Abstract
In in vivo experiments, DOI (a 5-HT2 receptor agonist), MK-212 (a 5-HT2C receptor agonist), and BW-723C86 (a 5-HT2B receptor agonist) were applied by ionophoresis to neurones in the rat nucleus tractus solitarius (NTS) receiving vagal afferent input.
The majority of the putative ‘monosynaptically' vagal activated cells were inhibited by both MK-212 (4/6) and DOI (2/4), but unaffected by BW-723C86 (12/14). In contrast, ‘polysynaptically' activated NTS cells were excited by both BW-723C86 (13/19) and DOI (9/10). Inactive ‘intermediate' cells were inhibited by BW-723C86 (9/12), MK-212 (5/6) and DOI (3/4), whilst active cells of this group were excited by BW-723C86 (7/13) and DOI (5/5).
The selective 5-HT2B receptor antagonist LY-202715 significantly reduced the excitatory actions of BW-723C86 on ‘intermediate' and ‘polysynaptic' cells (13/13), but not the inhibitory effects observed on inactive Group 2 cells (n=5) whereas the selective 5-HT2C receptor antagonist RS-102221 reversed the inhibitory effects of MK-212 and DOI on ‘monosynaptic and ‘intermediate' neurones.
Cardio-pulmonary afferent stimulation inhibited two of four putative ‘monosynaptically' activated calls and all four inactive intermediate cells. These were also inhibited by DOI and MK-212. In contrast, cardio-pulmonary afferents excited all five active intermediate cells and all six putative ‘polysynaptically' activated NTS cells, while all were also previously excited by BW-723C86 and/or DOI.
In conclusion, these data demonstrate that neurones in the NTS are affected differently by 5-HT2 receptor ligands, in regard of their vagal postsynaptic location, the type of cardio-pulmonary afferent they receive and the different 5-HT2 receptors activated.
Keywords: 5-HT2 receptors, NTS, brainstem, vagal afferents
Introduction
The nucleus tractus solitarius (NTS) is the site of termination of cardiovascular and other visceral afferents (Jordan & Spyer, 1986; Kalia & Mesulam, 1980) and therefore plays a pivotal role in cardiovascular regulation and integration. It is densely innervated by serotonergic terminals (Steinbusch, 1981), and autoradiographic studies have revealed that NTS neurones express binding sites for 5-HT2 receptors (Dashwood et al., 1988). Microinjection of 5-HT2 receptor agonists into the NTS produces hypotension and bradycardia (Merahi et al., 1992), similar to those observed by activation of cardio-pulmonary vagal afferents (Verberne & Guyenet, 1992). Recent data suggested that these afferents and 5-HT2 receptor-mediated effects activate the same central pathways (Sévoz et al., 1996a).
In a previous study, Wang et al. (1997) reported that in vivo ionophoretic application of the non-selective 5-HT2 receptor agonist DOI could excite, inhibit or have no effect on NTS neurones receiving vagal afferent input. Subsequently, it was noted that second order NTS neurones were inhibited whilst higher-order neurones were excited by 5-HT2 activation, and that these effects were similar to those observed by cardio-pulmonary afferent stimulation (Sévoz-Couche et al., 2000c). Most recently, it was demonstrated that 5-HT2C receptors were responsible for these inhibitory effects (Sévoz-Couche et al., 2000a). The aims of the present study were 2 fold. First, to identify the 5-HT2 receptors responsible for the excitatory effects on NTS cells and second, to assess whether there was a relationship between the effects elicited by 5-HT2 receptor activation and that evoked by vagal afferents.
A preliminary account of some of these observations has been published (Sévoz-Couche et al., 2000b).
Methods
General preparation
Experiments were performed on 27 adult Male Sprague-Dawley rats (320–380 g body weight) and prepared as described in detail previously (Sévoz-Couche et al., 2000c). The rats were anaesthetized with pentobarbitone sodium (60 mg kg−1, i.p.). The depth of anaesthesia was assessed by pinching the hindpaw and monitoring the stability of the arterial blood pressure. In case of withdrawal reflex and/or significant variations of arterial pressure and heart rate, a supplementary dose of pentobarbitone was given (5–10 mg kg−1, i.v.). A cannula was inserted into the femoral vein for administration of drugs or supplemental anaesthesia. Arterial pressure was monitored through a catheter inserted into a femoral artery. A tracheotomy was performed low in the neck, and tracheal and arterial pressures were measured with pressure transducers (Statham P23Db). The animals were ventilated with room air enriched with oxygen using a positive pressure ventilator (Harvard Rodent Ventilator, model 683), with 1 cmH2O positive end-expiratory pressure. During the experiment, four or five arterial blood samples (75 μl each) were taken at regular intervals to monitor blood gases and pH using a Corning pH/blood gas analyser (model 238): PO2 was maintained between 100–140 mmHg, PCO2 between 35 and 45 mmHg and pH at 7.3, by adjustments of the rate and/or volume of the respiratory pump, the volume of O2 added to the inspired air, or by slow intravenous infusions of sodium bicarbonate (1 M). In some experiments, a cannula was placed into the right atrium via the right jugular vein. Stimulation of cardio-pulmonary afferents was performed by right atrial administration of phenylbiguanide (PBG), at a dose sufficient to induce a reflex hypotension of at least 15 mmHg (10–15 μg kg−1 in 10–20 μl).
Surgical procedures
The rats were placed in a stereotaxic frame with the head ventroflexed at an angle of about 45° from the horizontal. The left vagus nerve was dissected from the sympathetic trunk by a lateral approach and placed on bipolar silver electrodes for electrical stimulation (1 Hz, 0.1–0.3 mA (2×T), 1 ms) triggered with a digital programmer (Master 8, AMPI). The dorsal surface of the brainstem was exposed through a limited occipital craniotomy, between the neck muscles.
Experimental protocol
Before beginning the brainstem recordings, animals were neuromuscular blocked with gallamine triethiodide (Flaxedil 8 mg kg−1, i.v.). Supplemental doses (4 mg kg−1, i.v.) were given every hour. Extracellular recordings of NTS neurones (1–3 per rat) were made using a single-barrel microelectrode (tip diameter ∼1 μm, 5–15 MΩ) glued to a six-barrelled microelectrode (tip diameter ∼10 μm, 2–5 MΩ) so that the tips of the recording and ionophoretic electrodes were at the same level. The single recording barrel contained 4 M sodium chloride, and the six barrels were filled with a selection of Pontamine Sky Blue dye (2% in 0.5 M Na acetate), DL-homocysteic acid (DLH), DOI, MK-212, BW-723C86, ketanserin, RS-102221 and LY-272015. Drugs were ejected by ionophoresis (Neurophore, Medical Systems) using positive currents with a retaining current of −10 nA applied between ejection periods, except for DLH which was ejected using negative current. Possible current artefacts were overcome using the automatic current balancing available on the Neurophore. In some experiments, the possibility of current and/or pH artefacts were tested directly by passing current through saline of the same pH as the ejected drugs (pH4). No significant artefact was seen using this test. Neuronal recordings were amplified ×5000 (Dagan 2400) and filtered (0.1–10 kHz).
