Abstract
Since nonsteroidal anti-inflammatory drugs (NSAIDs) may impair the ability of the chondrocyte to repair its damaged extracellular matrix, we explored the changes in the metabolism of newly synthesized proteoglycan and hyaluronan (HA) molecules produced by aceclofenac, diclofenac and meloxicam in human osteoarthritic (OA) cartilage.
Explants were sampled from the medial femoral condyle and were classified by use of the Mankin's histological-histochemical grading system. Cartilage specimens exhibited moderate (M) OA in 20 subjects and had severe (S) OA in 20.
Cartilage explants were pulsed with [-3H]-glucosamine and chased in the absence or in the presence of 0.3–3 μg ml−1 of either aceclofenac, diclofenac or meloxicam. After papain digestion, the labelled chondroitin sulphate ([-3H]-proteoglycans) and [-3H]-HA molecules present in the tissue and media were purified by anion-exchange chromatography.
In cartilage with MOA and SOA, the metabolic balance of proteoglycan and HA was unaffected by diclofenac. In contrast, and in a dose-dependent manner, aceclofenac and meloxicam both increased the synthesis of proteoglycans and HA in explants with MOA and SOA; these two NSAIDs also reduced significantly the net loss of [-3H]-proteoglycans and [-3H]-HA molecules from cartilage explants.
The data obtained in short-term in vitro cultures indicate that, at the concentrations found in synovial fluid, aceclofenac and meloxicam may exert a favourable effect on the overall metabolism of proteoglycans and HA in cartilage with MOA and SOA.
Keywords: Nonsteroidal anti-inflammatory drugs, diclofenac, aceclofenac, meloxicam, proteoglycan, hyaluronan, osteoarthritic cartilage
Introduction
In the abundant extracellular matrix of articular cartilage, many polyanionic proteoglycan molecules bind non-covalently and with high affinity to a single filamentous molecule of hyaluronan (HA) to form huge multimolecular aggregates which then become unable to diffuse out of the collagenous meshwork (Hardingham, 1999). It is the presence of the large proteoglycan aggregates firmly entrapped within the collagenous meshwork that creates a high fixed charge density and gives articular cartilage its load bearing properties. Proteoglycan aggregation also increases dramatically the rheological properties of proteoglycan molecules and, in so doing, affects the dynamic behaviour of cartilage in compression (Hardingham et al., 1987). Therefore, any decrease in the cartilage concentration of proteoglycans and HA, as occurs in osteoarthritis (OA), compromises the functional properties of cartilage.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely prescribed in patients suffering from arthritides and it is the inhibition of cyclo-oxygenase (COX), and hence the inhibition of prostaglandin (PG) production, that accounts at least in part for the anti-inflammatory properties of these drugs (Vane, 1971). Two isoforms of COX have been identified thus far: COX-1 which is constitutively expressed in most tissues and COX-2 which is highly inducible in response to proinflammatory cytokines and mitogens (as reviewed in Smith et al., 1996). It is generally believed that the beneficial effects of NSAIDs are related to their ability to inhibit COX-2 whereas the gastrointestinal and renal toxicity of these drugs results from their inhibition of COX-1 (Vane, 1994; Warner et al., 1999), a contention that has provided the basis for the development of highly selective COX-2 inhibitors. It should be however pointed out that COX-1-derived PGs can contribute to the inflammatory response (Gilroy et al., 1998; Wallace et al., 1998) and that COX-2-derived PGs perform physiologically important roles such as the maintenance of normal renal function (Dinchuk et al., 1995; Morham et al., 1995) and the regulation of the female reproductive system (Lim et al., 1997). Furthermore, COX-2-derived PGs have been implicated in the protection of the gastrointestinal tract from injury (Schmassmann et al., 1998; Gretzer et al., 1998) and also might have anti-inflammatory properties (Gilroy et al., 1999).
Although NSAIDs undeniably produce relief of pain and improvement of joint mobility in patients suffering from arthritides, the use of these drugs might be detrimental to the joints since effective pain relief could lead to overuse of a disabled joint. Further, ex vivo and in vivo studies have shown that some NSAIDs inhibit the synthesis of cartilage proteoglycans whereas other do not (Brandt, 1987; Howell et al., 1991; Rainsford et al., 1997; Dingle, 1999). This differential effect of NSAIDs on cartilage metabolism is most relevant to clinical practice since any drug, that suppresses proteoglycan synthesis and impairs the chondrocyte to repair its damaged extracellular matrix, could potentially accelerate the breakdown of the cartilage tissue. On the other hand, although HA plays a central structural role in the supramolecular organization of proteoglycan and, hence on the biomechanical properties of articular cartilage, the possible effects of NSAIDs on the metabolism of this glycosaminoglycan has so far focused little investigative attention (Manicourt et al., 1994).
Aceclofenac is a phenylacetic acid derivative and meloxicam is an acidic enolic derivative that is moderately selective for COX-2 (Warner et al., 1999). Although these two recently marketed NSAIDs display good efficacy and tolerability in therapy for rheumatic disorders (Hunter et al., 1996; Distel et al., 1996), knowledge of their possible effects on the metabolism of articular cartilage is still fragmentary (Rainsford et al., 1997; Dingle, 1999). We therefore investigated the action of the two drugs on the metabolism of newly synthesized HA and proteoglycan molecules in explant cultures from human OA cartilage. Results were compared with those obtained with diclofenac, a non-selective COX inhibitor (Warner et al., 1999).
Methods
Materials
[-3H]-glucosamine was from Amersham Pharmacia Biotech (Roosendaal, The Netherlands). Culture plates and media were from Gibco BRL (Merelbeke, Belgium). Dialysis membranes (mol. wt. cut-off: 3500) were from Spectrum (Los Angeles, CA, U.S.A.). Streptomyces hyaluronidase, chondroitinase ABC, hyaluronan, twice crystallized papain and diclofenac were from Sigma-Aldrich (Bornem, Belgium). Econo-Pac Q cartridges were from Bio-Rad (Nazareth, Belgium). Aceclofenac was kindly provided by UCB Pharma (Brussels, Belgium) whereas meloxicam (4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H-1,2-benzthiazine-3-carboxamide1, 1 dioxide) was a gift from Boehringer Ingelheim (Brussels, Belgium). All other reagents were from Merck (Darmstadt, Germany).
