Abstract
The Ca2+-sensor protein troponin C (TnC) exerts a key role in the regulation of muscle contraction, and constitutes a target for Ca2+ sensitizer compounds, such as bepridil, known to increase its apparent Ca2+ affinity. Moreover, bepridil has been reported to exert a differential effect in slow and fast skeletal muscle fibres, which express the slow/cardiac and fast TnC isoform, respectively.
The role of the TnC isoform in establishing the differential effect of bepridil was assessed in slow soleus and fast tibialis rat skinned fibres, by extraction of endogenous TnC and consecutive reconstitution with either slow or fast recombinant TnC. A mutant (VG2), lacking the regulatory site II, was also used to distinguish the role of each regulatory site.
Fast tibialis fibres reconstituted with cardiac TnC exhibited a typical slow bepridil reactivity, while slow soleus fibres reincorporated with fast TnC displayed a typically fast reactivity to bepridil. These results indicated that the differential effect of bepridil in slow and fast fibres is related to the TnC isoform predominantly expressed in a fibre.
Experiments with the VG2 mutant demonstrated that BPD can achieve an increase in the Ca2+ affinity in the absence of a functional site II. Thus, site I was necessary for the BPD effect to be independent of the Ca2+ concentration. Moreover, the amplitude of the reinforcement in the Ca2+ affinity, induced by the binding of bepridil to the TnC molecule, is dependent on the number of functional regulatory sites, the larger affinity reinforcement being detected when only one regulatory site (either site I or II) is functional.
Keywords: Ca2+-sensitizer drug, bepridil, TnC substitution, TnC mutant, skinned fibres
Introduction
Troponin C (TnC), a dumbell shaped protein, is one of the three subunits of the regulatory complex that acts as a Ca2+ sensor, and initiates a cascade of events leading to muscle fibre contraction when Ca2+ concentration rises. The TnC protein represents a major target for molecules called ‘Ca2+ sensitizers', which are able to enhance the Ca2+ responsiveness of the contractile system. Bepridil (BPD) belongs to this class of compounds, and was demonstrated to increase the apparent Ca2+ affinity of cardiac muscle fibres (Solaro et al., 1986; Herzig & Quast, 1992), as well as skeletal muscle fibres (Kischel et al., 1999). However, a differential effect of BPD was observed between fast and slow skeletal fibres which express distinct TnC isoforms: a fast skeletal and a slow skeletal/cardiac one, respectively (Burtnick & Kay, 1977). The fast skeletal isoform (TnCf) has four Ca2+ binding sites: two low affinity amino terminal sites I and II, that regulate muscle contraction (Potter & Gergely, 1975), plus two high affinity carboxyterminal sites III and IV that maintain TnC in the regulatory complex (Zot & Potter, 1982). Ca2+ fixation to site II occurs primarily and induces minor but essential conformational changes for contraction trigger, while Ca2+ binding to site I induces a structural opening of the N-lobe via subsequent conformational changes (Sia et al., 1997). Due to an insertion as well as a double substitution of critical amino acids, site I of the slow/cardiac isoform (TnCc) is rendered non-functional, leaving the amino-terminal lobe partially closed upon activation of only site II.
The sensitizing effect of BPD on the fast isoform in the presence of Ca2+ was attributed to a stabilization of the Ca2+-induced conformational changes by fixation of the drug on the hydrophobic amino acids exposed by the opened N-lobe, inducing a decrease of the Ca2+ off-rate (MacLachlan et al., 1990). Recently, it has been shown, in vitro, that BPD itself could induce an opening of the N-lobe of the cardiac isoform (similar to the N-lobe of 4Ca2+-skeletal TnC), and a stabilization of this N-lobe (Li et al., 2000).
However, in vivo, it has been previously shown that slow skeletal fibres were more drug reactive than the fast ones, and showed a BPD reactivity dependent on the Ca2+ concentration, the higher tension reinforcement being obtained at low activation levels. On the contrary, the sensitizing effect of the drug in fast fibres was quite independent of the Ca2+ concentration (Kischel et al., 1999). Therefore, it appeared relevant to determine whether this differential effect of BPD in slow and fast fibres was due to the TnC isoform only, or resulted from integrated effects within the myofibrillar lattice downstream of TnC.
