Abstract
The prostate function is regulated by androgens and α-adrenergic activity. Clinically, antiandrogens and/or α1-adrenergic antagonists are commonly used to treat symptomatic prostatic hypertrophy. To elucidate the effects of androgen deprivation on prostate contractility via α1-adrenoceptor, the characteristics and expression of α1-adrenoceptors were examined in castrated rats.
Isolated prostate strips from intact and castrated rats were subjected to a phenylephrine stimulated contraction. Prazosin (10 nM), [3H]-prazosin and phenoxybenzamine (3–300 nM) were used for inhibition assay, receptor characterization and partial alkylation of α-adrenoceptor, respectively. The mRNA content of three subtypes of α-adrenoceptors was determined by reverse transcription combined with polymerase chain reaction (RT–PCR).
Contractile response to phenylephrine increased in castrated rats, which could be explained by a relative increase of the stromal component. A lowered contraction potency was also noted in castrated rats. Receptor binding assay indicated minimal changes in the affinity or density of α1-adrenoceptor. Escalating alkylation of the α1-adrenoceptor population resulted in a rightward shift in the contraction-response curves before depressing maximal contractile force, and the suppression was detected at lower doses in castrated rats. RT–PCR study confirmed the expression of three types of α1-adrenoceptor, α1a, α1b and α1d-adrenoceptors, in intact rat prostate, and revealed that α1a-adrenoceptor, but not α1b or α1d-adrenoceptors, was down-regulated in castrates.
The results show that androgen deprivation suppressed α1-adrenergic contractility of rat prostate strips, and the suppression was associated with down-regulation of receptor reserve for the α1a-adreneroceptor population expressed in intact rat prostate.
Keywords: Prostate, α-adrenoceptor, α1a-adrenoceptor, phenylephrine, castration, rat
Introduction
The sex hormone environment is known to influence the contraction of smooth muscle in response to adrenergic stimulation. For example, in the female rabbit the sensitivity of urethral smooth muscle to α-adrenergic stimulation is increased after bilateral oophorectomy (Andersson et al., 1984). Estradiol treatment of immature rabbits enhances sensitivity of the uterus to norepinephrine-induced contraction (Roberts et al., 1981).
The prostate is a contractile organ that responds to α-adrenergic stimulation (Furuya et al., 1982). Although there are substantial differences in prostate stromal contents among species (Lepor et al., 1994), stromal smooth muscle cells that express α1-adrenoceptors (Lepor et al., 1987) are responsible for contractility with α-adrenergic agonists. Three distinct subtypes of α1-adrenoceptor have been cloned and characterized: α1a (Schwinn et al., 1990), α1b (Cotecchia et al., 1988), and α1d-adrenoceptors (Lomasney et al., 1991). Although all three α1-adrenoceptor subtypes have been detected in the prostate, the α1a-adrenoceptor subtype predominates in prostatic stromal tissue (Hiraoka et al., 1999; Walden et al., 1999). This was evidenced at RNA level (Hirasawa et al., 1993; Nasu et al., 1996; Price et al., 1993) as well as at the protein level and in smooth muscle contraction assays (Faure et al., 1994; Lepor et al., 1994; Marshall et al., 1995). Another pharmacologically distinct α1-adrenoceptor that mediates prostatic contraction has been suggested as a receptor with low affinity for prazosin (α1L-adrenoceptor) (Muramatsu et al., 1994); it may represent a functional phenotype of α1a-adrenoceptor (Daniels et al., 1999).
Contraction of the prostate increases intraurethral pressure and bladder outlet resistance (Furuya et al., 1982; Caine, 1990) and, in turn, relaxation of muscle tone by α-adrenergic antagonists has been shown to be effective in treating symptomatic benign prostatic hyperplasia (BPH) (Chapple, 1995; Kawabe & Niijima, 1987; Matyus & Horvath, 1997). On the other hand, the prostate is an androgen-dependent organ. Biological functions such as prostate fluid secretion and morphological characteristics are affected considerably by androgen deprivation (Cheng et al., 1993). This androgen dependency is a theoretical basis for clinical utility of antiandrogens or five α-reductase inhibitors in benign and malignant diseases of the prostate (Gormley et al., 1992).
It could, therefore, be hypothesized that the contractile properties of prostate smooth muscle cells under α-adrenergic stimulation might be affected by changes in androgen levels. This is clinically relevant in that an anti-androgen and an α-adrenergic antagonist may be simultaneously prescribed for BPH patients, which resulted in no synergy (Lepor et al., 1996) or modest synergy (Okada et al., 1996). However, the interaction between androgen deprivation and adrenergic contractile response of the prostate has been investigated in only a few studies (Akiyama et al., 1999; Lacey et al., 1996; Lin et al., 1995). The present study explored the effects of castration on the contractility and/or pharmacological characteristics of α-adrenoceptors in rat prostate. Receptor expression was evaluated using reverse transcription combined with polymerase chain reaction.
Methods
Animals
Male Sprague-Dawley rats weighing 300–500 g (Japan SLC Co., Shizuoka, Japan) were used throughout. Rats were housed in groups of four in a room maintained at 21–25°C and 45–65% humidity, controlled at an alternating 12 h light/dark cycle with lights on at 0700 h. Food and water were given ad libitum. Two weeks after bilateral orchidectomy under anaesthesia, the following studies were conducted.