Sampling and evaluation of cartilage tissue
Cartilage was obtained from knee joints of 40 patients undergoing arthroplasty for OA. Three weeks before surgery, NSAIDs were stopped, the patients being allowed to take paracetamol and/or dextropropoxyphen HCl as needed. Exclusion criteria were infections, articular injection of steroids within 2 months before surgery, immobilization for several weeks as well as known hereditary or congenital defects.
Joint pieces were immediately soaked into sterile phosphate-buffered saline (PBS) solution and transported to culture facilities. Full thickness cartilage was sampled from the medial femoral condyle and the cartilage from osteophytes was avoided. For each individual, three cartilage slices were taken at random, fixed (PBS containing 10% formalin and 5% cetylpyridinium chloride) and embedded in paraffin prior to slicing. After staining with either Safranin-O, Toluidine blue or hematoxylin and eosin, cartilage slices were examined for the severity of the OA disease process according to Mankin's grading system (Mankin et al., 1971) which explores the structure (grade 0 for normality; 1 for surface irregularities; 2 for pannus and surface irregularities; 3 for clefts to transitional zone; 4 for clefts to radial zone; 5 for deeper clefts; and 6 for complete disorganization), the cellularity (grade 0 for normality; 1 for diffuse hypercellularity; 2 for cloning; and 3 for hypo-cellularity) as well as the intensity of safranin-O-staining (grade 0 for normality; 1 for slight reduction; 2 for moderate reduction; and 3 for severe reduction). Since the subchondral bone was not harvested, the integrity of the tide-mark (grade 0 for normality and 1 for vascular invasion) was not taken into account.
Mankin's grading system ranged from 2–5 in 20 individuals (age range: 51–70 years) who were classified as having moderate (M) OA whereas the grading system ranged from 6–9 in the 20 other patients (age range: 52–72) who were classified as having severe (S) OA. The median age was similar in both groups (63 versus 62 years). Since the total amount of cartilage obtained from each patient was insufficient to conduct pulse and chase studies in the presence of different concentrations of NSAIDs, patients with MOA as well as patients with SOA were randomly distributed into two subgroups (n=10, each), the pulse studies being carried out in one subgroup and the chases studies in the other subgroup.
General culture procedures
Tissue specimens obtained from each donor were cut into pieces of 3–6 mg in Dulbecco's modified Eagle's medium (DMEM) supplemented with penicillin (5000 i.u. ml−1) and streptomycin (5000 μg ml−1). The tissue was washed several times with this medium and aspirated free from liquid. Cartilage pieces were taken at random, weighted and distributed into the different wells of multiwell culture plates (typically between 30–60 mg tissue - well). Extra pieces were not cultured but lyophilized in order to assess the initial content in collagen, proteoglycans and HA of the cartilage. Culture medium supplemented with 20% v v−1 foetal calf serum (culture medium A) was then added to each well and the culture plates were incubated for 48 h at 37°C.
For each experiment the cartilage from one individual was used and tissue cultures were conducted in triplicate: that is, for the control culture as well for each NSAID concentration, three cartilage explants were cultured separately. Reported values are the mean of the triplicate cultures.
Pulse studies
After two days of culture, the culture medium was aspirated and the explants were washed three times with 1 ml of DMEM. Explants were resuspended in culture medium A (1 ml–50 mg tissue) supplemented with [-3H]-glucosamine (50 μCi ml−1) (culture medium B). To each well, a solution of NSAID dissolved in dimethylsulphoxide (DMSO) was added (10 μl ml−1 culture medium) to achieve the concentrations of 0.3, 1, and 3 μg ml−1. Control cultures received DMSO that did not contain any NSAID (10 μl ml−1). Culture wells were then incubated for 12 h.
Chase studies
After 2 days of culture in medium A, the cartilage pieces were aspirated free of medium, washed three times with 1 ml of DMEM, resuspended in culture medium B (1 ml–50 mg tissue) and cultured for 12 h. After pulse labelling, the cartilage pieces were washed with DMEM and resuspended in culture medium A. DMSO alone or the appropriate NSAID dissolved in DMSO was added to each well (10 μl ml−1) as stated above and a non-radioactive chase period was conducted for 24 h.
Isolation and purification of proteoglycans and hyaluronan
At the end of the pulse labelling and non-radioactive chase periods, the culture media were removed and the cartilage pieces were washed with 0.15 M sodium chloride, 0.05 M sodium acetate, pH 6.0 (buffer A). Media and corresponding washes were combined. Bovine nasal proteoglycan monomers (500 μg ml−1) and HA (10 μg ml−1) were added to the mixtures which were then dialysed against buffer A before being incubated with papain (10 μg ml−1) for 24 h at 60°C. Cartilage specimens were lyophilized to obtain their dry weight and then resuspended in buffer A. Papain was added to each vial (0.1 mg ml−1) and the tissues were digested for 24 h at 60°C. Digests of cartilage and media were aliquoted. Samples were subjected either to biochemical determinations or to ion-exchange chromatography on Econo-Pac Q cartridge as previously reported (Manicourt et al., 1994).
Typically, two [-3H]-radiolabelled peaks were eluted from the Econo-Pac column: peak A at 0.23 M NaCl and peak B at about 1 M NaCl. Fifty to 70 per cent of the material present in peak A was sensitive to streptomyces hyaluronidase and thus identified to [-3H]-HA whereas the radiolabelled material present in peak B was identified as chondroitin sulphate, and thus as [-3H]-proteoglycan, since it was resistant to digestion with streptomyces hyaluronidase and completely digested by chondroitinase ABC.
The rates of biosynthesis of HA and proteoglycans were determined by the summation of [-3H]-HA and [-3H]-proteoglycan disintegrations per min. (d.p.m.) found in papain-digested tissues and media at the end of the 12-h pulse period.
At the end of the 24-h non-radioactive chase period, the total incorporation of [-3H]-glucosamine into HA and proteoglycans was determined by the summation of [-3H]-HA and [-3H]-proteoglycans d.p.m. found in the media and corresponding papain-digested tissue specimens. The radiolabelled material that accumulated in the medium during this non-radioactive chase period represents not only degraded HA and proteoglycan molecules but also intact HA and proteoglycan molecules that were being synthesized at the end of the pulse period and that were not incorporated into the matrix and lost in the medium during the subsequent chase period. Since the material present in the chase medium was not characterized in the present study, it is difficult to assess the respective proportion of these two processes and, accordingly, the radiolabelled material recovered in the medium during the 24-h chase period was described as net loss rather than catabolism. This net loss was expressed as the percentage of total incorporated d.p.m. found in the medium samples of the 24-h period.