For this purpose, we used extraction-replacement experiments of TnC. Following extraction of native slow (TnCs) and fast (TnCf) skeletal TnC from slow and fast rat fibres respectively, recombinant TnC were reincorporated: cardiac mouse TnC (identical to TnCs) was added in fast tibialis anterior muscle fibres, while chicken fast TnC was substituted for TnCs in soleus fibres. In an attempt to correlate more precisely the effect of BPD to the number of active regulatory sites and possibly attribute a specific role to each site, we also used a previously described mutant called ‘VG2' (Sheng et al., 1990), which is unable to bind Ca2+ in site II but retains the ability to bind Ca2+ in site I. This latter was shown to allow partial activation of fast fibres (Sheng et al., 1990; Putkey et al., 1991): therefore, the VG2 mutant was substituted for TnCf in a fast regulatory environment.
All the results indicated that TnC appeared as the protein responsible for the differential bepridil reactivity of slow and fast fibres, the role of each regulatory site being distinguishable. Moreover, our data provided evidence that the TnC isoform regulated the fibre sensitivity to Sr2+ ions as well as the Ca2+ sensitivity in the skeletal rat fibres.
Methods
Animals and muscle preparation
The experiments as well as the maintenance conditions of the animals received authorizations from the Ministry of Agriculture and the Ministry of Education (veterinary service of health and animal protection, authorization 03805). Experiments were carried out on soleus and tibialis anterior muscles of adult male Wistar rats (Iffa Credo, l'Arbresle, France). These muscles were chosen for their homogeneity in fibre types: according to the myosin ATPase activity, the relative proportions of slow oxidative, fast oxidative and fast glycolytic fibres are 87, 13, 0% in the soleus and 1, 27, 72% in the white tibialis anterior, respectively (Armstrong & Phelps, 1984).
Muscles were removed from animals anaesthetized with an intraperitoneal injection of pentobarbitone sodium (3 mg kg−1). Chemically skinned fibres were prepared as previously described (Wood et al., 1975). The skinned muscles were stored at −20°C up to 3 weeks in a 50/50 glycerol/skinning solution, containing protease inhibitor leupeptin (10 μg ml−1), known to prevent loss of contractile proteins and preserve the fibre tension (Reiser et al., 1988).
Solutions
All reagents were provided by Sigma (St Louis, U.S.A.). The composition of all the solutions was calculated by the Fabiato computer program (Fabiato, 1988). The program calculation was used with stability constants listed for Ca2+ (Orentlicher et al., 1977) and for Sr2+ (Moisescu & Thieleczek, 1979), to keep final ionic strength at 200 mM. pH was adjusted to 7.0 and ATP at 2.5 mM was added to each solution, except extraction solution. Activating solutions were made up of 3-(N-morpholino) propanesulphonic acid (MOPS, 10 mM), potassium propionate (K Prop, 185 mM), magnesium acetate (MgAc, 2.5 mM) and various concentrations of free Ca2+ from CaCO3, buffered with ethylene glycol bis (β-aminoethyl ether) N,N,N′, N′ tetraacetic acid (EGTA) and added in proportions to obtain the different pCa values (7.0 to 4.2, with pCa=−log[Ca2+]). The pSr (pSr=−log[Sr2+]) solutions were similar to the pCa solutions except free Sr2+ from SrCl2. Relaxing solution (R) was identical to the skinning solution and was composed of 10 mM MOPS, 170 mM K Prop, 2.5 mM MgAc and 5 mM EGTA. Prior to submaximal pCa exposure, EGTA traces from the previously applied relaxing solution were rinsed with washing (W) solution, identical to pCa solutions except EGTA and CaEGTA free.
The extraction solution contained 2.5 mM ethylenediamine-N,N,N′,N′-tetraacetic acid (EDTA), and was adjusted to pH 7.8.
BPD was prepared fresh each day and used as a 20 mM stock solution in absolute ethanol. At concentrations used, ethanol itself had no effect on the developed tensions (data not shown).