Recorded cells were located at ≈amp;−14.5 mm from Bregma (Paxinos & Watson, 1998). NTS cells were found between 300 and 600 μm, from the surface of the brainstem. In some experiments, NTS recording sites were marked by deposition of Pontamine Sky Blue dye. At the end of these experiments, brains were removed and fixed in 10% formal saline. Frozen coronal sections (70 μm) were cut using a microtome and stained with 1% neutral red.
Analysis of data
Arterial blood pressure, tracheal pressure and neuronal activity were recorded on video tape via a digital interface (Instrutech, VR-100A). Analysis of the recorded data was made with commercially available software (CED Spike 2) on a computer accessed via an A–D interface (CED 1401 plus). Single unit activity was discriminated with a window discriminator (Digitimer 130) and analysed as described previously (Sévoz-Couche et al., 2000c). Rate histograms (1 s bins) and peri-stimulus time histograms (PSTHs) were constructed. Cells were classed as affected when application of a ligand produced changes of at least 20% of the baseline levels. The effects were analysed using a nonparametric Mann-Whitney test (paired, significant at P<0.05). All values are expressed as mean±s.e.mean.
Drugs and solutions
All drugs were freshly dissolved and pH adjusted by addition of drops of either 0.1 M HCl or 0.1 M NaOH. DL-Homocysteic Acid (DLH: 100 mM, pH 8.5, Sigma Chemicals) was dissolved in 0.159 M saline; (±)-2,5-dimethoxy-4-iodoamphetamine HCl (DOI: 20 mM, pH 4, Research Biochemicals), was dissolved in 1 mM saline; 6-chloro-2-(1-piperazinyl)pyrazine (MK-212 hydrochloride: 20 mM, pH 4, Tocris), ketanserin tartrate (10 mM, pH 4, Tocris) and LY-202715 (20 mM, pH 4, a generous gift from Dr J. Audia, Lilly) were dissolved in deionized water; α-methyl-5-(2-thienylmethoxy)-1H-indole-3-ethanamine (BW-723C86 : 20mM, pH4, Tocris) and 8-(5-(2,4-Dimethoxy-5-(4-trifluoromethylphenylsulphonamido)phenyl-5-oxopentyl)-1,3,8-triazaspiro(4,5)decane-2,4-dione (RS-102221 : 20 mM, pH 4, Tocris) were dissolved in DMSO and made to volume with deionized water.
Results
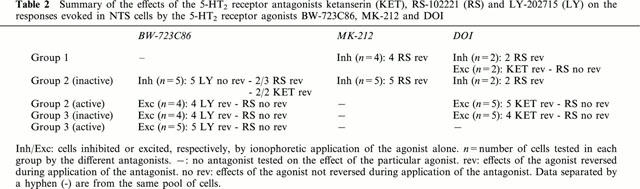
A total of 66 neurones was recorded from the left NTS, between +0.5 to −0.5 mm with reference to the calamus scriptorius and 0.2–0.5 mm from the midline. They were identified by their orthodromic responses to cervical vagus nerve stimulation. Of these 66 vagal-identified cells, 58 cells were activated at long-latency (mean: 33.8±2.6 ms, Figures 1 and 2), giving a calculated conduction velocity of 0.77±0.08 m s−1. The eight remaining NTS cells were activated at much shorter latency (mean: 6.5±1.5 ms, Figure 3), giving a calculated conduction velocity of 4.2±0.8 m s−1. The activated neurones were divided into three groups on the basis of the variability of the onset latency of their excitatory response to vagal stimulation (Sévoz-Couche et al., 2000c): Group 1 cells had onset latency variabilities <3 ms (Figure 1) which would include second order neurones. Group 3 cells had onset latency variations >5 ms (Figure 2) indicating that they were mainly polysynaptically activated. Group 2 cells had onset latency variations between 3 and 5 ms (Figure 1).
Figure 1.
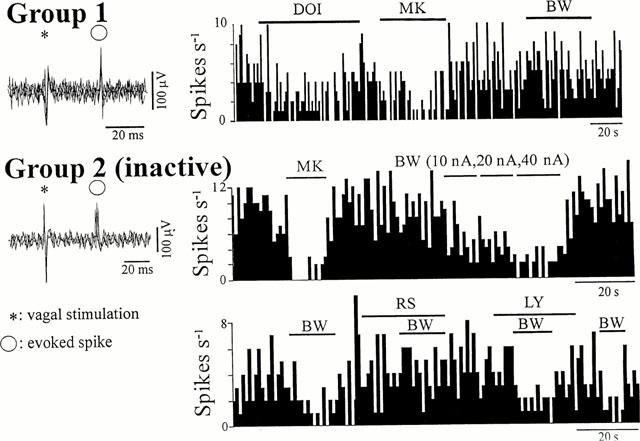
Effects of 5-HT2 receptor ligands on Group 1 and Group 2 cells. The two Group 2 cells were two different inactive cells in which activity was evoked by previous ionophoretic application of DLH. Left panels: five superimposed sweeps showing the variability in the onset latency of the vagal-evoked discharge of <1 ms in Group 1 and 4 ms in Group 2 cells. Right panels: Continuous ratemeter records (1 s bins) of the activity of NTS neurones excited by vagal nerve stimulation. Group 1: Histogram illustrating the inhibitory effects of MK-212 (MK, 50 nA) and DOI (20 nA) applied during the bars. BW-723C86 (BW, 40 nA) had no effect on the cell. Group 2: (top) Both MK-212 (MK, 40 nA) and BW-723C86 (BW) applied during the bars with the stated ejection current, have inhibitory effects on the firing rate of the cell. (Bottom) The BW-723C86 (40 nA)-induced inhibitory effect was prevented during application of RS-102221 (RS, 30 nA) but not LY-272015 (LY, 50 nA).
Figure 2.