Contraction study
Animals were killed and the ventral and dorsal lobes of the prostate removed. Two strips of about 2 mm in width and 5–10 mm in length were prepared from each lobe. Each strip was weighed and mounted in an organ bath containing 10 ml Krebs solution (mM: NaCl 111 KCl 5.9, MgCl2 1.2, CaCl2 2.5, NaHCO3 25.0, NaH2PO4 1.2, glucose 11.5), gassed with 95% O2 and 5% CO2, maintained at 37°C. The strip was stretched to a resting tension of 1 g and equilibrated for 60 min. Changes in tension were isometrically detected with a force-displacement transducer connected to a polygraph recorder (T7-30-240, Toyo Baldwin, Tokyo, Japan) via a strain-gauge pressure amplifier (AP-620G, Nihon Kohden, Tokyo, Japan) and recorded on a pen recorder (RJG-4024, Nihon Kohden). K+ (100 mM) induced contraction was obtained by exchange of Krebs solution to high K+ Krebs solution (mM: NaCl 17, KCl 100, MgCl2 1.2, CaCl2 2.5, NaHCO3 25.0, NaH2PO4 1.2, glucose 11.5). When maximal contraction was obtained, the strip was washed with normal Krebs solution until the tone returned to the basal level. The concentration-response curves were generated by cumulative addition of phenylephrine (0.01–1000 μM) and repeated for the same strip at 60 min intervals. The first curve was taken as the control response. For the inhibition study, prazosin (10 nM) was added to the bath 15 min before the initiation of phenylephrine application, and reproducibility of the curve was confirmed in the absence of antagonist. The maximal contraction was obtained from the concentration-response curve and the pEC50 value for phenylephrine was calculated from a regression line between logarithmic concentrations and logit-converted response rate based on the Hill equation by the least square method (Jenkinson et al., 1995). The pKB values for antagonists were determined according to the method of van Rossum (1963).
Alkylation of α-adrenoceptors
After a control concentration-response curve for phenylephrine had been determined, the strip was equilibrated with phenoxybenzamine at 3–300 nM for 10 min and then washed for 2 h in Krebs solution. During the first hour of washing, sodium thiosulphate (100 μM) was added to prevent further alkylation of α-adrenoceptors. After washing for 2 h, phenylephrine was applied cumulatively. Reproducibility of the response to phenylephrine without phenoxybenzamine treatment was confirmed in other strips. Maximal contraction and pEC50 values were determined as described above.
Receptor preparation and binding assay
Prostate tissue was minced into small pieces and homogenized in 20 volumes of ice-cold Tris-HCl buffer (50 mM, pH 7.4) with a Polytron homogenizer (Kinematica, Littau, Switzerland) for 30 s. The homogenate was centrifuged at 39,000×g for 20 min at 4°C. The pellet was suspended in 20 volumes of ice-cold buffer, and the suspension was centrifuged at 39,000×g for 20 min at 4°C. The pellet was then resuspended in 10 to 40 volumes of ice-cold buffer and the suspension was filtered through gauze. The filtrate was used for the binding assay. Protein concentration was determined by the method of Bradford (1976). The binding assay was performed at six different concentrations (in the range 0.02–3 nM) of [3H]-prazosin (2671.4 GBq mmol−1). The membrane preparation (0.4 ml) was incubated with [3H]-prazosin in the presence and absence of 10 μM phentolamine at 25°C for 30 min in a total volume of 0.5 ml. The reaction was terminated by the addition of 3 ml of ice-cold buffer and the reaction mixture was rapidly filtered under a vacuum through a glass-fibre filter (Whatman GF/B, Maidstone, U.K.) presoaked with 0.1% polyethyleneimine. Filtres were washed three times with 3 ml ice-cold buffer and then placed in vials with 10 ml scintillation fluid. Radioactivity was determined in a liquid scintillation counter (Tri-Carb4640, Packard, Downers Glove, IL, U.S.A.). The dissociation constant (Kd) and the binding capacity (Bmax) were obtained by non-linear regression analysis of logistic equation (De Lean et al., 1978). Inhibitory effects of phenylephrine on [3H]-prazosin (0.2 nM) binding to membrane preparations were examined at nine concentrations (in range 0.1–100 μM) of phenylephrine. IC50 values for phenylephrine were determined by regression analysis of logarithmic concentration versus logit-converted inhibition rate. Inhibition constant (Ki) was calculated by the method of Cheng & Prusoff (1973).