Force measurements and recording
The experiments were carried out in a thermostatically controlled room (19±1°C). For each experiment, a single fibre (7–8 mm long) was isolated from the skinned muscle, and divided into three segments called ‘CONT' (control), ‘EXT' (extracted) and ‘REC' (reconstituted). The CONT segment was dissolved in 20 μl sodium dodecyl sulphate (SDS) lysis sample buffer and stored until electrophoretic analysis. EXT and REC segments (2–2.5 mm each) were mounted in experimental chambers and connected to strain-gauges (Fort 10, World Precision Instruments, Aston, U.K.). A micrometer allowed fibre diameter measurements assuming the cross-section was circular, fibres with a high degree of ellipticity being discarded. The resting sarcomere length (SL) was determined by diffraction using a Helium/Neon laser (Spectra Physics, Carlsbad, U.S.A.). The SL was set to 2.6 μm (120% of resting SL) to allow optimal isometric tension development upon ionic activation: it was subsequently regularly controlled and readjusted if necessary during the experiment. Sarcoplasmic reticulum was rendered non-functional, each fibre being bathed for 15 min in a solution made up of 2% Brij 58 (polyoxyethylene 20 cetyl ether) and 0.5% Triton X-100 in R solution under constant stirring.
Measurements of Sr2+ and Ca2+ activated tensions
Single fibres were first immersed in a pCa 4.2 solution to measure the initial force. REC segments were then checked for their strontium reactivity by successive exposure to pSr solutions: steady state tensions obtained with pSr 5.8, 5.4, 5.0, 4.6 were normalized to maximal Sr2+ activated tension (pSr3.4), in order to deduce the half maximal activation by strontium from the linear part of the T/pSr. REC fibres were then activated with various pCa (from 7.0 or 6.6 to 4.2 for slow and fast fibres, respectively). The steady state submaximal tensions P were expressed as a percentage of the maximal tension P0 (induced by the saturating pCa 4.2 solution), and reported as Tension/pCa (T/pCa) relationships. Fibre type was determined and based on the difference between Ca2+ and Sr2+ activation characteristics for fast and slow fibres, the fast muscle fibres being less sensitive to Sr2+ than slow fibres (Kerrick et al., 1980). Fibre type was therefore determined by establishing the Δ value, the difference between the respective Ca2+ and Sr2+ affinity criteria, pCa50 and pSr50 (Ca2+ and Sr2+ concentration needed to elicit 50% of P0). Typically, Δ of slow fibres is less than 0.30 pCa units, while Δ of fast fibres is higher than 1.00 pCa unit. This functional determination was later checked by the structural analysis of the fibres. Two other important parameters were extracted from the T/pCa relationships: the threshold for activation by Ca2+ reflected the sensitivity of the contractile system, and the steepness of the T/pCa curve reflected the cooperativity between the different regulatory proteins within the thin filament. Fast type fibres could be distinguished from slow type ones by a higher Ca2+ threshold (lower pCa value) and a steeper T/pCa curve, pCa50 being usually not significantly different (Stevens et al., 1993). The steepness of the T/pCa curve was determined by the Hill coefficients nH, either n1 or n2, calculated according to the following equation (Brandt et al., 1982):
where P/P0 is the normalized tension and K is the apparent dissociation constant (pK=−log K=pCa50). n1 corresponded to P/P0>50%; and n2 to P/P0<50%.
For the T/pCa+BPD relationship determination, 100 μM BPD were added in each pCa solution, as previously described (Kischel et al., 1999). To quantify the shift that occurred when BPD was added to the activating solution, we defined a Δ′n, which represented the shift expressed in pCa units at n per cent of relative tension. In our analyses, we measured Δ′10, Δ′50 and Δ′90.
