Abstract
In urinary bladder, M2-muscarinic receptors predominate, but it is the smaller population of M3-receptors which mediate detrusor contraction. This study examines the M2 : M3 ratio and the role of M2-receptors in contraction of pig urinary bladder.
Competition experiments with [3H]-QNB determined the ratio of M2 : M3. In functional studies, affinity values (pKB) for 4-DAMP, darifenacin and methoctramine were calculated. Similar experiments were performed on tissues following selective M3-inactivation (incubation with 40 nM 4-DAMP mustard in the presence of 1 μM methoctramine to protect M2-receptors), precontraction with 50 mM KCl and relaxation with isoprenaline (30 μM) or forskolin (1 μM).
In competition binding, displacement of [3H]-QNB by 4-DAMP, darifenacin and methoctramine best fitted a two-site model suggesting a predominant (70–80%) population of M2-receptors.
On normal detrusor in vitro, 4-DAMP and methoctramine caused surmountable antagonism of responses to carbachol with pKB values of 9.37±0.07 and 6.05±0.05 respectively. Darifenacin caused unsurmountable antagonism, the apparent pKB value being 8.61±0.10.
In tissues where the M3-receptors had been inactivated and cyclic AMP levels elevated, 4-DAMP and darifenacin were less potent, with apparent pKB values of 8.72±0.08 and 6.74±0.07. In contrast, methoctramine was more potent, the apparent pKB value increasing significantly to 6.86±0.06.
These data suggest that the pig bladder possesses a similar muscarinic receptor population to the human bladder and that the M3-receptor subtype mediates contraction of the normal detrusor muscle. However an involvement of M2-receptors in contraction can be observed following pharmacological manipulation of the receptor population.
Keywords: Muscarinic receptor, urinary bladder, radioligand, in vitro
Introduction
The urinary bladder, like the majority of other smooth muscles from many species, exhibit heterogeneous populations of muscarinic receptors (Hegde et al., 1997; Reddy et al., 1995). A predominance of the M2 muscarinic receptor subtype, with a minor population of M3-receptors, has been reported for urinary bladder smooth muscle for several species. Immunoprecipitation data indicate that the proportion of muscarinic M2 and M3-receptors is 3 : 1 in bladders from humans, guinea-pigs and rabbits, and 9 : 1 in the rat bladder (Wang et al., 1995). Radioligand binding studies also have revealed a population of 87% M2-receptors and 13% M3-receptors in rat urinary bladder (Monferini et al., 1988). However, pharmacological characterization of muscarinic receptors mediating contraction of detrusor muscle in rat (Longhurst et al., 1995), rabbit (Mutoh et al., 1997; Choppin et al., 1998) and guinea-pig bladder (Noronha-Blob et al., 1989) suggest the singular involvement of M3 receptors. These observations suggest that the M3-receptor is the predominant muscarinic receptor subtype responsible for detrusor contraction to muscarinic agonists, and the M2 receptors are not directly involved in contraction, thus a role for the majority M2 receptor in detrusor contraction is unclear.
The lack of discriminating pharmacological tools (particularly a selective M2 receptor agonist) poses considerable difficulties in pharmacologically isolating a muscarinic receptor subtype (Eglen et al., 1994). Although no direct contractile response to M2 receptor activation can be demonstrated, an indirect influence on contraction via inhibition of cyclic AMP-mediated smooth muscle relaxation by β-adrenoceptors, forskolin, 5-hydroxytryptamine or vasoactive intestinal peptide has been reported (Griffin & Ehlert, 1992; Caulfield, 1993; Reddy et al., 1995; Eglen et al., 1996). M2-receptors have also been suggested to be able to inhibit smooth muscle relaxation through decreasing the opening probability of K+-channels (Caulfield, 1993; Kume & Kotlikoff, 1991).
Recently, an M2-mediated contraction to muscarinic stimulation has been demonstrated following selective M3-receptor inactivation and elevation of cyclic AMP levels in guinea-pig ileum (Thomas et al., 1993; Reddy et al., 1995) and in the rat bladder (Hegde et al., 1997), an effect which manifests as a re-contraction.