Determination of α1 adrenoceptor mRNAs by RT–PCR
Total cytoplasmic RNA was extracted from rat prostate tissues with ISOGEN and subjected to reverse transcription and quantitative competitive PCR. Briefly, 5.0 μg total RNA was converted to cDNA using SuperScript II reverse transcriptase in the presence of d (N) 6 random primers. To confirm that all samples contained equivalent amounts of RNA, total RNA from samples was separately co-amplified in the presence of serial dilutions of an RNA internal standard (mimic) prepared for β-actin. The β-actin RNA mimic shares the same primer template sequence but contains a smaller intervening sequence. Samples containing 5.0 μg of prostate total RNA were co-reverse-transcribed with 2 fold serial dilutions of β-actin mRNA. The β-actin DNA fragments that share the same primer template sequence with the target cDNA but contain a completely different, smaller intervening sequence were prepared and used as DNA internal standards. Aliquots of sample cDNA mixed with serial dilutions of DNA mimics were co-amplified as templates in the presence of 10.0 μmol μl−1 downstream and upstream primers for β-actin (downstream primers: 5′-CAG GAG ATG GCC ACT GCC GCA-3′, upstream primers: 5′-TCC TTC TGC ATC CTG TCA GCA-3′). The PCR mixture consisted of 2.5 mM of each deoxyribonucleotide, 10×ExTaq buffer and 0.015 units of ExTaq DNA polymerase. The DNA was denatured by heating at 95°C for 5 min, followed by 28 cycles of 30 s at 95°C, annealing at 56°C for 30 s and extension at 72°C for 1 min (Perkin-Elmer thermocycler). The amplified products were analysed by electrophoresis on 3.0% agarose gels followed by staining with ethidium bromide. The stained products were scanned and quantified, and all samples were prepared to contain 3 ng β-actin cDNA. To determine the amounts of α1 adrenoceptor mRNAs, samples were amplified as templates in the presence of 10.0 μmol μl−1 downstream and upstream primers for α1a, α1b, α1d (downstream primers; α1a: 5′-AGG CTG CTC AAG TTT TCTCG-3′, α1b: 5′-ATC CTG AGC CTA TGT GCC AT-3′-3′, α1d: 5′-CGC TGT GGT GGG AAC CGG CAG CGG A-3′, upstream primers; α1a: 5′-ACT TCC TCC CCG TTT TCACC-3′, α1b: 5′-TTG GTA CTG CTG AGG GTG TC-3′, α1d: 5′-ACA GCT GCA CTC AGT AGC AGG TCA-3′) in a PCR mixture. The DNA was denatured by heating at 95°C for 5 min, followed by 28 cycles of 30 s at 95°C, annealing at 56°C (α1d: 58°C) for 30 s and extension at 72°C for 1 min. This reaction was followed by a final elongation step for 7 min at 72°C. The amplified products were analysed by electrophoresis on 3.0% agarose gels, stained with ethidium bromide and quantified.
Drugs
Phenoxybenzamine (Nacalai Tesque Inc., Kyoto, Japan); [3H]-prazosin (New England Nuclear, Boston, MA, U.S.A.); ISOGEN (Nippon Gene, Toyama, Japan); SuperScript II reverse transcriptase (Gibco BRL); ExTaq DNA polymerase (TaKaRa, Shiga, Japan). All other compounds were purchased from Sigma, St Louis, MO, U.S.A.
Statistics
Results are expressed as mean±s.e.mean. Unpaired t-tests were used to assess the difference between intact and castrated rats. Multiple comparison of contraction variable values at various doses of phenoxybenzamine with control values was done with Dunnett's test. P values less than 0.05 by two-tailed analysis were considered significant.
Results
Castration caused a dramatic decrease in prostate weight from 525±37 mg (n=4) to 74±3 mg in ventral lobes, and 350±15 mg (n=4) to 115±10 mg in dorsal lobes, respectively. On the other hand, the maximal contraction force of prostatic strips provoked by 100 mM K+, which was adjusted for strip weight, was greater in castrated rats: 271% of control for ventral lobes and 157% for dorsal lobes (Table 1). Similarly, the phenylephrine-induced contraction force per mg tissue was higher in castrates than in controls: 341% of control for ventral lobes, and 200% for dorsal lobes, whereas pEC50 values for phenylephrine were slightly but significantly lowered in castrates (Table 2). The antagonist study of phenylephrine-induced contraction by prazosin revealed comparable pKB values for both prostatic lobes of castrated rats (Table 3). The representative contraction-response curves for phenylephrine with or without prazosin in ventral lobe strips are shown in Figure 1.
Table 1.
K+-induced contractions of rat prostatic strips

Table 2.
Phenylephrine-induced contractions of rat prostatic strips
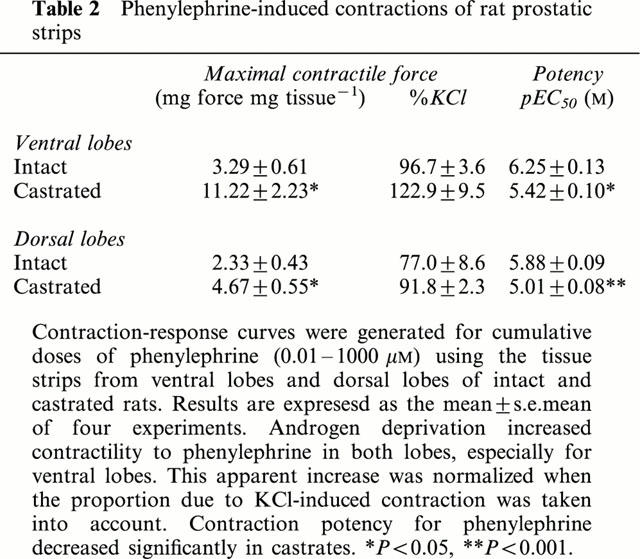
Table 3.
Antagonistic effects of prazosin on phenylephrine-induced contractions of rat prostate strips
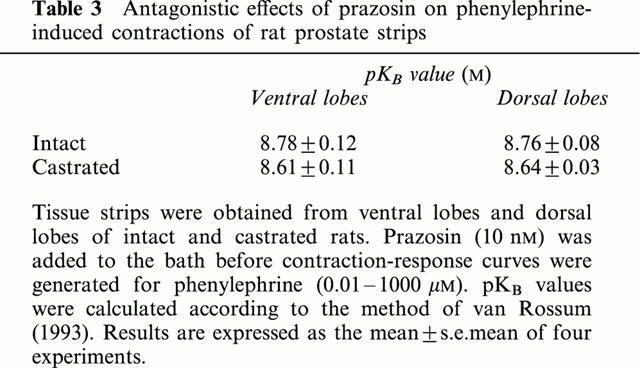
Figure 1.
