Abstract
Anti-inflammatory effects of a novel derivative of the glucocorticoid prednisolone were investigated. NCX-1015 (prednisolone 21-[(4′-nitrooxymethyl)benzoate]) incubation in human platelet-rich plasma produced a time (0–60 min) and concentration (3–300 μM) dependent release of nitrite, that was mirrored by accumulation of cyclic guanosine monophosphate in the human platelets. Intraperitoneal injection of NCX-1015 to mice (up to 27.7 μmol kg−1) produced nitrite accumulation in the peritoneal cavity maximal at 60 min.
NCX-1015 dose-dependently induced the steroid sensitive cell surface marker CD163 in human peripheral blood mononuclear cells (PBMCs). NCX-1015 was more potent than prednisolone in inducing CD163. Similarly, lipopolysaccharide induced interleukin-1β release from these cells was inhibited by NCX-1015 with higher potency than prednisolone.
In the zymosan peritonitis model, NCX-1015 was more active than prednisolone in suppressing neutrophil extravasation (ED50 of 5.5 and 25.8 μmol kg−1, respectively), nitrite accumulation (ED50 of 1.38 and 22.2 μmol kg−1, respectively) and release of the chemokine KC (ED50 of 5.5 and 27.7 μmol kg−1, respectively) as determined at the 4 h time-point. No differences were measured for the levels of interleukin-1β or prostaglandin E2. NCX-1015 administered orally was also found to be equally active. Co-administration of the nitric oxide donors NOC-18 ((z)-1-[(2-aminoethyl)-N-(2-aminoethyl)amino] diazen-1-ium-1, 2-diolate; 7.9 μmol kg−1) or sodium nitroprusside (13.8 μmol kg−1) with prednisolone resulted in an additive anti-migratory action.
In a chronic model of granulomatous tissue inflammation, administration of NCX-1015 (13.9 μmol kg−1) from day 1 (i.e. after induction of inflammation) was more effective than prednisolone in reducing the granuloma dry weight, and this was associated to a lower anti-angiogenic effect.
In conclusion we show that NCX-1015 is more potent than prednisolone in controlling several, though not all, parameters of acute and chronic inflammation, and propose that this effect may be due to a co-operation between the steroid moiety and nitric oxide or related species released in biological fluids. Whereas this aspect needs to be further clarified, we propose NCX-1015 as the first member of a novel class of anti-inflammatory compounds, the nitro-steroids.
Keywords: Inflammation, cell trafficking, cytokines, nitric oxide, in vivo animal models
Introduction
Glucocorticoids (GCs) are potent anti-inflammatory drugs with a therapeutic efficacy in most inflammatory conditions. Their wide spectrum of effects ranges from inhibition of the synthesis of pro-inflammatory cytokines and other mediators (including specific adhesion molecules) (Panés & Granger, 1998; Henricks & Nijkamp, 1998; Fantuzzi & Ghezzi, 1993) to promotion of leukocyte apoptosis and subsequent removal (Liu et al., 1999). At a molecular level, GCs interfere with the activation and nuclear translocation of several distinct transcription factors, including nuclear factor-κB (NF-κB): such an effect would explain their ability to inhibit de novo synthesis of inflammatory mediators (Barnes & Karin, 1997). More recently, it has also been clear that GCs exert potent actions within minutes of application possibly through a direct alteration of protein-protein interactions and these actions are referred to as non-genomic effects (Buttgereit et al., 1998). Overall, these pharmacological actions account for the large application that GC have in the clinical management of inflammatory diseases, including rheumatoid arthritis and inflammatory bowel disease (Kirwan, 1995; Bickston & Cominelli, 1998). However, the use of GCs is often hampered since long-term treatment is associated with a multiplicity of side effects, particularly at the level of the bone compartment and hypothalamo-pituitary axis, with the latter resulting in abnormalities in hormone release (Kimberly, 1991). In addition, use of high doses of GCs is accompanied by serious side effects such as diabetes-like syndrome and hypertension. Therefore, modified forms of steroids active at lower therapeutic doses are an urgent need.
A novel class of anti-inflammatory drugs obtained by the addition of a chemical moiety able to release nitric oxide (NO) to non-steroidal anti-inflammatory drugs (NSAIDs) has been recently described (Wallace et al., 1995). Examples are the nitroso-butyl esters of the NSAID flurbiprofen or of acetylsalycilic acid (aspirin™): these novel chemical entities not only lack the gastrointestinal damaging effect of classical NSAIDs, but the slow release of NO in a biological microenvironment confers novel activities not shared by the parent compound (Wallace & Cirino, 1994; Wallace et al., 1994, 1995). For instance, NO released from NO-aspirin as well as from other NO-releasing molecules is able to interact with caspases (Dimmeler et al., 1997), preventing the damage to the gastric mucosa produced by the non-nitrosylated NSAID alone (Fiorucci et al., 1999a). This molecular mechanism is also likely to be responsible for the reported capacity of NO-NSAIDs to diminish the synthesis of Th1-derived cytokines, IL-1β, IL-18 and IFNγ in models of experimental inflammation (Fiorucci et al., 1999a,1999b, 2000).
In the present study we have used a similar approach to produce a novel GC with additional properties. We have synthesized a new compound, the nitrooxy-methylbenzoate derivative of the GC prednisolone (thereafter referred to as NCX-1015 or NO-prednisolone) and compared its efficacy with the parent compound on: (i) a rodent model of acute inflammation and (ii) in a murine model of chronic granulomatous tissue formation. We propose that NCX-1015 is the first member of a novel class of anti-inflammatory drugs, the nitro-steroids (or NO-steroids) that has increased anti-inflammatory actions compared to its parent coticosterone. A preliminary account of this work was presented to a recent meeting of the British Pharmacological Society (Paul-Clark et al., 2000).
Methods
Synthesis of NCX-1015 (NO-prednisolone)
NCX-1015 (prednisolone 21-[(4′nitrooxymethyl)benzoate]) was synthetized at NicOx S.a. laboratories, in Milan (Italy). The batches were prepared following a two-step synthesis with a overall yield of about 75%. A solution of prednisolone (33.3 mmol) in tetrahydrofurane was added to 49.9 mmol of 4-(chloromethyl)benzoyl chloride and triethylamine. The reaction was stirred for 24 h, and the solvent evaporated under vacuum. Following treatment of the residue with ethyl acetate and water, and removal of the insoluble material, the intermediate I (prednisolone 21-(4′chlorobenzoate) was obtained by anidrification with sodium sulphate and concentrated under reduced pressure (31.19 mmol, yield 97%). This intermediate was then treated with silver nitrite (43.66 mmol), acetonitrile (100 ml) and tetrahydrofurane (200 ml) in the dark for 35 h. The precipitate was filtered off, the solvent evaporated under vacuum and the residue purified by silica gel chromatography, and crystalized from tetrahydrofurane. The final product, (prednisolone 21-[(4′-nitrooxymethyl)benzoate) was obtained as a white powder, with a molecular weight of 539.59 and a melting point of 231–235°C (Figure 1). The structure of NCX-1015 was confirmed by nuclear magnetic resonance (with 1H and 13C) and infrared analyses. This preparation was tested for endotoxin and found to have less than 1 endotoxin unit per mg as determined by the Limulus assay (Whittaker, Walkersville, MS, U.S.A.).
