Abstract
The present study was undertaken to investigate the effects of the novel nociceptin receptor antagonist, [Nphe1]-Nociceptin (1-13)-NH2 (bilateral intrahippocampal injection, 50 nmole rat−1) on purported nociceptin-induced (bilateral intrahippocampal injection, 5 nmole rat−1) deficits in spatial learning in the rat Morris water maze task. In addition, experiments were performed in an ‘open field' to investigate possible peptide-induced changes in exploratory behaviour.
Nociceptin significantly impaired the ability of the animal to locate the hidden platform throughout training (P<0.001 versus control group).
Pretreatment with [Nphe1]-Nociceptin (1-13)-NH2 significantly blocked nociceptin-induced impairment of spatial learning (P<0.001 versus nociceptin group).
A probe trial revealed that vehicle-treated animals spent more time in the quadrant that had previously contained the hidden platform, whereas nociceptin-treated animals did not spend more time in any one quadrant.
Learning impairments were not attributable to non-specific deficits in motor performance or change in exploratory behaviour.
Taken together, our results reveal that [Nphe1]-Nociceptin (1-13)-NH2 represents an effective and useful in vivo antagonist at the nociceptin receptors involved in learning and memory.
Keywords: Nociceptin/orphanin FQ, nociceptin receptor antagonist, spatial learning, hippocampus
Introduction
A G-protein-coupled receptor, previously known as ORL 1 (Opioid Receptor-Like 1) now known as OP4/nociceptin (Hamon, 1998; Calo' et al., 2000a,2000b), which is sequentially homologous with opioid (OP) receptors but not activated by known opioid ligands, has recently been identified and cloned (Bunzow et al., 1994; Chen et al., 1994; Mollereau et al., 1994). An endogenous agonist for OP4/nociceptin receptors was later identified (Meunier et al., 1995; Reinscheid et al., 1995) and named orphanin FQ or nociceptin.
Nociceptin is a 17-amino acid peptide structurally related to opioid peptides, in particular, to dynorphin A. However, nociceptin is devoid of any activity at OP1, OP2 or OP3 opioid receptor subtypes (Meunier et al., 1995; Reinscheid et al., 1995). Since its discovery, a surge of scientific investigation has implicated a role for nociceptin in pain, fear and anxiety, feeding circadian rhythms (see Harrison & Grandy, 2000; Calo' et al., 2000b for recent reviews).
Moreover, in situ hybridization and immunohistochemical studies, in rodents, have demonstrated that both OP4/nociceptin mRNA and receptor protein are found in high concentrations in the hippocampus, brainstem and cortex, areas of the brain involved in higher functions including learning and memory processing (Henderson & McKnight, 1997; Meunier, 1997; Darland et al., 1998).
Several behavioural studies have suggested a role for nociceptin in learning and memory using different animal models. For example, the hippocampal administration of nociceptin impaired learning of a spatial memory task in rats (Sandin et al., 1997) and mice (Hiramatsu & Inoue, 1999), whereas nociceptin receptor knockout mice presented enhanced performance in spatial learning/attention tasks (Mamiya et al., 1998; Manabe et al., 1998; Nabeshima et al., 1999).
Recent studies concerning the possible role played by nociceptin in learning and memory have mainly concentrated on gene knockout (KO) experiments, as specific nociceptin receptor antagonists have not been available. While KO approaches are certainly most useful at present, it is difficult to exclude compensatory developmental effects of gene deletion. Recently one antagonist, [Nphe1]-Nociceptin (1-13)-NH2, which is devoid of any residual agonist activity has been developed (Calo' et al., 2000a). This novel molecule selectively antagonized the effects of nociceptin in vitro in various isolated tissues and in CHO cells expressing the human recombinant nociceptin receptor (Rizzi et al., 1999; Calo' et al., 2000b; Hashimoto et al., 2000). [Nphe1]-Nociceptin (1-13)-NH2 is also active in vivo to prevent the pro-nociceptive and anti-morphine actions of nociceptin in the mouse tail withdrawal assay and the hyperphagic effect of nociceptin in the rat (Calo'et al ., 1998; Polidori et al., 2000).