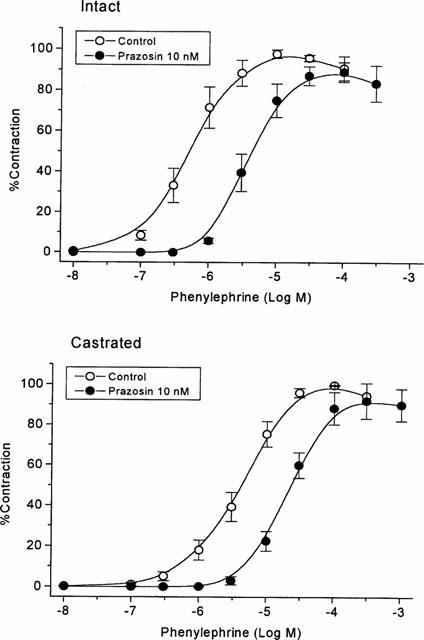
Contraction-response curves for phenylephrine with or without prazosin (10 nM) in isolated strips from prostate ventral lobes of intact and castrated rats. Each point and bar represents mean±s.e.mean of four experiments.
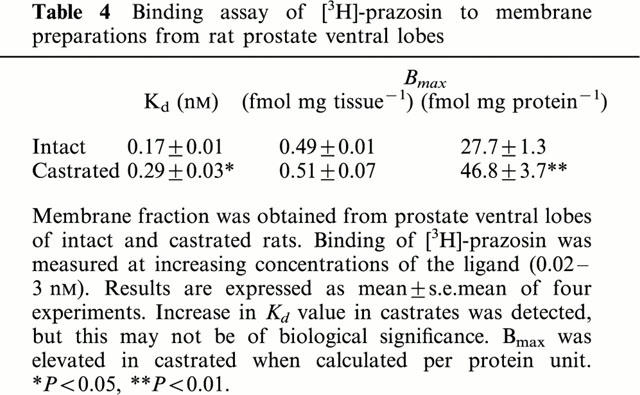
The characteristics of [3H]-prazosin binding to membrane preparations obtained from ventral lobes are shown in Table 4. Saturation curves of [3H]-prazosin binding appeared monophasic in tissue from both control and castrated rats (data not shown). The Kd value was significantly increased in castrated rats, although it would not be physiologically significant. An increase in Bmax values was also apparent when expressed per mg protein, but not on a tissue weight basis. The study of competitive inhibition by phenylephrine of [3H]-prazosin (0.2 nM) binding to membrane preparations gave pK1 values (M) for phenylephrine of 5.66±0.06 (n=4) and 5.67±0.09 (n=3) in intact and castrated rats, respectively.
Table 4.
Binding assay of [3H]-prazosin to membrane preparations from rat prostate ventral lobes
The effect of various doses of phenoxybenzamine pre-treatment on phenylephrine contraction-response curves are shown in Figure 2, and the related parameter values are summarized in Table 5. In intact rats, extending alkylation by phenoxybenzamine at 3–30 nM produced a decrease in pEC50 without a change in the maximal response size, and further extended alkylation at 100 nM and 300 nM suppressed the maximal response. Furthermore, the suppression of maximal response was more readily detected at the lower doses of phenoxybenzamine (30 nM) in castrated rats. In both intact and castrated rats, pEC50 values were relatively unchanged at lower doses of phenoxybenzamine and decreased when the suppression of maximal contraction started, but again stabilized despite further suppression by increasing doses of phenoxybenzamine.
Figure 2.
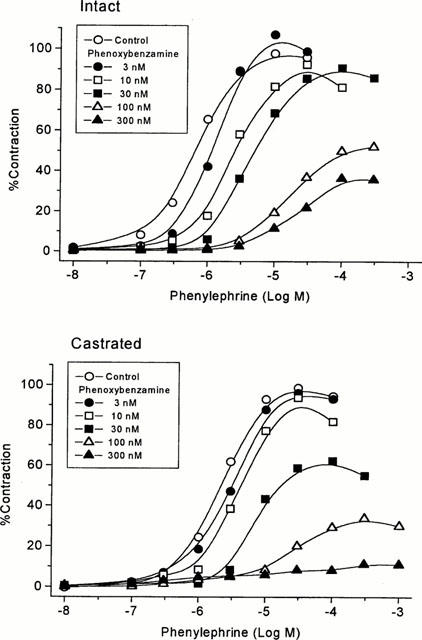
The effects of partial alkylation of α-adrenoceptor population on phenylephrine-induced contractions of isolated rat prostatic strips. Tissue strips from ventral lobes of intact and castrated rats were equilibrated with phenoxybenzamine at 3–300 nM for 10 min. After washing for 2 h, with sodium thiosulphate (100 μM) added during the first hour, contraction-response curves for phenylephrine were produced. Each point represents the mean of four to 20 experiments. Error bars are not shown for simplicity. Parameter values calculated from the curves are given in Table 5. A rightward shift of curves was observed before maximal contraction size was suppressed. The suppression started at lower doses of phenoxybenzamine in castrates.
Table 5.
Effect of phenoxybenzamine pre-treatment on phenylephrine-induced contractions in strips of rat prostate ventral lobes
The expression of mRNAs for α1 adrenoceptor subtypes in the prostate was measured by RT–PCR (Figure 3). RT–PCR using three different subtypes of α1 adrenoceptor primers resulted in a single band at the expected length. Three fold dilutions of DNA internal standards were used to determine the concentration range necessary to compete for the PCR amplification of reverse-transcribed sample cDNAs. Cycle number was also varied to determine the linear amplification range for sample cDNA and internal standard DNA. Each cDNA sample was prepared at a concentration of 3 ng μl−1 β-actin cDNA. The expression of adrenoceptor mRNA was reduced in castrated rats for α1a adrenoceptor, but not for α1b or α1d adrenoceptors. The reduction of α1a adrenoceptor expression was more pronounced in ventral lobes than in dorsal lobes.