Thus an M2-mediated contraction can be demonstrated in the rat bladder where the M2 : M3 ratio is approximately 9 : 1. Whether a role for M2-receptors can be demonstrated in other species where the ratio is significantly lower e.g. human, has yet to be investigated. The present study examines the M2 : M3 ratio (radioligand binding experiments) and the role of M2-receptors in mediating contraction (isolated tissue experiments) of the pig urinary bladder
Methods
In vitro functional studies
Female pig urinary bladder was collected from the abattoir and immediately placed in cold Krebs solution (4°C). Strips of tissue (10×3 mm) were cut from the bladder dome, and mucosa and serosa removed. The tissues were mounted in 30 ml organ baths containing Krebs solution which was maintained at 37°C and continuously gassed with 95% O2 and 5% CO2. The tissues were subjected to a resting tension of 1 g and allowed to equilibrate for 60 min, during which time they were washed every 10 min and the resting tension was adjusted. Isometric tension generated by the tissue was measured from UF1 force transducers to PC via a Cambridge Electronic Design (CED) interface using CHART software.
Effects of muscarinic antagonists on concentration-response curve to carbachol in normal tissues
Cumulative concentration-response curves (CRCs) to carbachol were obtained. Tissues were then washed for about 45 min until a steady resting level of tension was attained, and were then equilibrated for 30 min with Krebs solution containing the appropriate concentration of the antagonist or vehicle (time control). After incubation for 30 min, a second CRC to carbachol was constructed in the continued presence of antagonist or vehicle. In this way, four CRCs to carbachol were obtained from the same strip, three in the presence of increasing concentrations of 4-DAMP (3, 10, 30 nM), darifenacin (3, 10 and 30 nM) and methoctramine (1, 3 and 10 μM) or in the presence of vehicle. Antagonists were added in increasing concentrations after washing for 45 min. Control experiments were performed with the addition of vehicle instead of antagonist and these were used to correct for any tachyphylaxis or time-dependent changes in tissue sensitivity and responsiveness. The correction factors of 1.0–2.0 for EC50 values (negligible on a logarithmic scale) and 1.0–0.8 for maximum responses were small, but were still applied in all experiments.
Effects of increasing cyclic AMP activity on concentration-response curves to carbachol in normal and M3-inactivated tissues
After construction of the first CRCs to carbachol, tissues were washed with Krebs solution for 60 min. The tissues were then precontracted with KCl (50 mM). Once a stable contractile tone had been attained, the tissues were relaxed with isoprenaline (30 μM) to stimulate adenylate cyclase activity. Twenty minutes later, a second CRC to carbachol was obtained. Using separate tissues, selective M3-inactivation was achieved (Eglen & Harris, 1993; Hegde et al., 1997) after the first CRCs to carbachol by incubating with the alkylating agent 4-DAMP mustard (40 nM) for 60 min in the presence of methoctramine (1 μM) to protect M2-receptors, the methoctramine being added 30 min before the 4-DAMP mustard. The drugs were then removed from the tissues by washing for 90 min with Krebs solution. The second curve to carbachol was obtained under conditions of stimulated adenylate cyclase activity i.e., isoprenaine-induced relaxation of KCl precontracted tissue as described below (Figure 1).
Figure 1.
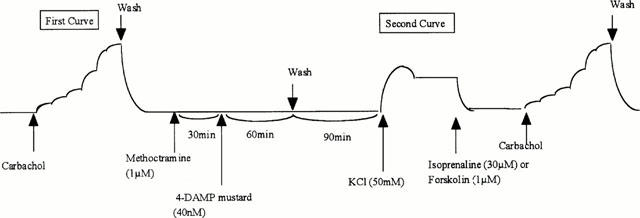
Schema of the procedures and time course for selective M3-inactivation and cyclic AMP elevation in in vitro functional studies. Selective M3-inactivation was achieved after the first CRCs to carbachol by incubating with 4-DAMP mustard (40 nM) for 60 min in the presence of methoctramine (1 μM) to protect M2-receptors, the methoctramine being added 30 min before the 4-DAMP mustard. The drugs were then removed from the tissues by washing for 90 min with Krebs solution. The second curve to carbachol was obtained under conditions of stimulated adenylate cyclase activity i.e., pre-contraction with KCl (50 mM) and relaxation with isoprenaline (30 μM) or forskolin (1 μM) (for methoctramine).