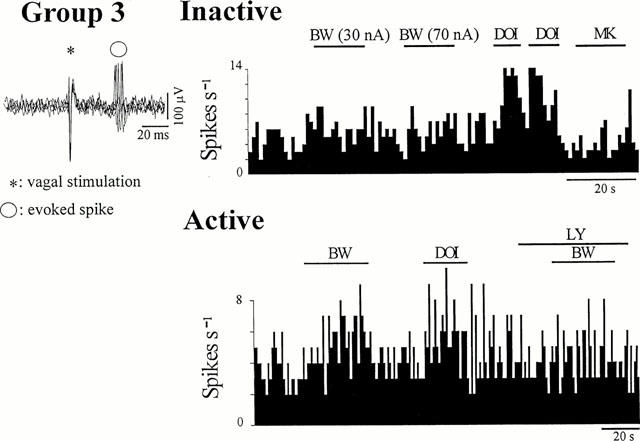
Effects of 5-HT2 receptor ligands on Group 3 cells. Activity was evoked in the inactive cell by previous ionophoretic application of DLH. Left panels: five superimposed sweeps showing a variability in the onset latency of the vagal-evoked discharge of 8 ms. Right panels: continuous ratemeter records (1 s bins) of the activity of NTS neurones excited by vagal nerve stimulation. (Top): no effect was observed on this particular inactive cell after ionophoretic application of either BW-723C86 (BW, with the stated ejection current) or MK-212 (MK, 40 nA) during the bars, but application of DOI (20 nA) produced excitation. (Bottom): both BW-723C86 (30 nA) and DOI (30 nA), applied during the bars, excited this cell. The BW-723C86-induced excitation was blocked during application of LY-272015 (LY, a selective 5-HT2B receptor antagonist, 30 nA) applied during the bar.
Figure 3.
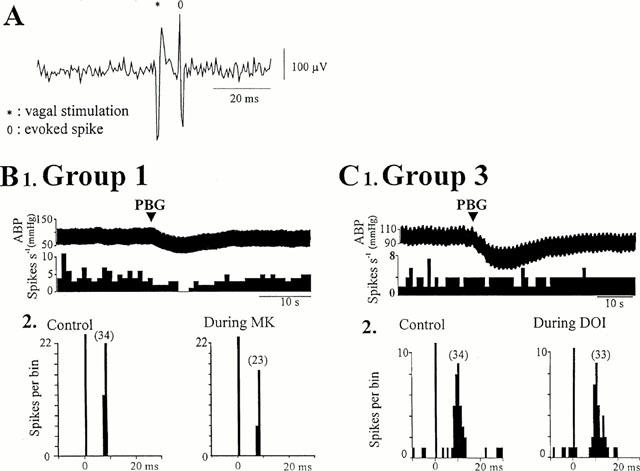
Effects of 5-HT2 receptor agonists on cells activated by myelinated fibres. (A) Single sweep showing the short latency of the spike evoked by vagal stimulation, as expected for fast conducting fibres (myelinated). (B1) Ratemeter record (1 s bins) showing that administration of phenylbiguanide (PBG, 10 μg kg−1, arrow) inhibited the firing of a Group 1 cell, (B2) peri-stimulus time histograms (PSTH, 1 ms bins, 40 sweeps) of the same cell showing that application of MK-212 (MK, 30 nA, right) reduced the number of evoked spikes compared to pre-drug (control, left). (C1) Ratemeter record (1 s bins) showing that administration of PBG (10 μg kg−1, arrow) did not affect the firing of this Group 3 cell, and (C2) PSTHs (1 ms bins, 40 sweeps) of the same cell showing that ionophoretic application of DOI (20 nA, right) had no effect compared to pre-drug (control, left). (Vertical bars at t=0 ms represent the stimulus artefacts and numbers in brackets are the number of evoked discharges counted). ABP: arterial blood pressure.
Ongoing activity (mean 3.5±0.8 spikes s−1) was present in 29 of the 66 NTS cells. In the other cells (inactive cells, n=37), activity was evoked (in a range of 4–10 spikes s−1) by ionophoretic application of the excitatory amino acid DLH (2–10 nA), in order to observe effects of serotonergic ligands on the firing rate of these cells. The effects of the application of serotonergic ligands were analysed on the ongoing discharge of NTS cells and the latency and number of the vagal-evoked spikes. Since in the majority of cells the effects on ongoing and evoked discharge were in the same direction, the data were not separated on these criteria.
Effects of 5-HT2 receptor agonists on NTS cells activated by non-myelinated vagal afferents
The effects of BW-723C86, a selective 5-HT2B receptor agonist (Duxon et al., 1997b), was tested on 58 cells. The ejection currents were usually in a range of 10–50 nA, and when present, the sign of response did not change with further increase of current (up to 150–200 nA). The effects of this ligand appeared within the first 5 s of application, and returned to control after the application. In some cells, DOI (20–50 nA, 23/58) and/or MK-212 (10–50 nA, 20/58), a selective 5-HT2C receptor agonist (Gommans et al., 1998; Halford et al., 1997), were applied in addition to BW-723C86 (in order to identify the receptor(s) involved in the 5-HT2 receptor activation) (Table 1).
Table 1.
Effects of BW-723C86 (a selective 5-HT2B receptor agonist), MK-212 (a selective 5-HT2C receptor agonist), and DOI, a non-selective 5-HT2 receptor agonist, on the three different Groups of NTS cells
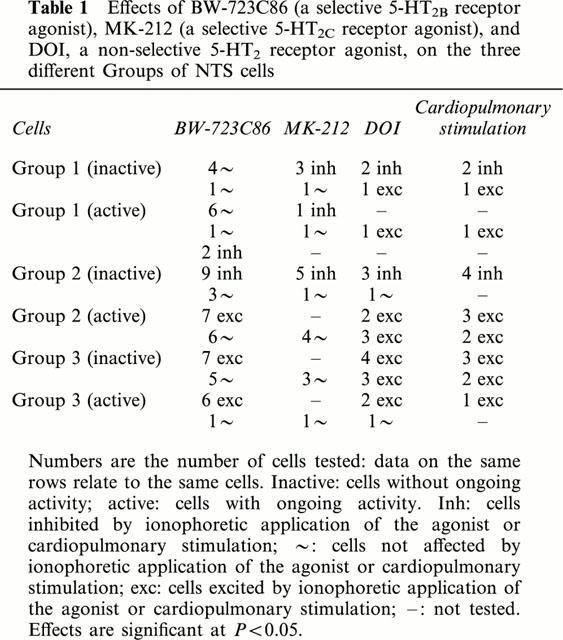
BW-723C86 was without effect on most (12/14) Group 1 cells tested (Figure 1), the remaining two cells being inhibited. MK-212 inhibited four and was without effect on two of the cells unaffected by BW-723C86 (Figure 1). DOI inhibited only cells that had been inhibited by MK-212 (2/2) (Figure 1) whereas it excited Group 1 cells unaffected by MK-212 or BW-723C86 (2/2).