Figure 3.
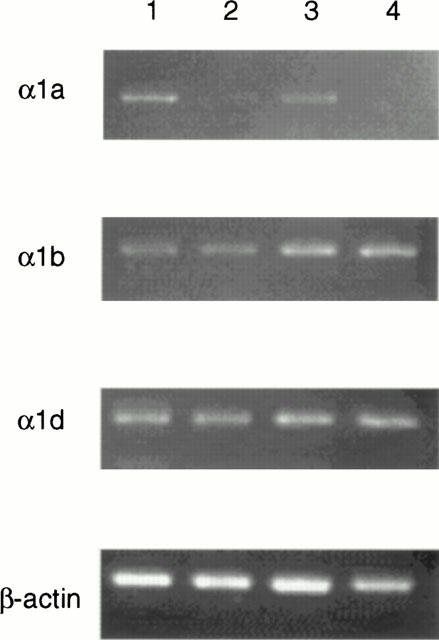
The effect of castration on α1-adrenoceptor mRNA expression in rat prostate. The expression of subtypes of α1-adrenoceptor mRNAs was evaluated by RT–PCR of total RNA from intact and castrated rat prostate using the primer set as described in Methods. In each panel PCR products using cDNA from rat prostate tissues as a template are shown. The band of specific primers for β-actin was used as a positive internal control, and assay without RNA or RT as a negative control. Lane 1; dorsal lobe of intact rat, lane 2; dorsal lobe of castrated rat, lane 3; ventral lobe of intact rat, lane 4; ventral lobe of castrated rat. Castration caused reduction in expression of α1a-adrenoceptor mRNA but not α1b or α1d. The change was more pronounced in ventral lobes.
Discussion
Both androgen receptors and α-adrenoceptors are present in the prostate, and their physiological, pathological and therapeutic implications have been extensively documented (Cheng et al., 1993; Furuya et al., 1982; Gormley et al., 1992; Lepor et al., 1987; Matyus & Horvath, 1997). In principle, secretory and proliferative activities of the prostate are regulated by androgens, whilst the contractile properties of stromal cells are mediated via α-adrenoceptors, with α1a-adrenoceptor predominating over other subtypes. There are, however, only a few reports of investigations on the interaction between prostate contractility and androgen level.
Using the rat castration model, Lacey et al. (1996) found an apparent 2 fold increase in prostatic α1-adrenoceptor density as a consequence of androgen deprivation in rats. These authors speculated that this increase was an artefact due to a relative increase in the ratio of smooth muscle, which is mostly responsible for prostatic contraction, to epithelium in the prostate rather than from up-regulation of α1-adrenoceptors, and they concluded that α1-adrenoceptors are not influenced by androgen deprivation. Lin et al. (1995) reported a similar observation in dogs: α-adrenergic contractility was increased in castrates, which was attributed to elevation in the relative proportion of stromal tissue by the hormonal intervention. These authors also found no discernible changes in in vivo measurement of prostatic urethral pressure as a result of androgen deprivation. Conversely, testosterone supplementation to rats resulted in subtype non-specific reduction in α1-adrenoceptor density of the prostate, and again the reduction was explained simply by the decreased proportion of fibromuscular element in the entire prostate (Auger-Pourmarin et al., 1998). However, castration dramatically reduced phenylpherine-induced increase in urethral pressure in rats (Akiyama et al., 1999), and a neurohormonal treatment with dihydrotestosterone and prazosin increased prostate weight, its stiffness and urodynamic obstruction by the prostate (Lee et al., 1998).
In our experiments, the effects of castration on α-adrenergic contractile response of the rat prostate were examined. Surgical castration resulted in a marked weight loss in both ventral and dorsal lobes of the prostate. Regarding contractile function, the maximal contractile response to K+ and phenylephrine expressed as force per unit of tissue weight was several fold greater in castrated rats. This apparent increase in contractility is most likely explained by the change in tissue component of the prostate (Lacey et al., 1996; Lin et al., 1995). Upon castration, prostate epithelium undergoes degeneration, whilst stromal elements, the compartment responsible for the contractile property of the prostate, are preserved. Thus, the contraction force is greater in castrates when expressed per unit weight. Interestingly, however, we found that contraction potency, or pEC50 values, for phenylephrine were lowered in castrated rats. This could not be explained by preferential degeneration of epithelium, or by lower binding affinity of α-adrenoceptors, since pKB values for prazosin in phenylephrine-induced contraction were not influenced by castration. Furthermore relatively high pKB values for prazosin (8.78), when compared with canine (7.94, Suzuki et al., 2000) or rabbit (8.15, Martin et al., 1997) prostate, would indicate that α1A-adrenoceptor rather than a putative subtype, α1L-adrenoceptor, is functionally important in rat prostate.
In the characterization and quantitative assay of α1-adrenoceptors of prostate membrane preparations, the Kd values for [3H]-prazosin binding were 0.17 and 0.29 nM in intact and castrated rats, respectively, although this difference would not be of biological significance. The apparent increase in Bmax values in castrates was also ambiguous because they were normalized to tissue weight or protein content, and these denominators were profoundly affected by the hormonal intervention. Inhibition assay of phenylephrine against [3H]-prazosin binding indicated almost identical pKi values for both treatment arms, although the functional data showing reduced sensitivity to phenylpherine by castration are more reliable and relevant to receptor affinity (Hieble et al., 1997).
