Abstract
Exposure to midrange ultraviolet radiation (UVB) is known to produce skin inflammation similar to sunburn. The aim of this study was to characterize the hyperalgesia and cytokine upregulation induced by UVB and their modulation by antiinflammatory cytokines.
Acute exposure of the dorsal skin of mice to UVB (200, 250 and 300 mJ cm2) resulted in a dose-dependent decrease in the latencies of the hot plate and tail flick tests, without evident signs of skin lesions.
The observed hyperalgesia displayed a biphasic temporal evolution with an acute phase (3–6 h) and a late (48–96 h) phase.
Exposure to UVB (300 mJ cm2) elicited significant upregulation of interleukin (IL)-1β, tumour necrosis factor (TNF)-α and nerve growth factor (NGF), determined by ELISA in the exposed skin. This upregulation was more important during the acute phase of hyperalgesia.
Daily treatment of mice, with i.p. injections of either IL-10 or IL-13 (1.5, 7.5 and 15 ng in 100 μl saline) produced a dose-dependent attenuation of the UVB-induced hyperalgesia.
Treatment with the highest doses of either IL-10 or IL-13, produced significant attenuation of the levels of the cytokines and NGF by UVB, with relatively more pronounced effects by IL-13.
Acute exposure to moderate amounts of UVB results in a systemic hyperalgesia related to the upregulation of cytokine and NGF levels, since both were prevented by treatment with antiinflammatory cytokines.
Keywords: Inflammatory hyperalgesia, interleukins, NGF, ultraviolet radiations, TNF-α, pain-tests
Introduction
Acute exposure to ultraviolet radiations (UVR) has been shown to produce a biphasic reaction in the mammalian skin with an immediate phase (within a few minutes) and a late phase lasting 2 to 3 days (Cotran & Pathak, 1968). Mid range UVB radiations (280–320 nm) have been considered the most effective in simulating sunburn reaction (Hruza & Pentland, 1993) and in inducing inflammatory reactions characterized by erythema (Cotran & Pathak, 1968), and oedema and pain in the exposed area of the skin (Benrath et al., 1995; Eschenfelder et al., 1995; Gillardon et al., 1992; and for review, see Hruza & Pentland, 1993). Accumulated evidence on the effects of UVB exposure during the past two decades, can be summarized under two main headings which include skin inflammation and immunosuppression leading to carcinogenesis.
Skin inflammation due to acute exposure to UVR has been shown to be characterized by the release of neuropeptides, histamine, prostaglandins, serotonin and oxygen radicals (Benrath et al., 1995; Eschenfelder et al., 1995; Greaves & Søndergaard, 1970; Hawk et al., 1983; Hruza & Pentland, 1993) and the upregulation of proinflammatory cytokines, such as interleukin (IL)-1 (Araneo et al., 1989; Gahring et al., 1984; Kupper et al. 1987) and IL-6 (Urbanski et al., 1990) and tumour necrosis factor alpha (TNF-α) (Köck et al., 1990; Oxholm et al., 1988). Prolonged or repetitive exposure to UVB, however, has been reported to lead to generalized immunosuppression (Streilein et al., 1994) through the production/secretion of antiinflammatory cytokines such as IL-4, and IL-10 (Araneo et al., 1989; Rivas & Ullrich, 1992; Shreedhar et al., 1998).
Direct neural involvement in the effects of skin exposure to UVR was first reported by Szolcsányi (1987) who showed increased activity of polymodal nociceptors by UVR. A large proportion of these nociceptors, has been known to constitute a group of capsaicin sensitive primary afferents, characterized by their ability to secrete neuropeptides from their peripheral and central terminals. Subsequent studies have established the contribution of neuropeptides (Substance P, CGRP) in the inflammatory reaction induced by UVR (Benrath et al., 1995; Gillardon et al., 1992; Eschenfelder et al., 1995; Scholzen et al., 1999). Furthermore, cytokines and nerve growth factor (NGF), increased during UVB-induced inflammation, have been shown to produce hyperalgesia following either local or systemic injections (Andreev et al., 1995; Cunha et al., 1992; Ferreira et al., 1988; Lewin & Mendell 1993; Watkins et al., 1994).
Based on the above mentioned evidence, UVB exposure has been used as a rodent model for inflammation-induced hyperalgesia. In this model, unilateral UV exposure of one hind leg induces bilateral mechanical and thermal hyperalgesia and alteration of the levels of neuropeptides in the spinal cord (Perkins et al., 1993; Polgár et al., 1998; Urban et al., 1993).
In the present study, we investigated the acute and long term alterations of nociception in mice following acute exposure to UVB. The results show a sustained and systemic hyperalgesia assessed in areas remote to that exposed to UVR. This hyperalgesia is paralleled by a sustained upregulation of IL1-β, TNF-α and nerve growth factor (NGF). We also show that pretreatment of mice with the antiinflammatory cytokines IL-10 and IL-13 prevents the UVB induced hyperalgesia and the upregulation of the levels of proinflammatory cytokines and NGF.
Methods
Animals and experimental protocols
All experiments were performed on Balb/c mice housed in cages of 5–6 under standard colony conditions (To 22±2°C, 12 h dark/light cycle) with free access to water and food. Invasive procedures were performed under deep anaesthesia (pentobarbitone 50 mg kg, i.p.) and all tests involving nociceptive reactions were carried with strict adherence to the ethical guidelines for experimental work on animals (Zimmerman, 1983).