It was therefore of significant interest to investigate the effects of this new antagonist on purported nociceptin-induced deficits in spatial learning in the Morris water maze task, a behavioural model in which the ability of a rat to locate a hidden platform is dependent on the integrative functioning of the hippocampal formation (Morris et al., 1982). In addition, experiments were performed to investigate any peptide-induced changes in exploratory behaviour that may have interfered with the interpretation of Morris water maze data.
Methods
Subjects
Forty-eight young adult male Sprague Dawley rats (Charles River, St-Constant, Quebec, Canada), weighing 350–400 g, were housed under standard laboratory conditions (12 h light/12 h dark cycle, lights on at 0700 h, food and water ad libitum). Animal care was provided according to protocols and guidelines approved by McGill University and the Canadian Council of Animal Care.
Surgery
Prior to administration of anaesthetic, animals received an intraperitoneal injection (IP) of sterile atropine sulphate (0.04 mg kg−1) to minimize respiratory difficulties that may have been invoked by the anaesthetic. Following a delay of 15 min, animals were carefully weighed and administered sodium pentobarbital (50 mg kg−1, IP). The respiratory rate was monitored throughout the entire surgical procedure. Animals were then implanted bilaterally with permanent 26 gauge steel guide cannula (Plastics One, Roanoke, VA, U.S.A.) in the CA3 region of the dorsal hippocampus. Coordinates were taken from the atlas of Paxinos & Watson (1986): anterior-posterior, −3.3 mm with respect to bregma; lateral, ±2.7 mm with respect to the midsagittal suture line; ventral, −4.3 mm (injection site) with respect to the surface of the skull. The animals were then placed under a warming lamp until they had sufficiently recovered from the anaesthetic, before being individually housed for a period of 7 days post-surgery.
Treatment
The rats were randomly divided into four groups (n=12) and treated with: (1) isotonic NaCl→5 nmole nociceptin (control/nociceptin group); (2) 50 nmole [Nphe1]-Nociceptin (1-13)-NH2→isotonic NaCl (antagonist/control group); (3) 50 nmole [Nphe1]-Nociceptin (1-13)-NH2→5 nmole nociceptin (antagonist/nociceptin group) or (4) isotonic NaCl→isotonic NaCl (control/control group), injected bilaterally (total injection volume: 1 μl; flow-rate: 0.5 μl min−1) with a microinfusion pump (Carnegie Medicine, Stockholm, Sweden). Pretreatment was performed 5 min before the second injection, and testing began 15 min after the second intrahippocampal injection. Doses (considered intermediate) were chosen on the basis of previously reported in vivo studies, together with binding data of nociceptin and [Nphe1]-Nociceptin (1-13)-NH2 in transfected CHO cells expressing recombinant human OP4 receptors (Calo' et al., 2000a,2000b; Sandin et al., 1997).
Spatial learning
A circular pool, 160 cm in diameter, was used for Morris water maze experiments (Quirion et al., 1995). The pool was filled up with tap water, made opaque with powdered milk, and maintained at 22±1°C. The escape platform (diameter 15 cm) was placed in the pool so that it was submerged 2 cm below the water surface. All animals were given four trials per day over three consecutive days. For each trial, the rat was placed in the pool (facing pool wall) at one of four selected starting points (North, South, East or West pole). On locating the platform, the rat was allowed to remain there for 30 s before being returned to its cage. If the rat did not find the platform within 120 s, it was guided to the platform by hand and allowed to remain there for 30 s. An inter-trial period of 5 min was employed. The escape latency and swim speed were measured by a video tracking system connected to a computer equipped with the commercially available HVS image system (HVS, U.K.) for the analysis of Morris water maze performance. On the morning after the last training trial, animals were subjected to a probe trial in which the platform was removed. Animals were placed in the pool at the same pole and allowed to swim for 2 min. The time that each animal spent in the quadrant that had previously contained the hidden platform was measured. This trial is necessary to confirm that the spatial navigation task was learned using environmental cues. Afterwards, animals were subjected to another trial in which the platform was made visible, thus eliminating any misinterpretation of results due to drug-induced impairment of vision.