These results indicate that castration caused no essential changes in the affinity of phenylephrine to α1-adrenoceptor. We then hypothesized that spare receptors for α1-adrenoceptor, which have been demonstrated in the prostate (Hiraoka et al., 1999), arteries (Fox & Friedman, 1987, Ruffolo & Yaden, 1984) and vas deferens (Fox & Friedman, 1987), would be present in intact rat prostate and might be down-regulated in castrated rats, explaining the lowered potency in castrates. To confirm this assumption, irreversible alkylation of α1-adrenoceptor was semi-quantitatively induced by phenoxybenzamine. Escalating alkylation of α1-adrenoceptor population from 3 nM to 300 nM produced a rightward shift in the contraction-response curves before depressing the maximal contractile force, and the suppression was readily detected at lower doses of phenoxybenzamine in castrated rats than in intact rats. The response characteristics to phenoxybenzamine treatment and the difference between intact and castrated rats may indicate that a receptor reserve for α1-adrenoceptor is present in intact prostate and that the reserve diminishes after castration. Subsequent RT–PCR study confirmed the expression of three types of α1-adrenoceptor, α1a, α1b and α1d-adrenoceptors, in rat prostate, as has been reported previously (Roskosh et al., 1994, Scofield et al., 1995). It was also shown that the expression of α1a-adrenoceptor, but not α1b- or α1d-adrenoceptors, was down-regulated by castration. Considering the predominance of α1a-adrenoceptor in the prostate in functional terms (Auger-Pourmarin et al., 1998), down-regulation of α1a-adrenoceptor may be of biological significance. To our knowledge this is the first report suggesting that spare receptors of α-adrenoceptors in the prostate are down-regulated by androgen deprivation and that the down-regulated spare receptors are α1a-adrenoceptors.
The clinical implication of the presence of spare receptors in the prostatic smooth muscle cells, and its androgen dependency, is unclear. As far as α-adrenoceptor blocking agents in clinical use are concerned, since they are not irreversible inhibitors, the presence or absence of spare receptors should not alter the efficacy of these drugs on the contractility of prostate smooth muscle cells. In turn, the efficacy of contractility by adrenoceptor agonists may be affected by androgen deprivation. If affected, the proportion of the two components accounting for symptoms in BPH patients, a static component due to compression by the increased prostate volume and a dynamic component related to contraction of prostate smooth muscle cells (Caine, 1990), is influenced by androgen level. For example, the dynamic component of infravesical obstruction by the prostate is less important in men with a lower androgen level, i.e., in older men or men receiving antiandrogen treatment. However, extrapolating rat studies to human may be misleading, since rat prostate contains far less stromal components compared to human hypertrophied prostatic tissue, and this might have accentuated the effects of castration in rats. Clinically there was no discernible effect of a 5α-reductase inhibitor on the therapeutic efficacy of an α1-adrenoceptor antagonist (Lepor et al., 1996).
In conclusion, androgen deprivation suppressed α1-adrenergic contractility of rat prostate strips, and this suppression was associated with down-regulation of receptor reserves for the α1a-adrenoceptor population. Further studies on androgen dependency of prostate contractility and the evaluation of its clinical relevance are warranted.
Abbreviations
- BPH
benign prostatic hyperplasia
- RT–PCR
reverse transcription combined with polymerase chain reaction
References
- AKIYAMA K., HORA M., TATEMICHI S., MASUDA N., NAKAMURA S., YAMAGISHI R., KITAZAWA M. KMD-3213, a uroselective and long-acting α1a -adrenoceptor antagonist, tested in novel rat model. J. Pharmacol. Exp. Ther. 1999;291:81–91. [PubMed] [Google Scholar]
- ANDERSSON K.-E., LARSSON B., SJOGREN C. Characterization of the alpha-adrenoreceptors in the female rabbit urethra. Br. J. Pharmacol. 1984;81:293–300. doi: 10.1111/j.1476-5381.1984.tb10078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUGER-POURMARIN L., ROUBERT P., CHABRIER P.E. α1-Adrenoceptors in testosterone-induced prostatic hypertrophy. Eur. J. Pharmacol. 1998;341:119–126. doi: 10.1016/s0014-2999(97)01432-5. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CAINE M. Alpha-adrenergic blockers for the treatment of benign prostatic hyperplasia. Urol. Clin. North Am. 1990;17:641–649. [PubMed] [Google Scholar]
- CHAPPLE C.R. Alpha-adrenergic blocking drugs in bladder outflow obstruction: what potential has alpha 1-adrenoceptor selectivity. Br. J. Urol. 1995;1:47–55. [PubMed] [Google Scholar]