Effects of muscarinic antagonists on concentration-response curves to carbachol in M3-inactivated tissues
After CRCs to carbachol had been obtained with selective M3-inactivated tissue, the tissues were washed and incubated for 30 min with Krebs solution containing antagonists (4-DAMP 3, 10 and 30 nM, darifenacin 1, 3 and 10 μM or methoctramine 0.3, 1 and 3 μM), or vehicle. The tissues were then contracted with KCl (50 mM) and relaxed with isoprenaline (30 μM) or forskolin (1 μM) (for methoctramine) before CRCs to carbachol were again constructed. As with normal tissues, four CRCs to carbachol were obtained from the same strip, except for experiments with darifenacin in which CRCs to carbachol were obtained from separate strips from the same animal because the maximum contraction was significantly decreased by darifenacin.
Radioligand binding studies
Tissues were homogenized at 0.2 g ml−1 in 50 mM Tris-HCl buffer (pH 7.4), and filtered through muslin. Homogenates were centrifuged at 45,000×g for 10 min at 4°C. The pellets were re-suspended in 5 ml of Tris-HCl buffer. Homogenates were again centrifuged and the pellets re-suspended in 5 ml of Tris-HCl buffer. Membranes were used immediately in radioligand binding assays performed in duplicate.
Saturation experiments were conducted using seven concentrations (0.0625 to 4 nM) of 1-quinuclidinyl [phenyl-4-3H] benzilate ([3H]-QNB) (Goepel et al., 1998). Binding was determined in a final volume of 0.25 ml and non-specific binding determined with 10 μM atropine. The assay tubes were incubated at 37°C for 60 min before filtration through a cell harvester (Brandel M30) and the radioactivity of filters determined by liquid scintillation spectrometry. Protein content was determined by the method of Lowry et al. (1951) using bovine serum albumin (BSA) as a standard.
Competition experiments with [3H]-QNB were used to determine the ratio of M2 : M3 receptors. Cell membranes were incubated (37°C) with 0.3 nM [3H]-QNB and a range of concentrations of unlabelled antagonists (4-DAMP 0.1 nM–1 μM, darifenacin 0.25 nM–0.5 μM and methoctramine 25 nM–0.25 mM).
Statistical analysis
In functional studies, agonist potencies and maximum responses were expressed as mean pEC50±s.e.mean (−logarithm of the molar concentration of agonist resulting in 50% of the maximum response) and mean maximum contraction±s.e.mean, respectively. Antagonist dissociation constants (KB) were determined from the following equation: KB=antagonist concentration (molar) (dose ratio-1)−1. PA2 values for the muscarinic receptor antagonists were determined by Schild regression and taken as the x-intercept on the Schild plot (Arunlakshana & Schild, 1959).
Data are normalized to the maximal response generated by the first (control) curve, and are expressed as means±s.e.mean. A non-paired Student's t-test was used for statistical analysis to compare normal and M3-inactivated tissues. Radioligand binding data was analysed using Graphpad ‘PRISM' software and data compared using paired Student's t-test.
Drugs and chemicals
1-Quinuclidinyl [phenyl-4-3H] benzilate ([3H]-QNB) (specific activity 42 Ci mmol−1) was purchased from NEN Life Science Products, Inc. (Boston, MA, U.S.A.), and was stored at −20°C. Atropine, 4-DAMP and methoctramine were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). Darifenacin was a gift kindly donated by Pfizer Ltd. (Sandwich, Kent, U.K.).
Results
Radioligand binding studies
Radioligand binding experiments were performed on paired tissues following incubation in gassed Krebs-bicarbonate solution at 37°C (vehicle) or selective M3-inactivation (see Methods), and the membranes prepared from these tissues.
Binding of [3H]-QNB was saturable and displaceable by atropine. Specific binding was 82.3±1.8% of the total binding. Scatchard analysis of [3H]-QNB binding (n=8) demonstrated a single population of binding sites with a mean dissociation constant (KD) of 0.30±0.06 nM and a density of 89.3±12.9 fmol mg protein−1 in normal tissues. After selective M3-receptor inactivation the density of receptors was significantly reduced (P<0.05) to 61.1±10.1 fmol mg protein−1, but the affinity of binding was unchanged (KD=0.24±0.04 nM).
Competition binding with muscarinic antagonists
Displacement experiments were performed using 4-DAMP (n=9), methoctramine (n=9) and darifenacin (n=8). All experiments on normal tissue had low Hill slopes and fitted both one-site and two-site models. Correlation coefficients (r2 values) were nearer unity with the two-site model and this model yielded a statistically significant better fit in four of the 4-DAMP, six of the methoctramine and one of the darifenacin experiments. The two-site model of competition data for these antagonists are summarized in Table 1.
Table 1.