Analytical methods
Hydroxyproline (OH-pro) was determined by the method of Woessner (1961) and hexuronate by the method of Bitter & Muir (1962). HA was quantified by a specific enzyme-linked immunosorbent assay (Li et al., 1989).
Statistics
The statistical significance of the differences observed between the group of MOA and the group of SOA was evaluated by the Mann–Whitney U-test whereas, in each group, the significance of the differences in HA and proteoglycan metabolism in the presence of different NSAID concentrations were evaluated by the Wilcoxon signed rank test. P values <0.05 were considered as statistically significant.
Results
Biochemical characterization of cartilage explants
Values found for the content of OH-pro, hexuronate and HA in cartilage explants from the two groups are given in Figure 1. In close agreement with previous studies (Mankin et al., 1971; Muir, 1986), the groups with MOA and SOA had a similar median content of OH-pro (70.5 versus 66.5 μg mg−1 tissue dry weight, respectively; P=0.1718). On the other hand, as reported previously (Mankin et al., 1971) the median content of hexuronate, and thus of proteoglycan, was higher in the group with MOA than in the group with SOA (41.5 versus 31.5 μg mg−1 tissue dry weight, respectively, P<0.0001). The group with MOA also had a higher content of HA (0.47 versus 0.83 μg mg−1 tissue dry weight, respectively; P<0.0001) and this in close agreement with a previous report (Manicourt et al., 1994). This overall cartilage chemistry was not significantly affected by standard culture conditions and treatment with the NSAIDs examined over the 72-h period of culture (results not shown).
Figure 1.
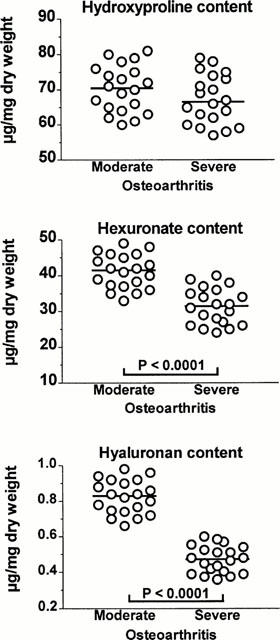
Distribution of values found for the hydroxyproline content, the total hexuronate (and thus proteoglycan) content and the total hyaluronan content of cartilage explants with moderate and severe osteoarthritis that were cultured in the absence of nonsteroidal anti-inflammatory drugs. The horizonal line present in each column scatter corresponds to the median value. The Mann–Whitney U-test disclosed that the two groups had a similar median content of hydroxyproline (U=149; P=0.1718) but differed in their median content of hexuronate (U=34; P<0.0001) and in their median content of hyaluronan (U=0; P<0.0001).
Metabolic characterization of cartilage explants in the absence of NSAIDs
Since cartilage specimens with MOA and SOA had a similar OH-pro content and since the loss of OH-pro from tissue specimens into the medium over a 72-h culture period was less than 5% of the amount present in cartilage pieces before culture, the rates of proteoglycan and HA biosynthesis were expressed as d.p.m. [-3H]-HA and [-3H]-proteoglycan per h and per mg OH-pro. Values were distributed over a wide range in the two groups (Figure 2, upper panels). The group with MOA and the group with SOA had a similar median rate of proteoglycan synthesis (P=1). There was also no statistically significant difference in the median rate of HA synthesis between the two groups (P=0.395).
Figure 2.
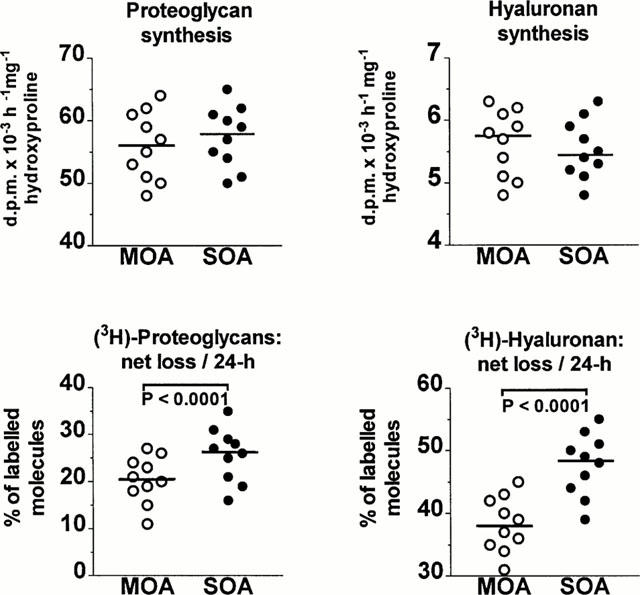
Cartilage explants with moderate (M) and severe (S) osteoarthritis (OA) were cultured in the absence of nonsteroidal anti-inflammatory drugs. The distribution of values found for the rates of proteoglycan and hyaluronan (HA) synthesis is illustrated in the upper panels whereas the distribution of values found for the net loss of newly synthesized proteoglycan and HA molecules over a 24-h non-radioactive chase period is given in the lower panels. The horizontal line present in each column scatter corresponds to the median value. The Mann–Whitney U-test disclosed that the groups with MOA and SOA had a similar median rate of proteoglycan synthesis (56 versus 58, respectively; U=43, P=1) as well as a similar median rate of HA synthesis (5.8 versus 5.5, respectively; U=44.5, P=0.395). On the other hand, during the 24-h non-radioactive chase period, the median net loss of labelled proteoglycans from cartilage tissue was higher in the group with SOA (26.5 versus 20.5, respectively; U=23, P<0.0001); the group with SOA also exhibited a higher median net loss of labelled HA molecules (48.5 versus 38; U=8, P<0.0001).
The net loss of [-3H]-proteoglycan and [-3H]-HA molecules from radiolabelled cartilage explants (Figure 2, lower panels) was also distributed over a wide range of values in the two groups. The median loss of [-3H]-proteoglycans was significantly higher in the group with SOA than in the group with MOA (P<0.0001). Likewise, the group with SOA had a higher median loss of [-3H]-HA than the group with MOA (P<0.0001).
