Abstract
In the present study, the effects of the novel vanilloid agonist, 12-phenylacetate 13-acetate 20-homovanillate (PPAHV), on oxygen consumption (VO2) and vascular resistance (perfusion pressure, PP) were investigated in the constant flow, perfused rat hindlimb. The acute desensitizing properties of this novel synthetic agent were also examined.
Maximum stimulation of VO2 was produced by 0.2 μM PPAHV (ΔVO2, 0.83±0.06 μmol g−1 h−1) and was accompanied by mild vasoconstriction (increase in PP; 8.0±1.1 mmHg). The highest concentration of PPAHV tested (2 μM) caused inhibition of VO2 (ΔVO2, −2.73±0.51 μmol g−1 h−1) and strong vasoconstriction (ΔPP, 42.0±1.2 mmHg).
Capsazepine (10 μM) caused a parallel shift to the right of both VO2 and PP concentration-response curves for PPAHV (pKb=5.00), indicative of competitive binding to vanilloid receptors.
The stimulation of VO2 produced by 0.2 μM PPAHV decreased, but was not completely abolished, after repeated infusion of PPAHV (change in VO2, first infusion, 0.66±0.18 μmol g−1 h−1; sixth infusion, 0.29±0.08 μmol g−1 h−1, P<0.05), an acute tachyphylactic response not previously seen with the repeated infusion of other vanilloid analogues. Conversely, the PP response to repeated PPAHV infusion increased (ΔPP, first infusion, 5.8±0.7 mmHg; sixth infusion, 9.0±0.6 mmHg, P<0.05).
In conclusion, PPAHV produces vasoconstriction and a biphasic effect on VO2 in the perfused rat hindlimb very similar to that induced by naturally occurring vanilloids. Both effects are blocked by the competitive antagonist capsazepine. Since, the metabolic response to low concentrations of PPAHV (stimulation of VO2) undergoes tachyphylaxis, the present data suggest that PPAHV desensitizes putative vanilloid receptors in the hindlimb.
Keywords: Vanilloids, vanilloid receptors, perfused rat hindlimb, oxygen consumption, perfusion pressure, tachyphylaxis
Introduction
The nociceptive neuron stimulant capsaicin produces a refractory state of desensitization with prolonged or repeated administration (tachyphylaxis). This property has promoted intense interest in its possible use as a non-steroidal analgesic and anti-inflammatory agent (Maggi, 1992; Dray & Urban, 1996). Recent attention has focused on other natural ‘vanilloid' molecules including resiniferatoxin (RTX), an ultrapotent vanilloid analogue that shows high potency and a greater efficacy for inducing desensitization than for causing pain (Szallasi et al., 1996; Szallasi & Blumberg, 1996; Appendino & Szallasi, 1997). However, the promotion of RTX as a therapeutic agent has been hampered by lack of availability since Euphorbia resinifera is a protected species. More recently, the development of synthetic phorbol-based vanilloids has yielded encouraging compounds such as phorbol 12-phenylacetate 13-acetate 20-homovanillate (PPAHV), which is only mildly noxious and is devoid of unwanted side effects at doses that protect against neurogenic inflammation (Appendino et al., 1996).
Vanilloid-like properties of PPAHV have been demonstrated in recent radioligand binding (Appendino et al., 1996) and patch clamp studies (Liu et al., 1998). However, there have been very few studies examining its functional vanilloid-like effects in whole tissue preparations. In the perfused rat hindlimb, several natural vanilloid analogues have been shown to produce unique metabolic and vascular effects mediated by pharmacologically distinct (putative) vanilloid receptor subtypes (VN1 and VN2) (Colquhoun et al., 1995; Griffiths et al., 1996). Using the perfused rat hindlimb as a functional assay system, the present study examines the actions of PPAHV on resting muscle oxygen consumption (VO2) and vascular resistance (determined by perfusion pressure, PP). In addition, the effects of repeated infusion of PPAHV were studied to determine the desensitizing properties of this agent in muscle. We provide solid evidence that PPAHV has strong vanilloid-like effects in this preparation and promotes rapid tachyphylaxis where other natural vanilloid analogues have failed to do so in our previous studies.
Methods
All experimental procedures used in this study were approved by the University of Tasmania Animal Ethics Committee under the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (Australian Government, 1990).
Male 180–200 g hooded Wistar rats were housed at 21±1°C under a 12 h light : dark cycle and fed a commercial rat chow diet containing 21.4% protein, 4.6% lipid, 68% carbohydrate and 6% crude fibre with added vitamins and minerals. Water was supplied ad libitum.