Figure 1.
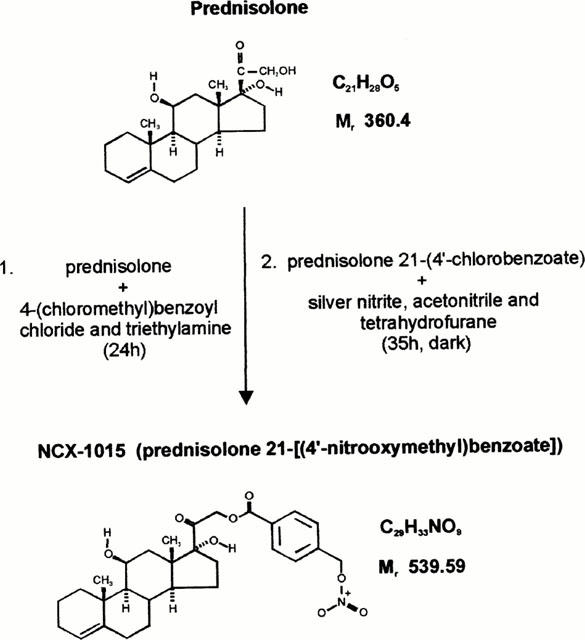
Chemical structure and a schematic summary of NCX-1015 synthesis from prednisolone.
Animals
Male or female Swiss Albino mice were purchased from Banton and Kingsman (Hull, U.K.). The animals were fed on a standard chow pellet diet and had free access to water. All animals were maintained on a 12 h light/dark cycles and housed for 1 week prior to experimentation. Mice weighed between 26–30 g on the day of the peritonitis experiment. Animal work was performed according to Home Office regulations (guidance on the operation on animals was from the Scientific Procedures act 1986).
Release of nitrite from NCX-1015 in biological microenvironments
Monitoring liberation of NO from the prednisolone structure in human platelet rich plasma (PRP) was carried out according to a published method (Wallace et al., 1995). Initially, blood collected from healthy volunteers was spun at 400×g for 30 min. PRP was collected and aliquots (1 ml) incubated with 100 μM NCX-1015, prednisolone, the NO donor sodium nitroprusside (SNP) or vehicle (PBS+0.01% DMSO) at 37°C. At different times aliquots (0.2 ml) were collected, platelets removed by centrifugation and supernatants tested for nitrite levels (see below). In another set of experiments, 5–500 μM of NCX-1015 or prednisolone were added to PRP for 45 min. At the end of the incubation, samples were centrifuged and supernatants assayed for nitrite levels. Pellets containing platelets were tested for cyclic GMP content using a commercially available enzyme immunoassay (Amersham, Little Chalfont, U.K.).
The enzymatic release of NO from NCX-1015 was tested in vitro. A commercially available mixture of esterases (10 u ml−1 final concentration; Sigma, Poole, U.K.) was added to sterile PBS containing prednisolone or NCX-1015 (5–500 μM) and incubated at 37°C for 30 min. At the end of the incubation period, samples were snap-frozen in liquid N2 and assayed for nitrite content (see below).
In vivo, NCX-1015 was injected to mice i.p. at doses of 7.5 and 15 mg kg−1 (13.8 and 27.7 μmol kg−1). Control groups received 100 μl peanut oil, 8.3 mg kg−1 SNP (27.7 μmolkg−1) or 10 mg kg−1 prednisolone (27.7 μmol kg−1). At the reported time, mice were sacrificed and their peritoneal cavities washed with 1 ml PBS containing 3 mM EDTA and 25 u ml−1 heparin. The lavage fluid was then tested for nitrite release as detailed below.
PBMC preparation and release of IL-1β
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized human whole venous blood using Ficoll-Hypaque (d=1.077 g) as recently described (Perretti et al., 1999). PBMCs were then washed three times with HEPES buffered RPMI 1640 containing 0.05% gentamycin and 10% donor serum. Cells (1×106, ∼20% monocytes, the remainder having been lymphocytes) were transferred to a 48-well plate and pre-incubated for 1 h with prednisolone, NCX-1015 (10−5–10−10 M) or vehicle, followed by activation with LPS (1 μg ml−1) for 24 h at 37°C and 5% CO2. Supernatants were removed and IL-1β production measured using a commercially available human IL-1β ELISA (R&D systems, Abingdon, U.K.).
CD163 staining and FACS analysis
All staining procedures were performed at 4°C as previously described (Morganelli & Guyre, 1988). Briefly, human peripheral blood mononuclear cells (PBMCs) were incubated for 24 h at 37°C and 5% CO2 with prednisolone, NCX-1015 (10−5–10−10 M) or vehicle. Cells were washed then blocked with normal human IgG (6 mg ml−1) and 30 μg ml−1 of the isotype control monoclonal antibody P3 or a saturating amount of monoclonal antibody Mac 2–48 (20 μg ml−1, anti-CD163 monoclonal antibody), provided by Dr N.J. Goulding (William Harvey Research Institute) for 1 h. Cells were then washed and stained for 1 h with 17.5 μg ml−1 FITC labelled goat F(ab′)2 anti-mouse IgG. FACS analysis was then performed, and cell fluorescence of monocytes gated using forward and side scatter measured in the FL1 channel using a Becton Dickenson FACScan (San Jose, CA, U.S.A.). Mean fluorescence intensity (MFI) was calculated by subtracting the MFI of the P3 stained mononuclear cells from the corresponding Mac 2-28 stained cells.
Model of acute inflammation: zymosan peritonitis
Zymosan peritonitis was induced as previously reported (Getting et al., 1997; Ajuebor et al., 1999). Briefly, male Swiss Albino mice were injected i.p. with zymosan A (1 mg in 0.5 ml sterile saline). Animals were sacrificed 4 h later by carbon-dioxide exposure and peritoneal cavities were lavaged with 3 ml PBS containing 3 mM EDTA. Aliquots of the lavage fluid were then stained with Turk's solution (0.01% crystal violet in 3% acetic acid) and differential cell counts performed using a Neubauer hemocytometer and a light microscope (Olympus B061). At this time-point between 80 and 90% of the cells recovered were granulocytes, of which the large majority were neutrophils, with less than 3% being eosinophils, as determined on cytospin preparations following staining with hematoxilyn/eosin (H/E, not shown). The lavage fluids were centrifuged at 400×g for 10 min and cell free supernatants stored at −20°C prior to mediator measurements, including evaluation of chemokine and cytokine by ELISA (see below). Cell pellets were then prepared for protein extraction and Western blotting analysis as described below.
NCX-1015 and prednisolone were dissolved in peanut oil and injected i.p. or in PEG 400 and administered by oral gavage, 1 h prior to zymosan. In view of the different molecular weights (see Figure 1) doses were adjusted to give equivalent number of moles. Control animals received peanut oil or PEG 400 alone (100 μl i.p. or p.o.).
The contribution of NO to the effects of NCX-1015 in vivo was assessed by injecting the NO donors SNP (13.8 μmol kg−1) and NOC-18 (7.9 μmol kg−1) with or without prednisolone (13.8 μmol kg−1).