Exploratory behaviour
Seven days after the last trial, rats received isotonic NaCl, nociceptin or [Nphe1]-Nociceptin (1-13)-NH2 as described above, 15 min before examination of exploratory behaviour in an ‘open field' (Gray & Lalljee, 1974). Each animal was placed into the centre of the apparatus, which consisted of a square base (90×90 cm) divided into 10 cm squares by faint black lines. The wall surrounding the base consisted of a 75 cm high wall covered with aluminium foil. Illumination was provided by a 60 W bulb, positioned 90 cm above the floor of the apparatus. The number of squares crossed, rearing, grooming and faecal boli deposited were measured over a 3 min test period by an investigator unaware of the treatment regime. Such a testing period has been shown to be sufficient to detect changes in exploratory behaviour in a novel environment. Immediately after the experiments, all rats were decapitated and the brain was removed and sliced to visually confirm the position of the cannulae in fresh tissue. No subjects were excluded from the data analysis because of improper cannula placement, and tissue damage was minimal.
Statistics
Statistical analyses were performed using a two-way analysis of variance where treatment and trial were first and second factors, followed by post hoc Bonferroni tests to assess individual group differences. Data were considered statistically significant when P<0.05.
Results
As shown in Figure 1A, bilateral intrahippocampal injection of 5 nmole nociceptin significantly impaired the rat's ability to locate the hidden platform throughout the 3 days training, compared with control performance (F1,66=177.5, P<0.001). Fifty nmole [Nphe1]-Nociceptin (1-13)-NH2 did not display any effect per se but significantly antagonized nociceptin-induced impairment of spatial learning (F1,66=64.13, P<0.001). It is noteworthy that nociceptin-treated animals failed to show any trial-to-trial improvement in spatial learning, whereas animals that were pretreated with [Nphe1]-Nociceptin (1-13)-NH2 presented learning patterns that were not significantly different from control animals. Escape latencies of animals that were treated with [Nphe1]-Nociceptin (1-13)-NH2 alone were similar to that of the isotonic NaCl-treated control group (Figure 1B).
Figure 1.

Effect of intrahippocampal injection of nociceptin and [Nphe1]-Nociceptin (1-13)-NH2 on escape latency in the Morris water maze task (hidden platform). Animals were microinjected with isotonic NaCl, 5 nmole nociceptin, 50 nmole [Nphe1]-Nociceptin (1-13)-NH2 or [Nphe1]-Nociceptin (1-13)-NH2/nociceptin. (A) Data are expressed as mean latency±s.e.mean (averaged over four trials per session; n=12). (B) Data are expressed as mean latency±s.e.mean of each trial (12 trials during training; n=12). **P<0.01, ***P<0.001 versus NaCl; +P<0.05, ++P<0.01, +++P<0.001 versus nociceptin.
Results from the probe trial indicated that vehicle-treated control animals had learned the spatial navigation task using environmental cues as they spent more time in the quadrant (goal area) that had contained the hidden platform during training (Figure 2). In contrast, nociceptin-treated animals did not spend more time in any one specific quadrant. Animals treated in combination with nociceptin/[Nphe1]-Nociceptin (1-13)-NH2, or [Nphe1]-Nociceptin (1-13)-NH2 alone, displayed similar activity to vehicle-treated control animals, i.e., they spent more time in the goal area of the pool.
Figure 2.

Effect of intrahippocampal injection of nociceptin and [Nphe1]-Nociceptin (1-13)-NH2 on escape latency in the Morris water maze task (probe trial). Animals were microinjected with isotonic NaCl, 5 nmole nociceptin, 50 nmole [Nphe1]-Nociceptin (1-13)-NH2 or [Nphe1]-Nociceptin (1-13)-NH2/nociceptin. Data are expressed as mean latency± s.e.mean (n=12). **P<0.01 versus NaCl.