- CHENG E., LEE C., GRAYHACK J.Endocrinology of the prostate Prostate Diseases 1993Philadelphia: Saunders; 57–71.ed. Lepor, H. & Lawson, R.K. pp [Google Scholar]
- CHENG Y., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- COTECCHIA S., SCHWINN D.A., RANDALL R.R., LEFKOWITZ R.J., CARON M.G., KOBILKA B.K. Molecular cloning and expression of the cDNA for the hamster α1-adrenergic receptor. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7159–7163. doi: 10.1073/pnas.85.19.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIELS D.V., GEVER J.R., JASPER J.R., KAVA M.S., LESNICK J.D., MELOY T.D., STEPAN G., WILLIAMS T.J., CLARKE D.E., CHANG D.J., FORD A.P.D.W. Human cloned alpha1A-adrenoceptor isoforms display alpha1L-adrenoceptor pharmacology in functional studies. Eur. J. Pharmacol. 1999;370:337–343. doi: 10.1016/s0014-2999(99)00154-5. [DOI] [PubMed] [Google Scholar]
- DE LEAN A., MUNSON P.J., RODBARD D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am. J. Physiol. 1978;40:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- FAURE C., PIMOULE C., VALLANCIEN G., LANGER S.Z., GRAHAM D. Identification of α1-adrenoceptor subtypes present in the human prostate. Life Sci. 1994;54:1595–1605. doi: 10.1016/0024-3205(94)90031-0. [DOI] [PubMed] [Google Scholar]
- FOX A.W., FRIEDMAN P.A. Evidence for spare α1-adrenoceptors for the accumulation of inositol phosphates in smooth muscle. J. Pharm. Pharmacol. 1987;39:68–71. doi: 10.1111/j.2042-7158.1987.tb07169.x. [DOI] [PubMed] [Google Scholar]
- FURUYA S., KUMAMOTO Y., YOKOYAMA E., TSUKAMOTO T., IZUMI T., ABIKO Y. Alpha-adrenergic activity and urethral pressure in prostatic zone in benign prostatic hypertrophy. J. Urol. 1982;128:836–839. doi: 10.1016/s0022-5347(17)53216-4. [DOI] [PubMed] [Google Scholar]
- GORMLEY G.J., STONE R.E., BRUSKEWITZ R.C., IMPERATO-MCGINLEY J., WALSH P.C., MCCONNELL J.D., ANDRIOLE G.L., GELLER J., BRACKEN B.R., TENOVER J.S., VAUGHAN E.D., PAPPAS F., TAYLOR A., BINKOWITZ B., NG J. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N. Engl. J. Med. 1992;327:1185–1191. doi: 10.1056/NEJM199210223271701. [DOI] [PubMed] [Google Scholar]
- HIEBLE J.P., HEHR A., LI Y.-O., NASELSKY D.P., RUFFOLO R.R., JRCharacterization of stereoselective interactions of catecholamines with the α2a-adrenoceptor via site directed mutagenesis α2-Adrenorgic Receptors Structure, Function and Therapeutic Implications 1997New York: Harwood; 43–51.eds Lanier, S.M. & Limbird, L.E. pp [Google Scholar]
- HIRAOKA Y., OHMURA T., OSHITA M., WATANABE Y., MORIKAWA K., NAGATA O., KATO H., TANIGUCHI T., MURAMATSU I. Binding and functional characterization of α1-adrenoceptor subtypes in the rat prostate. Eur. J. Pharmacol. 1999;366:119–126. doi: 10.1016/s0014-2999(98)00895-4. [DOI] [PubMed] [Google Scholar]
- HIRASAWA A., HORIE K., TANAKA T., TAKAGAKI K., MURAI M., YANO J., TSUJIMOTO G. Cloning, functional expression and tissue distribution of human cDNA for the α1c-adrenergic receptor. Biochem. Biophys. Res. Commun. 1993;195:902–909. doi: 10.1006/bbrc.1993.2130. [DOI] [PubMed] [Google Scholar]
- JENKINSON D.H., BARNARD E.A., HOYER D., HUMPHREY P.P.A., LEFF P., SHANKLEY N.P. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. IX. Recommendations on Terms and Symbols in Quantitative Pharmacology. J. Pharmacol. Exp. Ther. 1995;47:255–266. [PubMed] [Google Scholar]
- KAWABE K., NIIJIMA T. Use of an alpha 1-blocker, YM-12617, in micturition difficulty. Urol. Int. 1987;42:280–284. doi: 10.1159/000281956. [DOI] [PubMed] [Google Scholar]
- LACEY J.P., DONATUCCI C.F., PRICE D.T., PAGE S.O., BENNETT S.A.L., TENNISWOOD M.P., SCHWINN D.A. Effects of androgen deprivation on prostate α1-adrenergic receptors. Urology. 1996;48:335–341. doi: 10.1016/S0090-4295(96)00183-5. [DOI] [PubMed] [Google Scholar]
- LEE J.Z., OMATA S., TILLIG B., PERKASH I., CONSTANTINOU C.E. Chronology and urodynamic characterization of micturition in neurohormonally induced experimental prostate growth in the rat. Neurourol. Urodyn. 1998;17:55–69. doi: 10.1002/(sici)1520-6777(1998)17:1<55::aid-nau8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- LEPOR H., BAUMANN M., SHAPIRO E. Identification and characterization of alpha 1 adrenergic receptors in the canine prostate using [125I]-Heat. J. Urol. 1987;138:1336–1339. doi: 10.1016/s0022-5347(17)43594-4. [DOI] [PubMed] [Google Scholar]
- LEPOR H., WILLIFORD W.O., BARRY M.J., BRAWER M.K., DIXON C.M., GORMLEY G., HAAKENSON C., MACHI M., NARAYAN P., PADLEY R.J. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. Veterans affairs cooperative studies benign prostatic hyperplasia study group. N. Engl. J. Med. 1996;335:533–539. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- LEPOR H., ZHANG W., KOBAYASHI S., TANG R., WANG B., SHAPIRO E. A comparison of the binding and functional properties of alpha-1 adrenoceptors and area density of smooth muscle in the human, canine and rat prostates. J. Pharmacol. Exp. Ther. 1994;270:722–727. [PubMed] [Google Scholar]