These wide variations in metabolism that, in each group, were exhibited by the cartilage explants cultured in the absence of drug were likely to hamper the assessment of the effect of NSAIDs on proteoglycan and HA metabolism. Therefore, for each cartilage specimen of the two groups, the rates of proteoglycan and HA metabolism that were obtained in the presence of different concentrations of NSAID were divided by the values observed in the absence of drug to yield percentage changes.
Effects of NSAIDs on the total synthesis of proteoglycan and HA
In both groups, diclofenac at the three concentrations tested (Figure 3, upper left panel) as well as aceclofenac at a concentration of 0.3 μg ml−1 (Figure 3, middle left panel) and meloxicam at the concentrations of 0.3 and 1 μg ml−1 (Figure 3, lower left panel) did not change significantly the total amounts (tissue and medium) of newly synthesized proteoglycan molecules. In contrast, a significant increase in proteoglycan synthesis was observed when cartilage explants from two groups were incubated with either aceclofenac at the concentrations of 1 and 3 μg ml−1 or with meloxicam at a concentration of 3 μg ml−1 (P=0.002). In each group, the increase in proteoglycan synthesis was always stronger when explants were incubated with aceclofenac than with meloxicam (P=0.002). Further comparison between explants with MOA and SOA disclosed that the increase in proteoglycan synthesis was significantly higher in explants with less advanced OA lesions at a meloxicam concentration of 3 μg ml−1 (P<0.001) and at an aceclofenac concentration of 1 μg ml−1 (P<0.001) and 3 μg ml−1 (P<0.001).
Figure 3.
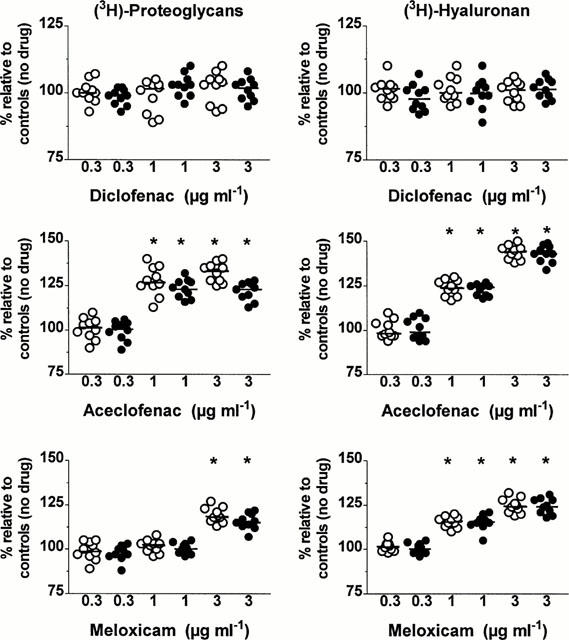
Effects of different concentrations (μg ml−1 of diclofenac, aceclofenac and meloxicam on the total amounts (tissue+medium) of newly synthesized proteoglycan and hyaluronan molecules in cartilage explants with moderate (open circles) and severe (closed circles) osteoarthritis. Results are expressed as the negative percentage (%) of values observed in corresponding explants cultured in the absence of drug. The horizontal line present in each column scatter corresponds to the median value. *P: comparison by the Wilcoxon signed-rank test.
In explants with MOA and SOA, the total amounts of newly synthesized HA molecules were unaffected by diclofenac at a concentration of 0.3, 1 and 3 μg ml−1 (Figure 3, upper right panel) as well as by 0.3 μg ml−1 of either aceclofenac (Figure 3, middle right panel) or meloxicam (Figure 3, lower right panel). On the other hand, at the concentrations of 1 and 3 μg ml−1, both aceclofenac and meloxicam enhanced HA synthesis in a relatively dose-dependent manner in the two groups (P=0.002). The increase in HA synthesis observed at these two concentrations was however stronger with aceclofenac than with meloxicam (P=0.002) and this in the two groups. No statistically significant difference in the increase of HA synthesis could be disclosed between the two groups at the concentrations of 1 and 3 μg ml−1 of either aceclofenac or meloxicam.
Effect of NSAIDs on the relative amounts of newly synthesized proteoglycan and HA molecules incorporated within cartilage matrix
During the pulse studies conducted in the absence of drug, a relative proportion of newly synthesized [-3H]-proteoglycan and [-3H]-HA molecules were not incorporated into the matrix and were lost into the culture medium. Further, in both groups with MOA and SOA, this relative loss of radiolabelled molecules varied from one donor to another. Therefore, in each experiment, the amount of [-3H]-proteoglycans and [-3H]-HA (as expressed in d.p.m. h–1 mg−1 of OH-pro) found in the explants at the end of the pulse-labelling period conducted in the presence of different concentrations of NSAIDs were divided by the amounts observed in the control explants pulse-labelled in the absence of drug to yield percent changes.
The changes in the tissue content of [-3H]-proteoglycans obtained at each NSAID concentration are illustrated in Figure 4. In the two groups, the relative amounts [-3H]-proteoglycan molecules remaining in cartilage matrix were unaffected by diclofenac over the range of concentrations studied whereas a significant increase was already observed with both aceclofenac and meloxicam at a concentration of 1 μg ml−1. The increase induced by these two NSAIDs became even stronger at a concentration of 3 μg ml−1.
Figure 4.
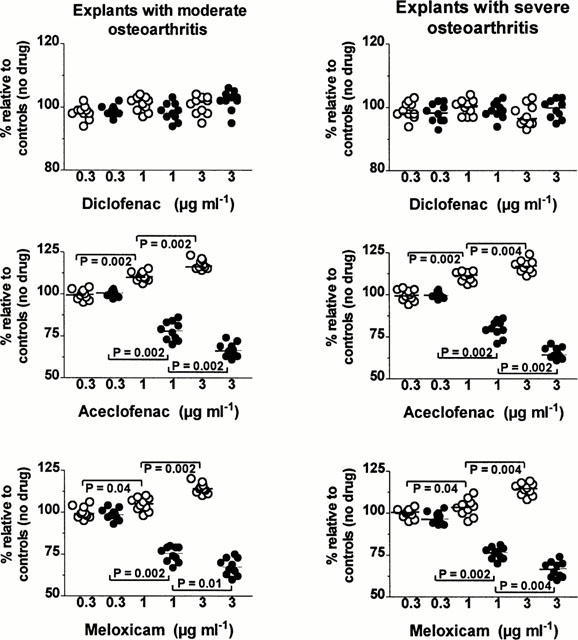
Effects of different concentrations (μg ml−1) of diclofenac, aceclofenac and meloxicam on the net loss of labelled proteoglycans from the tissue during the 24-h non-radioactive chase period (closed circles) and on the relative amounts of newly synthesized proteoglycan (open circles) molecules remaining within the matrix at the end of the pulse labelling period in cartilage explants with moderate (left panels) and severe (right panels) osteoarthritis. Results are expressed as the relative percentage (%) of values observed in corresponding explants cultured in the absence of drug. The horizontal line present in each column scatter corresponds to the median value. P: comparison by the Wilcoxon signed-rank test.