Analysis of swim speed indicated that there were no significant differences in swimming ability between control group and drug-treated groups (Figure 3). When animals were presented with a visible platform, there were no significant differences in the time taken to locate the platform between vehicle-control and drug-treated animals (Figure 4).
Figure 3.
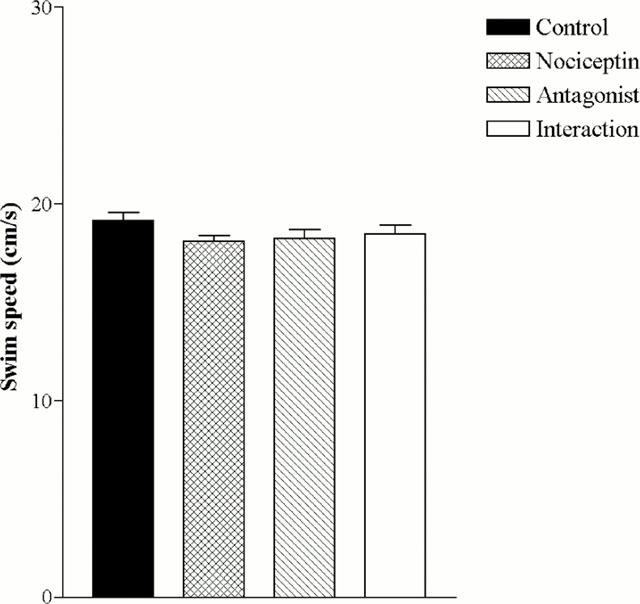
Effect of intrahippocampal injection of nociceptin and [Nphe1]-Nociceptin (1-13)-NH2 on swim speed in the Morris water maze task (hidden platform). Animals were microinjected with isotonic NaCl, 5 nmole nociceptin, 50 nmole [Nphe1]-Nociceptin (1-13)-NH2 or [Nphe1]-Nociceptin (1-13)-NH2/nociceptin. Data are expressed as cumulative mean latency±s.e.mean (averaged over 12 trial training sessions; n=12).
Figure 4.

Effect of intrahippocampal injection of nociceptin on escape latency in the Morris water maze task (visible platform). Animals were microinjected with isotonic NaCl, 5 nmole nociceptin, 50 nmole [Nphe1]-Nociceptin (1-13)-NH2 or [Nphe1]-Nociceptin (1-13)-NH2/nociceptin. Data are expressed as mean latency±s.e.mean (n=12).
The results from the ‘open field' test are shown in Figure 5. Analysis of the data revealed no significant differences in ambulation, exploratory rearing, grooming or faecal boli deposited between the different groups.
Figure 5.

Effect of intrahippocampal injection of nociceptin and [Nphe1]-Nociceptin (1-13)-NH2 on exploratory behaviour. Animals were microinjected with isotonic NaCl, 5 nmole nociceptin, 50 nmole [Nphe1]-Nociceptin (1-13)-NH2 or [Nphe1]-Nociceptin (1-13)-NH2/nociceptin. Data are expressed as mean±s.e.mean (n=12).
Discussion
The present study demonstrates that bilateral injection of nociceptin into the CA3 region of the hippocampus induced a significant impairment in the learning of a spatial memory task, the Morris water maze, which is dependent on the integrative functioning of the hippocampal formation (Morris et al., 1982). Such impairments were not attributable to non-specific deficits in motor performance or change in exploratory behaviour. Most importantly, pretreatment with [Nphe1]-Nociceptin (1-13)-NH2, a nociceptin receptor antagonist, prevented nociceptin-induced deficits. To our knowledge this is the first evidence using a pure pharmacological approach, i.e. nociceptin receptor blockade, that indicates a role for the nociceptin and its receptor in learning and memory. Other studies have used a molecular strategy, i.e., gene knockout. The present approach rules out the possibility of developmental interference following gene deletion. Our data support a role for nociceptin and its receptor in cognitive processes.