Mouse chronic croton oil-induced chronic granulomatous air pouch
Female Swiss Albino mice were lightly anaesthetized with halothane and 3 ml of air injected subcutaneously into the dorsal area (day −1). On day 0, 0.5 ml Freund's complete adjuvant supplemented with 0.1% croton oil was injected into this pre-formed air pouch. Animals were sacrificed 3, 7 and 14 days later for assessment of pouch vascularity using the carmine red vascular casting technique (Colville-Nash et al., 1995). Animals were anaesthetized with hypnorm/hypnovel and warmed on a heated box (40°C) for 10 min, then injected with 1 ml 25% carmine red dye in 10% gelatine (40°C). Cadavers were chilled for 24 h, then the granuloma dissected and dried (56°C). Once dry, the granulomatous tissue was weighed then digested (12 u ml−1 papain in 0.05 M phosphate buffer, pH 7.0 supplemented with 0.33 gl−1 N-acetyl cystine). The extracted carmine dye was solubilized with 0.5 M sodiun hydroxide then quantified, in a 96-well plate at 405 nm, against a carmine standard curve (1–100 μgml−1).
Measurement of nitrite levels
Nitrite formation was measured by a modification of the Griess method (Verdon et al., 1995). Briefly, 10 μl of NADPH (10 μM), glucose-6-phosphate (5 mM), glucose-6-phosphate dehydrogenase (0.16 u) and PBS (10 mM) was added to 50 μl of cell-free lavage fluids or in plasma samples in a 96-well plate. Nitrate was converted to nitrite by addition of 10 μl (0.08 u) nitrate reductase and incubated for 45 min. 200 μl of Griess reagent (equal volumes of 1% w v−1 sulphanilamide in 5% H3PO4 and 0.1% w v−1 (1-napthyl)ethylenediamine) was added and incubated for a further 10 min. Nitrite concentrations were measured at 570 nm with a reference filter at 620 nm and results expressed as μM nitrite in cell-free exudates.
Western blot analysis
Cell pellets were sonicated using a micro-probe (Sonics and Materials Inc. U.S.A.) on ice in protease inhibitory buffer (1 mM phenylmethylsulphonylfluoride, 1.5 mM pepstatin A and 0.2 mM leupeptin in 50 mM Tris buffer, pH 7.4) and centrifuged (4000×g, 5 min, 4°C). Protein concentrations of the supernatants were determined by the Bradford assay. Samples were adjusted to 2 mg ml−1 concentration, mixed 1 : 1 with 2× loading buffer (125 mM Tris base, 2 mM ethylenediaminetetra-acetic acid, 10% mercaptoethanol, 4% sodium dodecyl sulphate (SDS), 20% glycerol and 0.1% Coomassie brilliant blue, pH 6.8) and boiled for 5 min. Samples (30–50 μg lane−1), iNOS positive control antigen and molecular weight colour markers were resolved by gel electrophoresis on 7.5% SDS-polyacrylamide gels, and transferred onto nitrocellulose membranes. iNOS protein was detected using a specific polyclonal rabbit anti-mouse antibody (1 : 5000; Santa Cruz Biotechnology, CA, U.S.A.), the signal was amplified with a horseradish peroxidase linked goat anti-rabbit secondary antibody (1 : 2000; Santa Cruz, CA, U.S.A.) and visualized using a BioMax MR-1 film (Kodak, NY, U.S.A.) after the application of Luminol (ECL, Amersham, U.K.).
Measurement of PGE2 in cell-free exudates
Prostaglandin E2 (PGE2) levels were measured in peritoneal lavage fluids 4 h after the injection of zymosan, using a commercially available enzyme-immunoassay system and following the manufacturer's instructions (Amersham Pharmacia Biotech, Buckinghamshire, U.K.). PGE2 concentrations in unknown samples (50 μl) were calculated from the standard curve and results expressed as pg PGE2 ml−1. The kit was specific and showed neglibile cross-reactivity with several other eicosanoids (data furnished by the manufacturer).
Measurement of chemokines and cytokine levels
Immunoreactive murine IL-1β and KC in the inflammatory exudates were quantified using commercially available ELISAs (R&D Systems, Abingdon, U.K.) according to manufacturer's protocol. The ELISAs showed negligible (<1%) cross-reactivity with several other murine cytokines and chemokines (data as furnished by manufacturer).
Data analysis
All values in the figures and text are expressed as mean±s.e.mean of n mice per group. Comparisons of more than two groups were calculated on original values using one-way ANOVA followed by Bonferroni post-hoc test. A P value <0.05 was considered significant.
Results
NCX-1015 releases NO in biological microenvironments
Incubation of human PRP with 100 μM NCX-1015 resulted in a time-dependent accumulation of nitrite maximal between 30 and 60 min (Figure 2A). An equimolar concentration of the NO donor SNP produced a much faster accumulation of nitrite, whereas prednisolone did not increase values above those measured in control tubes. The NCX-1015 effect was concentration-dependent as shown in Figure 2B, whereas prednisolone was again without effect. Finally, cyclic GMP accumulation into human platelets mirrored nitrite accumulation in the plasma, with detectable amounts above controls only in SNP (data not shown) and NCX-1015 samples (Figure 2C). In a final experiment the potential sensitivity of the nitrooxymethylbenzoate side chain to esterases was determined. Addition of a commercially available mixture of esterases to NCX-1015, but not prednisolone, produced a concentration dependent accumulation of nitrite in sterile PBS in the NCX-1015 group, whereas prednisolone was without effect (Figure 3). Some spontaneous release of nitrite was observed by NCX-1015 alone at the highest dose tested (500 μM).
Figure 2.
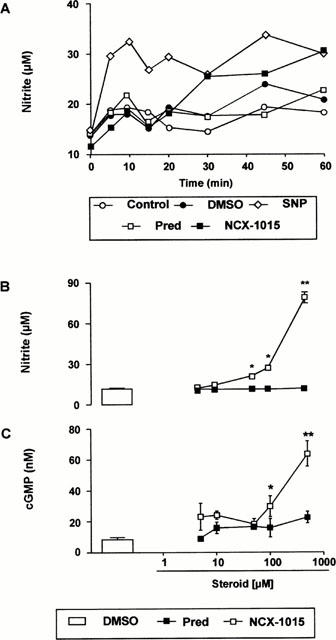
NCX-1015 releases nitrite in human platelet-rich plasma. Time-course (A) and dose-dependency (B) of nitrite accumulation following NCX-1015 incubation in human platelet rich plasma. Cyclic guanosine monophosphate (cyclic GMP) accumulation in platelets present in the samples of (B) was also measured (C). Data are mean±s.e.mean of n=8 determinations per group. *P<0.05 and **P<0.01 vs vehicle group.
Figure 3.
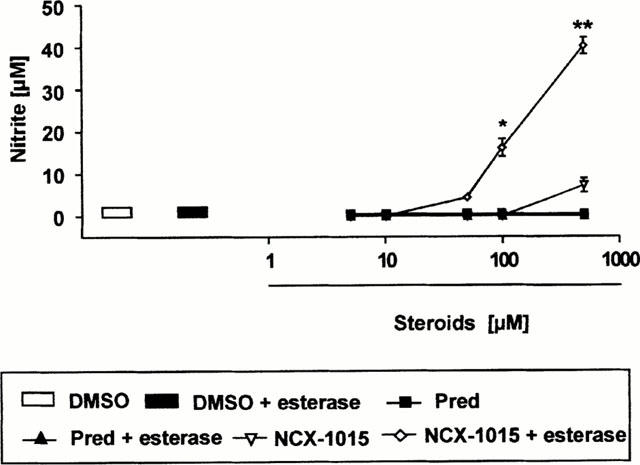
Nitrite release from NCX-1015 after incubation with esterases. Known concentrations of NCX-1015, prednisolone or vehicle (DMSO) were incubated for 30 min at 37°C with a mixture of commercially available esterases (10 u ml−1). Data are mean±s.e.mean of n=8 determinations per group. *P<0.05 and **P<0.01 vs vehicle group.