In explants with MOA, the tissue levels of [-3H]-proteoglycans were higher in the presence of aceclofenac than in the presence of meloxicam at the concentrations of 1 μg ml−1 (P=0.0195) and 3 μg ml−1 (P=0.0137). At the end of the pulse labelling period of explants with SOA, the specimens bathed with aceclofenac had also a higher tissue content in [-3H]-proteoglycans than the specimens exposed to meloxicam at a concentration of either 1 μg ml−1 (P=0.0137) or 3 μg ml−1 (P=0.0078).
The changes in the tissue content of [-3H]-HA observed at each NSAID concentration are shown in Figure 5. In the two groups, 1 μg ml−1 of either aceclofenac or meloxicam had already produced a significant increase in the tissue content of [-3H]-HA and this increase became even stronger at a concentration of 3 μg ml−1. Further, in explants with SOA, the tissue content in [-3H]-HA observed at the end of the pulse labelling period was significantly higher in specimens incubated with aceclofenac than in specimens bathed with meloxicam at a concentration of 3 μg ml−1 (P=0.0156). On the other hand, over the range of concentrations tested, diclofenac did not change significantly the amount of [-3H]-HA molecules remaining within the matrix of cartilage specimens with MOA and SOA.
Figure 5.
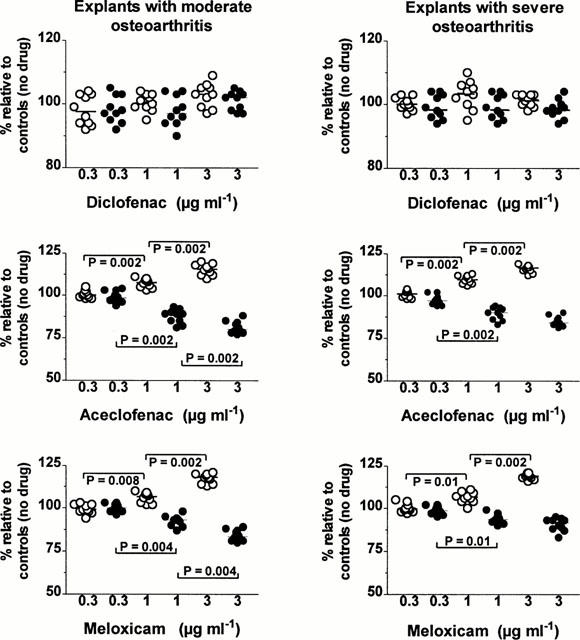
Effects of different concentrations (μg ml−1) of diclofenac, aceclofenac and meloxicam on the net loss of labelled hyaluronan from the tissue during the 24-h non-radioactive chase period (closed circles) and on the relative amounts of newly synthesized hyaluronan (open circles) molecules remaining within the matrix at the end of the pulse labelling period in cartilage explants with moderate (left panels) and severe (right panels) osteoarthritis. Results are expressed as the relative percentage (%) of values observed in corresponding explants cultured in the absence of drug. The horizontal line present in each column scatter corresponds to the median value. P: comparison by the Wilcoxon signed-rank test.
Comparison between the group of MOA and the group of SOA showed that, at the end of the pulse labelling period, the tissue content in [-3H]-HA molecules was higher in explants with SOA cultured in the presence of aceclofenac and meloxicam at the concentrations of 1 and 3 μg ml−1 (P<0.0001).
Effects of NSAIDs on the net loss of newly synthesized proteoglycan and HA
In both groups, diclofenac did not change significantly the net loss of [-3H]-proteoglycan molecules at the three concentrations tested (Figure 4). On the other hand, in the two groups, 1 μg ml−1 of aceclofenac and meloxicam had already significantly reduced the net loss of [-3H]-proteoglycan molecules and 3 μg ml−1 of the two NSAIDs produced a further decrease in the net loss of labelled proteoglycans. At each concentration examined, there was no statistically significant difference between the effect of aceclofenac and that of meloxicam; there was also no statistically significant difference between explants with MOA and explants with SOA.
At the three concentrations tested, diclofenac did not change significantly the net loss of [-3H]-HA molecules from explants of both groups (Figure 5). In contrast, 1 μg ml−1 of either aceclofenac or meloxicam inhibited significantly the net loss of labelled HA from explants with MOA and SOA. At a concentration of 3 μg ml−1, the two NSAIDs reduced further the net loss of [-3H]-HA molecules from explants with MOA, but not from cartilage specimens with more advanced OA lesions.
At a concentration of 1 μg ml−1, aceclofenac was stronger than meloxicam in inhibiting the net loss of [-3H]-HA from explants with MOA (P=0.0039) and SOA as well (P=0.0156). Three μg ml−1 of aceclofenac exhibited also a stronger inhibition than 3 μg ml−1 of meloxicam in the group with MOA (P=0.0488) and in the group with SOA (P=0.0273).
Comparison between the two groups disclosed that the effect of aceclofenac was stronger in explants with MOA at 1 μg ml−1 (P<0.0001) and 3 μg ml−1 (P<0.0001); the effect of meloxicam was also stronger in explants with MOA, but only at the concentration of 3 μg ml−1 (P<0.0001).
Discussion
The data presented herein are the first to describe the effects of aceclofenac, diclofenac and meloxicam on the metabolism of HA in explants of human OA cartilage.
Cartilage composition and metabolism vary widely in the different topographical areas of the same joint and between the different joints of the same individual (Muir, 1986; Holmes et al., 1988). Therefore, the articular tissue was sampled from the same region of the knee joint to restrict variations in the concentration and metabolism of both proteoglycan and HA. Further, as cartilage composition and metabolism also change with age, the donors had a very similar range of age distribution in both groups, so that differences observed in response to NSAIDs could be more closely related to the OA disease process rather than ageing.