Behavioural studies, in which the influence of OP4/nociceptin receptors is removed by gene knockout, have suggested that nociceptin may have some tonic control over learning and memory processing (Mamiya et al., 1998; Manabe et al., 1998; Hiramatsu & Inoue, 1999; Nabeshima et al., 1999). In the present experiments, intrahippocampal administration of [Nphe1]-Nociceptin (1-13)-NH2 alone failed to enhance learning of the task when compared to control performance. Hence additional events (occurring during embryogenesis and development) invoking compensatory adaptation following gene deletion, could explain the apparent tonic effect of nociceptin on learning observed in previous studies.
The results of the present study cannot be explained on the basis of motor impairments in the Morris water maze apparatus, as analysis of swim speed did not reveal any differences in swimming ability between control or drug-treated groups. In addition, possible nociceptin-induced impairment of visual capacity was ruled out by the results obtained when the escape platform was made visible. Nociceptin-treated animals located the platform with an escape latency comparable to that of NaCl-treated control animals. Hence, nociceptin-induced impairment of learning is a specific cognitive effect and is truly mediated by nociceptin receptors as these effects are antagonized by [Nphe1]-Nociceptin (1-13)-NH2.
Testing in the ‘open field', revealed that drug treatment did not significantly affect ambulation in a novel environment or exploratory rearing. Previous studies have suggested a role for nociceptin in the mediation of exploratory locomotor behaviour. Such changes in locomotor activity were detected in the first 5 min of testing, and support the use of a 3 min testing period in the present study. However, this subject remains controversial with both decreases (Reinscheid et al., 1995; Devine et al., 1996; Sandin et al., 1997) and increases (Florin et al., 1996) in locomotion being reported. A possible explanation is related to doses, with lower doses of nociceptin inducing increased locomotor activity while higher doses decreased exploratory locomotor behaviour. The dose of nociceptin used in the present study would be considered intermediate compared to those previously reported in in vivo studies (Calo' et al., 2000b). A further explanation may be that the animals became tolerant to nociceptin-induced changes in locomotion. Indeed, it has been demonstrated that rats rapidly developed tolerance to the locomotor-inhibiting effects of nociceptin, and this tolerance was still apparent 7 days after the final injection (Devine et al., 1996). In addition, previous studies have used intracerebroventricular injections of nociceptin, after which the peptide may be rather freely distributed possibly acting on brain regions involved in motor behaviours. In contrast, the results of the present study were obtained after discrete injections of nociceptin into the CA3 region of the hippocampus, and suggest that this region may not play an important role in the mediation of exploratory locomotor activity induced by nociceptin and its receptors. However, firm conclusions are difficult to substantiate as the present study used only one dose of each peptide.
Recent studies have suggested possible cellular mechanisms involved in nociceptin-induced amnesia. It has been shown that nociceptin inhibited the release of glutamate from cerebral cortical slices (Nicol et al., 1996) and inhibited Ca2+ currents in hippocampal pyramidal cells (Knoflach et al., 1996). In addition, electrophysiological studies have demonstrated that OP4/nociceptin receptors can couple to inwardly rectifying K+ channels in hippocampal preparations, and that nociceptin induces the activation of such channels (Ikeda et al., 1997). Furthermore, nociceptin inhibited synaptic transmission and synaptic plasticity such as long-term potentiation (LTP) in the rat hippocampus and dentate gyrus (Yu et al., 1997; Yu & Xie, 1998). [Nphe1]-Nociceptin (1-13)-NH2, by blocking nociceptin receptors, may prevent such agonist-induced effects and hence antagonize nociceptin-induced impairments of learning and memory.