The standard design of the experiments was based on the following steps. In the first week, mice were brought to the laboratory, to be familiarized with the new environment. Pain tests were performed in daily sessions during the morning and at the end of the first week the skin of the back of each mouse was shaved 2 days before exposure to UVB. At the beginning of the second week, the mice were placed, in individual compartments, in a special plastic cage covered with a wire mesh and subjected to one session of UVB radiation. Naives or sham mice were placed in the same cage without receiving UVB radiation. After the UVB sessions, the mice were either subjected to pain tests or sacrificed, under deep anaesthesia, and at different time intervals, for surgical isolation of the irradiated skin tissues.
A bank of two UVB lamps (FS40 T12) was used. Mice were placed in individual compartments (12×5.5 cm) of a special cage (36×22×4.5 cm) covered with a special wire mesh, at a distance of 20 cm from the lamps. Intensity of irradiation was measured by a UVB radiometer (National Biological Corporation, Ohio, U.S.A.) placed in the centre of the exposed area. The exposure times of 4 min 42 s, 5 min 52 s and 7 min 3 s, used were selected to provide 150, 200 or 300 mJ cm2, respectively. The maximum amount (300 mJ cm2) of irradiation produced evident erythema without any signs of skin burn that leads to visible skin lesion and scar formation.
Assays of thermal hyperalgesia
The tail flick and the hot plate tests were used to assess the changes in thermal nociception. The hot plate test is known to be coordinated at supraspinal level, while the tail flick is mainly coordinated at the spinal level. For the hot plate test, each animal was placed on a metal pad heated at 52.8±0.2°C, and the time for paw licking or jumping was measured as the latency of the test. A hot plate analgesia instrument (Stoelting, Illinois, U.S.A.) was used to perform this test. For the tail flick, each animal was restrained in a plastic cone and the tip of its tail (2 cm) was dipped in a water bath heated at 48±0.2°C. The time for tail curling or flicking was measured as the latency of the test. Each animal was subjected to three trials separated by a minimum interval of 3 min. The average of three measurements was considered as the latency of tail flick test for each mouse (for more details, see Kanaan et al., 1998).
The baseline of each pain test for each group (n=5) was established during 1 week before the UVB exposure. Variations of the latencies of these tests were assessed at 3, 6, 24, 48, 96 and 120 h after UVB or sham exposure. The obtained values for each group were compared to the baseline established before UVB exposure, using each animal as its own control, and to the measurements made on a sham group (n=5), to account for any possible variation in the nociceptive thresholds produced by stress due to frequent manipulation. Four groups (n=5 each) were used, one for each UVB intensity exposure and one for sham.
Administration of IL-10 and IL-13
Both IL-10, human rDNA (WHO reference 92/516) and IL-13 (WHO reference 94/622) produced in CHO cells (a gift from Dr Stephen Poole, National Institute of Biological Standards and Control [NIBSC], Blanche Lane, Potters Bar, U.K.) were dissolved in sterile saline and injected intraperitoneally (i.p.) at one of each of the following three concentrations: 1.5, 7.5 and 15 ng in 100 μl. Individual animals from each group received daily injection of one concentration of either cytokine, starting at 1 h before UVB exposure and over the following days of observation. Other sham groups (n=5 each) received daily injections of either the highest dose of each cytokine or saline.
Preparation of skin samples and assay for IL-1β, TNF-α and NGF
Skin tissues from the exposed areas were isolated, under deep anaesthesia, from different groups (n=5 each) of mice for the determination of the cytokine and NGF levels.
For the determination of the effects of UVB exposure, tissues were sampled from the following groups: one naïve group, one saline injected group and one group per time interval sampled at 2, 5, 24, 48 and 96 h following UVR.
Determination of the effects of pretreatment with IL-10 and IL-13, on cytokine and NGF levels, was made on the following groups: one group for each cytokine with tissues sampled at 5 h following the injection without UVB exposure and one group for each cytokine pretreatment, at each of the following time intervals after UVR: 2, 5, 24, 48 and 96 h.
Removed skin tissues were weighed and stored at −70°C until the processing for cytokine measurement by a two-site Enzyme-linked Immunosorbant Assay (ELISA) as described in detail by Kanaan et al. (1998). Briefly, tissues were homogenized in phosphate buffered saline containing 0.4 M NaCl, 0.05% Tween-20, 0.5% bovine serum albumin, 0.1 mM phenylmethylsulphonyl floride, 0.1 mM benzethonium, 10 m EDTA and 20 KI ml−1 aprotinin. The homogenates were centrifuged at 12,000×g for 60 min at 4°C and the supernatant was used for the determination of cytokine levels.
A two-site sandwich Enzyme-linked immunosorbant assay (ELISA) was used to assay for the cytokines and NGF in the supernatants, as described in detail previously (Kanaan et al., 1998).
For IL-1β assay, immunoaffinity-purified polyclonal sheep anti-mouse IL-1β antibodies (S5/150799/JW supplied by the NISBC, England) were used to coat high binding plates (Nunc) at a concentration of 1 μg ml−1 and incubated overnight at 4°C. The following day plates were washed and after blocking for non-specific binding, standards in 100 μl (preparation 93/668, supplied by NISBC) and samples (100 μl) were added to the plates and incubated overnight at 4°C. Following the washing of the plates, biotinylated immunoaffinity-purified polyclonal antibodies (S329B4/190799/JW) diluted 1/4000 were added in (100 μl) and the colour developed with the chromagen 3,3′,5,5′-tetramethyl-benzidine (Sigma) after the development of the colour the reaction was stopped with 1 M sulphuric acid and the optical density measured at 450 nm (Kanaan et al., 1998; 2000).
