with incubation buffer (+0.25 M sucrose) and centrifuged at 1200×g for 10 min at 0°C. The supernatant was filtered through four layers of cheesecloth and centrifuged at 20 000×g for 20 min at 4°C. The resulting pellet was resuspended in 20 ml incubation buffer (+0.25 M sucrose) and recentrifuged at 20 000×g for 20 min at 4°C. The final crude membrane pellets were resuspended in ice-cold incubation buffer (without sucrose) yielding a protein concentration of 250 μg ml−1. Protein content was determined according to Bradford (1976) using bovine γ-globulin as a standard.
[3H]-nisoxetine saturation analysis was performed by incubating 100 μg protein per assay with six concentrations of [3H]-nisoxetine ranging from 0.3125 to 10 nM in a final assay volume of 500 μl for 3 h at 4°C. Incubation was terminated by adding 5 ml of ice-cold incubation buffer to the reaction mixture followed by rapid filtration through Whatman GF/C filters. Each filter was washed two times with 5 ml incubation buffer. The filters were transferred to scintillation vials and dried overnight. Thereafter 4 ml scintillation cocktail (Lumasafe™ Plus, Lumac; Groningen, Netherlands) was added, and the radioactivity was determined in a Packard TRI-Carb 2250CA scintillation counter at an efficiency of 61%. ‘Unspecific' binding of [3H]-nisoxetine was defined as binding in the presence of 1 μM desipramine. Specific binding was defined as total binding minus unspecific binding and amounted to approximately 85% at 2 nM [3H]-nisoxetine.
For assessment of the ability of uptake-inhibitors desipramine, nisoxetine, cocaine and GBR 12909 to inhibit specific [3H]-nisoxetine binding membranes (100 μg protein/assay) were incubated with 2 nM [3H]-nisoxetine and with eight different concentrations of the competing agents for 3 h at 4°C, and specific binding was determined as described above. The competition curves were analysed using the iterative curve fitting programme Prism (GraphPad Software, San Diego, CA, U.S.A.) from which the individual IC50-values (concentration of the competing agent to inhibit specific binding by 50%) were obtained. Six separate experiments were performed for each drug.
Data analysis
The experimental data given in text, figures and tables are expressed as the means±s.e.mean of n experiments. The equilibrium dissociation constants (KD) and the maximal number of binding sites (Bmax) for [3H]-nisoxetine binding were calculated by use of the iterative curve fitting programme Prism (GraphPad Software). Statistical significance of differences was analysed by non-paired two-tailed Student's t-test. A P-value <0.05 was considered to be significant. All statistical calculations were performed with the Prism program (GraphPad Software).
Drugs used
D,L-[7-3H(N)]-Noradrenaline hydrochloride (specific activity: 13.5 Ci mmol−1) and [N-methyl-3H]-Nisoxetine (specific activity: 80 Ci mmol−1) were purchased from NEN™ Life Science Products Inc., Boston, MA, U.S.A.; GBR 12909 dihydrochloride from TOCRIS, Bristol, U.K.; nisoxetine HCl and desipramine HCl from RBI, Natick, MA, U.S.A. and corticosterone, [−]-noradrenaline, polyethylenimine and nialamide from Sigma, Deisenhofen, Germany.
Results
Characterization of MCT-treated rats with experimental induced right heart failure
Alterations in body weight and heart weight of MCT-treated rats are given in Table 1. Between 4 and 6 weeks after MCT-injection the body weight was markedly reduced vs saline-treated rats. The MCT-treated rats exhibited a significant enlargement of the right ventricle (about 2 fold) and the ratio RV/body weight was significantly increased; moreover the LV/RV-ratio, as an indicator of right ventricular hypertrophy independent of body weight, was significantly reduced in MCT-treated rats vs controls. On the other hand, the LV/body weight ratio was only slightly (but significantly) increased in MCT-treated rats vs controls (Table 1).
Characterization of [3H]-noradrenaline uptake1 in ventricular slices of the rat heart
[3H]-noradrenaline uptake1 in right and left ventricular slices was directly related to the concentration of [3H]-noradrenaline (1–25 nM) present in the incubation medium (Figure 1A). At the highest [3H]-noradrenaline concentration (25 nM) right ventricular slices accumulated specifically 145.1±6.2 pmol NA mg−1 tissue 15 min−1 and left ventricular slices 116.1±5.2 pmol NA mg−1 tissue 15 min−1. Non-specific noradrenaline-accumulation at 4°C was less than 5% (data not shown).