The pharmacokinetics of nociceptin have not yet been studied in detail. Few experiments have been performed investigating the metabolism of the peptide in vitro in human blood (Yu et al., 1996), in cell culture (Vlaskovska et al., 1999), and in mouse brain cortical slices (Montiel et al., 1997). In vivo experiments in the rat hippocampus (Sandin et al., 1997) and in the mouse brain after intracerebroventricular administration (Noble & Roques, 1997) have also been performed. In the latter study, it was demonstrated that the association of different peptidase inhibitors potentiated the behavioural effects of nociceptin on motor behaviour, suggesting that as for other neuropeptides, interruption of the responses induced by nociceptin is ensured by enzymes which cleave the peptide into inactive fragments. To our knowledge, there are no data related to the pharmacokinetics of [Nphe1]-Nociceptin (1-13)-NH2. The few experiments performed in vivo with this molecule indicated that its duration of action is similar to that of nociceptin, around 30 min (See Calo' et al., 2000b for review).
In summary, the results from the present study support and extend those of Sandin et al. (1997) in that bilateral intrahippocampal (CA3 region) injection of nociceptin provoked marked impairments in spatial learning in the Morris water maze task, results which are consistent with the dense localization of OP4/nociceptin receptors in the hippocampal formation (Mollereau et al., 1994). Most interestingly, this study demonstrates that such learning impairments are truly mediated by OP4/nociceptin receptors, as pretreatment with the recently developed nociceptin receptor antagonist, [Nphe1]-Nociceptin (1-13)-NH2, reversed nociceptin-induced deficits in escape latency. By itself, this antagonist was devoid of any activity. Taken together, this study supports the idea that [Nphe1]-Nociceptin (1-13)-NH2 represents an effective in vivo antagonist at the nociceptin receptors involved in learning and memory.
Acknowledgments
This study was supported by a Medical Research Council of Canada grant to R. Quirion.
Abbreviations
- Ca2+
calcium ion
- CHO
Chinese hamster ovary
- KO
knockout
- NaCl
sodium chloride
- OP
opioid
- ORL
opioid receptor-like
References
- BUNZOW J.R., SAEZ C., MORTRUD M., BOUVIER C., WILLIAMS J.T., LOW M., GRANDY D.K. Molecular cloning and tissue distribution of a putitive member of the rat opioid receptor gene family that is not mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- CALO' G., GUERRINI R., BIGONI R., RIZZI A., MARZOLA G., OKAWA H., BIANCHI C., LAMBERT D.G., SALVADORI S., REGOLI D. Characterization of [Nphe1]Nociceptin (1-13)-NH2, a new selective nociceptin antagonist. Br. J. Pharmacol. 2000a;129:1183–1193. doi: 10.1038/sj.bjp.0703169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALO' G., GUERRINI R., RIZZI A., SALVADORI S., REGOLI D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br. J. Pharmacol. 2000b;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALO' G., RIZZI A., MARZOLA G., GUERRINI R., SALVADORI S., BEANI L., REGOLI D., BIANCHI C. Pharmacological characterization of the nociceptin receptor mediating hyperalgesia in the mouse tail withdrawal assay. Br. J. Pharmacol. 1998;125:373–378. doi: 10.1038/sj.bjp.0702087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Y., FAN Y., LIU J., MESTEK A., TIAN M., KOZAK C.A., YU L. Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 1994;347:279–283. doi: 10.1016/0014-5793(94)00560-5. [DOI] [PubMed] [Google Scholar]