Injection of NCX-1015 in the mouse peritoneal cavity led to the accumulation of nitrite into the lavage fluids collected between 0 and 60 min later (Figure 4). Injection of SNP (i.p.) produced an immediate increase (15 min) in nitrite recovered into the peritoneal washing. Prednisolone (27.7 μmol kg−1) had no effect above control values, whereas NCX-1015 produced a time- and dose-dependent increase in nitrite levels (Figure 4). At the highest dose tested of 27.7 μmol kg−1, NCX-1015 produced an effect comparable to SNP, whereas a slower onset of nitrite release was measured after administration of the lower dose tested of 13.8 μmol kg−1, being significant at 60 min.
Figure 4.
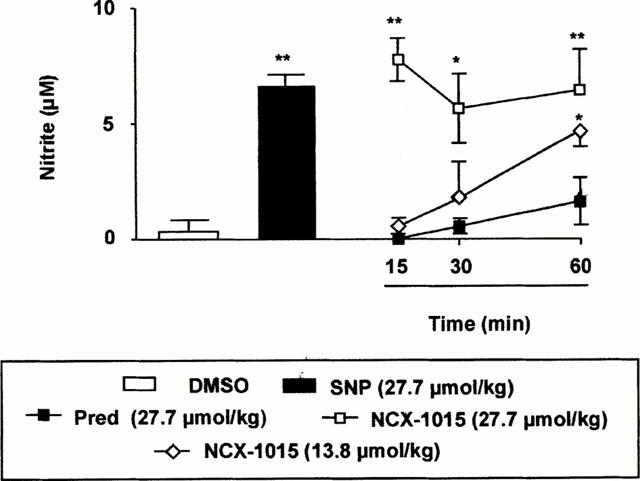
NCX-1015 releases nitrite in the mouse peritoneal cavity. NCX-1015, prednisolone (pred), sodium nitroprusside (SNP) or DMSO (0.01%) when injected i.p. into naïve mice at time 0 and lavage fluids collected from 15 to 60 min later (15 min only for SNP). Data are mean±s.e.mean of n=56 determinations or animals per group. *P<0.05 and **P<0.01 vs vehicle group.
NCX-1015 effect on CD163 expression and IL-1β release from human PBMCs
Expression of the human mononuclear phagocyte-specific antigen CD163 has recently been demonstrated to be under exquisite control by glucocorticoids (Morganelli & Guyre, 1988; Hogger et al., 1998b). GCs have long been shown to inhibit IL-1β production from stimulated PBMCs (Lew et al., 1988). Therefore, both parameters were used to assess the effect of NCX-1015 on glucocorticoid receptor activation. Incubation of PBMCs with NCX-1015 and prednisolone produced a concentration-dependent activation of CD163 (Figure 5A). NCX-1015 was more potent than prednisolone at inducing CD163 cell surface expression. The increased efficacy of NCX1015, compared with the parent molecule prednisolone, was also observed when assessing inhibition of LPS induced IL-1β production (Figure 5B).
Figure 5.
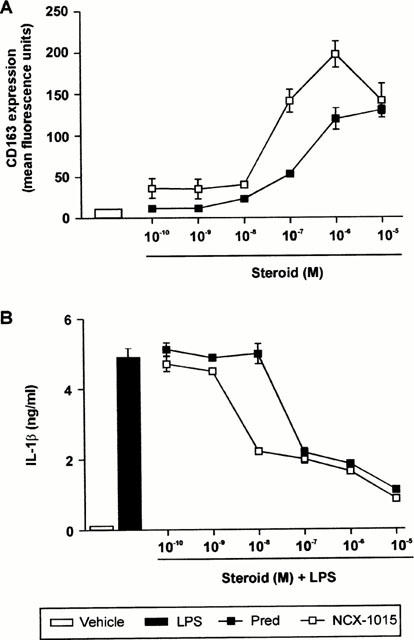
Effects of NCX-1015 on the expression of CD163 and IL-1β production in human peripheral blood mononuclear cells (PBMC). (A) Shows concentration-dependent induction of CD163 expression in PBMC after 24 h incubation with prednisolone, NCX-1015 or vehicle (DMSO). (B) Shows inhibition of LPS-stimulated IL-1β production after treatment with the reported concentrations of prednisolone, NCX-1015 or vehicle (DMSO). Data are mean±s.e.mean of n=6 distinct determinations per group.
NCX-1015 and prednisolone in the zymosan-induced peritonitis model
Treatment of mice with NCX-1015 produced a potent anti-migratory action on the peritoneal extravasation of neutrophils, which was significant even at the lowest dose tested of 1.38 μmol kg−1 (Figure 6A). Prednisolone produced a significant anti-inflammatory effect at doses⩾13.8 μmol kg−11. Approximate ED50s calculated from the dose-responses curves shown in Figure 5 were 25.8 μmol kg−1 for prednisolone and 5.5 μmol kg−1 for NCX-1015. A marked difference between the two steroid derivatives was also seen with respect to iNOS expression and nitrite levels in the exudates. Figure 6B shows these data, with a potent reduction in nitrite levels been measured at all doses of NCX-1015, whereas prednisolone was active only at the highest dose tested of 27.7 μmol kg−1 (an approximate ED50 of 22.2 μmol kg−1 could be calculated). The potent inhibitory action of NCX-1015 on nitrite production was clearly paralleled by a strong attenuation of iNOS expression (Figure 6B).
Figure 6.
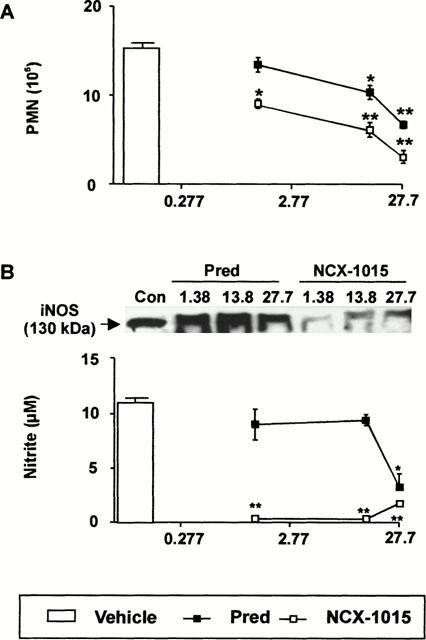
Effect of NCX-1015 in the zymosan peritonitis model. Mice were treated i.p. with the reported doses of NCX-1015, prednisolone or vehicle (100 μl peanut oil) 1 h prior to zymosan (1 mg i.p.). Peritoneal cavities were washed 4 h later and the following determinations obtained: (A) Neutrophil counts; (B) nitrite levels in the lavage fluids and iNOS protein expression in the cell pellets as determined by Western blotting analysis. Data are mean±s.e.mean of n=6–8 animals per group. *P<0.05 and **P<0.01 vs vehicle group.