Extraction and replacement of TnC in skinned fibres
Removal of endogenous TnC from both slow soleus and fast tibialis fibres was derived from a previously described method (Sheng et al., 1990). Two segments (EXT and REC) of the same fibre were used: for the REC segment, functional data were collected prior to extraction and subsequently to TnC reconstitution, which permitted each fibre to serve as its own control. The EXT and REC segments were incubated in the low salt EDTA solution (20 min for the fast fibres, and up to 2 h for the slow ones). Maximal tensions were evaluated with pCa4.2 solutions, and extraction was stopped when residual tension dropped to 5–10% of P0 before extraction. The EXT segment was then dissolved in lysis sample buffer for evaluating the TnC extraction. The REC TnC depleted segment was reincorporated with either 0.1 mg ml−1 R rabbit fast TnC, 0.2 mg ml−1 R cardiac mouse TnC, or 1 mg ml−1 R VG2 mutant for 10 min. Cardiac mouse TnC or VG2 mutant was thus reintroduced in fast tibialis anterior fibres, while fast chicken TnC was reincorporated in slow soleus fibres. Maximal tension after TnC reconstitution was checked after removing any excess of unbound TnC with R solution, and T/pCa with and without bepridil were established.
Electrophoresis
All fibre segments (CONT, EXT and REC) were dissolved in 20 μl sodium dodecyl sulphate (SDS) lysis sample buffer, heated at 90°C for 3 min and stored at −80°C until electrophoretic analysis. Separation was performed by SDS–PAGE using 10–20% linear gradient gels, which permitted from the same gel a good separation of the two TnC isoforms, and an accurate analysis of the MLC isoforms (Toursel et al., 2000). Proteins were stained with Sypro Orange dye and revealed under a 315 nm UV transilluminator before immunoblot.
Immunoblotting
Electrotransfer was performed to a 0.2 μm nitrocellulose sheet (Schleicher & Schuell, Dassel, Germany). The membranes were blocked with a phosphate buffered saline solution (PBS, pH 7.4) containing 5% nonfat dry milk and 0.2% sodium azide. The TnC isoforms were localized by a polyclonal antibody, incubated overnight. This antibody reacted equally well with fast and slow TnC isoforms (Leeuw et al., 1994). TnC antibodies were detected by an extravidin-biotin peroxidase staining kit. TnC isoforms were visualized by an enhanced chemi-luminescence (ECL) kit (Amersham Pharmacia Biotech, Piscataway, U.S.A.) and hyperfilms ECL (Amersham International, Little Chalfont, U.K.), to ensure optimal protein detection. Signal intensities were evaluated by densitometry (GS-700 Imaging Densitometer, Biorad, Ivry s/ Seine, France).
Only pure slow soleus and fast tibialis fibres (i.e. expressing only slow and fast MLC/TnC, respectively) were retained, hybrid fibres being discarded. This ensured the reliability of the extraction-replacement experiments. Moreover, these pure slow or fast fibres expressed, respectively, only slow and fast isoforms of other regulatory proteins such as TnT and TnI (controlled by immunoblots, data not shown). Therefore, the BPD effects could be directly related to the TnC content of the fibres.
The profile of TnC expression was determined by measuring the relative proportions of the TnC signals of fast and slow isoforms, respectively.
Statistical analysis
All the data were reported as means±s.e.mean. The statistical significance of the difference between means was determined using the Student's t-test or paired t-test when data were obtained from the same fibre in different experimental conditions. Differences at or above the 95% confidence level were considered significant.
Results
Fibre characteristics before extraction
Diameter and maximal isometric tensions are reported in Figure 1. Soleus and tibialis anterior fibre diameters were not different, but tibialis fibres developed higher absolute and normalized tensions. As previously shown (Kischel et al., 1999), BPD had no effect on these tensions (values not reported). Ca2+ activation characteristics of both fibre types are reported in Table 1 and illustrated in Figure 2a,b. In the slow soleus fibres, bepridil increased submaximal tensions as a function of the Ca2+ concentration, the sensitizing effect being more efficient at low Ca2+ levels, as attested by the significantly different Hill coefficients (n1 and n2) of the T/pCa curves with and without bepridil. In contrast, this effect was quite independent of the pCa range in fast tibialis fibres, and the apparent affinity reinforcement estimated by the shift in the pCa50 values was lower than that measured for slow fibres.
Figure 1.