- DARLAND T., HEINRICHER M.M., GRANDY D.K. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- DEVINE D.P., TAYLOR L., REINSCHEID R.K., MONSMA F.J., CIVELLI O., AKIL H. Rats rapidly develop tolerance to locomotor-inhibiting effects of the novel neuropeptide orphanin FQ. Neurochem. Res. 1996;21:1387–1396. doi: 10.1007/BF02532380. [DOI] [PubMed] [Google Scholar]
- FLORIN S., SUAUDEAU C., MEUNIER J.C., COSTENTIN J. Nociceptin stimulates locomotion and exploratory behavior in mice. Eur. J. Pharmacol. 1996;317:9–13. doi: 10.1016/s0014-2999(96)00707-8. [DOI] [PubMed] [Google Scholar]
- GRAY J.A., LALLJEE B. Sex differences in emotional behaviour in the rat: correlation between ‘open field' defaecation and active avoidance. Anim. Behav. 1974;22:856–861. doi: 10.1016/0003-3472(74)90008-6. [DOI] [PubMed] [Google Scholar]
- HAMON M. The new approach to opioid receptors. N.S. Arch. Pharmacol. 1998;358:SA 5.3. [Google Scholar]
- HARRISON L.M., GRANDY D.K. Opiate modulating properties of nociceptin/orphanin FQ. Peptides. 2000;21:151–172. doi: 10.1016/s0196-9781(99)00185-0. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO Y., CALO' G., GUERRINI R., SMITH G., LAMBERT D.G. Antagonistic effects of [Nphe1]Nociceptin (1-13)-NH2 on nociceptin mediated inhibition of cAMP formation in Chinese ovary cells stably expressing the recombinant human nociceptin receptor. Neurosci. Lett. 2000;278:109–112. doi: 10.1016/s0304-3940(99)00915-5. [DOI] [PubMed] [Google Scholar]
- HENDERSON G., MCKNIGHT A.T. The orphan opioid receptor and its endogenous ligand–nociceptin/orphanin FQ. Trends Pharmacol. Sci. 1997;18:293–300. [PubMed] [Google Scholar]
- HIRAMATSU M., INOUE K. Effects of nocistatin on nociceptin-induced impairment of learning and memory in mice. Eur. J. Pharmacol. 1999;367:151–155. doi: 10.1016/s0014-2999(99)00003-5. [DOI] [PubMed] [Google Scholar]
- IKEDA K., KOBAYASHI K., KOBAYASHI T., ICHIKAWA T., KUMANISHI T., KISHIDA H., YANO R., MANABE T. Functional coupling of the nociceptin/orphanin FQ receptor with the G-protein-activated K+ (GIRK) channel. Mol. Brain Res. 1997;45:117–126. doi: 10.1016/s0169-328x(96)00252-5. [DOI] [PubMed] [Google Scholar]
- KNOFLACH F., REINSCHEID R.K., CIVELLI O., KEMP J.A. Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. J. Neurosci. 1996;16:6657–6664. doi: 10.1523/JNEUROSCI.16-21-06657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAMIYA T., NODA Y., NISHI M., TAKESHIMA H., NABESHIMA T. Enhancement of spatial attention in nociceptin/orphanin FQ receptor-knockout mice. Brain Res. 1998;783:236–240. doi: 10.1016/s0006-8993(97)01406-6. [DOI] [PubMed] [Google Scholar]
- MANABE T., NODA Y., MAMIYA T., KATAGIRI H., HOUTANI T., NISHI M., NODA T., TAKAHASHI T., SUGIMOTO T., NABESHIMA T., TAKESHIMA H. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature. 1998;394:577–581. doi: 10.1038/29073. [DOI] [PubMed] [Google Scholar]
- MEUNIER J.C. Nociceptin/orphanin FQ and the opioid receptor-like ORL 1 receptor. Eur. J. Pharmacol. 1997;340:1–15. doi: 10.1016/s0014-2999(96)00919-3. [DOI] [PubMed] [Google Scholar]
- MEUNIER J.C., MOLLEREAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.C., FERRARA P., MONSERRAT B., MAZARGUIL H., VASSART G., PARMENTIER M., COSTENTIN J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL 1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MOLLEREAU C., PARMENTIER M., MAILLEUX P., BUTOUR J.L., MOISAND C., CHALON P., CAPUT D., VASSART G., MEUNIER J.C. ORL 1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- MONTIEL J.L., CORNILLE F., ROQUES B.P., NOBLE F. Nociceptin/orphanin FQ metabolism: role of aminopeptidase and endopeptidase 24.15. J. Neurochem. 1997;68:354–361. doi: 10.1046/j.1471-4159.1997.68010354.x. [DOI] [PubMed] [Google Scholar]