Figure 1.
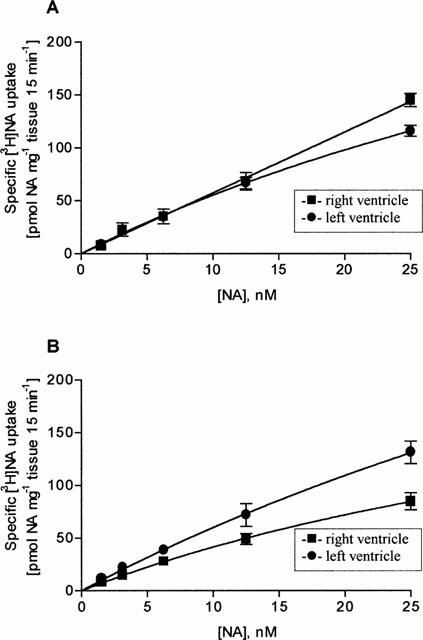
Specific [3H]-noradrenaline uptake into tissue slices taken from right and left ventricles of saline- (A) and MCT-treated (B) rats (Values are means±s.e.mean of six (A) and 12 (B) experiments).
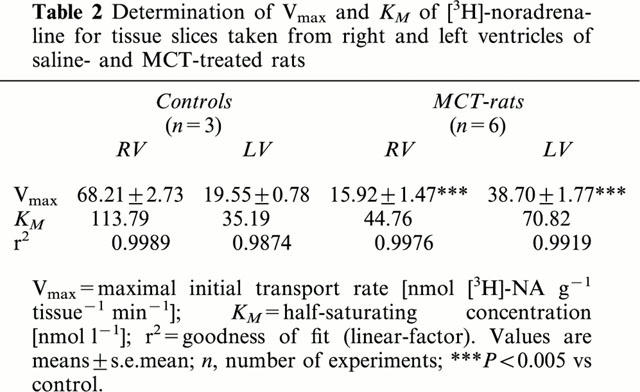
Since uptake1 is governed by saturation kinetics of the Michaelis-Menten type (for review see Graefe & Bönisch, 1988), we next assessed the half-saturating concentration (KM) and the maximal initial transport rate (Vmax) of [3H]-noradrenaline uptake1 in slices from right and left ventricle. As shown in Figure 2 and Table 2 Vmax- and KM-values were in right ventricular slices about 3 fold higher than in left ventricular slices.
Figure 2.
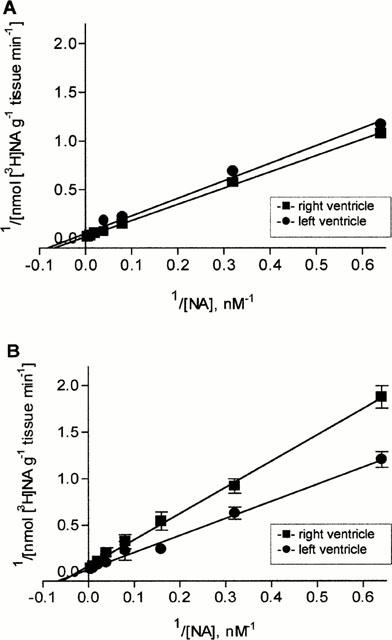
Kinetics (Lineweaver-Burk plot) of specific [3H]-noradrenaline uptake as a function of external noradrenaline concentration into tissue slices of right and left ventricles of saline- (A) and MCT-treated (B) rats (Values are means±s.e.mean of three (A) and six (B) experiments).
Table 2.