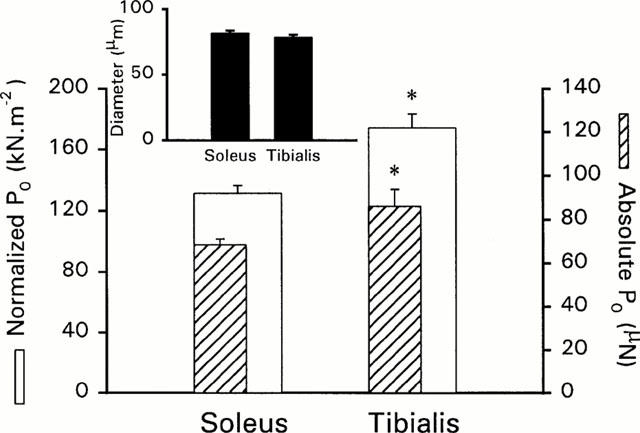
Diameter, absolute and normalized maximal tensions (P0) of slow soleus and fast tibialis anterior fibres in CONT conditions. Normalized tensions are represented with empty bars, and absolute tensions are represented in hatched bars. Inset represents diameters of both fibre types. *represents significant difference between soleus and tibialis anterior fibres (symbols were reported on tibialis values).
Table 1.
Calcium activation characteristics of soleus and tibialis muscle fibres
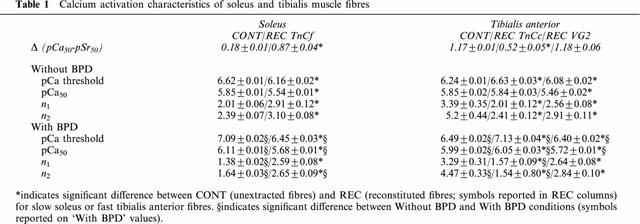
Figure 2.
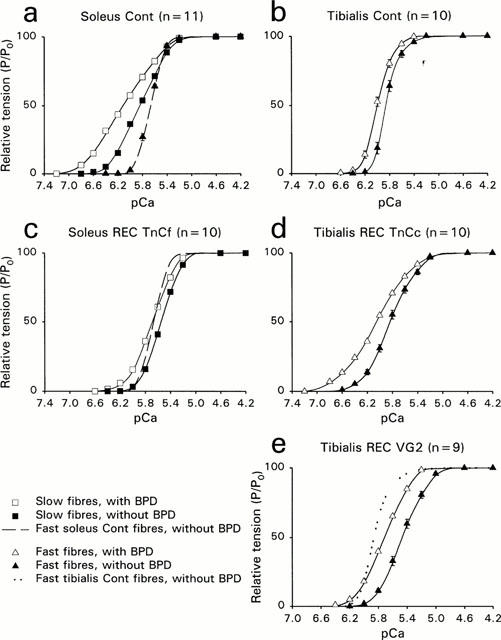
T/pCa relationships T/pCa relationships of soleus and tibialis anterior muscle fibres, before extraction (a,b) and after reconstitution with recombinant TnC (c–e). n represents the number of fibres. Squares represent slow fibres, and triangles represent fast fibres. Filled symbols are used in absence of BPD, and empty symbols are used in the presence of BPD. s.e.m were not reported when they merged with the mean points. The dashed line in graphs (a) and (c) represents the T/pCa relationship of fast soleus muscle fibres. In (e), the dotted line represents the T/pCa relationship of tibialis fibres, already shown in (b). Curves were fitted with the Hill parameters (n1 for P/P0>50% and n2 for P/P0<50%).
T/pCa relationship of fast soleus fibres was also established (Figure 2a,c, dashed line) and presented lower pCa threshold, pCa50 and higher Hill coefficients than the slow fibres from the same muscle. These fibres were found to coexpress both TnC isoforms, the fast one being always largely predominant (67.3±5.6% of the total TnC content, Figure 3, lane 4).
Figure 3.