Figure 7A shows that a highly significant reduction in KC levels was observed only in the animals treated with NCX-1015. An ED50 of 5.5 μmol kg−1 could be calculated from this dose-response curve, which interestingly is identical to that measured on neutrophil extravasation. However, the lowest dose of 1.38 μmol kg−1 NCX-1015 significantly inhibited neutrophil extravasation but not KC release (compare Figure 6A with Figure 7A). In this model, both drugs were equipotent in reducing exudate levels of PGE2 (ED50s around 13.8 μmol kg−1) (Figure 7B). Similarly, no significant differences were detected with respect to IL-1β release (Figure 7C).
Figure 7.
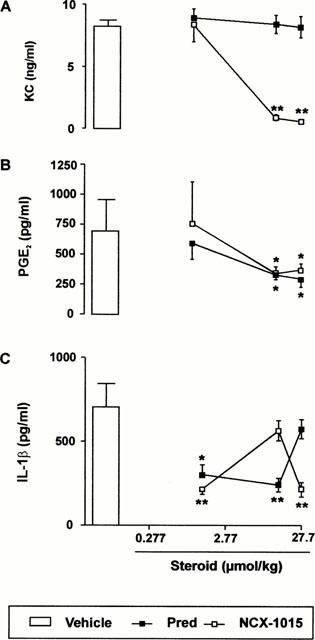
Effect of NCX-1015 on the release of soluble mediators in the zymosan peritonitis model. Mice were treated i.p. with the reported doses of NCX-1015, prednisolone or vehicle (100 μl peanut oil) 1 h prior to zymosan (1 mg i.p.). Peritoneal cavities were washed 4 h later and the following determinations obtained: (A) KC; (B) PGE2; (C) IL-1β. Data are mean±s.e.mean of n=6–8 animals per group. *P<0.05 and **P<0.01 vs vehicle group.
Oral efficacy of NCX-1015 and prednisolone in the zymosan-induced peritonitis model
From a therapeutic point of view, it was also important to establish whether or not NCX-1015 was orally active. As observed in Figure 8, both NCX-1015 and prednisolone had similar potentency profiles when administered either i.p. or p.o., again NCX-1015 being more effective than prednisolone at reducing inflammatory cell influx, nitrite and KC levels in lavage fluids.
Figure 8.

Effect of oral administration of NCX-1015 in the zymosan peritonitis model. Mice were treated p.o. with the reported doses of NCX-1015, prednisolone or vehicle (100 μl PEG 400) 1 h prior to zymosan (1 mg i.p.). Peritoneal cavities were washed 4 h later and the following determinations obtained: (A) Neutrophil counts; (B) nitrite levels and (C) KC levels in the lavage fluids. Data are mean±s.e.mean of n=68 animals per group. *P<0.05 and **P<0.01 vs vehicle group.
Nitric oxide contributes to the increased anti-inflammatory profile of NCX-1015
The effect of co-administration of prednisolone with two distinct NO donors SNP and NOC-18 was established. As observed in Figure 9, either prednisolone, SNP or NOC-18 alone reduced PMN influx into the peritoneal cavity inflamed with zymosan. An additive effect was seen with the combination of prednisolone and slow NO releasing compound NOC-18. The combination of this particular NO donor and prednisolone best reflects the profile of NO release from NCX-1015, since NO is released from SNP in a rapid manner (see also Figures 2A and 4).
Figure 9.
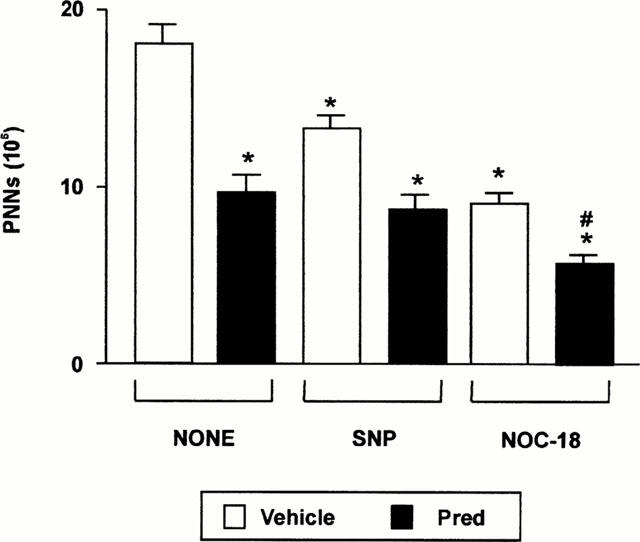
The cumulative anti-inflammatory effect of prednisolone and NO donors in the zymosan peritonitis model. Mice were treated i.p. with the reported doses of SNP and NOC-18 with either prednisolone or vehicle (100 μl peanut oil) 1 h prior to zymosan (1 mg i.p.). Peritoneal cavities were washed 4 h later and neutrophil counts performed. Data are mean±s.e.mean of n=6 animals per group. *P<0.05 vs vehicle group alone and #P<0.05 vs prednisolone group alone.
Therapeutic administration of NCX-1015 is more effective than prednisolone in reducing granulomatous tissue formation in a chronic air pouch model
NCX-1015 was more potent anti-inflammatory than prednisolone in a model of acute inflammation, however since steroids are given over extended periods of time and are used mainly for the control of chronic inflammation, it was important to assess the effects of this drug in a chronic inflammatory model. NCX-1015 significantly reduced granulomatous tissue formation at every time point measured, whereas prednisolone was only effective at day 7, the peak of inflammation (Figure 10A). Again carmine content, a measure of blood vessel formation in the air pouch, was also significantly reduced at all time points, with prednisolone only being effective at day 3 (Figure 10B). However, when the data from Figure 10A,B are combined to produce a vascular index (Figure 10C), there was an overall increase in vascularity in the NCX-1015 group.
Figure 10.
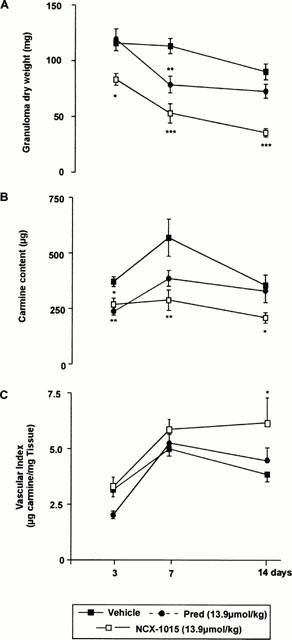
Effect of therapeutic administration of prednisolone and NCX-1015 on the murine croton oil-induced chronic granulomatous tissue air pouch. After injection of CO/CFA on day 0, mice were injected i.p. daily with the reported doses of NCX-1015, prednisolone or vehicle (peanut oil) from days 2 to 14. Distinct groups were sacrificed at days 3, 7 or 14. (A) Granuloma dry weight; (B) carmine content and (C) vascular index were measured. Data are mean±s.e.mean of n=8–10 animals per group. *P<0.05 and **P<0.01 vs vehicle group.