Hindlimb perfusion
Animals were anaesthetized with pentobarbitone sodium (60 mg kg−1) and their left hindlimbs perfused according to the method described previously (Colquhoun et al., 1988). In brief, flow was isolated to the left hindlimb by the cannulation of the abnormal aorta, posterior to the renal vessels, and the ligation of the tail, right common iliac and cutaneous blood vessels. The hindlimb was perfused under constant flow conditions (4 ml min−1) with a modified Krebs-Ringer bicarbonate buffer containing 8.3 mM glucose, 1.27 mM CaCl2 and 2% bovine serum albumin (Fraction V). All perfusions were conducted at 25°C and the perfusate was continually gassed with carbogen (95% O2/5% CO2) to ensure a constant arterial PO2. The oxygen content of the venous effluent (venous oxygen partial pressure, PVO2) was measured continuously by directing outflow from the cannulated vena cava through an in-line 0.5 ml Clark-type oxygen electrode. Perfusion pressure (PP) was monitored by means of a pressure transducer adjoining the cannulated abdominal aorta.
The method of calculation of VO2 has been described previously (Colquhoun et al., 1988). Values for VO2 calculation and PP were taken only after steady state conditions were obtained either under basal or drug-induced conditions.
Agent infusion
The infusion of various agents into the hindlimb commenced only after steady state VO2 and PP had been reached. All agents were either freshly prepared before each experiment, or prepared and then stored at 4°C if chemically stable. Due to the lipophilic nature of vanilloids and their affinity for silicon-based tubing, PPAHV was infused using a syringe pump (Model 2620, Harvard apparatus, U.S.A.) driving a 1.0 ml glass syringe (SGE, Australia) equipped with teflon tubing. All other agents were infused with a second pump (Model 355, Sage instruments, Orion Research Inc., U.S.A.) using an identical 1.0 ml glass syringe and teflon tubing.
Concentration-response curves for PPAHV were constructed in a cumulative manner with at least two low (nanomolar) and two high (micromolar) concentrations. Each concentration was infused only after VO2 and PP had reached steady state with the preceding concentration. In experiments using capsazepine (10 μM), the antagonist was first infused alone for approximately 5 mins, then co-infused with increasing concentrations of PPAHV.
In further experiments, PPAHV (0.2 μM) was infused repeatedly (six times) to determine the ability of PPAHV to cause acute desensitization in this preparation. On each occasion, the drug was infused for a sufficient length of time to achieve steady state VO2 and PP responses (10–12 min). Sufficient time (approximately 5 min) was also allowed in between each infusion to allow VO2 and PP to return to basal (pre-stimulus) values.
Drugs and chemicals
Capsaicin was purchased from Sigma-Aldrich Chemical Co and capsazepine from Research Biochemicals International (U.S.A.). Phorbol 12-phenylacetate 13-acetate 12-homovanillate (PPAHV) was a generous gift from Dr Giovanni Appendino (University of Torino, Torino, Italy; commercially available from Alexis Corporation, California, U.S.A.). Capsazepine was dissolved in ethanol (70–80%), as was PPAHV (50%). All other chemicals were of analytical grade.
Data and statistical analysis
The pKb for capsazepine was determined from the perfusion pressure concentration response curves for PPAHV alone and in the presence of capsazepine (Figure 1) according to the Gaddum-Schild equation: pKb=log (CR−1)/[antagonist], where CR is the concentration ratio.
Figure 1.
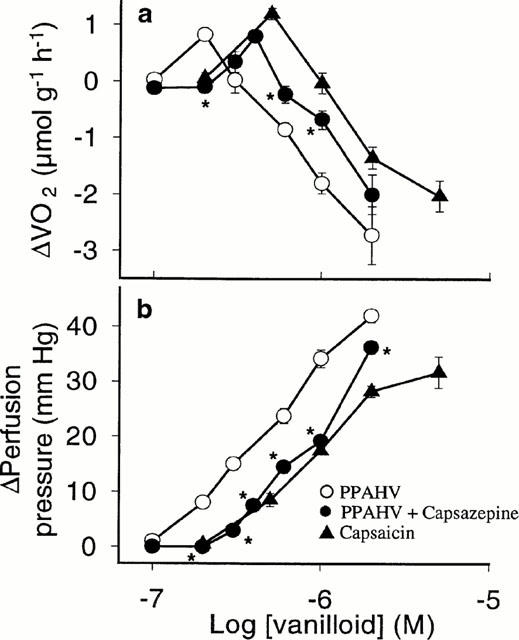
Concentration-response curves for (a) oxygen consumption (VO2) and (b) perfusion pressure (PP) in the perfused rat hindlimb for 12-phenylacetate 13-acetate 20-homovanillate (PPAHV) alone and in the presence of 10 μM capsazepine, compared to previously published data for capsaicin (Griffiths et al., 1996). Values are mean±s.e.mean of 4–5 experiments. *P<0.05, response significantly different from PPAHV alone (ANOVA).