Competition data for [3H]-QNB binding to normal pig detrusor membranes

After selective M3-inactivation, 4-DAMP exhibited one-site binding in five of six experiments and the Hill slopes were significantly (P<0.05) nearer unity (0.81±0.04). Darifenacin exhibited one-site displacement with Hill slopes close to unity (1.06±0.08) in all experiments (n=6) and a mean pKi of 6.9, as did methoctramine (n=5, Hill slopes of 0.77±0.11 and mean pKi value of 7.9). Thus in no experiment, except one on M3-inactivated tissue, was a two-site binding model statistically better fit than a one-site model.
Effects of muscarinic antagonists in normal tissues in functional in vitro studies
Carbachol produced concentration-dependent contraction of the pig urinary bladder with mean pEC50 values and maximum responses of 5.86±0.02 and 16.0±2.02 g, respectively (n=12). 4-DAMP and methoctramine produced parallel, rightward displacement of the CRCs to carbachol without affecting maximum responses and yielded mean (±s.e.mean) pKB values of 9.37±0.07 and 6.05±0.05, respectively. Schild slopes for 4-DAMP and methoctramine were not significantly different from unity (Figures 2 and 4, Table 2) Darifenacin, however, produced unsurmountable antagonism, maximum responses being significantly reduced at 30 nM (Figure 3, Table 2).
Figure 2.

Concentration-response curve to carbachol in normal (upper panel) and M3-inactivated (middle panel) pig detrusor muscle strips in the absence and presence of 4-DAMP (3–30 nM). Data are normalized to the maximal response generated during the first (control) curve. Lower panel shows Schild slopes for normal and M3-inactivated bladder tissues.
Figure 4.

Upper panel: concentration-response curve to carbachol in normal pig detrusor muscles in the absence and presence of methoctramine (1–10 μM). Middle panel: concentration-response curve to carbachol in M3-inactivated pig detrusor muscles in the absence and presence of methoctramine (0.3–3 μM). Data are normalized to the maximal response generated during the first (control) curve. Lower panel shows Schild slopes of normal and M3-inactivated bladder tissues.
Table 2.
Affinity of antagonists determined using isolated pig detruscor muscle strips

Figure 3.

Upper panel: concentration-response curve to carbachol in normal pig detrusor muscles in the absence and presence of darifenacin (3–30 nM). Middle panel: concentration-response curve to carbachol in M3-inactivated pig detrusor muscles in the absence and presence of darifenacin (1 μ–10 μM). Data are normalized to the maximal response generated during the first (control) curve. Lower panel shows Schild slopes for normal and M3-inactivated bladder tissues.
Effects of increasing cyclic AMP activity in normal and M3-inactivated tissues
There was a slight, but significant decrease (P<0.05) in pEC50 values (5.61±0.05) to carbachol in the presence of isoprenaline and KCl compared with those (6.06±0.01) in the absence of these agents (n=6). Maximum responses in the presence of isoprenaline and KCl (9.7±0.26 g) were also significantly (P<0.0001) decreased by 40% compared with those in the absence of isoprenaline and KCl (23.7±0.15 g). In tissues where the M3-receptors had been inactivated, carbachol produced contractile responses in KCl-precontracted/isoprenaline-relaxed tissues (pEC50=5.23±0.14, n=12). Maximum responses after M3 inactivation were 6.06±0.41 g, which was 33.7% of control values (15.8±5.32 g).
Effects of muscarinic antagonists in M3-inactivated tissues
In tissues where the M3-receptors had been inactivated and cyclic AMP levels elevated (isoprenaline plus KCl), 4-DAMP produced antagonism of the contractile responses to carbachol, but was less potent, the pKB value being 8.72±0.08, significantly (P<0.01) lower than in the normal tissues. Maximum responses were unaffected by the antagonist and the Schild slope was 1.17±0.09 (Figure 2, Table 2). Darifenacin showed unsurmountable antagonism of the responses to carbachol, and was also less potent, the apparent pKB value being 6.74±0.07, significantly (P<0.0001) lower than in the normal tissues (Figure 3, Table 2). In contrast, methoctramine was more potent after M3-inactivation (KCl and isoprenaline), the apparent pKB value increasing significantly (P<0.0001) to 6.59±0.11. Maximum responses were unaffected by the antagonist but the Schild slope for methoctramine was variable (0.90±0.58). In M3-inactivated tissues where cyclic AMP levels were increased by forskolin (1 μM), the apparent pKB value for methoctramine again was increased significantly (P<0.0001) to 6.86±0.06. The Schild slope was less variable and was similar to unity (0.90±0.12), but maximum responses were reduced by all concentrations of methoctramine (P<0.0001 (Figure 4, Table 2).