- MORRIS R.G.M., GARRUD P., RAWLINS J.N.P., O'KEEFE J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- NABESHIMA T., NODA Y., MAMIYA T. The role of nociceptin in cognition. Brain Res. 1999;848:167–173. doi: 10.1016/s0006-8993(99)01906-x. [DOI] [PubMed] [Google Scholar]
- NICOL B., LAMBERT D.G., ROWBOTHAM D.J., SMART D., MCKNIGHT A.T. Nociceptin induced inhibition of K+ evoked glutamate release from rat cerebrocortical slices. Br. J. Pharmacol. 1996;119:1081–1083. doi: 10.1111/j.1476-5381.1996.tb16007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOBLE F., ROQUES B.P. Association of aminopeptidase N and endopeptidase 24.15 inhibitors potentiate behavioural effects mediated by nociceptin/orphanin FQ in mice. FEBS Lett. 1997;401:227–229. doi: 10.1016/s0014-5793(96)01476-7. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The rat brain in stereotaxic coordinates. Orlando FL: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- POLIDORI C., CALO' G., CICCOCIOPPO R., GUERRINI R., REGOLI D., MASSI M. Pharmacological characterization of the nociceptin receptor mediating hyperphagia: identification of a selective antagonist. Psychopharmacology. 2000;148:430–437. doi: 10.1007/s002130050073. [DOI] [PubMed] [Google Scholar]
- QUIRION R., WILSON A., ROWE W., AUBERT I., RICHARD J., DOODS H., PARENT A., WHITE N., MEANEY M.J. Facilitation of acetylcholine release and cognitive performance by an M(2)-muscarinic receptor antagonist in aged memory-impaired. J. Neurosci. 1995;15:1455–1462. doi: 10.1523/JNEUROSCI.15-02-01455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J. , Jr, CIVELLI O. Orphanin FQ: a neuropeptide that activates an opioid-like G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- RIZZI A., BIGONI R., CALO' G., GUERRINI R., SALVADORI S., REGOLI D. [Nphe1]Nociceptin (1-13)-NH2 antagonizes nociceptin effects in the mouse colon. Eur. J. Pharmacol. 1999;385:R3–R5. doi: 10.1016/s0014-2999(99)00730-x. [DOI] [PubMed] [Google Scholar]
- SANDIN J., GEORGIEVA J., SCHÖTT P.A., ÖGREN S.O., TERENIUS L. Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur. J. Neurosci. 1997;9:149–197. doi: 10.1111/j.1460-9568.1997.tb01367.x. [DOI] [PubMed] [Google Scholar]
- VLASKOVSKA M., KASAKOV L., SUDER P., SILBERRING J., TERENIUS L. Biotransformation of nociceptin/orphanin FQ by enzyme activity from morphine-naïve and morphine treated cell cultures. Brain Res. 1999;818:212–220. doi: 10.1016/s0006-8993(98)01266-9. [DOI] [PubMed] [Google Scholar]
- YU T.P., XIE C.W. Orphanin FQ/nociceptin inhibits synaptic transmission and long-term potentiation in rat dentate gyrus through postsynaptic mechanisms. J. Neurophysiol. 1998;80:1277–1284. doi: 10.1152/jn.1998.80.3.1277. [DOI] [PubMed] [Google Scholar]
- YU T.P., CHAIT B.T., TOLL L., KREEK M.J. Nociceptin in vitro biotransformation in human blood. Peptides. 1996;17:873–876. doi: 10.1016/0196-9781(96)00079-4. [DOI] [PubMed] [Google Scholar]
- YU T.P., FEIN J., PHAN T., EVANS C.J., XIE C.W. Orphanin FQ inhibits synaptic transmission and long-term potentiation in rat hippocampus. Hippocampus. 1997;7:88–94. doi: 10.1002/(SICI)1098-1063(1997)7:1<88::AID-HIPO9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]