With the exception of the typical tracings, all data are presented as mean±s.e.mean. Statistical analysis was performed using one-way repeated measures analysis of variance (ANOVA) with post-hoc Student-Newman-Keuls analysis. P<0.05 was considered statistically significant.
Results
Figure 1 shows concentration-response curves for the effect of the synthetic vanilloid analogue PPAHV on VO2 and PP in the perfused rat hindlimb. PPAHV produced a biphasic effect on VO2, in association with concentration-dependent vasoconstriction, that is also characteristic of the infusion of other natural vanilloids into this preparation. Maximum stimulation of VO2 was observed at 0.2 μM PPAHV (ΔVO2, 0.83±0.06 μmol g−1 h−1) and was accompanied by a mild increase in PP (ΔPP, 8.0±1.1 mmHg). Higher (micromolar) concentrations inhibited VO2 and caused pronounced vasoconstriction. At the highest concentration of PPAHV tested (2 μM), VO2 and PP changes were −2.73±0.51 μmol g−1 h−1 and 42.0±1.2 mmHg, respectively.
In the presence of capsazepine (10 μM), concentration-response curves for PPAHV-induced changes in VO2 and PP were shifted (parallel) to the right (Figure 1). Maximum stimulation of VO2 (similar to that observed in the absence of capsazepine) was attained using 0.4 μM PPAHV (change in VO2, 0.80±0.10 μmol g−1 h−1). However, the highest observed PP response to PPAHV was significantly (P<0.05) lowered by capsazepine (change in PP, 36.3±1.1 mmHg), although this could be overcome by increasing the concentration of PPAHV. Using the Gaddum-Schild equation, the estimated pKb for capsazepine in the present studies was five.
Comparison of the VO2 and PP concentration response curves for PPAHV and those for capsaicin reveals that PPAHV is slightly more potent than the natural analogue (Figure 1). This could not be confirmed by estimating the half-maximal effect concentration (EC50) for these ligands as maximum responses for the PPAHV-induced PP change and inhibition of VO2 were not observed at the concentrations used.
A typical tracing of the effects of repeated infusion of 0.2 μM PPAHV on venous PO2 and PP is shown in Figure 2. PPAHV rapidly induced a fall in venous PO2 (thus increasing VO2) to a new steady state value, and increased PP. The fall in venous PO2 gradually diminished with successive infusions of PPAHV, while PP increased further. Mean data from five experiments are shown in Figure 3. The first PPAHV infusion produced a typical stimulation of VO2 (change in VO2, 0.66±0.18 μmol g−1 h−1). However, a decrease in the VO2 stimulatory effect was observed with subsequent infusions which achieved statistical significance (P<0.05) with the fourth (0.29±0.09 μmol g−1 h−1), fifth (0.31±0.07 μmolg−1 h−1) and sixth infusions (0.29±0.08 μmol g−1 h−1; Figure 3a). In contrast, repeated infusion of PPAHV was associated with a significant (P<0.05) increase in PP (first infusion, 5.8±0.7; fourth infusion, 8.6±0.05; fifth infusion, 9.0±0.7; and sixth infusion, 9.0±0.7 mmHg, Figure 3b).
Figure 2.