The concentrations of the three NSAIDs used in our culture system are similar to the range of total (bound and free) concentrations of these drugs that has been observed in human synovial fluid (Bort et al., 1996; Turck et al., 1996). It is however difficult to assess the actual effective concentration of drug acting on the tissue since this depends upon various factors such as the pH of the synovial fluid, the integrity of the surface of the cartilage, the partition coefficient of the drug in the articular tissue as well as protein binding. Most NSAIDs are extensively bound to plasma proteins and our culture media contained a relatively small proportion (20%) of foetal calf serum. Therefore, it is likely that, at the total concentration of 3 μg ml−1, the concentration of the free drug in our culture media was higher than that present in synovial fluid bathing cartilage in vivo.
Diclofenac unaffected the HA metabolism of OA cartilage, whereas, in a dose dependent manner, both aceclofenac and meloxicam were able concomitantly to increase HA synthesis and reduce the loss of newly synthesized HA molecules from the articular tissue. Further, the action of aceclofenac was stronger than that of meloxicam. That these two NSAIDs had a positive effect on the metabolic balance of HA is worth stressing since the progressive reduction in the HA content of OA cartilage (Manicourt et al., 1988; Rizkalla et al., 1992) is likely to contribute, at least in part, to the apparent irreversibility of the OA disease process (Pita et al., 1992) and further contrasts with the age-related increase in the HA content of normal articular cartilage (Holmes et al., 1988).
Our knowledge of the synthesis and degradation of HA in articular cartilage is still fragmentary. Chondrocytes do express two different HA synthases (HAS) which are localized to the cell surface, but the enzymatic characteristics of the two HAS isoforms as well as their mechanisms of regulation, including the possible effects of NSAIDs, remain to be clarified (Hiscock et al., 2000). On the other hand, as no hyaluronidase has been identified thus far in the cartilage matrix, it has been suggested that the degradation and loss of HA molecules may result from the action of oxygen-derived free radicals (ODFR) (Ng et al., 1995). Although NSAIDs may block the production of ODFR (Minta & Williams, 1985), it is likely that no single factor accounts for the favourable effect of aceclofenac and meloxicam on the overall metabolism of HA in the OA cartilage. Therefore, the exact mechanisms of action of the two drugs should be elucidated in further studies as they might be of great biological and therapeutic significance in OA.
Aceclofenac and meloxicam also reduced the loss labelled proteoglycan molecules from the articular tissue and both drugs concomitantly enhanced proteoglycan synthesis whereas diclofenac unaffected the overall metabolism of sulphated glycosaminoglycans. Previous in vitro studies have indeed shown that, at concentrations within therapeutic range, the effect of NSAIDs on the ability of chondrocytes to synthesize proteoglycans may be either stimulatory, inhibitory or neutral (Brandt, 1987; Dingle, 1999). Therefore, it is likely that a mechanism other than COX inhibition accounts for these marked differences in the effects of these drugs on proteoglycan synthesis, a contention further strengthened by the report that the PG E1 analogue, misoprostol, does not protect against the suppression of proteoglycan synthesis caused by NSAIDs (Brandt et al., 1991). Inhibition of IL-1 production and consequent expression of growth factor activity have been recently proposed as possible stimulatory mechanisms (Dingle, 1999). On the other hand, some NSAIDs do have toxic effects on chondrocyte metabolism such as inhibition of glucuronyltransferase, an enzyme responsible for the elongation of chondroitin sulphate chains on the nascent proteoglycan molecules (Hugenberg et al., 1993).
That meloxicam enhanced the rate of proteoglycan synthesis contrasts with the report of Rainsford et al. (1997) who found that this drug unaffected cartilage proteoglycan production. The reasons for this apparent discrepancy are unknown, but it is possible they reflect, in part, differences in the histological-histochemical grade of cartilage explants. Indeed, Dingle (1999) has reported that, in contrast to OA cartilage, normal cartilage shows no evidence of proteoglycan stimulation with aceclofenac. This heightened susceptibility of pathological articular cartilage has already been observed in vivo and in vitro with several NSAIDs (Brandt, 1987). Although its exact mechanism is unknown at present, data in the literature suggest that the uptake of NSAID by cartilage is inversely related to the proteoglycan content of the matrix (Brandt, 1987). Accordingly, any decrease in the concentration of the negatively charged proteoglycans, which is proportional to the gravity of the OA process, would increase the permeability of the matrix to the acidically charged NSAID.
The reduction in the net loss of proteoglycans produced by aceclofenac and meloxicam might be, at least in part, related to the positive effect of these drugs on the overall metabolism of HA since any decrease in the HA content of cartilage is likely to limit the aggregation of proteoglycans and, in so doing, favours the loss of newly synthesized proteoglycan molecules by diffusion or by proeolytic degradation (Heinegard & Hascall, 1974). On the other hand, reports have shown that, at concentrations within the therapeutic range, several NSAIDs inhibit the proteoglycanase and collagenase activities present in OA cartilage (Vignon et al., 1992; Barracchini et al., 1998). It remains to determine whether this suppressive effect of NSAIDs on the loss of proteoglycans is due either to an inhibition of the production of ODFR (Halliwell, 1995), or to a reduction in the synthesis of metalloproteinases (MMPs) and other proteolytic enzymes and/or to a stimulation of the synthesis and secretion of tissue inhibitors of proteolytic enzymes (Poole et al., 1995). Recent studies also suggest that NSAIDs could act either as reversible enzymatic inhibitors (Barracchini et al., 1998) or by inhibiting COXs which mediate the induction of membrane-type metalloproteinase-1 (MMP-14), an enzyme able to activate gelatinase A (MMP-2) and collagenase-3 (MMP-13) (Takahashi et al., 1999).
In conclusion, although it remains to be established whether changes observed in cartilage metabolism over short-term in vitro cultures would also occur in vivo as a result of long-term administration, the results presented herein show that, in contrast to diclofenac, aceclofenac and, to a lesser extent, meloxicam at the concentrations found in synovial fluid both exert a favourable effect on the overall metabolism of proteoglycans and HA in OA cartilage. Accordingly, the two drugs should not hamper the biomechanical properties of the articular tissue and might delay joint failure in OA. Aceclofenac and meloxicam, however, did not normalize the changes in cartilage metabolism seen in the OA tissue.