Discussion
Previous studies indicate that the contractile response of detrusor muscle to muscarinic agonists is wholely mediated via the M3-receptor subtype in all species so far examined (Claufield 1993; Eglen et al., 1994; Longhurst et al., 1995). This is surprising since immunoprecipitation studies suggest that in most species the density of M2 receptors exceeds that of the M3 population by a ratio of 3 : 1 and in the rat by a ratio of 9 : 1 (Wang et al., 1995). This conclusion is supported in radioligand binding studies where the ratio of M2 : M3 receptors has been shown to be about 7 : 1 in the rat (Monferini et al., 1988). A previous study of the pig and human bladder concluded that only one subtype exists in both these species and it was identified as being of the M2-receptor subtype (Goepel et al., 1998). This highlights the problems found when using drugs without a great selectivity between the various subtypes of muscarinic receptor. In the present study some of the most selective agents available were used, and we were able to demonstrate that, as found in other species, it is a minor population of M3-receptors which mediates contraction of the pig detrusor muscle to carbachol. The M3 selective agents 4-DAMP and darifenacin had relatively high affinities for the detrusor muscarinic receptor, the values of 9.4 and 8.6 being similar to those reported for these antagonists at the M3-receptor, whilst the M2-selective antagonist methoctramine had a relatively low apparent affinity, which again was similar to values reported for this antagonist at M3-receptors (Hegde et al., 1997).
As found by other laboratories working in this area (Goepel et al., 1998), demonstrating the M3-receptor population in radioligand binding assays was difficult, although we had more selective antagonists available. In competition experiments, displacement of [3H]-QNB was usually best fitted by a two-site model for the antagonists, but there was only a statistically better fit with the two-site model in four of the 4-DAMP experiments, one of the darifenacin and six of the methoctramine experiments. Hill slopes for all three antagonists were low, which supports the concept of two-site binding and this was further supported in tissues following M3-alkylation, where only one binding site (M2) could be observed. The radioligand binding data therefore demonstrated a minority M3-receptor population of about 25% in the pig bladder, and this ratio is similar to that reported previously for the human bladder (Wang et al., 1995; Yamaguchi et al., 1996). For this reason we investigated the receptor subtype mediating functional responses in this species.
In the normal pig isolated detrusor strips, Schild slopes for the antagonists were close to unity, suggesting that only one receptor subtype was mediating the contractile responses to carbachol in these tissues. Other tissues have also been identified which have a high M2-receptor density, but where responses in vitro appear to be mediated solely via the M3-subtype (Eglen et al., 1994). It has been suggested that the conditions in vitro are not favourable to the demonstration of an M2-response which involves the inhibition of adenylate cyclase. Indeed in guinea-pig ileum, responses normally mediated via M3-receptor can involve M2-activity following M3 inactivation and elevation of cyclic AMP levels, the response then being regarded as a re-contraction or reversal of relaxation (Thomas et al., 1993; Reddy et al., 1995). This has also been shown in the rat bladder, but in this species the M2-receptor population is large (Hegde et al., 1997; Braverman & Ruggieri, 1999) and the M2 : M3 ratio is far greater than found in other species. The present study investigated whether an M2-mediated response could be demonstrated in the pig bladder where the M2 : M3 ratio is lower than that found in the rat. The M2 : M3 ratio of 3 : 1 obtained in the present study is significantly lower than that found in the rat, but is similar to the value recorded for the human bladder (Wang et al., 1995; Yamaguchi et al., 1996).