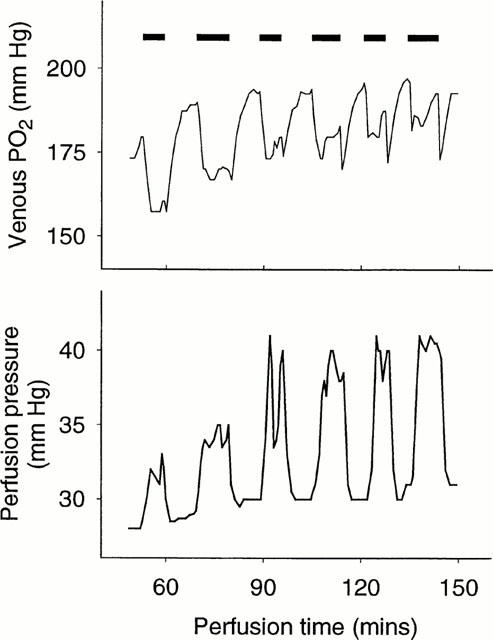
Typical tracing for the actions of 12-phenylacetate 13-acetate 20-homovanillate (PPAHV; 0.2 μM) on venous PO2 and perfusion pressure (PP) in the perfused rat hindlimb. The first infusion of PPAHV caused a strong reduction in venous PO2 (and hence stimulation of VO2) and a mild increase in hindlimb PP reflecting a vasoconstrictor effect. Removal of PPAHV resulted in the return of venous PO2 and PP to basal levels. With subsequent infusion of the same concentrations of PPAHV, the effect on venous PO2 was markedly reduced while that for PP was enhanced.
Figure 3.
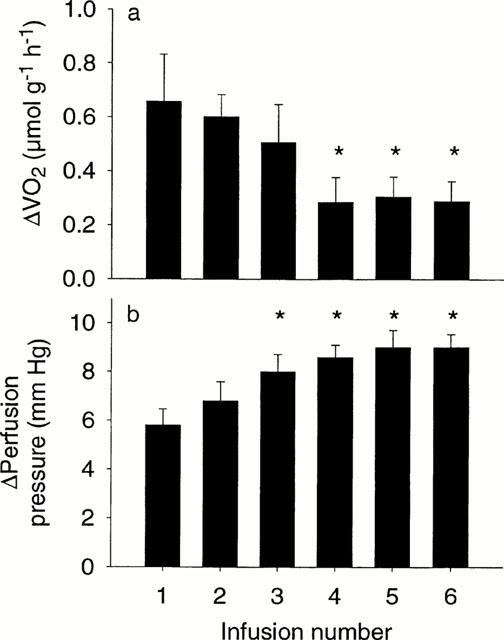
Effect of six repeated infusions of 12-phenylacetate 13-acetate 20-homovanillate (PPAHV; 0.2 μM) on rat hindlimb (a) change in oxygen consumption (VO2), (b) change in perfusion pressure (PP). Values are the mean±s.e.mean of five experiments. *P<0.05, response significantly different from first infusion (one-way repeated measures ANOVA).
Discussion
A variety of naturally occurring vanilloids, including capsaicin, RTX and piperine, produce a biphasic effect on hindlimb VO2, namely stimulation at low concentrations and inhibition at higher concentrations, with both actions associated with vasoconstriction (Eldershaw et al., 1994; Colquhoun et al., 1995; Griffiths et al., 1996; 1998). Similarly, in the present study, submicromolar concentrations of the synthetic vanilloid agonist PPAHV stimulated VO2 which was associated with mild vasoconstriction, whereas higher (micromolar) concentrations of the agonist inhibited VO2 and produced marked vasoconstriction. Thus, PPAHV produced the same metabolic and vascular effects that other natural analogues produce in this functional assay preparation. In addition, we demonstrated for the first time, the powerful desensitizing properties of PPAHV which, unlike other vanilloid agonists, caused pronounced acute tachyphylaxis with repeated infusion.
It is very likely that PPAHV produces its effects by binding to specific vanilloid receptors (Caterina et al., 1997), since the vanilloid antagonist capsazepine caused a parallel shift to the right of the concentration-response curves for PPAHV, with little change to the maximum. The competitive inhibition of PPAHV by capsazepine was very similar to that observed for capsaicin (Griffiths et al., 1996), suggesting that all three compounds interact with the same vanilloid recognition sites. The pKb for capsazepine estimated from the concentration-response curves for PPAHV in Figure 1 (pKb=5.00) was consistent with our previously published findings when capsaicin was used as the agonist (pKb=5.07, Griffiths et al., 1996). In addition, the potency of PPAHV in this preparation, relative to that of capsaicin and resiniferatoxin (RTX) (Eldershaw et al., 1994), is in excellent agreement with the affinity of this compound for vanilloid receptors previously determined in radioligand binding assays (Szallasi et al., 1996).