Discussion
There are a number of reasons supporting the hypothesis that addition of a NO releasing moiety to a GC structure will provide beneficial ‘added value' to the anti-inflammatory actions of the GC nucleus. Chief amongst these is that the anti-inflammatory actions of GCs require ongoing RNA and protein (Barnes & Karin, 1997; Buttgereit et al., 1998), therefore many of GC effects have a long (hours) latency. In contrast the effects of NO are more acute, with rapid alterations of the microcirculation being observed within minutes of application of a NO donor. For instance, arteriole dilation and an anti-adhesive effect on circulating leukocytes are both phenomena requiring NO dependent cyclic GMP (Kubes & Grainger, 1996). Although it is clear that inappropriate production of NO by iNOS can lead to tissue damage, there is a growing body of evidence suggesting that reactive nitrogen intermediates produced during inflammation are protective in various models of pathology. NOS inhibitors increased organ damage and mortality following endotoxic shock in rats by NO dependent maintenance of vascular tone (Harbrecht et al., 1992; Park et al., 1996). Acetic acid-induced colitis in iNOS-deficient mice resulted in increased PMN-associated tissue damage in comparison to wild type (McCafferty et al., 1997). Accumulation of inflammatory cell in the hepatic microvasculature of these animals during experimental endotoxaemia has also been observed (Hickey et al., 1997). Overall these and other findings support the concept for a protective role of endogenously produced NO in several experimental pathologies.
There are a number of possible molecular mechanisms by which NO can produce an anti-inflammatory effect. One possibility is that NO interacts directly with the haeme prosthetic group within NADPH oxidase to inhibit superoxide production (Fukahori et al., 1994; Wang et al., 1995) thereby reducing tissue damage associated with production of this free radical (Vega et al., 1998). Elevated levels of superoxide radicals produce mediator release from mast cells (Mannaioni et al., 1991), whereas NO has an important stabilising effect on this cell type (Gaboury et al., 1996). In addition, raised levels of superoxide activate NF-κB (Sen & Packer, 1996) whereas NO prevents activation of this transcription factor. This can occur via superoxide-dependent and independent pathways. In fact, NO can also decrease NF-κB activation through inhibition of IκB-phosphorylation and degradation (Katsuyama et al., 1998). Finally, it can also inhibit direct NF-κB binding to DNA (Park et al., 1997). Together with the finding that micromolar concentrations of NO inhibits the release of Th1-dependent cytokines (IL-1β, IL-18 and IFN-γ), T lymphocyte proliferation and T cell-mediated diseases (Dimmeler et al., 1997; Fiorucci et al., 2000; Kim et al., 1998; Kolb & Kolb-Bacofen, 1998), these data clearly provide a mechanistic support to NO anti-inflammatory profile.
To test our hypothesis we linked NO to prednisolone, a glucocorticoid of moderate potency (Schimmer & Parker, 1996), at position 21 in a ratio of 1 : 1 to obtain NCX-1015. Position 21 was chosen because substitution of this position does not inhibit the biological activity of the molecule (Schimmer & Parker, 1996). NO was linked to prednisolone through a methyl benzoate side chain. This side chain was chosen because sensitive to esterase action as demonstrated for an aspirin derivative (Wallace et al., 1995). Therefore, we initially demonstrated that incubation of NCX-1015 with plasma liberated nitrite in a time- and concentration-dependent fashion, and that this process was associated to increased formation of cyclic GMP in platelets. These observations could only have risen through the liberation of NO from the parent compound. This data is in line with that reported for NO-aspirin (Wallace et al., 1994, 1995). As expected SNP produced a much faster accumulation of nitrite, whereas at least 30 min were required to reach 80–90% of maximal release of nitrite from the benzoic nitroso esther moiety. Finally, nitrite accumulation was also detected in vivo following i.p. administration of NCX-1015. Therefore, NCX-1015 fulfilled the first criteria of our hypothesis, since it released a biologically active NO species.
To compare the glucocorticoid activity of NCX-1015 with that of the parent compound prednisolone, the effect on CD163 expression was measured. This marker has recently been characterized (Morganelli & Guyre, 1988) and shown to genuinely correlate with activation of the glucocorticoid receptor (Hogger et al., 1998a,1998b). In this system NCX-1015 was more potent (shift of the dose response curve to the left) and more efficacious (higher maximal effect) than prednisolone. Though studies examining the molecular interactions between NCX-1015 and the glucocorticoid receptor, as well as glucocorticoid receptor activation are required (and are in progress), the data obtained on CD163 expression are strongly supportive of the notion that NCX-1015 possesses higher glucocorticoid activity. This hypothesis is well corroborated by the analysis of the drug's inhibitory effect upon IL-1β synthesis. This cytokine expression is exquisitely sensitive to glucocorticoids (Lew et al., 1988) and in this experimental condition NCX-1015 was again more potent than prednisolone. In line with what was discussed above, NO inhibition of the enzyme synthesizing IL-1β (Dimmeler et al., 1997; Kim et al., 1998) could easily explain the higher potency displayed by NCX-1015.
To test the profile of anti-inflammatory activities, the zymosan peritonitis model was initially employed, since we have already demonstrated the sensitivity of the process of cell recruitment to synthetic glucocorticoids (Getting et al., 1997). With the exception of PGE2 and IL-1β release, NCX-1015 was found to be approximately between two and 20 times more potent than the parent compound in this experimental set up. In particular, neutrophil extravasation and KC release were much more sensitive to NCX-1015, with inhibition of nitrite formation being essentially abolished by the NO-steroid. The latter effect was linked to a dramatic suppression of iNOS expression. Higher potency of NCX-1015 on neutrophil migration is likely to be NO-dependent in view of the anti-adhesive activity of this molecule (Kubes & Granger, 1996; Kubes et al., 1991). It is now clear that this specific action of NO occurs via mast cell stabilization (and consequent reduction in endothelial P-selectin expression) (Kubes & Granger, 1996; Gaboury et al., 1996). Such an inhibitory loop may also be operating in our model, since peritoneal mast cells greatly contribute to the release of KC (Ajuebor et al., 1999), a parameter greatly affected by NCX-1015. The potent inhibitory action of NCX-1015 on iNOS expression and nitrite formation are likely the result of NO-dependent inhibition of NF-κB as discussed above. Similarly, it is likely that NF-κB-dependent NO suppression of cytokine and adhesion molecule expressions may contribute, together with the mast cell stabilizing effect discussed above, to the higher potency displayed by NCX-1015 compared to prednisolone in both the peritonitis model and the murine croton oil-induced chronic granulomatous tissue air pouch. Targeting the formation of these mediators by NCX-1015-released NO provides a molecular explanation for the higher anti-inflammatory potency of this compound.
Finally, we tested the efficacy of NCX-1015 in a chronic model of inflammation. The chronic inflammatory air pouch induced by croton oil has histological similarities to invading inflammatory tissue seen in RA (Colville-Nash et al., 1995), therefore was chosen as an appropriate model to assess the effects of NCX-1015 not only on granulomatous tissue formation but also on angiogenesis (Colville-Nash et al., 1995). It was found that therapeutic administration of NCX-1015, but not prednisolone, reduced inflammation at day 3, with the nitro-derivative being more effective at day 7. Since intense accumulation of blood-borne leukocytes is the predominant characteristic of this inflammatory reaction at these time-points (Appleton et al., 1993), it is clear that NCX-1015 is more potent than prednisolone in inhibiting neutrophil and monocyte extravasation also in this experimental condition. The injection of the viscous suspension of Carmine red in gelatine has been successfully used as a marker of angiogenesis in this chronic air-pouch model (Colville-Nash et al., 1995). At day 14, which is the most relevant to neo-vessel formation (Colville-Nash et al., 1995). NCX-1015 appeared to have a more favourable vascular index, that takes into account the extent of vascularity per gram of granuloma tissue. Though waiting for confirmation in more focused studies, it seems possible to suggest that NO release from NCX-1015 may occur in a manner to reduce the anti-angiogenic effect of the steroid moiety. Interestingly, the pro-angiogenic action of NO and NO donor has been reported in a few studies (Morbidelli et al., 1996; Ziche et al., 1997).