For TNF-α assay, a similar procedure was utilized. The coating antibody was FPLC-purified sheep polyclonal anti-mouse TNF-α antibody (NIBSC, H92/090899/JW) and the detecting antibody was biotinylated FPLC-purified (NIBSC, H92/120899/JW). The standard was a recombinant mouse TNF-α (NIBSC preparation 88/532). Both assays were validated by Dr Stephen Poole, and no cross-reactivity between cytokines and antibodies was reported.
For NGF assay, a commercial kit was purchased from Promega (U.S.A.), which was also based on a two-site sandwich ELISA. The assay was performed as recommended by the manufacturers (Safieh-Garabedian et al., 2000).
Data analysis
Data are presented as mean±standard error of the mean (s.e.mean) for each experimental group at each time interval and for the various experimental procedures. Differences between experimental groups and controls were analysed by one way ANOVA followed by the Bonferroni post hoc test, using Graph-Pad Instat and Prism 3 (California, U.S.A.).
Results
Effects of UVB exposure on nociceptive thresholds
All reported results are based on UVB exposure producing reversible erythema without any sign of skin lesion. In few exceptional cases, where signs of skin lesions were reported either in the exposed or non-exposed skin to UVR, animals were eliminated from the experimental groups.
UVB exposure, at 300 mJ cm2, produced a biphasic decrease in the latencies of the nociceptive tests, which was more pronounced for the hot plate test (Figure 1). A significant decrease of 32 and 22% in the latencies of the hot plate and tail flick tests (hyperalgesia), respectively, was observed at 3 h followed by a partial recovery at 24 h following the exposure. A more sustained hyperalgesia, however, was observed during the second and third days which was followed by a progressive recovery to control level at 4–5 days after UVR.
Figure 1.
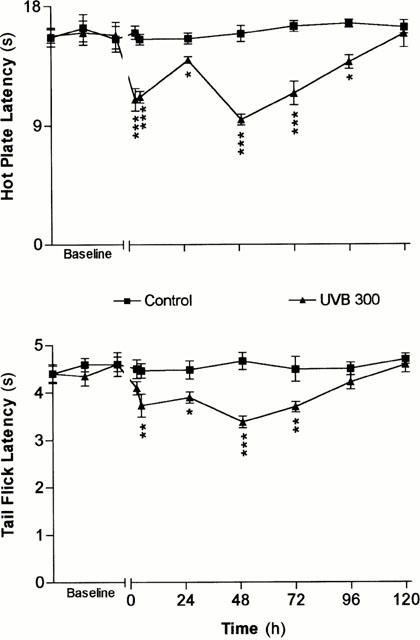
Time courses of the variations of the latencies of the nociceptive tests following UVB (300 mJ cm2 -▴- or sham (control -▪- ) exposures. The zero time on the X-axis indicates the time of exposure to UVR. Each point in each curve represents the average±s.e.mean of measurements conducted on five different animals. The values of significance of differences (*P<0.05; **P<0.01; ***P<0.001) are measured in reference to the average baseline values obtained on the same group of animals before the treatment, and to values measured in sham animals for the same time interval.
Exposures to increasing amounts of UVB, at 150, 200 and 300 mJ cm2, elicited progressive decrease of the nociceptive thresholds or increased hyperalgesia, which was more evident during the late phase (Figure 2). A more pronounced hyperalgesia was also observed following UVB exposure at 400 mJ cm2 (data not shown); however, delayed skin ulceration was observed in few cases. For this reason, UVB exposure at 300 mJ cm2 appeared to provide the optimum condition for the induction of hyperalgesia without skin lesion and, therefore, was selected for the remaining parts of this study.
Figure 2.
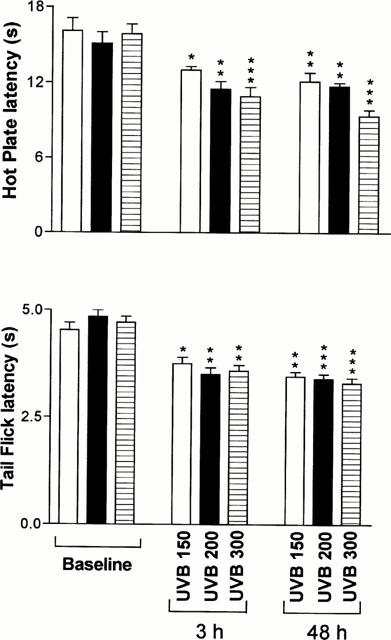
Dose-dependent effects of exposures to various amounts of UVB radiation. Each bar represents the average±s.e.mean of the latencies of nociceptive tests performed on a different group of mice (n=5) for each indicated exposure. Measurements were made at 6 and 48 h following exposure to UVB radiations, which correspond to the peak of hyperalgesia in the acute and late phases, respectively. *P<0.05; **P<0.01 and ***P<0.001 as compared to the baseline established before treatment in each experimental group.
Effects of pretreatment with IL-10 and IL-13 on UVB-induced hyperalgesia
Daily injections of either IL-10 or IL-13 (15 ng in 100 μl, i.p.) produced an almost complete reversal of UVB-induced hyperalgesia (Figure 3). Pretreatment with lower doses (1.5 and 7.5 ng in 100 μl, i.p.) of either IL-10 or IL-13 produced dose-dependent reduction in UVB-induced acute and late hyperalgesia, as illustrated in Figure 4. Injections of the highest doses of IL-10 and IL-1β (15 ng) did not elicit significant alteration of the latencies of nociceptive tests in mice not exposed to UVR (Figure 4).