The selective 5-HT2 receptor agonists had different actions on Group 2 cells, dependent on whether (n=13) or not (n=12) they exhibited ongoing activity. As described previously (Sévoz-Couche et al., 2000c), DOI inhibited inactive Group 2 cells (3/4) and excited active cells (5/5). The inhibitory effects on these inactive cells were also seen with BW-723C86 (9/12, Figure 1), and by MK-212 (5/6, Figure 1). All three cells inhibited by DOI were also inhibited by MK-212 and BW-723C86 (Table 1). In contrast, BW-723C86 excited seven and had no effect on six of 13 active Group 2 cells (four of these six were also unaffected by MK-212). The cells activated by DOI included two cells excited by BW-723C86, and three cells unaffected by both BW-723C86 and MK-212 (Table 1).
Finally, BW-723C86 excited 13 of the 19 Group 3 cells, and this was independent of whether they were active (6/7) or inactive (7/12). MK-212 was without effect on all four of these cells tested that were unaffected by BW-723C86 (Figure 2, Table 1). DOI excited the majority of Group 3 cells (9/10), and it is important to note that three of these cells were inactive cells previously unaffected by both BW-723C86 and MK-212 (Figure 2, Table 1).
Effects of 5-HT2 receptor antagonists on DOI, MK-212 and BW-72386-induced effects on NTS cells activated by non-myelinated vagal afferents
Ketanserin, a 5-HT2A/2B/2C receptor antagonist, RS-102221, a selective 5-HT2C receptor antagonist (Bonhaus et al., 1997) and LY-272015, a selective 5-HT2B receptor antagonist (Cohen et al., 1996), were used to confirm the selective effects of the agonist ligands. Application of these antagonists produced no effect on the ongoing or evoked activity of the cells.
The excitatory effects of BW-723C86 on active Group 2 (4/4) and both active (5/5, Figure 2) and inactive (4/4) Group 3 cells were reversed by application of LY-272015 (30–50 nA) but not by application of RS-102221 (30–50 nA) (n=4) (Table 2). In contrast, the inhibitory effect of BW-723C896 observed on inactive Group 2 cells was not significantly reduced after application of LY-272015 (5/5) but these inhibitions were reversed by application of ketanserin (30–50 nA) (2/2) or RS-102221 (30–50 nA) (2/3) (Figure 1; Table 2). Application of RS-102221 (30–60 nA) also antagonized the inhibitory effect of MK-212 on Group 1 (4/4) and inactive Group 2 (5/5) cells (Figure 1) and the inhibitory effects of DOI on the two Group 1 cells and two inactive Group 2 cells tested (Table 2). In contrast, the DOI-evoked excitation of both Group 1 cells, all five spontaneously active Group 2 cells and four of five inactive Group 3 cells were reversed by ketanserin (30 nA) but not by RS-102221 (Table 2).
Table 2.
Summary of the effects of the 5-HT2 receptor antagonists ketanserin (KET), RS-102221 (RS) and LY-202715 (LY) on the responses evoked in NTS cells by the 5-HT2 receptor agonists BW-723C86, MK-212 and DOI
Effects of 5-HT2 receptor ligands on NTS cells receiving myelinated vagal afferents
The 5-HT2 receptor agonists were also tested on NTS neurones receiving excitatory inputs from myelinated vagal afferents (latency 6–8 ms, see Figure 3). On the basis of calculated conduction velocity, eight cells were found to receive myelinated afferent input. Five were classified as Group 1, and these were all inhibited by DOI (10–50 nA). All three of these five cells tested were also inhibited by MK-212 (10–50 nA) (Figure 3) but were unaffected by application of BW-723C86 (10–50 nA). The inhibitory effect of MK-212 was reversed during application of RS-102221 (30–50 nA) (n=3). The remaining three cells, two active Group 3 (Figure 3) and one inactive Group 2, were unaffected by DOI.
Stimulation of cardiopulmonary NTS afferents
The effect of cardiopulmonary afferent stimulation by right atrial administration of PBG was tested in 24 of the 58 NTS neurones receiving non-myelinated vagal afferent inputs. Administration of PBG either increased (6.5±0.5 spikes s−1 from a baseline firing rate of 3.5±0.5 spikes s−1, P<0.05, n=13, Figures 4 and 5) or decreased (0.8±0.5 spikes s−1 from a baseline firing rate of 3.6±0.6 spikes s−1, P<0.05, n=6, Figures 4 and 5) the firing rate of 19 cells. There was no effect of PBG on the other five cells tested though a hypotensive response was evoked (Figure 3). In the majority of these cells (17/19, Figures 4 and 5), right atrial administration of PBG had the same effect as ionophoretic application of DOI and/or agonists selective for different 5-HT2 receptors (Table 1). Two of four Group 1 neurones were inhibited by administration of PBG and ionophoretic application of DOI and MK-212 (Figure 4). The remaining two cells were excited by both PBG and DOI, but not by MK-212 or by BW-723C86. In contrast, all six Group 3 cells tested were excited both by administration of PBG and ionophoresis of DOI, and four of these six were also excited by BW-723C86 (Figure 4). Spontaneously active Group 2 cells were excited by both PBG and DOI (5/5), and three of these were also excited by BW-723C86 (Figure 5). The DLH-evoked discharge of four inactive Group 2 cells was inhibited by both cardiopulmonary stimulation and MK-212 application (Figure 5).
Figure 4.