The potential clinical implications of these findings are important, for steroids are amongst the most potent anti-inflammatory drugs that we possess and yet their chronic use is limited by their burden of side effects which are themselves related to the dose of the steroid in use. If a way could be found to reduce the overall dosage of such compounds whilst maintaining their potency it would be possible to remove many of the concerns which surround the clinical usage of these most useful drugs. We believe that our data points to a method whereby this might be done and through which even the great potency exhibited by glucocorticoids may be increased in a safe and efficacious manner.
Acknowledgments
M. Paul-Clark is a post-doctoral fellow of the Arthritis Research Campaign (U.K.), whereas R.J. Flower is a principal research fellow of the Wellcome Trust (U.K.). S. Fiorucci is partially supported by a grant from MURST (Rome, Italy).
Abbreviations
- CFA
Complete Freund's adjuvant
- CO
croton oil
- ED50
dose giving 50% of maximal effect
- FACS
fluorescence activated cell sorter
- cyclic GMP
cyclic guanosine monophosphate
- GCs
glucocorticoids
- H/E
hematoxylin and eosin
- IL-1β
interleukin-1β
- LPS
lipopolysaccharide
- NOC-18
(z)-1-[(2-aminoethyl)-N-(2-aminoethyl)amino] diazen-1-ium-1, 2-diolate
- iNOS
inducible nitric oxide synthase
- NCX-1015
nitro-prednisolone or prednisolone 21-[(4′nitrooxymethyl)benzoate]
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- NSAIDs
non-steroidal anti-inflammatory drugs
- PGE2
prostaglandin E2
- PRP
platelet rich plasma
- SNP
sodium nitroprusside
References
- AJUEBOR M.N., DAS A.M., VIRÁG L., FLOWER R.J., SZABÓ C., PERRETTI M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J. Immunol. 1999;162:1685–1691. [PubMed] [Google Scholar]
- APPLETON I., TOMLINSON A., COLVILLE-NASH P.R., WILLOUGHBY D.A. Temporal and spatial immunolocalization of cytokines in murine chronic granulomatous tissue: implications for their role in tissue development and repair process. Lab. Invest. 1993;69:369–372. [PubMed] [Google Scholar]
- BARNES P.J., KARIN M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. New Eng. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- BICKSTON S.J., COMINELLI F. Inflammatory bowel disease: short- and long-term treatments. Adv. Intern. Med. 1998;43:143–174. [PubMed] [Google Scholar]
- BUTTGEREIT F., WEHLING M., BURMESTER GR. A new hypothesis of modular glucocorticoid action. Arth. Rheum. 1998;41:761–767. doi: 10.1002/1529-0131(199805)41:5<761::AID-ART2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- COLVILLE-NASH P.R., ALAM C.A.S., APPLETON I., BROWN J.R., SEED M.P., WILLOUGHBY D.A. The pharmacological modulation of angiogenesis in chronic granulomatous inflammation. J. Pharm. Exp. Ther. 1995;78:1463–1472. [PubMed] [Google Scholar]
- DIMMELER S., HAENDELER J., NEHLS M., ZEIHER A. M. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1β-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J. Exp. Med. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANTUZZI G., GHEZZI P. Glucocorticoids as cytokine inhibitors: role in neuroendocrine control and therapy of inflammation. Med. Inflamm. 1993;2:263–270. doi: 10.1155/S0962935193000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIORUCCI S., ANTONELLI E., SANTUCCI L., MORELLI O., MIGLIETTI M., FEDERICI B., MANNUCCI R., DEL SOLDATO P., MORELLI A. Gastrointestinal safety of nitric oxide-derived aspirin is related to inhibition of ICE-like cysteine proteases in rats. Gastroenterology. 1999a;116:1089–1106. doi: 10.1016/s0016-5085(99)70012-0. [DOI] [PubMed] [Google Scholar]
- FIORUCCI S., SANTUCCI L., FEDERICI B., ANTONELLI E., DISTRUTTI E., MORELLI O., DI RENZO G., CIRINO G., DEL SOLDATO P., MORELLI A. Nitric oxide-releasing NSAIDs inhibit interleukin-1β converting enzyme-like cysteine proteases and protect endothelial cells from apoptosis induced by TNF. Aliment. Pharmacol. Ther. 1999b;13:421–435. doi: 10.1046/j.1365-2036.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- FIORUCCI S., SANTUCCI L., ANTONELLI E., DISTRUTTI E., DEL SERO G., MORELLI O., ROMANI L., FEDERICI B., DEL SOLDATO P., MORELLI A. NO-aspirin protects from T-cell mediated liver injury by inhibiting caspase-dependent processing of Th1-like cytokines. Gastroenterology. 2000;118:404–421. doi: 10.1016/s0016-5085(00)70223-x. [DOI] [PubMed] [Google Scholar]
- FUKAHORI M., ICHIMORI K., ISHIDA H., NAKAGAWA H., OKINO H. Nitric oxide reversible suppresses xanthine oxidase activity. Free Radic. Res. 1994;21:203–212. doi: 10.3109/10715769409056572. [DOI] [PubMed] [Google Scholar]
- GABOURY J.P., NIU X.-F., KUBES P. Nitric oxide inhibits numerous features of mast cell-induced inflammation. Circulation. 1996;93:318–326. doi: 10.1161/01.cir.93.2.318. [DOI] [PubMed] [Google Scholar]
- GETTING S.J., FLOWER R.J., PERRETTI M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br. J. Pharmacol. 1997;120:1075–1082. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARBRECHT B.G., BILLIAR T.R., STADLER J. Nitric oxide synthesis serves to reduce hepatic damage during acute murine endotoxemia. Crit. Care Med. 1992;20:1568–1579. doi: 10.1097/00003246-199211000-00015. [DOI] [PubMed] [Google Scholar]
- HENRICKS P., NIJKAMP F.P. Pharmacological modulation of cell adhesion molecules. Eur. J. Pharmacol. 1998;344:1–13. doi: 10.1016/s0014-2999(98)00036-3. [DOI] [PubMed] [Google Scholar]
- HICKEY M.J., SHARKEY K.A., SIHOTA E.G., REINHARDT P.H., MACMICKING J.D., KUBES P. Inducible nitric oxide synthase-deficient mice have enhanced leukocyte-endothelium interactions in endotoxemia. FASEB J. 1997;11:955–964. doi: 10.1096/fasebj.11.12.9337148. [DOI] [PubMed] [Google Scholar]
- HOGGER P., DREIER J, , DROSTE A. , BUCK F., SORG C. Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible member of the scavenger receptor cysteine-rich family (CD163) J. Immunol. 1998a;161:1883–1890. [PubMed] [Google Scholar]
- HOGGER P., ERPENSTEIN U., ROHDEWALD P., SORG C. Biochemical characterisation of a glucocorticoid-induced membrane protein (RM3/1) in human monocytes and its application as model system for ranking glucocorticoid potency. Pharm. Res. 1998b;15:296–302. doi: 10.1023/a:1011931021743. [DOI] [PubMed] [Google Scholar]