Figure 3.
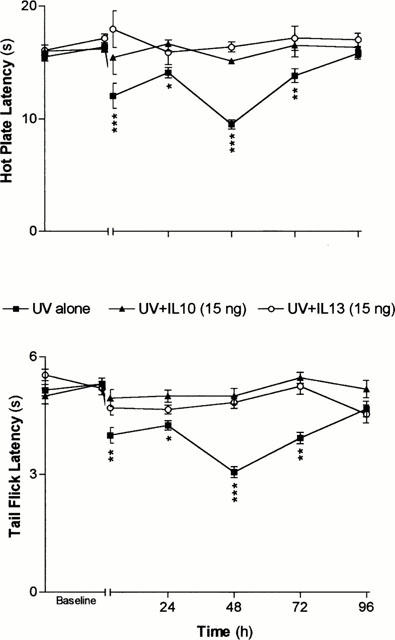
Time courses of the effects of pretreatments with IL-10 (▴) and IL-13 (○) on the hyperalgesia induced by UVB exposure (300 mJ cm2, ▪). Each point in each curve represents the average±s.e.mean of measurement performed on a different group of mice (n=5) for each indicated type of treatment. *P<0.05; **P<0.01 and ***P<0.001 as calculated in reference to the average latency measured before the treatment on each group.
Figure 4.
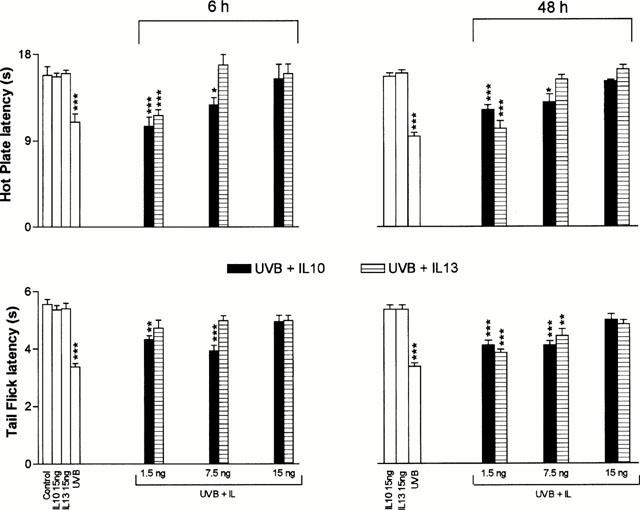
Dose-dependent effects of pretreatment with IL-10 (▪) or IL-13 ([Vertical lined box]) on the hyperalgesia induced by UVB exposure at 300 mJ cm2. Each bar represents the average±s.e.mean of measurements performed on a different group of mice for each indicated treatment at 6 and 48 h following exposure to UVB or treatment with each cytokine alone. The degree of significance of differences (*P<0.05; **P<0.01 and ***P<0.001) was calculated with reference to values obtained from animals receiving injections of either cytokines without exposure to UVB. Injections of IL-10 or IL-1β did not elicit significant alteration of nociceptive thresholds when compared to control animals.
Effects of UVB on cytokines and NGF levels
Skin tissues were isolated from one group of sham mice and from groups subjected to UVB 300 mJ cm2. The level of IL-1β increased from 9.76±1.16 pg mg−1 in sham to 27.42±2.91 at 2 h (P<0.05) and to a peak of 40.07±5.75 pg mg−1 at 5 h (P<0.001) and was still increased at 24–48 h then returned to sham value at 96 h following the UV exposure (Figure 5).
Figure 5.
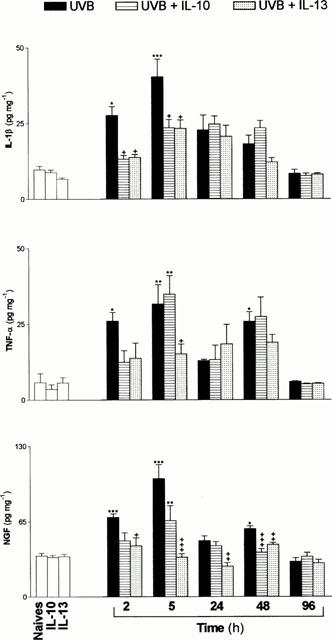
Time courses of the variations of the levels of cytokines and NGF following exposure to UVB (300 mJ cm2, ▪) or pretreatment with either IL-10 ([Horizontal lined box]) or IL-13 ([Dotted]) (15 ng). Each bar represents the average of measurements made on a different group of mice (n=5) subjected to each type of treatment for each indicated time interval. Measurements on naives or on animals receiving IL-10 or IL-13 without exposure to UVB were determined on animals 5 h following sham exposure. The degree of significance was determined in comparison to naives (*) or to values obtained with UVB exposure for each time interval (+). *+P<0.05; **++P<0.01 and ***+++P<0.001.
TNF-α levels displayed a biphasic variation with an early increase from 5.75±3 pg mg−1 in sham to 25.82±2.85 at 2 h (P<0.05) and a peak of 31.4±6.37 pg mg−1 at 5 h (P<0.01) following UVB exposure. During the second phase, however, TNF-α level returned to baseline level at 24 h and showed a second peak at 48 h (P<0.05) which was followed by recovery to the basal level at 96 h following the exposure (Figure 5). NGF levels, elicited the same pattern of temporal variation, with an increase from 35.6±2.4 pg mg−1 in sham to 78.15±3.4 at 2 h (P<0.001) and to 116.5±13.5 pg mg−1 at 5 h (P<0.001), but were still significantly increased at 48 h post exposure (Figure 5).