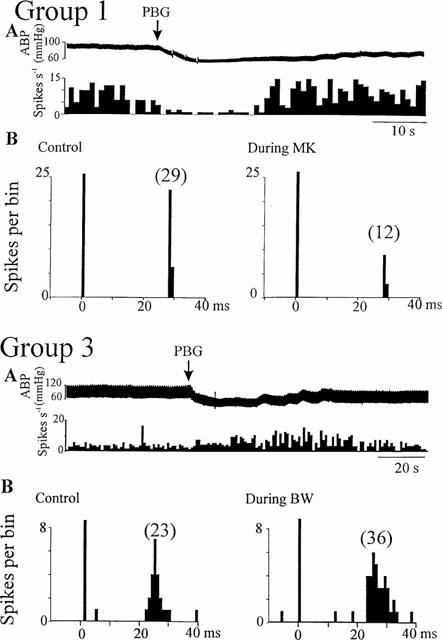
Activity of NTS neurones activated by non-myelinated vagal nerve stimulation during cardio-pulmonary afferent stimulation and ionophoretic application of 5-HT2 receptor ligands. Group 1 cell: (A) Ratemeter record (1 s bins) showing the inhibitory effects of cardio-pulmonary afferent stimulation (PBG, arrows), (B) peri-stimulus time histograms (PSTH, 1 ms bins, 40 sweeps) on the same cell showing that application of MK-212 (30 nA, right) reduced the number of evoked spikes compared to pre-drug (left). Group 3 cell: (A) Ratemeter record (1 s bins) showing the excitatory effect of cardio-pulmonary afferent stimulation (PBG, arrows), (B) peri-stimulus time histograms (PSTH, 1 ms bins, 40 sweeps) of the same cell showing that application of BW-723C86 (40 nA, right) increased the number of evoked spikes compared to pre-drug (left). ABP: arterial blood pressure. Vertical bars at t=0 ms represent the stimulus artefacts and numbers in brackets are the number of evoked discharges counted.
Figure 5.
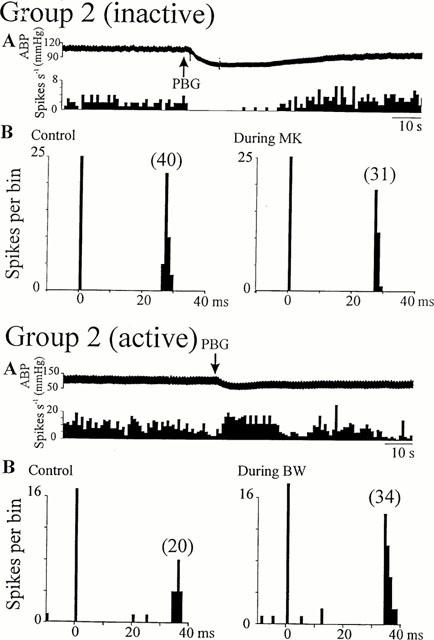
Activity of Group 2 NTS neurones activated by non-myelinated vagal nerve stimulation during cardio-pulmonary afferent stimulation and ionophoretic application of 5-HT2 receptor ligands. The activity of the inactive cell was increased by ionophoretic application of DLH. Top, inactive cell excited by application of DLH: (A) Ratemeter record (1 s bins) showing the inhibitory effect of cardio-pulmonary afferent stimulation (PBG, arrows), (B) peri-stimulus time histograms (PSTH, 1 ms bins, 40 sweeps) of the same cell showing that application of MK-212 (30 nA, right) reduced the number of evoked spikes compared to pre-drug (left). Bottom, cell with ongoing activity: (A) Ratemeter record (1 s bins) showing the excitatory effect of cardio-pulmonary afferent stimulation (PBG, arrows), (B) peri-stimulus histograms (PSTH, 1 ms bins, 40 sweeps) of the same cell showing that application of BW-723C86 (40 nA, right) increased the number of evoked spikes compared to pre-drug (left). ABP: arterial blood pressure. Vertical bars at t=0 ms represent the stimulus artefacts and numbers in brackets are the number of evoked discharges counted.
On six of the eight cells receiving myelinated vagal afferents, PBG inhibited the three of the five Group 1 cells inhibited by both DOI and MK-212 (Figure 3), but had no effect on all three cells from the Group 3 (Figure 3) or Group 2.
Discussion
The aim of the present study was to identify in vivo the presence of different 5-HT2 receptors on NTS neurones receiving vagal afferent input. The data confirm that the inhibitory effects of DOI on cells receiving putative ‘monosynaptic' vagal inputs and on inactive neurones receiving vagal afferents through a higher degree of synapses involve activation of 5-HT2C and 5-HT2A receptors (Sévoz-Couche et al., 2000a). In contrast, the majority of active neurones receiving an intermediary degree as well as the most ‘polysynaptic' vagal afferent input have both 5-HT2B and 5-HT2A receptors, which mediate excitation of these cells. On these vagally-activated cells, there was a high degree of correlation between the responses evoked by 5-HT2 receptors and cardio-pulmonary afferent activation.
In a previous study vagal-identified NTS cells were excited or inhibited by application of DOI, and 5-HT2 receptors were shown to play a role in transmission of cardiovascular inputs mediated by the vagus nerve (Wang et al., 1997). The variable effects of DOI can be explained since a heterogenous group of NTS neurones was recorded in that study whereas DOI has now been shown to inhibit cells close to the vagal input but excite those with a more polysynaptic input (Sévoz-Couche et al., 2000c). Both effects of DOI are likely due to activation of 5-HT2 receptors, since application of ketanserin, a 5-HT2A/2B/2C receptor antagonist (Van Nueten et al., 1981), prevented both. The central aim of the present study was to examine if different 5-HT2 receptors could be responsible for the dual effect of DOI on NTS cells. Also, since we reported that 5-HT2C receptors are probably responsible for inhibition in Group 1 and inactive Group 2 NTS cells (Sévoz-Couche et al., 2000a), in the present study, we applied MK-212 and DOI to cells also affected by BW-723C86 to test whether different 5-HT2 receptors are present on the same population of neurones.
Group 1 cells were usually inhibited by DOI and/or MK-212 but unaffected by BW-723C86. RS-102221 antagonized these inhibitions but not any excitatory actions of DOI on Group 1 cells, suggesting that Group 1 cell inhibition involves 5-HT2C but not 5-HT2B receptors, and that 5-HT2A receptors are likely to mediate their excitations. 5-HT2A and 5-HT2C receptors do not appear to coexist as DOI excitations were only observed on cells unaffected by MK-212 and DOI inhibitions were never converted to excitations by application of RS-102221.