The biphasic effect of PPAHV and other vanilloids on perfused muscle VO2 is an interesting, but unexplained, phenomenon. One possibility is that vanilloids bind to their muscle receptors in an allosteric fashion such as that seen for RTX and other vanilloid ligands which produce a biphasic effect on [3H]-RTX binding (an initial increase in binding followed by inhibition) on dorsal root ganglion (DRG) vanilloid receptors (Szallasi et al., 1993; Acs & Blumberg, 1994). That is, the biphasic competition curves for capsaicin and RTX observed in [3H]-RTX binding studies show striking resemblance to the biphasic nature of VO2 concentration-response curves for capsaicin and RTX in the perfused rat hindlimb (Colquhoun et al., 1995; Griffiths et al., 1996; 1998), suggesting that vanilloids may bind to hindlimb receptors in a cooperative (allosteric) fashion. If this hypothesis were correct, it might be expected that PPAHV would produce monophasic curves for VO2 in the hindlimb preparation since it produces monophasic competition curves in radioligand binding studies on DRG neurons (non-cooperativity) (Szallasi et al., 1996). However, the data presented in Figure 1 demonstrate that this is not the case and provides preliminary evidence that the biphasic VO2 curves observed with vanilloid infusion into this preparation are not the result of positive cooperativity.
We have previously shown that acute tachyphlaxis with repeated infusion of natural vanilloid analogues could not be achieved in this preparation (Eldershaw et al., 1994), and systemic capsaicin pretreatment was required to cause a reduced response to infused capsaicin (Griffiths et al., 1998). In the present study, the VO2 response to a submicromolar concentration of PPAHV declined with repeated infusion, without the requirement of chronic vanilloid pretreatment. The concentration of the drug used to achieve this was more than 10 fold lower than that used to eliminate rapidly activating currents in rat trigeminal ganglia (Liu et al., 1998), and the observed dissociation constant for PPAHV (Szallasi et al., 1996). The tachyphylaxis observed in the perfused hindlimb is not likely to be related to a decrease in the viability of the preparation since the vasoconstrictor response increased.
The PP response was enhanced, rather than attenuated, by repeated infusion of PPAHV and could be regarded as evidence of sensitization rather than desensitization. However, we recently demonstrated that while systemic capsaicin pretreatment can abolish the stimulation of VO2 by vanilloid infusion into the hindlimb, the vasoconstrictor response was enhanced (Griffiths et al., 1998). It is unlikely that these vascular resistance changes can explain the observed decrease in VO2 responsiveness (tachyphylaxis) since the elevated PP response was first observed after the third infusion when the VO2 response was normal (Figure 3). Conversely, a similar elevated PP response was observed with the fourth infusion, but the VO2 response was dramatically reduced.
In conclusion, the RTX-like synthetic vanilloid PPAHV produced characteristic capsiacin-like VO2 and PP responses in the perfused rat hindlimb, and was able to induce acute tachyphylaxis preparation with repeated infusion. The ability of PPAHV to cause tachyphylaxis in this preparation, where other natural vanilloids cannot, is not surprising given that its structure is based on the ultrapotent vanilloid RTX, a compound renowned for its powerful desensitizing properties. The mechanism of this desensitization is not clear from these studies but may be related to neuropeptide depletion and/or internalization of neuropeptide or vanilloid receptors. Despite the lack of knowledge regarding the mechanism of tachyphylaxis, PPAHV and more recently described phorbol-based compounds (Szallasi et al., 1999) remain exciting prospects for the development of anti-inflammatory-analgesic agents with few side effects.