Pretreatment with IL-10 and IL-13
The effects of pretreatments with either antiinflammatory cytokines (15 ng in 100 μl, i.p.) were assessed on different groups of mice for each time interval following UVB exposure. The obtained results are illustrated in Figure 5. Both pretreatments produced significant attenuations of the upregulated levels, by UVR, of IL-1β and NGF. However, IL-13 exerted a more potent effect than IL-10 on NGF levels.
A different pattern of effects was elicited by IL-10 and IL-13 on the upregulation of TNF-α by UVR. Both cytokines reduced TNF-α levels at 2 h post exposure, while IL-10 showed further increase of its level at 5 h with maintained decreasing effects elicited by IL-13. Furthermore, during the late phase, IL-13 did not alter the level of TNF-α, while IL-10 pretreatment maintained its increasing effect.
Finally, injections of either cytokines at 15 ng dosage did not alter the basal levels of IL-1β, TNF-α and NGF in sham treated mice.
Discussion
This study presents a detailed characterization of the hyperalgesia induced by UVB radiation that produces erythema without resulting in a real burn or evident skin lesion. Furthermore, the hyperalgesia was assessed in areas (the paws in the hot plate test) which were not directly exposed to UVR and not necessarily receiving the same amount of radiation like the exposed back skin of the animal. It is also interesting to note that the tail, used in the tail flick test, did not show evident signs of erythema or late desquamation, which suggests that it was not exposed, like the skin of the back of the animal, in its relatively restrained position during UV session. These observations suggest that the hyperalgesia could be a kind of ‘illness induced hyperalgesia' which was described by (Watkins et al. 1994; 1995) following i.p. injection of endotoxin and attributed to the action of cytokines (IL-1β, in particular) on the brain. This possibility will be discussed in more details later.
The observed hyperalgesic effects were biphasic with a short acute phase (3–5 h) and a latent sustained (48–96 h) phase. This observation correlates well with the early description of the UV-induced erythema by Cotran & Pathak (1968). On the other hand, cytokines (IL-1β and TNF-α) and NGF levels showed significant increase during the acute phase and a less pronounced increase during the late phase. This temporal evolution (mainly for NGF and TNF-α) is comparable to that of the observed hyperalgesia.
Upregulation of IL-1β, TNF-α and NGF during inflammation, induced by various agents, has been well documented by several authors and by reports from our laboratory (Kanaan et al., 2000; Safieh-Garabedian et al., 1997; 2000; Woolf et al., 1997). Furthermore, the key role of each of these factors in the induction of inflammatory hyperalgesia has also been demonstrated (Andreev et al., 1995; Cunha et al., 1992; Ferreira et al., 1988; Lewin & Mendell, 1993).
Despite differences in animal species and in the amounts and methods used for UVR, several authors have reported long lasting changes in neuropeptides (Benrath et al., 1995; Eschenfelder et al., 1995; Gillardon et al., 1992; Greaves & Søndergaard, 1970; Polgár et al., 1998), histamine and prostaglandins (Greaves & Søndergaard, 1970; Hawk et al., 1983) and other mediators known to contribute to the inflammatory reaction (for review, see Hruza & Pentland, 1993). Increased levels of proinflammatory and antiinflammatory cytokines in the skin or in the plasma have also been observed following UVR. As illustration, increases in proinflammatory cytokines such as IL-1β (Araneo et al., 1989; Gahring et al., 1984; Kupper et al., 1987; Oxholm et al., 1988), TNF-α (Köck et al., 1990; Oxholm et al., 1988; Streilein et al., 1994) and in antiinflammatory cytokines, mainly IL-4 and IL-10 (Araneo et al., 1989; Rivas & Ullrich, 1992; Shreedhar et al., 1998) were reported. Thus our observation, of increased levels of IL-1β and TNF-α, correlates well with previous reports and are in line with established role of these mediators in inflammatory reactions.
Epidermal keratinocytes have been shown to constitute the major source of NGF in the skin (Tron et al., 1990). UV exposure has been shown to increase NGF production by keratinocytes (Bull et al., 1998; Gillardon et al., 1995; Tron et al., 1990) and this modulation appears to rescue skin cells from UV-induced apoptosis (Gilchrest et al., 1996; Marconi et al., 1999; Zhai et al., 1996). The mentioned reports on its origins and upregulation by UV are in line with considerable evidence suggesting its key role in the hyperalgesia associated with inflammation of various origin (Kanaan et al., 1998; 2000; Safieh-Garabedian et al., 1997; Woolf et al., 1997 and for review, see Lewin & Mendell, 1993).
Pretreatment with anti-inflammatory cytokines IL-10 and IL-13 prevented, in a dose-dependent manner, the hyperalgesia induced by UVB. This inhibition can be characterized by the following (see Figure 4): first, at equal dosage, IL-13 had more potent effects than IL-10; second, the effects were more pronounced on the hot plate test (supraspinally coordinated) than on the tail flick test; third, the effects on the tail flick were more pronounced during the acute phase (6 h) than during the late phase (48 h). These observations provide supportive evidence for the inflammatory origins of the hyperalgesia and its similarity with the syndrome of ‘illness-induced hyperalgesia' due to systemic increase of proinflammatory cytokine levels (Watkins et al., 1995). Furthermore, the antihyperalgesic effects of IL-10 and IL-13 are directly correlated with the concomitant downregulation of the increased levels of IL-1β, TNF-α and NGF by UVB.