Unlike Group 1, inactive Group 2 cells were inhibited by both MK-212 and BW-723C86 suggesting that both 5-HT2B and 5-HT2C receptors inhibit these cells. Whilst 5-HT2C receptors are undoubtably involved in the inhibition–it was reversed by the selective 5-HT2C receptor antagonist RS-102221, involvement of 5-HT2B receptors is more difficult to assess. Inhibitions evoked by BW-723C86 were not reduced by the selective 5-HT2B receptor antagonist LY-272015, but completely reversed in some cells tested by RS-102221. As BW-723C86 has only a 10 fold selectivity for 5-HT2B compared to 5-HT2C receptors, and there is a significant population of 5-HT2C receptors on these cells, BW-723C86 may have been acting relatively more at 5-HT2C than at 5-HT2B receptors, the inhibitory effect produced by 5-HT2C receptors masking any 5-HT2B-induced excitatory effect. Indeed, the structural differences between 5-HT2B and 5-HT2C receptors are small and could not be discriminated in the underlying mechanism of migraine (Kalkman, 1994). Finally, 5-HT2A receptors are unlikely to be present on these cells since DOI only inhibited inactive Group 2 cells that were also inhibited by MK-212 or/and BW-723C86, and this effect was antagonized by RS-102221.
The 5-HT2 receptor family is coupled to a G protein that activates a phospholipase C leading to excitation. However, previous reports suggest that 5-HT2C receptors could act on a Gi(1) protein (Chen et al., 1994) or inhibit forskolin-stimulated cyclic AMP production (Lucaites et al., 1996), that could explain this inhibitory effect of the 5-HT2C receptor activation. It is unclear whether the inhibitory effects reported here were direct. With ionophoresis it is impossible to avoid the possibility that drugs applied in the vicinity of recorded neurones may act also on antecedent neurones and/or presynaptic terminals, though at least the area of effect will be much more limited than bath applications used in vitro. Thus, we cannot rule out the possible activation of GABAergic interneurones in the 5-HT2C receptor induced inhibition. In addition, several studies have demonstrated co-localization of 5-HT and GABA in some neurones of the medulla oblongata, particularly in raphe magnus (Belin et al., 1983; Millhorn et al., 1988) and activation of 5-HT receptors modulates GABAA receptor function in neurones of the ventral tegmental area, substantia nigra and cerebellum (Pessia et al., 1994; Strahlendorf et al., 1991). Moreover, in co-expression studies in Xenopus oocytes Huidobro-Toro et al. (1996) demonstrated that 5-HT2C receptors could act on GABAA receptors.
5-HT2A and 5-HT2B but not 5-HT2C receptors seem to be present on active Group 2 and inactive Group 3 cells, and their activation leads to the excitation. The selective 5-HT2B receptor agonist BW-723C86 had only excitatory actions on these cells, a proportion of which were also excited by DOI. In addition, DOI excited some cells unaffected by the selective 5-HT2B and 5-HT2C agonists, an effect reversed only by ketanserin, suggesting that these cells exhibited only 5-HT2A receptors.
Finally, active Group 3 cells express mainly 5-HT2B receptors as only BW-723C86 could excite these cells, MK-212 having no effect and DOI exciting only those cells excited by BW-723C86. If 5-HT2A receptors are present on these cells, they must be on the same population of neurones than those expressing 5-HT2B receptors. Only selective ligands for the 5-HT2A receptor could help assess their involvement on the excitation of these cells.
Some cells were unaffected by the 5-HT2 receptor agonists, and whilst these may not express 5-HT2 receptors, it is possible that they are present, but at sites distant from the electrode and thus not accessible to effective agonist concentrations. However, neurones unresponsive to the 5-HT2 receptor ligands could always be excited by DLH.
The presence of 5-HT2 receptors within the NTS has been documented previously using autoradiographic techniques. Binding sites for ketanserin (Pazos et al., 1985; Dashwood et al., 1988) and both 5-HT2A and 5-HT2C receptors have been localized in the NTS (Pompeiano et al., 1994). However, the presence of 5-HT2B receptors in rat brain has been disputed, though they are found in the spinal cord (Kursar et al., 1992; Helton et al., 1994). However, a recent study did demonstrate expression of 5-HT2B receptors in some areas of rat brain (Duxon et al., 1997a) and these had functional effects (Duxon et al., 1997b). Although, no expression has been reported in the NTS, the present functional data suggests that they are present, at least on a restricted neuronal population, which may have been overlooked in these previous studies.
The vagus nerve contains aortic, cardiopulmonary, airway and gastro-intestinal afferent fibres so cells reported here will receive input from functionally diverse sources. To study a more homogenous population, the effect of 5-HT2 receptors on cells responding to activation of cardiopulmonary receptor afferents by right atrial injection of phenylbiguanide was investigated previously (Sévoz-Couche et al., 2000c). Cardiopulmonary stimulation mirrored the effects produced by activation of 5-HT2 receptors: excitation of putative polysynaptically activated cells and inhibition of those more likely to receive vagal monosynaptic inputs. In the present study, we confirmed and extended these results. Cardiopulmonary stimulation excited cells putative output neurones, consistent with a facilitatory role for 5-HT2 receptors in vagal cardiovascular reflexes (Sévoz et al., 1996a), and both 5-HT2B and 5-HT2A receptors seem to be those involved in this role. In contrast, cardiopulmonary afferents inhibited neurones closer to the afferent input and this seems to be mimicked by 5-HT2C receptors. Such inhibition might underlie a protective effect of this reflex on inputs from other vagal depressor cardiovascular reflexes such as the baroreflex. The effects of cardiopulmonary stimulation observed on cells with myelinated vagal afferent input support this hypothesis. Unlike fibres mediating cardiopulmonary reflexes which are non-myelinated, barosensitive fibres may be either myelinated or non-myelinated. In the present study most cells with myelinated afferent input were Group 1 cells, and these were inhibited both by MK-212 and by cardiopulmonary afferents.
The effects of the different 5-HT2 receptor antagonists on cardiopulmonary responses was not tested as it is unlikely that the cardiopulmonary pathway involves 5-HT2 activation since microinjections of ketanserin had no effect on the cardiopulmonary reflex (Sévoz et al., 1996b) and vagal-evoked responses, which would include cardiopulmonary afferents, were unaffected by these antagonists. It is more likely that the two inputs are independent but convergent since the cardiovascular responses evoked during cardiopulmonary reflexes and by 5-HT2 receptors are similar and involve the same medullary regions (Sévoz et al., 1996a). If, as seems likely, NTS 5-HT2 receptors play a facilitatory role in depressor reflexes, the site of origin of the 5-HT and the conditions under which it is released remains unclear. However, there are several possible sources including intrinsic neurones in the NTS itself (Calza et al., 1985) and the area postrema (Steinbusch, 1981). In addition, projections to the NTS from 5-HT-containing neurones in raphe magnus and nucleus paragigantocellularis at the medullary level and from the midline raphe and dorsal raphe nuclei in the pons. (Schaffar et al., 1988). Finally, some vagal afferents terminating in the NTS also contain 5-HT (Nosjean et al., 1990; Sykes et al., 1994). The physiological stimuli which activate these different inputs and whether they innervate different functional groups of NTS neurones is the basis of a further study.