Acknowledgments
This work was supported by the grants 3.4597.98 and 9.4580.97 of the Fonds de la Recherche Scientifique Médicale (Belgium). The authors thank Mr D. Winand and Dr A. de Patoul for their support.
Abbreviations
- COX
cyclo-oxygenase
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethylsulphoxide
- HA
hyaluronan
- HAS
hyaluronan synthase
- OH-pro
hydroxyproline
- M
moderate
- NSAID
nonsteroidal anti-inflammatory drug
- OA
osteoarthritis
- Mol. wt
molecular weight
- ODFR
oxygen-derived free radicals
- PBS
phosphate-buffered saline
- PG
prostaglandin
- S
severe
References
- BARRACCHINI A., FRANCESCHINI N., AMICOSANTE G., ORATORE A., MINISOLA G., PANTALEONI G., DI GIULIO A. Can non-steroidal anti-inflammatory drugs act as metalloproteinase modulators? An in-vitro study of inhibition of collagenase activity. J. Pharm. Pharmacol. 1998;50:1417–1423. doi: 10.1111/j.2042-7158.1998.tb03369.x. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. A modified uronic acid carbazole reaction. Anal. Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BORT R., PONSODA X., CARRASCO E., GOMEZ-LECHON M.J., CASTELL J.V. Metabolism of aceclofenac in humans. Drug Metab. Dispos. 1996;24:834–841. [PubMed] [Google Scholar]
- BRANDT K.D. Effects of nonsteroidal anti-inflammatory drugs on chondrocyte metabolism in vitro and in vivo. Am. J. Med. 1987;83 Suppl. 5A:29–34. doi: 10.1016/0002-9343(87)90848-5. [DOI] [PubMed] [Google Scholar]
- BRANDT K.D., ALBRECHT M., O'BRYAN-REAR G. Misoprostol does not protect articular cartilage from salicylate-induced suppression of proteoglycan synthesis. J. Clin. Pharmacol. 1991;31:673–676. doi: 10.1002/j.1552-4604.1991.tb03755.x. [DOI] [PubMed] [Google Scholar]
- DINCHUK J.E., CAR B.D., FOCHT R.J., JOHNSTON J.J., JAFFEE B.D., COVINGTON M.B., CONTEL N.R., ENG V.M., COLLINS R.J., CZERNIAK P.M., GORRY S.A., TRZASKOS J.M. Renal abnormalities in the altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- DINGLE J.T.Non-steroidal anti-inflammatory drug administration in the treatment of osteoarthritis Osteoarthritis. Clinical and Experimental Aspects 1999Berlin: Springer; 370–387.ed. J-Y Reginster, J-P Pelletier, J. Martel-Pelletier & Y Henrotin. pp [Google Scholar]
- DISTEL M., MUELLER C., BLUHMKI E., FRIES J. Safety of meloxicam: a global analysis of clinical trials. Br. J. Rheum. 1996;35 Suppl. 1:68–77. doi: 10.1093/rheumatology/35.suppl_1.68. [DOI] [PubMed] [Google Scholar]
- GILROY D.W., COLVILLE-NASH P.R., WILLIS D., CHIVERS J., PAUL-CLARK M.J., WILLOUGHBY D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- GILROY D.W., TOMLINSON A., WILLOUGHBY D.A. Differential effects of inhibitors of cyclooxygenase (cyclooxygenase 1 and cyclooxygenase 2) in acute inflammation. Eur. J. Pharmacol. 1998;355:211–217. doi: 10.1016/s0014-2999(98)00508-1. [DOI] [PubMed] [Google Scholar]
- GRETZER B., EHRLICH K., MARICIC N., LAMBRECHT N., RESPONDEK M., PESKAR B.M. Selective cyclo-oxygenase-2 inhibitors and their influence on the protective effect of a mild irritant in the rat stomach. Br. J. Pharmacol. 1998;123:927–935. doi: 10.1038/sj.bjp.0701673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLIWELL B.Free radicals and rheumatoid disease Mechanisms and Models in Rheumatoid Arthritis 1995London: Academic Press; 301–316.ed. Henderson, B., Edwards, J.C.W. & Pettipher, E.R. pp [Google Scholar]
- HARDINGHAM T.Proteoglycans and glycosaminoglycans Dynamics of Bone and Cartilage Metabolism 1999San Diego: Academic Press; 71–81.ed. Seibel, M.J., Robins, S.P. & Bilezikian, J.P. pp [Google Scholar]
- HARDINGHAM T.E., MUIR H., KWAN M.K., LAI W.N., MOW V.C. Viscoelastic properties of proteoglycan solutions with varying proportions present as aggregates. J. Orthop. Res. 1987;5:36–46. doi: 10.1002/jor.1100050107. [DOI] [PubMed] [Google Scholar]
- HEINEGARD D., HASCALL V.C. Aggregatin of cartilage proteoglycans. III Characteristics of the proteins isolated from trypsin digests of aggregates. J. Biol. Chem. 1974;249:4250–4256. [PubMed] [Google Scholar]
- HISCOCK D.R., CATERSON B., FLANNERY C.R. Expression of hyaluronan synthases in articular cartilage. Osteoarthritis Cartilage. 2000;8:120–126. doi: 10.1053/joca.1999.0280. [DOI] [PubMed] [Google Scholar]
- HOLMES M.W., BAYLISS M.T., MUIR H. Hyaluronic acid in human articular cartilage. Age-related changes in content and size. Biochem. J. 1988;250:435–441. doi: 10.1042/bj2500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWELL D.S., PITA J.C., MULLER F.J., MANICOURT D.H., ALTMAN R.D. Treatment of osteoarthritis with tiaprofenic acid: biochemical and histological protection against cartilage breakdown in the Pond-Nuki canine model. J. Rheumatol. 1991;18 Suppl. 27:138–142. [PubMed] [Google Scholar]
- HUGENBERG S.T., BRANDT K.D., COLE C.A. Effects of salicylate, aspirin and ibuprofen on enzymes required by the condrocyte for synthesis of chondroitin sulfate. J. Rheum. 1993;20:2128–2133. [PubMed] [Google Scholar]
- HUNTER J.A., PARNHAM M.J., BALAGUER X.G. Aceclofenac in rheumatoid arthritis: a useful and novel anti-inflammatory drug. Clin. Rheum. 1996;15:329–334. doi: 10.1007/BF02230353. [DOI] [PubMed] [Google Scholar]
- LI X.Q., THONAR E.J.-M.A., KNUDSON W. Accumulation of hyaluronate in human lung carcinoma as measured by a new hyaluronate ELISA. Connect. Tissue Res. 1989;19:243–253. doi: 10.3109/03008208909043899. [DOI] [PubMed] [Google Scholar]