Determination of Vmax and KM of [3H]-noradrenaline for tissue slices taken from right and left ventricles of saline- and MCT-treated rats
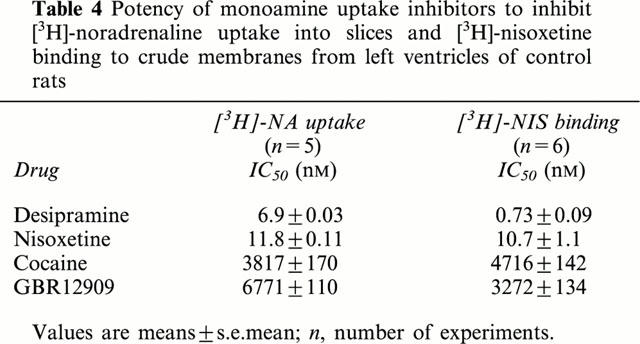
To further characterize the [3H]-noradrenaline uptake1 in right and left ventricular slices the effect of several uptake-inhibitors was determined. Forty μM corticosterone, an inhibitor of uptake2 (Grohmann & Trendelenburg, 1984) did not affect [3H]-noradrenaline uptake1 in right and left ventricular slices (Figure 3). On the other hand, the specific noradrenaline uptake1 inhibitors desipramine and nisoxetine were potent inhibitors of [3H]-noradrenaline uptake, with IC50-values in the low nanomolar range (Figure 4, Table 4), while the specific dopamine-uptake inhibitor GBR 12909 (Giros et al., 1992) was only a weak inhibitor (IC50-value >6 μM; Figure 4, Table 4). Finally, cocaine inhibited [3H]-noradrenaline uptake1 with an IC50-value (about 4 μM) that is in its range for its affinity to uptake1 (Jayanthi et al., 1993; Paczkowski et al., 1999).
Figure 3.
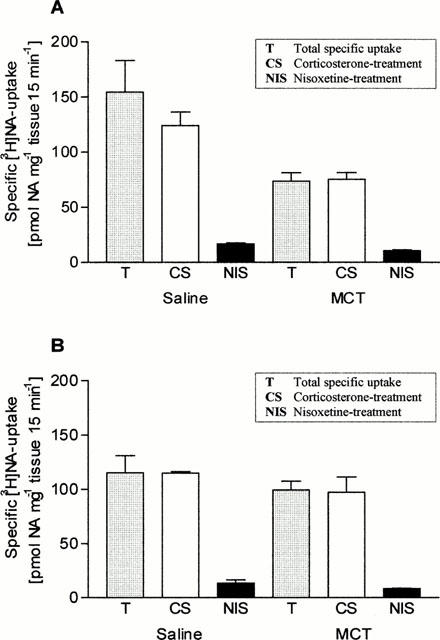
Specific [3H]-noradrenaline uptake (at 25 nM NA) into tissue slices from right (A) and left (B) ventricles of saline- and MCT-treated rats in the presence of corticosterone (40 μM) and nisoxetine (1 μM). Specific [3H]-noradrenaline uptake was defined as the difference between uptake at 37°C and that at 4°C (<15%) (values are means±s.e.mean of three experiments).
Figure 4.
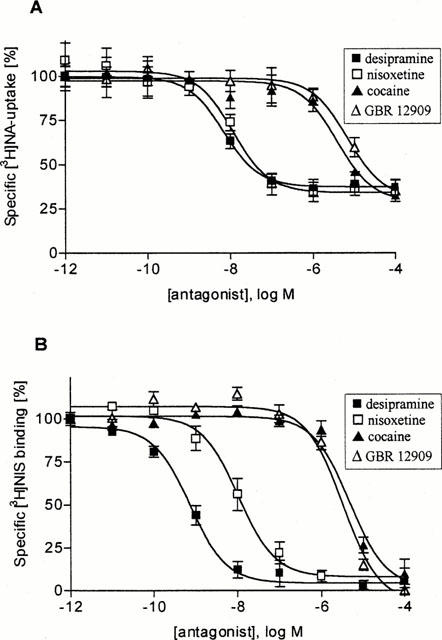
Inhibition of specific [3H]-noradrenaline uptake into tissue slices and [3H]-nisoxetine binding to crude membranes from left ventricles of saline-treated rats by several monoamine uptake inhibitors: (A) Specific [3H]-noradrenaline uptake into tissue slices, (B) Specific [3H]-nisoxetine binding to crude membranes (values are means±s.e.mean of five (A) and six (B) experiments.
Table 4.