In conclusion, previous data demonstrated that activating 5-HT2 receptors with DOI could excite or inhibit NTS neurones (Sévoz-Couche et al., 2000c). The present study showed that this dual effect appears to be correlated with the presumed nature of the vagal input to the cells, their response to cardiopulmonary afferent activation and the different 5-HT2 receptors activated. 5-HT2C receptors would be responsible for inhibitory responses whilst 5-HT2B and 5-HT2A receptors mediate the excitation. Activation of these different receptors on cells receiving specifically baro-sensitive and chemo-sensitive inputs will be tested in future studies.
Acknowledgments
This work was supported by grants from Fondation Pour la Recherche Médicale and The Wellcome Trust (Grant 055263).
Abbreviations
- BW-723C86
α-methyl-5-(2-thienylmethoxy)-1H-indole-3-ethanamine
- DLH
DL-Homocysteic Acid
- DMSO
dimethyl sulfoxide
- DOI
(±)-2,5-dimethoxy-4-iodoamphetamine HCl
- MK-212
6-chloro-2-(1-piperazinyl)pyrazine hydrochloride
- NTS
nucleus tractus solitarius
- PSTH
peristimulus time histogram
- RS-102221
8-(5-(2,4-Dimethoxy-5-(4-trifluoromethylphenylsulphonamido)phenyl-5-oxopentyl)-1,3,8-triazaspiro(4,5)decane-2,4-dione
References
- BELIN M.F., NANOPOULOS D., DIDIER M., AGUERA A., STEINBUSCH H., VERHOFSTAD A., MAITRE M., PUJOL J.F. Immunohistochemical evidence for the presence of gamma-aminobutyric acid and serotonin in one nerve cell. A study on the rpahe nuclei of the rat using antibodies to glutamate decarboxylase and serotonin. Brain Res. 1983;275:329–339. doi: 10.1016/0006-8993(83)90994-0. [DOI] [PubMed] [Google Scholar]
- BONHAUS D.W., WEINHARDT K.K., TAYLOR M., DE SOUZA A., MCNEELEY P.M., SZCZEPANSKI K., FONTANA D.J., TRINH J., ROCHA C.L., DAWSON M.W., FLIPPIN L.A., EGLEN R.M. RS-102221: a novel high affinity and selective 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:621–629. doi: 10.1016/s0028-3908(97)00049-x. [DOI] [PubMed] [Google Scholar]
- CALZA L., GIARDINO L., GRIMALDI R., RIGOLI M., STEINBUSCH H.W. Presence of 5-HT-positive neurons in the medial nuclei of the solitary tract. Brain Res. 1985;347:135–139. doi: 10.1016/0006-8993(85)90900-x. [DOI] [PubMed] [Google Scholar]
- CHEN Y., BAEZ M., YU L. Functional coupling of the 5-HT2C serotonin receptor to G proteins in Xenopus oocytes. Neurosci. Letts. 1994;179:100–102. doi: 10.1016/0304-3940(94)90944-x. [DOI] [PubMed] [Google Scholar]
- COHEN M.L., SCHENK K.W., MABRY T.E., NELSON D.L., AUDIA J.E. LY-272015, a potent, selective and orally active 5-HT2B receptor antagonist. J. Ser. Res. 1996;3:131–144. [Google Scholar]
- DASHWOOD M.R., GILBEY M.P., JORDAN D., RAMAGE A.G. Autoradiographic localisation of 5-HT1A binding sites in the brainstem of the cat. Br. J. Pharmacol. 1988;94:386P. [Google Scholar]
- DUXON M.S., FLANIGAN T.P., REAVLEY A.C., BAXTER G.S., BLACKBURN T.P., FONE K.C. Evidence for expression of the 5-hydroxytryptamine-2B receptor protein in the rat central nervous system. Neuroscience. 1997a;76:323–329. doi: 10.1016/s0306-4522(96)00480-0. [DOI] [PubMed] [Google Scholar]
- DUXON M.S., KENNETT G.A., LIGHTOWLER S., BLACKBURN T.P., FONE K.C. Activation of 5-HT2B receptors in the medial amygdala causes anxiolysis in the social interaction test in the rat. Neuropharmacology. 1997b;36:601–608. doi: 10.1016/s0028-3908(97)00042-7. [DOI] [PubMed] [Google Scholar]
- GOMMANS J., BOUWKNECHT J.A., HIJZEN T.H., BERENDSEN H.H., BROEKKAMP C.L., MAES R.A., OLIVIER B. Stimulus properties of fluvoxamine in a conditioned taste aversion procedure. Psychopharmacology. 1998;140:496–502. doi: 10.1007/s002130050794. [DOI] [PubMed] [Google Scholar]
- HALFORD J.C., LAWTON C.L., BLUNDELL J.E. The 5-HT2 receptor agonist MK-212 reduces food intake and increases resting but prevents the behavioural satiety sequence. Pharmacol. Biochem. Behav. 1997;56:41–46. doi: 10.1016/S0091-3057(96)00152-9. [DOI] [PubMed] [Google Scholar]
- HELTON L.A., THOR K.B., BAEZ M. 5-hydroxytryptamine2A, 5-hydroxytryptamine2B, and 5-hydroxytryptamine2C receptor mRNA expression in the spinal cord of rat, cat, monkey and human. NeuroReport. 1994;5:2617–2620. doi: 10.1097/00001756-199412000-00053. [DOI] [PubMed] [Google Scholar]
- HUIDOBRO-TORO J.P., VALENZUELA C.F., HARRIS R.A. Modulation of GABAA receptor function by G protein-coupled 5-HT2C receptors. Neuropharmacology. 1996;35:1355–1363. doi: 10.1016/s0028-3908(96)00084-6. [DOI] [PubMed] [Google Scholar]
- JORDAN D., SPYER K.M. Brainstem integration of cardiovascular and pulmonary afferent activity. Prog. Brain Res. 1986;67:295–313. doi: 10.1016/s0079-6123(08)62769-7. [DOI] [PubMed] [Google Scholar]
- KALIA M., MESULAM M. Brainstem projections of sensory and motor components of the vagus complex in the cat. J. Comp. Neurol. 1980;193:435–508. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- KALKMAN H.O. Is migraine prophylactic activity caused by 5-HT2B or 5-HT2C receptor blockade. Life Sci. 1994;54:641–644. doi: 10.1016/0024-3205(94)00546-x. [DOI] [PubMed] [Google Scholar]