Abbreviations
- Δ
change
- PP
perfusion pressure
- PPAHV
12-phenylacetate 13-acetate 20-homovanillate
- PVO2
venous oxygen partial pressure
- RTX
resiniferatoxin
- VO2
oxygen consumption
References
- ACS G., BLUMBERG P.M. [3H]-Resiniferatoxin binding to pig dorsal horn membranes displays positive cooperativity. Life Sci. 1994;55:337–346. doi: 10.1016/0024-3205(94)00643-1. [DOI] [PubMed] [Google Scholar]
- APPENDINO G., CRAVATTO G., PALMISANO G., ANNUNZIATA R., SZALLASI A. Synthesis and evaluation of phorboid 20-homovanillates: discovery of a class of ligands binding to the vanilloid (capsaicin) receptor with different degrees of cooperativity. J. Med. Chem. 1996;39:3123–3131. doi: 10.1021/jm960063l. [DOI] [PubMed] [Google Scholar]
- APPENDINO G., SZALLASI A. Euphorbium: modern research on its active principle, resiniferatoxon, revives an ancient medicine. Life Sci. 1997;60:681–696. doi: 10.1016/s0024-3205(96)00567-x. [DOI] [PubMed] [Google Scholar]
- AUSTRALIAN GOVERNMENT . Australian code of practice for the care and use of animals for scientific purposes. Australian Government Publishing Service, Canberra; 1990. [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- COLQUHOUN E.Q., ELDERSHAW T.P.D., BENNETT K.L., HALL J.L., DORA K.A., CLARK M.G. Functional and metabolic evidence for two different vanilloid (VN1 and VN2) receptors in perfused rat hindlimb. Life Sci. 1995;57:91–102. doi: 10.1016/0024-3205(95)00250-a. [DOI] [PubMed] [Google Scholar]
- COLQUHOUN E.Q., HETTIARACHCHI M., YE J.-M., RICHTER E.A., HNIAT A.J., RATTIGAN S., CLARK M.G. Vasopressin and angiotensin II stimulate oxygen uptake in the perfused rat hindlimb. Life Sci. 1988;43:1747–1754. doi: 10.1016/0024-3205(88)90487-0. [DOI] [PubMed] [Google Scholar]
- DRAY A., URBAN L. New pharmacological strategies for pain relief. Ann Rev. Pharmacol. Toxicol. 1996;36:253–280. doi: 10.1146/annurev.pa.36.040196.001345. [DOI] [PubMed] [Google Scholar]
- ELDERSHAW T.P.D., DORA K.A., BENNETT K., CLARK M.G., COLQUHOUN E.Q. Resiniferatoxin and piperine: capsaicin-like stimulators of oxygen uptake in the perfused rat hindlimb. Life Sci. 1994;55:389–397. doi: 10.1016/0024-3205(94)00650-4. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS C.D., ELDERSHAW T.P.D., GERAGHTY D.P., HALL J.L., COLQUHOUN E.Q. Capsaicin-induced biphasic oxygen uptake in rat muscle: antagonism by capsazepine and ruthenium red provides further evidence for peripheral vanilloid receptor subtypes (VN1/VN2) Life Sci. 1996;59:105–117. doi: 10.1016/0024-3205(96)00267-6. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS C.D., GERAGHTY D.P., ELDERSHAW T.P.D., COLQUHOUN E.Q. Acute and chronic effects of capsaicin in muscle: the role of tachykinins and calcitonin gene-related peptide. J. Pharmacol. Exp. Ther. 1998;287:697–704. [PubMed] [Google Scholar]
- LIU L., SZALLASI A., SIMON S.A. A non-pungent resiniferatoxin analogue, phorbol 12-phenylacetate 13-acetate 20-homovanillate, reveals vanilloid receptor subtypes on rat trigemminal ganglion neurons. Neuroscience. 1998;84:569–581. doi: 10.1016/s0306-4522(97)00523-x. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. Therapeutic potential of capsaicin-like molecules: studies in animals and humans. Life Sci. 1992;51:1777–1781. doi: 10.1016/0024-3205(92)90047-s. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., ACS G., CRAVOTTO G., BLUMBERG P.M., LUNDBERG J.M., APPENDINO G. A novel agonist, phorbol 12-phenylacetate 13-acetate 20-homovanillate, abolishes positive cooperativity of binding by the vanilloid receptor. Eur. J. Pharmacol. 1996;299:221–228. doi: 10.1016/0014-2999(95)00864-0. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid receptors; new insights enhance potential as a therapeutic target. Pain. 1996;68:195–208. doi: 10.1016/s0304-3959(96)03202-2. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., LEWIN N.A., BLUMBERG P.M. Vanilloid (capsaicin) receptor in the rat: positive cooperativity of resiniferatoxin binding and its modulation by reduction and oxidation. J. Pharmacol. Exp. Ther. 1993;266:678–683. [PubMed] [Google Scholar]
- SZALLASI A., SZABO T., BIRO T., MODARRES S., BLUMBERG P.M., KRAUSE J.E., CORTRIGHT D.N., APPENDINO G. Resiniferatoxin-type phorbol vanilloids display capsaicin-like selectivity at native vanilloid receptors on rat DRG neurons and at the cloned vanilloid receptor VR1. Br. J. Pharmacol. 1999;128:428–434. doi: 10.1038/sj.bjp.0702810. [DOI] [PMC free article] [PubMed] [Google Scholar]