Using a protocol previously used on the rat bladder to inactivate M3-receptors and raise cyclic AMP levels, the apparent affinities of the three antagonists were significantly altered and suggested that the M2-receptor was responsible for mediating responses under these conditions. The affinity of the M3-selective agents 4-DAMP and darifenacin were reduced, while that for the M2-selective methoctramine was increased, compared to control tissues. Affinity estimates for the antagonists under these conditions were similar to those reported in the literature for M2-mediated responses (Hegde et al., 1997) and Schild plots had slopes similar to unity for 4-DAMP and darifenacin. The Schild plots for methoctramine were variable, but when cyclic AMP levels were elevated using forskolin instead of isoprenaline, a similar affinity estimate was obtained and the Schild plot slope was more consistent and close to unity. The reason for the more reliable results obtained using forskolin are not known but presumably may result from a larger elevation of cyclic AMP levels (Griffin & Ehlert, 1992). In contrast to the normal tissues, the Schild plots suggested that responses after M3-inactivation and cyclic AMP elevation were mediated solely via M2-muscarinic receptors. Radioligand binding experiments supported this finding. Incubation of tissues with 4-DAMP mustard in the presence of methoctramine to protect M2-receptors resulted in a 30% decrease in total muscarinic receptor density, but competition curves had Hill slopes close to unity for all three antagonists, and no experiment could be fitted to a two-site model of binding, suggesting that the M3-receptor population had been almost completely removed.
These findings may have relevance in both physiological and pathological states. Hegde et al. (1997) have demonstrated a contribution by M2-receptors to bladder contraction in the rat in vivo and M2-muscarinic receptor density has been shown to be selectively elevated following denervation of the bladder, albeit again in the rat (Braverman et al., 1998). It has been suggested that M2-receptors may mediate the dominant parasympathetic control over smooth muscle tone under conditions of high sympathetic activity or where M3-receptors are dysfunctional (Eglen et al., 1994). Beta-adrenoceptors predominate over alpha-adrenoceptors in the urinary bladder body, where their tonic stimulation is thought to facilitate the storage phase of micturition by relaxing the detrusor smooth muscle (Levin et al., 1988). The cholinergic activity is inhibited during this filling phase. On the contrary, during the voiding phase, sympathetic nerve activity is inhibited and acetylcholine is released (deGroat, 1993). Activation of M2 receptors during the voiding phase may oppose inhibitory sympathetic activation of beta-adrenoceptors (Nilvebrant et al., 1997), resulting in more efficient bladder emptying or initiation of voiding.
In summary, pig detrusor muscle possesses a population of muscarinic receptor in which the M2 is the major subtype (75%), but where M3-receptors mediate all the contractile responses in isolated muscle strip in vitro. However, under altered pharmacological conditions M2-receptors can mediate contractile (re-contraction) responses to muscarinic agonists.
Since the pig has been shown to have a M2 : M3 ratio similar to that reported for human, the data suggest that less selective muscarinic agents with regard to M2/M3-selectivity might be more effective than M3-selective antagonists in treating detrusor over-activity.
Abbreviations
- cyclic AMP
adenosine 3′5′-cyclic monophosphate
- CRC
concentration-reponse curve
- 4-DAMP
4-diphenyl acetoxy-methyl piperidine methiodide
- [3H]-QNB
1-quinuclidinyl [phenyl-4-3H] benzilate
References
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAVERMAN A.S., LUTHIN G.R., RUGGIERI M.R. M2 muscarinic receptor contributes to contraction of the denervated rat urinary bladder. Am. J. Physiol. 1998;275:R1654–R1660. doi: 10.1152/ajpregu.1998.275.5.R1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAVERMAN A.S., RUGGIERI M.R. Selective alkylation of rat urinary bladder muscarinic receptors with 4-DAMP mustard reveals a contractile function for the M2 muscarinic receptor. J. Receptor & Signal Transduction Res. 1999;19:819–833. doi: 10.3109/10799899909042875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAULFIELD M.P. Muscarinic receptors-characterization, coupling and function. Pharmacol. Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- CHOPPIN A., EGLEN R.M., HEGDE S.S. Pharmacological characterization of muscarinic receptors in rabbit isolated iris sphincter muscle and urinary bladder smooth muscle. Br. J. Pharmacol. 1998;124:883–888. doi: 10.1038/sj.bjp.0701920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEGROAT W.C. Anatomy and physiology of the lower urinary tract. Urol. Clin. North. Am. 1993;20:383–401. [PubMed] [Google Scholar]