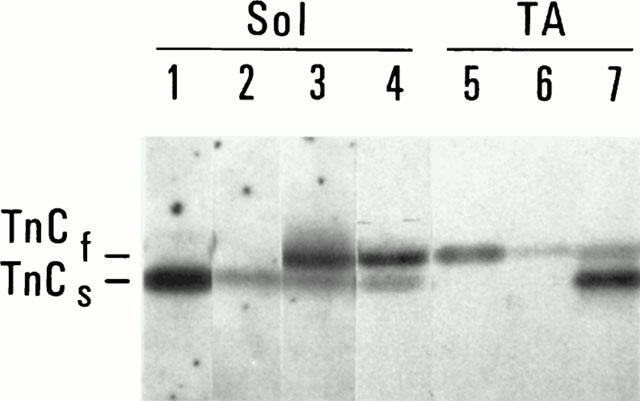
TnC expression profiles of single fibres from either soleus or tibialis muscles. ECL detection of TnC isoforms (only the region of TnC is shown). Lane 1: soleus CONT segment, lane 2: soleus EXT segment, lane 3: soleus REC TnCf segment; lane 4: fast soleus fibre; lane 5: tibialis anterior CONT segment; lane 6: tibialis anterior EXT segment, lane 7: tibialis anterior REC TnCc segment.
Effects of reconstitution
Estimations of endogenous TnC extraction and exogenous TnC reconstitution
Extraction was performed to obtain less than 10% residual tension: 8.9±1.3 for slow soleus and 5.1±1.6 for fast tibialis fibres, corresponding to 16.7±5.6% and 29.1±6.8% residual endogenous TnC signal, respectively (Figure 3, EXT segments). MLC content analysis of extracted fibres showed a slight decrease in MLC2 isoform content: 24.6±8.9% MLC2s and 21.4±6.5% of MLC2f were extracted simultaneously with TnC in slow and fast fibres, respectively. MLC1 and MLC3 were not affected by the extraction procedure.
Reconstitution with recombinant TnC was obvious in both fibre types, as judged by immunoblotting results (Figure 3, REC segments). From the TnC signal intensities, soleus fibres appeared constituted of 78.2±5.4% fast TnC, and acquired a profile close to that found in the fast soleus fibres (Figure 3, lane 4). After reconstitution with TnCc, tibialis fibres were constituted of 73.9±4.7% cardiac TnC.
P0 of reconstituted fibres were equal to 51 and 41% of P0 determined before TnC extraction in slow and fast fibres, respectively. Similar values were obtained with extended reconstitution time or higher concentration of recombinant proteins.
Reconstitution with the VG2 required higher concentrations of the mutant (see Sheng et al., 1990). Tension recovery was equal to 30.8±4.1% of the P0 measured in unextracted fibres.
Ca2+ activation properties of slow fibres reconstituted with TnCf
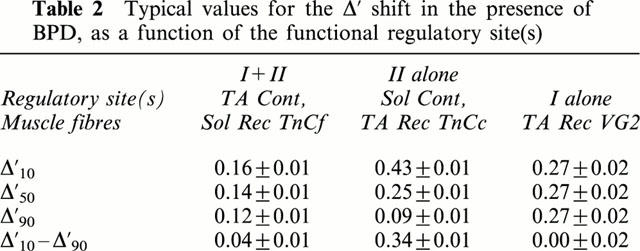
Subsequently to TnCf substitution in soleus fibres, strontium activation characteristics were modified: T/pSr relationships were shifted to higher Sr2+ concentrations (Δ was 0.18 before extraction and 0.87 after reconstitution). T/pCa curves were also changed (Figure 2c): pCa threshold and pCa50 were shifted towards higher Ca2+ concentrations, while Hill coefficients were significantly increased. When compared with values obtained in the presence of native TnC isoform, the apparent Ca2+ affinity increase induced by bepridil was largely reduced at low Ca2+ levels and bepridil exerted now its sensitizing effect whatever the Ca2+ concentration, the Δ10–Δ90 being equal to 0.04±0.01 (Table 2). As before extraction, BPD had no effect on the maximal tensions of reincorporated fibres.
Table 2.
Typical values for the Δ′ shift in the presence of BPD, as a function of the functional regulatory site(s)
Ca2+ activation properties of fast fibres reconstituted with TnCc
After TnCc reintroduction in TnCf depleted tibialis fast fibres, strontium activation characteristics were changed, T/pSr curves being shifted to lower Sr2+ concentrations (Δ was 1.17 before extraction and 0.52 after reconstitution). Two parameters of the T/pCa curve (Figure 2d) were also modified. The pCa threshold was displaced to higher pCa values and the Hill coefficients (n1 and n2) were significantly lowered (Table 1). The pCa50 value was not different, whichever the TnC isoform (native fast or recombinant slow). However, the bepridil effect was completely modified in the reconstituted fibres: the sensitizing effect was increased, submaximal tensions being all the more important as Ca2+ concentrations were low with a Δ′10–Δ′90 equal to 0.34±0.01.