- KURSAR J.D., NELSON D.L., WINSCOTT D.B., COHEN M.L., BAEZ M. Molecular cloning, functional expression, and pharmacological characterization of a novel serotonin receptor (5-hydroxytryptamine2F) from rat stomach fundus. Mol. Pharmacol. 1992;42:549–557. [PubMed] [Google Scholar]
- LUCAITES V.L., NELSON D.L., WAINSCOTT D.B., BAEZ M. Receptor subtype and density determine the coupling repertoire of the 5-HT2 receptor subfamily. Life Sci. 1996;59:1081–1095. doi: 10.1016/0024-3205(96)00423-7. [DOI] [PubMed] [Google Scholar]
- MERAHI N., ORER H.S., LAGUZZI R. 5-HT2 receptors in the nucleus tractus solitarius: characterization and role in cardiovascular regulation in the rat. Brain Res. 1992;575:74–78. doi: 10.1016/0006-8993(92)90425-9. [DOI] [PubMed] [Google Scholar]
- MILLHORN D.E., HOKFELT T., SEROOGY K., VERHOFSTAD A.A. Extent of colocalization of serotonin and GABA in neurons of the ventral medulla oblongata in rat. Brain Res. 1988;461:169–174. doi: 10.1016/0006-8993(88)90736-6. [DOI] [PubMed] [Google Scholar]
- NOSJEAN A., COMPOINT C., BUISSERET-DELMAS C., ORER H.S., MERAHI N., PUIZILLOUT J.J., LAGUZZI R. Serotonergic projections from the nodose ganglia to the nucleus tractus solitarius: an immunohistochemical and double labelling study in the rat. Neurosci. Letts. 1990;114:22–26. doi: 10.1016/0304-3940(90)90422-6. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The rat brain in stereotaxic coordinates. 4th edn. Academic Press, New York; 1998. [Google Scholar]
- PAZOS A., CORTES R., PALACIOS J.M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- PESSIA M., JIANG Z.G., NORTH R.A., JOHNSON S.W. Actions of 5-hydroxytryptamine on ventral tegmental area neurones of the rat in vitro. Brain Res. 1994;654:324–330. doi: 10.1016/0006-8993(94)90495-2. [DOI] [PubMed] [Google Scholar]
- POMPEIANO M., PALACIOS J.M., MENGOD G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Mol. Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- SCHAFFAR N., KESSLER J.P., BOSLER O., JEAN A. Central serotonergic projections to the nucleus tractus solitarii: evidence from a double labeling study in the rat. Neuroscience. 1988;26:951–958. doi: 10.1016/0306-4522(88)90111-x. [DOI] [PubMed] [Google Scholar]
- SÉVOZ C., HAMON M., LAGUZZI R. Medullary pathways of cardiovascular responses to 5-HT2 and 5-HT3 receptor stimulation in the rat nucleus tractus solitarius. NeuroReport. 1996a;7:1965–1969. doi: 10.1097/00001756-199608120-00021. [DOI] [PubMed] [Google Scholar]
- SÉVOZ C., NOSJEAN A., CALLERA J-C., MACHADO B., HAMON M., LAGUZZI R. Stimulation of 5-HT3 receptors in the NTS inhibits the cardiac Bezold-Jarisch reflex response. Am. J. Physiol. 1996b;271:H80–H87. doi: 10.1152/ajpheart.1996.271.1.H80. [DOI] [PubMed] [Google Scholar]
- SÉVOZ-COUCHE C., SPYER K.M., JORDAN D. Inhibition of rat nucleus tractus solitarius neurones by activation of 5-HT2C receptors. NeuroReport. 2000a;11:1785–1790. doi: 10.1097/00001756-200006050-00038. [DOI] [PubMed] [Google Scholar]
- SÉVOZ-COUCHE C., WANG Y., RAMAGE A.G., JORDAN D. Modulation of the nucleus tractus solitarii neurones by activation of 5-HT2 receptors in rats. J. Physiol. 2000b;523:266P. doi: 10.1016/s0028-3908(00)00055-1. [DOI] [PubMed] [Google Scholar]
- SÉVOZ-COUCHE C., WANG Y., RAMAGE A.G., JORDAN D. Modulation of neurones in the nucleus tractus solitarius (NTS) of the rat by 5-HT2 receptors. Neuropharmacology. 2000c;39:2006–2016. doi: 10.1016/s0028-3908(00)00055-1. [DOI] [PubMed] [Google Scholar]
- STEINBUSCH H.W.M. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- STRAHLENDORF J.C., LEE M.H., STRAHLENDORF H.K. Serotonin modulates muscimol- and baclofen- elicited inhibition of cerebellar Purkinje cells. Eur. J. Pharmacol. 1991;201:239–242. doi: 10.1016/0014-2999(91)90352-q. [DOI] [PubMed] [Google Scholar]
- SYKES R.M., SPYER K.M., IZZO P.N. Central distribution of substance P, calcitonin gene-related peptide and 5-hydroxytryptamine in vagal sensory afferents in the rat dorsal medulla. Neuroscience. 1994;59:195–210. doi: 10.1016/0306-4522(94)90110-4. [DOI] [PubMed] [Google Scholar]
- VAN NUETEN J.M., JANSSEN P.A., VAN BEEK J., XHONNEUX R., VERBEUREN T.J., VANHOUTTE P.M. Vascular effects of ketanserin (R 41468), a novel antagonist of 5-HT2 serotonergic receptors. J. Pharmacol. Exp. Ther. 1981;218:217–230. [PubMed] [Google Scholar]
- VERBERNE A.J.M., GUYENET P.G. Medullary pathway of the Bezold-Jarisch reflex in the rat. Am. J. Physiol. 1992;263:R1195–R1202. doi: 10.1152/ajpregu.1992.263.6.R1195. [DOI] [PubMed] [Google Scholar]
- WANG Y., RAMAGE A.G., JORDAN D. In vivo effects of 5-Hydroxytryptamine receptor activation on rat nucleus tractus solitarius neurones excited by vagal C-fibre afferents. Neuropharmacology. 1997;36:489–498. doi: 10.1016/s0028-3908(97)00063-4. [DOI] [PubMed] [Google Scholar]