Potency of monoamine uptake inhibitors to inhibit [3H]-noradrenaline uptake into slices and [3H]-nisoxetine binding to crude membranes from left ventricles of control rats
Characterization of [3H]-nisoxetine binding to membranes obtained from right and left ventricles of the rat heart
Binding of [3H]-nisoxetine to membranes from right and left ventricles was of high affinity (KD-values about 1 nM; Table 3) and saturable (Bmax about 125 fmol [3H]-nisoxetine specifically bound mg−1 protein). Non-specific binding, as defined by binding in the presence of 1 μM desipramine, was at 2 nM of [3H]-nisoxetine about 15% (see Methods).
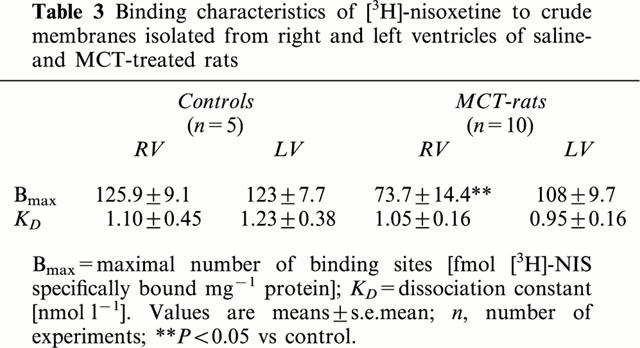
Table 3.
Binding characteristics of [3H]-nisoxetine to crude membranes isolated from right and left ventricles of saline- and MCT-treated rats
In order to characterize [3H]-nisoxetine binding sites in ventricular membranes inhibition of [3H]-nisoxetine binding by several uptake-inhibitors was assessed. Desipramine and nisoxetine were potent inhibitors of [3H]-nisoxetine binding with IC50-values in the low nanomolar range (Figure 4, Table 4), while GBR 12909 was only a weak inhibitor (IC50-value >3 μM; Figure 4, Table 4). Finally, cocaine inhibited [3H]-nisoxetine binding with a IC50-value (about 5 μM) that is well in its range of affinity to the noradrenaline uptake1 carrier site in several tissues (Tejani-Butt, 1992; Jayanthi et al., 1993).
Changes in noradrenaline uptake1 characteristics in MCT-treated rats
[3H]-noradrenaline uptake1 activity
Compared to saline-treated rats in right ventricular slices of MCT-treated rats [3H]-noradrenaline uptake1 activity was significantly reduced (Figure 1B); at the highest [3H]-noradrenaline concentration (25 nM) right ventricular slices accumulated only 84.7±8.2 pmol NA mg−1 tissue 15 min−1 (P<0.05). This was accompanied by a significantly reduced Vmax- and KM-value (Figure 2B, Table 2). In contrast, in left ventricular slices of MCT-treated rats [3H]-noradrenaline uptake1 activity was slightly but not significantly enhanced (131.2±10.5 pmol NA mg−1 tissue 15 min−1; Figure 1B); in addition Vmax- and KM-values were approximately doubled (Figure 2B, Table 2).
[3H]-nisoxetine binding
Compared to saline-treated rats the maximal number of [3H]-nisoxetine binding sites was significantly reduced in membranes from right ventricles of MCT-treated rats; KD-value for [3H]-nisoxetine, however, was not altered (Figure 3, Table 3). In contrast, in membranes from left ventricles of MCT-treated rats the maximal number of [3H]-nisoxetine binding sites was nearly identical with that determined in control rats (Figure 3, Table 3).
Discussion
It is well known that in several organs, including the heart, the major mechanism responsible for removal of neuronally released noradrenaline is the noradrenaline uptake transporter located on sympathetic nerve terminals (von Euler, 1972; Trendelenburg, 1991). In the present study we have assessed [3H]-noradrenaline accumulation in tissue slices and [3H]-nisoxetine binding to ventricular membranes in order to characterize the noradrenaline uptake1 transporter in right and left ventricles of the rat heart and to find out whether or not it might be changed in MCT-treated rats. Both [3H]-noradrenaline accumulation in tissue slices and [3H]-nisoxetine binding to membranes were inhibited by uptake-inhibitors with an order of potency desipramine>nisoxetine>>cocaine⩾GBR 12909, that is a typical one for noradrenaline uptake1 (Tejani-Butt, 1992; Jayanthi et al., 1993; Paczkowski et al., 1999); in addition, IC50-values were very well comparable in the two settings. Moreover, corticosterone, an inhibitor of uptake2, used in a concentration of 40 μM, did not at all affect [3H]-noradrenaline accumulation in right and left ventricular slices (of control as well as MCT-treated rats) although in this concentration it has been shown to completely suppress uptake2 activity (Starke et al., 1974). These data, therefore strongly support the view that with our approach we have assessed uptake1 activity and density of carrier sites in right and left ventricles of the rat heart.