After VG2 reintroduction, strontium activation characteristics were not changed (Δ was equal to 1.18±0.06). All parameters of the T/pCa relationship were modified with the mutant TnC: higher concentrations of Ca2+ were required to activate force, and the pCa50 was shifted from 5.84 to 5.46. There was also a significant decrease in the Hill coefficients (Table 2). The T/pCa curve in the presence of BPD (Figure 2e) was perfectly parallel to the T/pCa curve without BPD (n1 and n2 were identical), and shifted from 0.27 pCa units (vs 0.14 in control conditions) towards lower Ca2+ concentrations.
Additionally, BPD was found to increase maximal tensions of fast fibres reincorporated with either VG2 mutant or TnCc isoform, by 18±2.4 and 17.3±3.1%, respectively (Figure 4).
Figure 4.
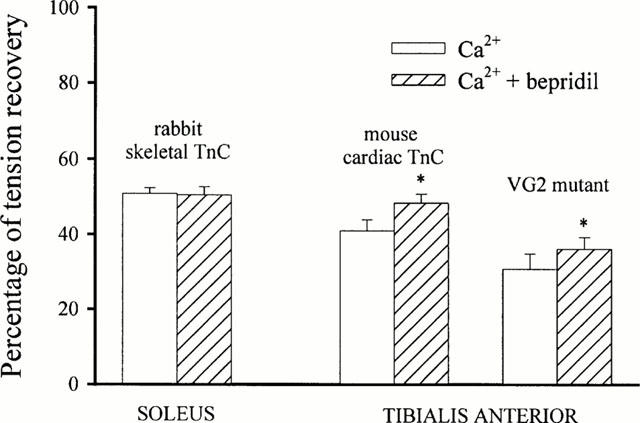
Tension recovery after reconstitution with recombinant TnC. Tension recovery was expressed in percentage of maximal tension elicited before extraction. *represents significant difference between absence and presence of BPD (symbols were reported on Ca2++BPD bars).
Discussion
We demonstrated in this study that the differential effect of bepridil in the slow and fast fibres could be attributed to the troponin C isoform. Moreover, we precisely studied the role of the regulatory sites of the troponin C towards the Ca2+ sensitizer BPD in vivo.
Our goal was to define the origin of the previously demonstrated differential effect of the Ca2+ sensitizer bepridil in slow and fast fibres. Slow soleus and fast tibialis fibres expressing only slow and fast TnC, respectively, were used to confirm or invalidate the hypothesis that TnC was implicated in the differential bepridil effect in slow and fast fibres. Functional data (Ca2+ activation characteristics, with and without bepridil) corroborated this choice: the Ca2+ affinity (pCa50) was not different, as usually reported (Stevens et al., 1993), fast fibres exhibited lower sensitivity (lower pCa threshold) and higher cooperativity (higher Hill coefficients), and BPD reactivity was typically different in these two fibre types, as previously reported (Kischel et al., 1999).
Extraction was performed to obtain a residual TnC signal intensity under 20% of initial TnC content, to avoid deleterious effects resulting from extended extraction. Only the MLC2 isoform was simultaneously slightly extracted, as previously reported (Moss et al., 1982). The loss of about 20% of these essential light chains could partly explain the fact that tension recovery was not higher than 50% in both fibre types. More presumably, incomplete tension recovery might be caused by species dependent differences (Hoar et al., 1988), i.e. the inability of exogenous mouse or chicken TnC to activate thin filaments of rat fibres, rather than incomplete TnC reconstitution or loss of MLC2. Indeed, if TnC reconstitution was not complete (not suggested by our immunoblot results), a decrease in cooperativity would be expected, since it has previously been shown that even limited TnC extraction caused cooperativity to drop (Brandt et al., 1984). This was not the case; soleus fibres with substituted TnCf showed an increase in the Hill coefficients when compared to the same fibres in the presence of endogenous TnC. Secondly, fibres with selective TnC extraction (without significant MLC2 decrease) were found among tibialis fibres (n=3), but tension recovery after TnCc reintroduction was not higher as observed for fibres which experienced significant MLC2 loss. Thus, although tension recovery was not as high as one would have expected, we were able to obtain reliable and coherent functional data.