- EGLEN R.M., HARRIS G.C. Selective inactivation of muscarinic M2 and M3 receptors in guinea-pig ileum and atria. in vitro. Br. J. Pharmacol. 1993;109:946–952. doi: 10.1111/j.1476-5381.1993.tb13712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., PEELLE B., PULIDO-RIOS M.T., LEUNG E. Functional interactions between muscarinic M2 receptors and 5-hydroxytryptamine (5-HT)4 receptors and β3-adrenoceptors in isolated oesophageal muscularis mucosae of the rat. Br. J. Pharmacol. 1996;119:595–601. doi: 10.1111/j.1476-5381.1996.tb15714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., REDDY H., WATSON N., CHALLIS R.A.J. Muscarinic acetylcholine receptor subtypes in smooth muscle. Trends. Pharmacol. Sci. 1994;15:114–119. doi: 10.1016/0165-6147(94)90047-7. [DOI] [PubMed] [Google Scholar]
- GOEPEL M., GRONEWALD A., KREGE S., MICHEL M.C. Muscarinic receptor subtypes in porcine detrusor: comparison with humans and regulation by bladder augmentation. Urol Res. 1998;26:149–154. doi: 10.1007/s002400050038. [DOI] [PubMed] [Google Scholar]
- GRIFFIN M.T., EHLERT F.J. Specific inhibition of isoproterenol-stimulated cyclic AMP accumulation by M2 muscarinic receptors in rat intestinal smooth muscle. J. Pharmacol. Exp. Therap. 1992;263:221–225. [PubMed] [Google Scholar]
- HEGDE S.S., CHOPPIN A., BONHAUS D., BRIAUD S., LOEB M., MOY T.M., LOURY D., EGLEN R.M. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br. J. Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUME H., KOTLIKOFF M.I. Muscarinic inhibition of single KCa channels in smooth muscle cells by a pertussis-sensitive G-protein. Am. J. Physiol. 1991;261:C1204–C1209. doi: 10.1152/ajpcell.1991.261.6.C1204. [DOI] [PubMed] [Google Scholar]
- LEVIN R.M., RUGGIERI M.R., WEIN A.J. Identification of receptor subtypes in the rabbit and human urinary bladder by selective radio-ligand binding. J. Urol. 1988;139:844–848. doi: 10.1016/s0022-5347(17)42659-0. [DOI] [PubMed] [Google Scholar]
- LONGHURST P.A., LEGGETT R.E., BRISCOE A.K. Characterization of the functional muscarinic receptors in the rat urinary bladder. Br. J. Pharmacol. 1995;116:2279–2285. doi: 10.1111/j.1476-5381.1995.tb15065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALLR J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MONFERINI E., GIRALDO E., LADINSKY H. Characterization of the muscarinic receptor subtypes in the rat urinary bladder. Eur. J. Pharmacol. 1988;147:453–458. doi: 10.1016/0014-2999(88)90180-x. [DOI] [PubMed] [Google Scholar]
- MUTOH S., LATIFPOUR J., SAITO M., WEISS R.M. Evidence for the presence of regional differences in the subtype specificity of muscarinic receptors in rabbit lower urinary tract. J. Urol. 1997;57:717–721. [PubMed] [Google Scholar]
- NILVEBRANT L., ANDERSSON K.E., GILLBERG P.G., STAHL M., SPARF B. Tolterodine–a new bladder-selective antimuscarinic agent. Eur. J. Pharmacol. 1997;327:195–207. doi: 10.1016/s0014-2999(97)89661-6. [DOI] [PubMed] [Google Scholar]
- NORONHA-BLOB L., LOWE V., PATTON A., CANNING B., COSTELLO D., KINNIER W.J.Muscarinic receptors: relationships among phosphoinositide breakdown, adenylate cyclase inhibition in vitro J. Pharmacol. Exp. Therap. 1989249843–851.detrusor muscle contractions and in vivo cystometrogram studies in guinea pig bladder [PubMed] [Google Scholar]
- REDDY H., WATSON N., FORD A.P.D.W., EGLEN R.M. Characterization of the interaction between muscarinic M2 receptors and β-adrenoceptor subtypes in guinea-pig isolated ileum. Br. J. Pharmacol. 1995;114:49–56. doi: 10.1111/j.1476-5381.1995.tb14904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS E.A., BAKER S.A., EHLERT F.J. Functional role for the M2 muscarinic receptor in smooth muscle of guinea pig ileium. Mol. Pharmacol. 1993;44:102–110. [PubMed] [Google Scholar]
- WANG P., LUTHIN G.R., RUGGIERI M.R. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J. Pharmacol. Exp. Therap. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]
- YAMAGUCHI O., SHISHIDO K., TAMURA K. , OGAWA T., FUJIMURA T., OHTSUKA M. Evaluation of mRNAs encoding muscarinic receptor subtypes in human muscle. J. Urol. 1996;156:1208–1213. [PubMed] [Google Scholar]