In the present study we have used the MCT-rat model of pulmonary hypertension to study the hypothesis (see Introduction) that cardiac neuronal noradrenaline uptake1 is regulated primarily by local mechanisms. A single i.p. injection of 60 mg kg−1 MCT resulted within 4–6 weeks in rapid development of right ventricular hypertrophy; part of these animals developed heart failure as indicated by signs of ascites and pleural effusions. On the other hand, left ventricular weight was nearly not altered in MCT-treated rats. In this study we did not measure right ventricular systolic pressure; however, we recently had determined right ventricular pressure in another group of MCT-treated rats with signs of heart failure and had found, that in these rats right ventricular systolic pressure (46.4±5.3 mmHg, n=7) was significantly (P<0.01) higher than in control rats (13.1±0.5 mmHg, n=13), whereas mean arterial blood pressure was not different in the two groups (86.3±5.5 vs 96.2±2.6 mmHg; Seyfarth et al. (submitted).
These data demonstrate that MCT-treatment caused primarily chamber-specific right ventricular hypertrophy (and right ventricular failure; for review see Doggrell & Brown, 1998), although in the present study a slight but significant increase in LV/body weight ratio was also observed (see Table 1). However, this increase might not indicate LV hypertrophy but is mainly due to the fact that in MCT-treated rats body weight was significantly lower than in control rats while left ventricular weight did not differ between the two groups.
In these MCT-treated rats right ventricular [3H]-noradrenaline accumulation (as a measure of uptake1 activity) was significantly reduced by about 42% (Figure 1A,B). This might be due to a decrease in the density of carrier protein, since the [3H]-nisoxetine binding sites were reduced in right ventricles of MCT-treated rats by about the same amount (41%; Table 3).
Correspondingly the maximal initial transport rate (Vmax) of noradrenaline was markedly reduced in right ventricles of MCT-treated rats as was the noradrenaline concentration that is necessary to half-saturates uptake1 (KM; see Table 2).
Liang et al. (1989) showed similar changes in noradrenaline uptake1 activity and carrier density in right ventricles of dogs with right heart failure produced by tricuspid avulsion and progressive pulmonary artery constriction. Himura et al. (1993) observed under the same conditions a chamber-specific loss of noradrenergic nerve terminals, as evidenced by a reduction in catecholaminergic histofluorescence- and tyrosine hydroxylase-immunoreactive profiles. The same group recently showed that chronic ACE inhibition attenuated these reductions of the noradrenaline clearance system possibly due to a protective effect of ACE inhibitors on the sympathetic nerve terminal integrity and function (Kawai et al., 1999).
On the contrary, no such changes could be observed in the left ventricle of MCT-treated rats. In comparison to left ventricles of saline-treated rats the noradrenaline uptake1 activity was not decreased, but rather elevated in MCT-treated rats. In addition, the uptake1 carrier protein density was not significantly altered compared to saline-treated rats. Moreover, an increase of the maximal transport rate (about 2 fold) could be measured in left ventricles of MCT-treated rats accompanied by an increase of KM to about the same extent. That leads to the assumption that the noradrenaline uptake1 activity might be upregulated in these ventricles by increasing the transport cycle of noradrenaline per carrier and not by altering the carrier density possibly to compensate system-specifically the reduced noradrenaline clearance in right ventricles of hypertrophied hearts.
In conclusion, in rats MCT-treatment resulted in right heart hypertrophy and right heart failure with significantly reduced uptake1 activity and noradrenaline transporter density in the failing right ventricle. On the other hand, in left ventricle of MCT-treated rats noradrenaline transporter density was unchanged and transporter capacity slightly upregulated.
Thus, the present results clearly demonstrate that also in MCT-treated rats with right ventricular hypertrophy and heart failure cardiac noradrenaline uptake1 is regulated by local rather than systemic influences.