Similar effects of IL-10 on the hyperalgesia and increased levels of IL-1β, TNF-α and NGF by endotoxin have been recently reported by our group on a mouse model of local inflammation induced by endotoxin (Kanaan et al., 1998). The antiinflammatory role of IL-10 has been demonstrated in various inflammatory models (Bogdan et al., 1991; De Waal Malefyt et al., 1991; Fiorentino et al., 1991; Oswald et al., 1992; Poole et al., 1995; Van der Poll et al., 1997). However, it is worth mentioning here that IL-10 exerted a consistent downregulation on NGF, while its effects on IL-1β and TNF-α were most evident during the acute phase of UVB effects (2 h). These observations are in line with several studies that established the key role of IL-10 in the persistent immunosuppression observed following chronic exposure to UVR (Araneo et al., 1989; Rivas et al., 1992; Shreedhar et al., 1998).
Finally, a relatively more potent effect on the hyperalgesia and cytokine and NGF upregulation was shown by IL-13. Despite many common features with IL-10, IL-13 has been shown to act through an IL-4 independent mechanism (Doherty et al., 1993; Kambayashi et al., 1996), which suggests that IL-13 and IL-4 are not redundant Th2 mechanisms (McKenzie et al., 1998). Reported differences between the effects of IL-10 and IL-13 on UVB-induced hyperalgesia and cytokine upregulation, provide further support to this suggestion.
In conclusion, this study demonstrates that acute exposure to relatively moderate amounts of UVB results in an upregulation of proinflammatory cytokines and NGF levels in the exposed skin area, which is paralleled by systemic hyperalgesia assessed on skin areas not directly exposed to UVB irradiation. The close relationship between the hyperalgesia and the inflammatory reaction is supported further by the action of the antiinflammatory cytokines. Furthermore, nuances or small differences between the effects of IL-10 and IL-13 are in favour of the existence of separate mechanisms mediating the action of each of these cytokines.
Acknowledgments
The authors thank Mrs Sawsan Sharrouf for her technical assistance. This project was supported by grants from the University Research Board and the Lebanese National Council for Scientific Research.
Abbreviations
- NGF
nerve growth factor
- IL
interleukin
- TNF-α
tumor necrosis factor-alpha
- UVB
ultraviolet, mid range
- UVR
ultraviolet radiation
References
- ANDREEV N.Y., DIMITRIEVA N., KOLTZENBURG M., MCMAHON S.B. Peripheral administration of nerve growth factor in the adult rat produces thermal hyperalgesia that requires the presence of sympathetic post-ganglionic neurons. Pain. 1995;63:109–115. doi: 10.1016/0304-3959(95)00024-M. [DOI] [PubMed] [Google Scholar]
- ARANEO B.A., DOWELL T., MOON H.B., DAYNES R.A. Regulation of murine lymphokine production in vivo. Ultraviolet radiation exposure depresses IL-2 and enhances IL-4 production by T cells through an IL-1-dependent mechanism. J. Immunol. 1989;143:1737–1744. [PubMed] [Google Scholar]
- BENRATH J., ESCHENFELDER C., ZIMMERMANN M., GILLARDON F. Calcitonin gene-related peptide, substance P and nitric oxide are involved in cutaneous inflammation following ultraviolet irradiation. Eur. J. Pharmacol. 1995;293:87–96. doi: 10.1016/0926-6917(95)90022-5. [DOI] [PubMed] [Google Scholar]
- BOGDAN C., VODOVOTZ Y., NATHAN C. Macrophage deactivation by interleukin-10. J. Exp. Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULL H.A., LESLIE T.A., CHOPRA S., DOWD P.M. Expression of nerve growth factor receptors in cutaneous inflammation. Br. J. Dermatol. 1998;139:776–783. doi: 10.1046/j.1365-2133.1998.02500.x. [DOI] [PubMed] [Google Scholar]
- COTRAN R.S., PATHAK M.A. The pattern of vascular leakage induced by monochromatic UV irradiation in rats, guinea pigs and hairless mice. J. Invest. Dermatol. 1968;51:155–164. doi: 10.1038/jid.1968.108. [DOI] [PubMed] [Google Scholar]
- CUNHA F.Q., POOLE S., LORENZETTI B.B., FERREIRA S.H. The pivotal role of tumour necrosis factor α in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE WAAL MALEFYT R., ABRAMS J., BENNETT B., FIGDOR C., DE VRIES J. IL-10 inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOHERTY T.M., KASTELEIN R., MENON S., ANDRADE S., COFFMAN R.L. Modulation of murine macrophage function by IL-13. J. Immunol. 1993;151:7151–7160. [PubMed] [Google Scholar]
- ESCHENFELDER C.C., BENRATH J., ZIMMERMANN M., GILLARDON F. Involvement of substance P in ultraviolet irradiation-induced inflammation in rat skin. Eur. J. Neurosci. 1995;7:1520–1526. doi: 10.1111/j.1460-9568.1995.tb01147.x. [DOI] [PubMed] [Google Scholar]