- LIM H., PARIA B.C., DAS S.K., DINCHUK J.E., LANGENBACH R., TRZASKOS J.M., DEY S.K. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- MANICOURT D.H., PITA J.C. Progressive depletion of hyaluronic acid in early experimental osteoarthritis in dogs. Arthritis Rheum. 1988;31:538–544. doi: 10.1002/art.1780310411. [DOI] [PubMed] [Google Scholar]
- MANICOURT D.H., DRUETZ-VAN EGEREN A., HAAZEN L., NAGANT DE DEUXCHAISNES C. Effects of tenoxicam and aspirin on the metabolism of proteoglycans and hyaluronan in normal and osteoarthritic human articular cartilage. Br. J. Pharmacol. 1994;113:1113–1120. doi: 10.1111/j.1476-5381.1994.tb17111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANKIN H.J., DORFMAN H., LIPPIELLO L., ZARINS A. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II Correlation of morphology with biochemical and metabolic data. J. Bone Joint Surg. 1971;53A:523–537. [PubMed] [Google Scholar]
- MINTA J.O., WILLIAMS M.D. Some nonsteroidal antiinflammatory drugs inhibit the generation of superoxide anions by activated polymorphs by blocking ligand-receptor interactions. J. Rheumatol. 1985;12:751–757. [PubMed] [Google Scholar]
- MORHAM S.G., LANGENBACH R., LOFTIN C.D., TIANO H.F., VOULOUMANOS N., JENNETTE J.C., MAHLER J.F., KLUCKMAN K.D., LEDFORD A., LEE C.A., SMITHIES O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- MUIR H.Current and future trends in articular cartilage research and osteoarthritis Articular Cartilage Biochemistry 1986New York: Raven Press; 423–440.ed. Kuettner, K., Schleyerbach, D.V.M. & Hascall, V.C. pp [Google Scholar]
- NG C.K., HANDLEY C.J., PRESTON B.N., ROBINSON H.C., BOLIS S., PARKER G. Effect of exogenous hyaluronan and hyaluronan oligosaccharides on hyaluronan and agrecan synthesis and catabolism in adult articular cartilage explants. Arch. Biochem. Biophys. 1995;316:596–606. doi: 10.1006/abbi.1995.1079. [DOI] [PubMed] [Google Scholar]
- PITA J.C., MULLER F.J., MANICOURT D.H., BUCKWALTER J.A., RADCLIFFE A.Early matrix changes in experimental osteoarthritis and joint disuse atrophy Articular Cartilage and Osteoarthritis 1992New York: Raven Press; 455–469.ed. Kuettner, K., Schleyerbach, D.V.M., Peyron, J.G. & Hascall, V.C. pp [Google Scholar]
- POOLE A.R., ALINI M., HOLLANDER A.P.Cellular biology of cartilage degradation Mechanisms and Models in Rheumatoid Arthritis 1995London: Academic Press; 163–204.ed. Henderson, B., Edwards, J.C.W. & Pettipher, E.R. pp [Google Scholar]
- RAINSFORD K.D., YING C., SMITH F.C. Effects of meloxicam, compared with other NSAIDs, on cartilage proteoglycan metabolism, synovial prostaglandin E2, and production of interleukins 1,6 and 8, in human and porcine explants in organ culture. J. Pharm. Pharmacol. 1997;49:991–998. doi: 10.1111/j.2042-7158.1997.tb06030.x. [DOI] [PubMed] [Google Scholar]
- RIZKALLA G., REINER A., BOGOCH E., POOLE A.R. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence of molecular heterogeneity and extensive molecular changes in disease. J. Clin. Invest. 1992;90:2268–2277. doi: 10.1172/JCI116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMASSMANN A., PESKAR B.M., STETTLER C., NETZER P., STROFF T., FLOGERZI B., HALTER F. Effects of inhibition of prostaglandin endoperoxide synthase-2 in chronic gastro-intestinal ulcer models in rats. Br. J. Pharmacol. 1998;123:795–804. doi: 10.1038/sj.bjp.0701672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH W.L., GARAVITO R.M., DEWITT D.L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI Y., KAWAHARA F., NOGUCHI M., MIWA K., SATO H., SEIKI M., INOUE H., TANABE T., YOSHIMOTO T. Activation of matrix metalloproteinase-2 in human breast cancer cells overexpressing cyclooxygenase-1 or 2. FEBS Lett. 1999;460:145–148. doi: 10.1016/s0014-5793(99)01328-9. [DOI] [PubMed] [Google Scholar]
- TURCK D., ROTH W., BUSCH U. A review of the clinical pharmacokinetics of meloxicam. Br. J. Rheumatol. 1996;35 Suppl 1:13–16. doi: 10.1093/rheumatology/35.suppl_1.13. [DOI] [PubMed] [Google Scholar]
- VANE J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- VANE J.R. Towards a better aspirin. Nature. 1994;367:215–216. doi: 10.1038/367215a0. [DOI] [PubMed] [Google Scholar]
- VIGNON E., MATHIEU P., LOUIZOT P., RICHARD M. In vitro effect of nonsteroidal antiinflammatory drugs on proteoglycanase and collagenase activity in human osteoarthritic cartilage. Arthritis Rheum. 1992;34:1332–1335. doi: 10.1002/art.1780341021. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., BAK A., MCKNIGHT W., ASFAHA S., SHARKEY K.A., MACNAUGHTON W.K. Cyclooxygenase I contributes to inflammation in rats and mice: implications for gastrointestinal toxicity. Gastroenterology. 1998;115:101–109. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]
- WARNER T.D., GIULIANO F., VOJNOVIC I., BUKASA A., MITCHELL J.A., VANE J.R. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclooxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro analysis. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESSNER J.F., JR The determination of hydroxyproline in tissue and protein samples containing a small portion of this amino acid. Arch. Biochem. Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]