Acknowledgments
This work was supported by a grant of the Deutsche Forschungsgemeinschaft (DFG Br 526/6-1).
Abbreviations
- CS
corticosterone
- LV
left ventricle
- MCT
monocrotaline
- NA
noradrenaline
- NIS
nisoxetine
- RV
right ventricle
References
- ANDERSON F.L., PORT D.J., REID B.B., LARRABEE P., HANSON G., BRISTOW M.R. Myocardial catecholamine and neuropeptide Y depletion in failing ventricles of patients with idiopathic dilated cardiomyopathy. Circulation. 1992;85:46–53. doi: 10.1161/01.cir.85.1.46. [DOI] [PubMed] [Google Scholar]
- BÖHM M., CASTELLANO M., FLESCH M., MAACK C., MOLL M., PAUL M., SCHIFFER F., ZOLK O. Chamber-specific alterations of norepinephrine uptake sites in cardiac hypertrophy. Hypertension. 1998;32:831–837. doi: 10.1161/01.hyp.32.5.831. [DOI] [PubMed] [Google Scholar]
- BÖHM M., LA ROSEE K., SCHWINGER R.H.G., ERDMANN E. Evidence for a reduction of norepinephrine uptake sites in the failing human heart. J. Am. Coll. Cardiol. 1995;25:146–153. doi: 10.1016/0735-1097(94)00353-r. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BRISTOW M.R., MINOBE W., RASMUSSEN R., LARRABEE P., SKERL L., KLEIN J.W., ANDERSON F.L., MURRAY J., MESTRONI L., KARAWANDE S.V., FOWLER M., GINSBURG R. β-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. J. Clin. Invest. 1992;89:803–815. doi: 10.1172/JCI115659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODDE O.-E., MICHEL M.C. Adrenergic and muscarinergic receptors in the human heart. Pharmacol. Rev. 1999;51:651–689. [PubMed] [Google Scholar]
- CHIDSEY C.A., BRAUNWALD E. Sympathetic activity and neurotransmitter depletion in congestive heart failure. Pharmacol. Rev. 1966;18:685–700. [PubMed] [Google Scholar]
- COHN J.N., LEVINE T.B., OLIVARI M., GARBERG V., LURA D., FRANCIS G.S., SIMON A.B., RECTOR T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- DOGGRELL S.A., BROWN L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc. Res. 1998;39:89–105. doi: 10.1016/s0008-6363(98)00076-5. [DOI] [PubMed] [Google Scholar]
- DUECKER S.R., LAME M.W., MORIN D., WILSON D.W., SEGALL H.J. Guinea pig and rat hepatic microsomal metabolism of monocrotaline. Drug Metab. Dispos. 1992;20:275–280. [PubMed] [Google Scholar]
- ESLER M., KAYE D., LAMBERT D., JENNINGS G. Adrenergic nervous system in heart failure. Am. J. Cardiol. 1997;80:7L–14L. doi: 10.1016/s0002-9149(97)00844-8. [DOI] [PubMed] [Google Scholar]
- GIROS B., EL MESTIKAWY S., GODINOT N., ZHENG K., HAN H., YANG-FENG T., CARON M.G. Cloning, pharmacological characterization, and chromosome assignment of the human dopamine transporter. Mol. Pharmacol. 1992;42:383–390. [PubMed] [Google Scholar]
- GRAEFE K.H., BÖNISCH H.The transport of amines across the axonal membranes of noradrenergic and dopaminergic neurons Catecholamines I 1988Berlin: Springer-Verlag; 193–245.eds. Trendelenburg U. & Weiner N. Chapter 4 pp [Google Scholar]
- GROHMANN M., TRENDELENBURG U. The handling of five catecholamines by the extraneuronal O-methylating system of the rat heart. Naunyn-Schmiedebergs Arch. Pharmacol. 1984;329:264–270. doi: 10.1007/BF00501878. [DOI] [PubMed] [Google Scholar]
- HIMURA Y., FELTEN S., KASHIKI M., LEWANDOWSKI T.J., DELEHANTY J.M., LIANG C.S. Cardiac noradrenergic nerve terminal abnormalities in dogs with experimental congestive heart failure. Circulation. 1993;88:1299–1309. doi: 10.1161/01.cir.88.3.1299. [DOI] [PubMed] [Google Scholar]