- FERREIRA S.H., LORENZETTI B.B., BRISTOW A.F., POOLE S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- FIORENTINO D.F., ZLOTNIK A., MOSSMANN T.R., HOWARD M., O'GARRA A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- GAHRING L., BALTZ M., PEPYS M.B., DAYNES R. Effect of ultraviolet radiation on production of epidermal cell thymocyte-activating factor/interleukin 1 in vivo and in vitro. Proc. Natl. Acad. Sci. U.S.A. 1984;81:1198–1202. doi: 10.1073/pnas.81.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILCHREST B.A., PARK H.Y., ELLER M.S., YAAR M. Mechanisms of ultraviolet light-induced pigmentation. Photochem. Photobiol. 1996;63:1–10. doi: 10.1111/j.1751-1097.1996.tb02988.x. [DOI] [PubMed] [Google Scholar]
- GILLARDON F., ESCHENFELDER C., RUSH R.A., ZIMMERMAN M. Increase in neuronal Jun immunoreactivity and epidermal NGF levels following UV exposure of rat skin. Neuroreport. 1995;6:1322–1324. doi: 10.1097/00001756-199506090-00023. [DOI] [PubMed] [Google Scholar]
- GILLARDON F., SCHRÖCK H., MORANO I., ZIMMERMAN M. Long-term increase in CGRP levels in rat spinal dorsal horn following skin ultraviolet irradiation. A mechanism of sunburn pain. An. NY. Acad. Sci. 1992;657:493–496. doi: 10.1111/j.1749-6632.1992.tb22810.x. [DOI] [PubMed] [Google Scholar]
- GREAVES M.W., SØNDERGAARD J. Pharmacologic agents released in ultraviolet inflammation studied by continuous skin perfusion. J. Invest. Dermatol. 1970;54:365–367. doi: 10.1111/1523-1747.ep12259058. [DOI] [PubMed] [Google Scholar]
- HAWK J.L.M., BLACK A.K., JAENICKE K.F., BARR R.M., SOTER N.A, , MALLETT A.I., GILCHREST B.A., HENSBY C.N., PARRISH J.A., GREAVES M.W. Increased concentrations of arachidonic acid, prostaglandins E2, D2, and 6-oxo-F1α, and histamine in human skin following UVA irradiation. J. Invest. Dermatol. 1983;80:496–499. doi: 10.1111/1523-1747.ep12535038. [DOI] [PubMed] [Google Scholar]
- HRUZA L.L., PENTLAND A.P. Mechanisms of UV-induced inflammation. J. Invest. Dermatol. 1993;100:35S–41S. doi: 10.1111/1523-1747.ep12355240. [DOI] [PubMed] [Google Scholar]
- KAMBAYASHI T., JACOB C.O., STRASSMANN G. IL-4 and IL-13 modulate IL-10 release in endotoxin-stimulated murine peritoneal mononuclear phagocytes. Cell. Immunol. 1996;171:153–158. doi: 10.1006/cimm.1996.0186. [DOI] [PubMed] [Google Scholar]
- KANAAN S.A., POOLE S., SAADÉ N.E., JABBUR S.J., SAFIEH-GARABEDIAN B. Interleukin-10 reduces the endotoxin-induced hyperalgesia in mice. J. Neuroimmunol. 1998;86:142–150. doi: 10.1016/s0165-5728(98)00027-7. [DOI] [PubMed] [Google Scholar]
- KANAAN S.A., SAADÉ N.E., KARAM M., KHANSA H., JABBUR S.J., JURJUS A.R. Hyperalgesia and upregulation of cytokines and nerve growth factor by cutaneous leishmaniasis in mice. Pain. 2000;85:477–482. doi: 10.1016/S0304-3959(99)00297-3. [DOI] [PubMed] [Google Scholar]
- KÖCK A., SCHWARZ T., KIRNBAUER R., URBANSKI A., PERRY P., ANSEL J.C., LUGER T.A. Human keratinocytes are a source for tumor necrosis factor α: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J. Exp. Med. 1990;172:1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUPPER T.S., CHUA A.O., FLOOD P., MCGUIRE J., GUBLER U. Interleukin 1 gene expression in cultured human keratinocytes is augmented by ultraviolet irradiation. J. Clin. Invest. 1987;80:430–436. doi: 10.1172/JCI113090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIN G.R., MENDELL L.M. Nerve growth factor and nociception. Trends in neuroscience. 1993;16:353–359. doi: 10.1016/0166-2236(93)90092-z. [DOI] [PubMed] [Google Scholar]
- MARCONI A., VASCHIERI C., ZANOLI S., GIANNETTI A., PINCELLI C. Nerve growth factor protects human keratinocytes from ultraviolet-B-induced apoptosis. J. Invest. Dermatol. 1999;113:920–927. doi: 10.1046/j.1523-1747.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- MCKENZIE G.J., BANCROFT A., GRENCIS R.K., MCKENZIE A.N.J. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Current Biol. 1998;8:339–342. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- OSWALD I.P., WYNN T.A., SHER A., JAMES S.L. Interleukin-10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor α required as a costimulatory factor for interferon γ-induced activation. Proc. Natl. Acad. Sci. U.S.A. 1992;89:8676–8680. doi: 10.1073/pnas.89.18.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OXHOLM A., OXHOLM P., STABERG B., BENDTZEN K. Immunohistological detection of interleukin 1-like molecules and tumour necrosis factor in human epidermis before and after UVB-irradiation in vivo. Br. J. Dermatol. 1988;118:369–376. doi: 10.1111/j.1365-2133.1988.tb02430.x. [DOI] [PubMed] [Google Scholar]