- LIN A.T.-L., CHEN M.-T., CHIANG H., YANG C.-H., CHANG L.S. Effect of orchiectomy on the alpha adrenergic contractile response of dog prostate. J. Urol. 1995;154:1930–1933. doi: 10.1016/s0022-5347(01)66828-9. [DOI] [PubMed] [Google Scholar]
- LOMASNEY J.W., COTECCHIA S., LORENZ W., LEUNG W.Y., SCHWINN D.A., YANG-FENG T.L., BROWNSTEIN M., LEFKOWITZ R.J., CARON M.G. Molecular cloning and expression of the cDNA for the α1A-adrenergic receptor - the gene for which is located on human chromosome 5. J. Biol. Chem. 1991;266:6365–6369. [PubMed] [Google Scholar]
- MARSHALL I., BURT R.P., CHAPPLE C.R. Noradrenaline contractions of human prostate mediated by α1A-(α1C-) adrenoceptor subtype. Br. J. Phamacol. 1995;115:781–786. doi: 10.1111/j.1476-5381.1995.tb15001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN D.J., LLUEL P., GUILLOT E., COSTE A., JAMMES D., ANGEL I. Comparative alpha-1 adrenoceptor subtype selectivity and functional uroselectivity of alpha-1 adrenoceptor antagonists. J. Pharmacol. Exp. Ther. 1997;282:228–235. [PubMed] [Google Scholar]
- MATYUS P., HORVATH K. α-Adrenegic approach in the medical management of benign prostatic hyperplasia. Med. Res. Rev. 1997;17:523–535. doi: 10.1002/(sici)1098-1128(199711)17:6<523::aid-med2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- MURAMATSU I., OSHITA M., OHMURA T., KIGOSHI S., AKINO H., GOBARA M., OKADA K. Pharmacological characterization of α1-adrenoceptor subtypes in the human prostate: Functional and binding studies. Br. J. Urol. 1994;74:572–578. doi: 10.1111/j.1464-410x.1994.tb09186.x. [DOI] [PubMed] [Google Scholar]
- NASU K., MORIYAMA N., KAWABE K., TSUJIMOTO G., MURAI M., TANAKA T., YANO J. Quantification and distribution of alpha 1-adrenoceptor subtype mRNAs in human prostate: comparison of benign hypertrophied tissue and non-hypertrophied tissue. Br. J. Pharmacol. 1996;119:797–803. doi: 10.1111/j.1476-5381.1996.tb15742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA H., KAWAIDA N., OGAWA T., ARAKAWA S., MATSUMOTO O., KAMIDONO S. Tamsulosin and chlormadinone for the treatment of benign prostatic hyperplasia. The Kobe University YM617 Study Group. Scand. J. Urol. Nephrol. 1996;30:379–385. doi: 10.3109/00365599609181314. [DOI] [PubMed] [Google Scholar]
- PRICE D.T., SCHWINN D.A., LOMASNEY J.W., ALLEN L.F., CARON M.G., LEFKOWITZ R.J. Identification, quantification, and localization of mRNA for three distinct alpha 1 adrenergic receptor subtypes in human prostate. J. Urol. 1993;150:546–551. doi: 10.1016/s0022-5347(17)35544-1. [DOI] [PubMed] [Google Scholar]
- ROBERTS J.M., INSEL P.A., GOLDFIEN A. Regulation of myometrial adrenoreceptors and adrenergic response by sex steroids. Mol. Pharmacol. 1981;20:52–58. [PubMed] [Google Scholar]
- ROSKOSH D.G., BAILEY B.A., STEWART A.F.R., KARNS L.R., LONG C.S., SIMPSON P.C. Distribution of α1C-adrenergic receptor mRNA in adult rat tissues by RNase protection assay and comparison with α1B and α1D. Biochem. Biophys. Res. Commun. 1994;200:1177–1184. doi: 10.1006/bbrc.1994.1575. [DOI] [PubMed] [Google Scholar]
- RUFFOLO R.R., JR, YADEN E.L. Existence of spare α1-adrenoceptors, but not α2-adrenoceptors, for the respective vasopressor effects of cirazoline and B-HT 933 in pithed rat. J. Cardiovasc. Pharmacol. 1984;6:1011–1019. [PubMed] [Google Scholar]
- SCHWINN D.A., LOMASNEY J.W., LORENZ W., SZKLUT P.J., FREMEAU R.T., JR, YANG-FENG T.L., CARON M.G., LEFKOWITZ R.J., COTECCHIA S. Molecular cloning and expression of the cDNA for a novel α1-adrenergic receptor subtype. J. Biol. Chem. 1990;265:8183–8189. [PubMed] [Google Scholar]
- SCOFIELD M.A., LIU F., ABEL P.W., JEFFRIES W.B. Quantification of steady state expression of mRNA for alpha-1 adrenergic receptor subtypes using reverse transcription and a competitive polymerase chain reaction. J. Pharmacol. Exp. Ther. 1995;275:1035–1042. [PubMed] [Google Scholar]
- SUZUKI Y., KANADA A., OKAYA Y., AISAKA K. Effect of JTH-601, a novel α1-adrenoceptor antagonist, on prostate function in dogs. Eur. J. Pharmacol. 2000;394:123–130. doi: 10.1016/s0014-2999(00)00159-x. [DOI] [PubMed] [Google Scholar]
- VAN ROSSUM J.M. Cumulative dose-response curves. II. Technique for making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch. Int. Pharmacodyn. 1963;143:299–330. [PubMed] [Google Scholar]
- WALDEN P.D., GERARDI C., LEPOR H. Localization and expression of the alpha1A-1, alpha1B and alpha1D-adrenoceptors in hyperplastic and non-hyperplastic human prostate. J. Urol. 1999;161:635–640. [PubMed] [Google Scholar]