- KATSUYAMA K., SHICHIRI M., MARUMO F., HIRATA Y. NO inhibits cytokine-induced iNOS expression and NF-κB activation by interfering with phosphorylation and degradation of IκB-α. J. Immunol. 1998;15:2889–2895. doi: 10.1161/01.atv.18.11.1796. [DOI] [PubMed] [Google Scholar]
- KIM Y-M, , TALANINIAN R.V., LI J., BILLIAR T.R. Nitric oxide prevents IL-1β and IFN-γ-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1β converting enzyme) J. Immunol. 1998;161:4122–4128. [PubMed] [Google Scholar]
- KIMBERLY R.P. Mechanism of action, dosage schedules, and side effects of steroid therapy. Curr. Opin. Rheumatol. 1991;3:373–379. doi: 10.1097/00002281-199106000-00008. [DOI] [PubMed] [Google Scholar]
- KIRWAN J.R. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. New Eng. J. Med. 1995;333:142–146. doi: 10.1056/NEJM199507203330302. [DOI] [PubMed] [Google Scholar]
- KOLB H., KOLB-BACHOFEN V. Nitric oxide in autoimmune disease: cytotoxic or regulatory mediator. Immunol. Today. 1998;19:556–561. doi: 10.1016/s0167-5699(98)01366-8. [DOI] [PubMed] [Google Scholar]
- KUBES P., GRANGER D.N. Leukocyte-endothelial cell interactions evoked by mast cells. Cardiovasc. Res. 1996;32:699–708. [PubMed] [Google Scholar]
- KUBES P., SUZUKI M., GRANGER D.N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEW W., OPPENHEIM J.J., MATSUSHIMA K. Analysis of the suppression of IL-1α and IL-1β production in peripheral blood mononuclear adherent cells by a glucocorticoid hormone. J. Immunol. 1988;140:3812–3816. [PubMed] [Google Scholar]
- LIU Y., COUSIN J.M., HUGHES J., VAN DAMME J., SECKL J.R., HASLETT C., DRANSFIELD I., SAVIL J., ROSSI A. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 1999;162:3639–3646. [PubMed] [Google Scholar]
- MANNAIONI P.F., MASINI E., PISTELLI A., SALVEMINI D., VANE J.R. Mast cells as a source of superoxide anions and nitric oxide-like factor: relevance to histamine release. Int. J. Tissue React. 1991;13:271–278. [PubMed] [Google Scholar]
- MCCAFFERTY D.M., MUDGETT J.S., SWAIN M.G., KUBES P. iNOS plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997;112:1022–1027. doi: 10.1053/gast.1997.v112.pm9041266. [DOI] [PubMed] [Google Scholar]
- MORBIDELLI L., CHANG C.H., DOUGLAS J.G., GRANGER H.J., LEDDA F., ZICHE M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am. J. Physiol. 1996;39:H411–H415. doi: 10.1152/ajpheart.1996.270.1.H411. [DOI] [PubMed] [Google Scholar]
- MORGANELLI P.M., GUYRE P.M. INF-γ plus glucocorticoids stimulate the expression of a newly identified human mononuclear phagocyte specific antigen. J. Immunol. 1988;140:2296–2304. [PubMed] [Google Scholar]
- PANÉS J., GRANGER D.N. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998;114:1066–1090. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- PARK J.H., CHANG S.H., LEE K.M., SHIN S.H. Protective effect of nitric oxide in an endotoxin-induced septic shock. Am. J. Surg. 1996;171:340–346. doi: 10.1016/S0002-9610(97)89638-9. [DOI] [PubMed] [Google Scholar]
- PARK S.K., LIN H.L., MURPHY S. Nitric oxide regulates nitric oxide synthase-2 gene expression by inhibiting NF-κB binding to DNA. Biochem. J. 1997;322:609–613. doi: 10.1042/bj3220609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUL-CLARK M.J., LIM L.H.K., DEL SOLDATO P., BURGAUD J.-L., FLOWER R.J., PERRETTI M. NCX-1015, a novel derivative of prednisolone with enhanced anti-inflammatory activity. Br. J. Pharmacol. 2000;129:98P. doi: 10.1038/sj.bjp.0703704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRETTI M., WHELLER S.K., FLOWER R.J., WAHID S., PITZALIS C. Modulation of cellular annexin I in human leukocytes infiltrating DTH skin reactions. J. Leukoc. Biol. 1999;65:583–589. doi: 10.1002/jlb.65.5.583. [DOI] [PubMed] [Google Scholar]
- SCHIMMER B.P., PARKER K.L.Adrenocortical steroids and their synthetic analogs The pharmacological basis of therapeutics 1996New York, McGraw-Hill; 1465–1481.ed. Hardman, J.G., Limbird, L.E., Molinoff, P.B., Ruddon, R.W. & Gilman, A.G. pp [Google Scholar]
- SEN C.K., PACKER L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- VEGA V.L., MALDONADO M., MARADONES L., MANRIQUEZ V., VIVALDI E., ROA J., WARD P.H. Inhibition of nitric oxide synthesis aggravates hepatic oxidative stress and enhances superoxide dismutase inactivation in rats subjected to tourniquet shock. Shock. 1998;9:320–328. doi: 10.1097/00024382-199805000-00002. [DOI] [PubMed] [Google Scholar]
- VERDON C.P., BURTON B.A., PRIOR R.L. Sample pretreatment with nitrate reductase and glucose-6-phosphate dehydrogenase quantitatively reduces nitrate while avoiding interference by NADP+ when the Griess reaction is used to assay for nitrite. Anal. Biochem. 1995;224:502–508. doi: 10.1006/abio.1995.1079. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., CIRINO G. The development of gastrointestinal-sparing nonsteroidal anti-inflammatory drugs. Trends Pharmacol. Sci. 1994;15:405–406. doi: 10.1016/0165-6147(94)90083-3. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., REUTER B., CICALA C., MCKNIGHT W., GRISHAM M.B., CIRINO G. Novel nonsteroidal anti-inflammatory drug derivatives with markedly reduced ulcerogenic properties in the rat. Gastroenterology. 1994;107:173–179. doi: 10.1016/0016-5085(94)90074-4. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., DEL SOLDATO P., BAYDOUN A.R., CIRINO G. Anti-thrombotic effects of a nitric oxide-releasing, gastric sparing aspirin derivative. J. Clin. Invest. 1995;96:2711–2718. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y., MATHEWS W.R., GUIDO D.M., FARHOOD A., JAESCHKE H. Inhibition of nitric oxide synthase aggrevates reperfusion injury after hepatic ischemia and endoxemia. Shock. 1995;4:282–288. doi: 10.1097/00024382-199510000-00009. [DOI] [PubMed] [Google Scholar]
- ZICHE M, , MORBIDELLI L., CHOUDHURI R., ZHANG H.-T., DONNINI S., GRANGER H.J., BICKNELL H. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J. Clin. Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]