- JAYANTHI L.D., PRASAD P.D., RAMAMOORTHY S., MAHESH V.B., LEIBACH F.H. &, GANAPATHY V. Sodium- and chloride-dependent, cocaine-sensitive, high-affinity binding of nisoxetine to the human placental norepinephrine transporter. Biochemistry. 1993;32:12178–12185. doi: 10.1021/bi00096a030. [DOI] [PubMed] [Google Scholar]
- KAWAI H., FAN T.H.M., DONG E., SIDDIQUI R.A., YATANI A., STEVENS S.Y., LIANG C.S. ACE inhibition improves cardiac NE uptake and attenuates sympathetic nerve terminal abnormalities in heart failure. Am. J. Physiol. 1999;277:H1609–H1617. doi: 10.1152/ajpheart.1999.277.4.H1609. [DOI] [PubMed] [Google Scholar]
- KAY J.M., HARRIS P., HEATH D. Pulmonary hypertension in rats produced by the ingestion of Crotalaria spectabilis seeds. Thorax. 1967;22:176–179. doi: 10.1136/thx.22.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIANG C.S., FAN T.H.M., SULLEBARGER T., SAKOMOTO S. Decreased adrenergic neuronal uptake activity in experimental right heart failure: A chamber-specific contributor to beta-adrenoceptor downregulation. J. Clin. Invest. 1989;84:1267–1275. doi: 10.1172/JCI114294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACZKOWSKI F.A., BRYAN-LLUKA L.J., PÖRZGEN P., BRÜSS M., BÖNISCH H. Comparison of the pharmacological properties of cloned rat, human, and bovine norepinephrine transporters. J. Pharmacol. Exp. Therap. 1999;290:761–767. [PubMed] [Google Scholar]
- PETCH M.C., NAYLER W.G. Uptake of catecholamines by human cardiac muscle in vitro. Br. Heart J. 1979;41:336–339. doi: 10.1136/hrt.41.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEYFARTH T., GERBERSHAGEN H.-P., GIESSLER C., LEINEWEBER K., HEINROTH-HOFFMANN J., PÖNICKE K., BRODDE O.-E. 2000(in press) [DOI] [PubMed]
- SHEARMAN L.P., MEYER J.S. Norepinephrine transporters in rat placenta labeled with [3H]-nisoxetine. J. Pharmacol. Exp. Therap. 1998;284:736–743. [PubMed] [Google Scholar]
- STARKE K., MONTEL H., GAYK W., MERKER R. Comparison of the effects of clonidine on pre- and postsynaptic adrenoceptors in the rabbit pulmonary artery. Naunyn-Schmiedeberg's Arch. Pharmacol. 1974;285:133–150. doi: 10.1007/BF00501149. [DOI] [PubMed] [Google Scholar]
- TEJANI-BUTT S.M. [3H]Nisoxetine: A radioligand for quantification of norepinephrine uptake sites by autoradiography or by homogenate binding. J. Pharmacol. Exp. Therap. 1992;260:427–436. [PubMed] [Google Scholar]
- THOMAS J.A., MARKS B.H. Plasma norepinephrine in congestive heart failure. Am. J. Cardiol. 1978;41:233–243. doi: 10.1016/0002-9149(78)90162-5. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG U. Functional aspects of the neuronal uptake of noradrenaline. Trends Pharmacol. Sci. 1991;12:334–337. doi: 10.1016/0165-6147(91)90592-g. [DOI] [PubMed] [Google Scholar]
- VON EULER U.S.Synthesis, uptake and storage of catecholamines in adrenergic nerves Catecholamines 1972Berlin: Springer-Verlag; 3–12.eds.: Blashko H & Musholl E., Handbuch der experimentellen Pharmakologie, 33, pp [Google Scholar]
- YOSHIKAWA T., HANDA S., SUSUKI M., NAGAMI K. Abnormalities in sympathoneuronal regulation are localized to failing myocardium in rabbit heart. J. Am. Coll. Cardiol. 1994;24:210–215. doi: 10.1016/0735-1097(94)90565-7. [DOI] [PubMed] [Google Scholar]