- PERKINS M.N., CAMPBELL E., DRAY A. Antinociceptive activity of the bradykinin B1 and B2 receptor antagonists, des-Arg9, [Leu8]-BK and HOE 140, in two models of persistent hyperalgesia in the rat. Pain. 1993;53:191–197. doi: 10.1016/0304-3959(93)90080-9. [DOI] [PubMed] [Google Scholar]
- POLGÁR E., SZÛCS P. , URBÁN L., NAGY I. Alerations of substance P immunoreactivity in lumbar and thoracic segments of rat spinal cord in ultraviolet irradiation induced hyperalgesia of the hindpaw. Brain Res. 1998;786:248–251. doi: 10.1016/s0006-8993(97)01434-0. [DOI] [PubMed] [Google Scholar]
- POOLE S., CUNHA F.Q., SELNIK S., LORENZETTI B.B., FERREIRA S.H. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-10. Br. J. Pharmacol. 1995;115:684–688. doi: 10.1111/j.1476-5381.1995.tb14987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIVAS J.M., ULLRICH S.E. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J. Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- SAFIEH-GARABEDIAN B., KANAAN S.A., ATWEH S.F., JABBUR S.J., SAADÉ N.E. Prostaglandin-E2 dependent cytokine upregulation and hyperalgesia induced by thymulin. Neuropharmacology. 2000;39:1652–1660. doi: 10.1016/s0028-3908(99)00247-6. [DOI] [PubMed] [Google Scholar]
- SAFIEH-GARABEDIAN B., KANAAN S.A., HADDAD J.J., ABOU JAOUDE P., JABBUR S.J., SAADÉ N.E. Involvement of interleukin-1β, nerve growth factor and prostaglandin E2 in endotoxin induced localized inflammatory hyperalgesia. Br. J. Pharmacol. 1997;121:1619–1626. doi: 10.1038/sj.bjp.0701313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOLZEN T.E., BRZOSKA T., KALDEN D.H., O'REILLY F., ARMSTRONG C.A., LUGER T.A., ANSEL J.C. Effect of ultraviolet light on the release of neuropeptides and neuroendocrine hormones in the skin: mediators of photodermatitis and cutaneous inflammation. J. Investig. Dermatol. Symp. Proc. 1999;4:55–60. doi: 10.1038/sj.jidsp.5640182. [DOI] [PubMed] [Google Scholar]
- SHREEDHAR V., GIESE T., SUNG V.W., ULLRICH S.E. A cytokine cascade including prostaglandin E2, IL-4, and IL-10 is responsible for UV-induced systemic immune suppression. J. Immunol. 1998;160:3783–3789. [PubMed] [Google Scholar]
- STREILEIN J.W., TAYLOR J.R., VINCEK V., KURIMOTO I., RICHARDSON J., TIE C., MEDEMA J.-P., GOLOMB C. Relationship between ultraviolet radiation-induced immunosuppression and carcinogenesis. J. Invest. Dermatol. 1994;103:107S–111S. doi: 10.1111/1523-1747.ep12399400. [DOI] [PubMed] [Google Scholar]
- SZOLCSÁNYI J. Selective responsiveness of polymodal nociceptors of the rabbit ear to capsaicin, bradykinin and ultra-violet irradiation. J. Physiol. 1987;388:9–23. doi: 10.1113/jphysiol.1987.sp016598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRON V.A., COUGHLIN M.D., JANG D.E., STANISZ J., SAUDER D.N. Expression and modulation of nerve growth factor in murine keratinocytes (PAM 212) J. Clin. Invest. 1990;85:1085–1089. doi: 10.1172/JCI114539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URBAN L., PERKINS M.N., CAMPBELL E., DRAY A. Activity of deep dorsal horn neurons in the anaesthetized rat during hyperalgesia of the hindpaw induced by ultraviolet irradiation. Neuroscience. 1993;57:167–173. doi: 10.1016/0306-4522(93)90118-y. [DOI] [PubMed] [Google Scholar]
- URBANSKI A., SCHWARZ T., NEUNER P., KRUTMANN J., KIRNBAUER R., KÖCK A., LUGER T.A. Ultraviolet light induces increased circulating interleukin-6 in humans. J. Invest. Dermatol. 1990;94:808–811. doi: 10.1111/1523-1747.ep12874666. [DOI] [PubMed] [Google Scholar]
- VAN DER POLL T., JANSEN P.M., MONTEGUT W.J., BRAXTON C.C., CALVANO S.E., STACKPOLE S.A., SMITH S.R., SWANSON S.W., HACK C.E., LOWRY S.F., MOLDAWER L.L. Effects of IL-10 on systemic inflammatory responses during sublethal primate endotoxemia. J. Immunol. 1997;158:1971–1975. [PubMed] [Google Scholar]
- WATKINS L.R., MAIER S.F., GOEHLER L.E. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63:289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- WATKINS L.R., WIERTELAK E.P., GOEHLER L., SMITH K.P., MARTIN D., MAIER S.F. Characterisation of cytokine-induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- WOOLF C.J., ALLCHORNE A., SAFIEH-GARABEDIAN B., POOLE S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumor necrosis factor α. Br. J. Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAI S., YAAR M., DOYLE S.M., GILCHREST B.A. Nerve growth factor rescues pigment cells from ultraviolet-induced apoptosis by upregulating BCL-2 levels. Exp. Cell. Res. 1996;224:335–343. doi: 10.1006/excr.1996.0143. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