Slow fibres reconstituted with rabbit fast TnC exhibited typical fast Ca2+ activation characteristics (higher cooperativity, decreased sensitivity, and pCa50 value shifted towards higher Ca2+ concentrations). It is noteworthy that TnCf substitution makes the T/pCa curves of slow fibres more characteristic of fast fibres from the same muscle. These results are in agreement with previous data showing the involvement of the TnC isoform in the T/pCa relationships (Babu et al., 1987). The effects of bepridil in these reconstituted fibres were also characteristic of fast fibres, i.e. lower BPD reactivity, that was independent of the activating Ca2+ concentration. Thus, despite the slow regulatory environment, bepridil reactivity was changed from a typical slow towards a typical fast effect. This indicated that the predominant TnC isoform was responsible for the bepridil effect and strongly suggested a role of site II in the Ca2+ dependence of the drug effect.
This result was confirmed by complementary experiments: TnC fast extraction/TnC ‘slow' (cardiac) substitution in fast tibialis fibres conferred a higher BPD reactivity that depended on the activating Ca2+ concentrations, characteristic of slow fibres. The latter result, obtained with a TnC isoform whose site I was inactive, suggested once again that the TnC isoform was responsible for the bepridil effect.
Study with the VG2 mutant, lacking the regulatory site II, substituted for endogenous fast TnC of tibialis fibres, was designated to further define the role of site I in the bepridil effect. Ca2+ activation of these reconstituted fibres was more difficult without the regulatory site II, since tension recovery was not higher than 30% of P0 before extraction. Indeed, this site is known to initiate the conformational changes at the origin of the TnC activation (Spyracopoulos et al., 1997). BPD could reinforce both submaximal and maximal tensions. This indicates that BPD can achieve an increase in the Ca2+ affinity in the absence of a functional site II. Thus BPD can induce a reinforcement of the Ca2+ affinity with only one functional regulatory site, either site I or II. It is interesting to note that BPD had no effect on the maximal tensions of soleus fibres (with either native or recombinant fast TnC) or tibialis fibres (with native TnC), while it enhanced maximal tensions of tibialis fibres reconstituted with TnC lacking either site I or site II. This suggested that BPD helped thin filament activation in a fast regulatory environment, which is known to need both Ca2+ specific sites for full functional activity (Sheng et al., 1990). The effect presumably resulted from stabilization and/or enhancement of partial conformational changes that occur upon Ca2+ occupation of either site I or II alone.
Ca2+ activation characteristics of VG2 reconstituted fibres were modified as previously described (Sheng et al., 1990), and the T/pCa+BPD relationship was shifted towards lower Ca2+ concentration, remaining exactly parallel to the T/pCa curve.
Thus, (i) when site I alone is active, BPD induces a Ca2+ affinity increase without altering the intrinsic cooperativity; (ii) when both sites are active (tibalis CONT or soleus REC), the cooperativity is not significantly different from that obtained in the absence of BPD; (iii) when only site II is active, whichever the regulatory environment, cooperativity is largely affected in the presence of BPD. Therefore, site I seems to be essential for keeping the cooperativity intact in the presence of BPD.
Moreover, the increase in Ca2+ affinity induced by BPD seems to be dependent on the number of regulatory sites, since a shift close to 0.25 pCa unit occurred at half maximal activation with only one active regulatory site (either site I or II), while an 0.14 pCa unit shift was generally observed with two regulatory active sites. This finding is in agreement with previous observations (Kischel et al., 1999) suggesting that BPD is all the more efficient as TnC activation is difficult.
Finally, since Sr2+ activation characteristics of slow and fast fibres were modified in extraction-replacement experiments (VG2 excluded), we can conclude from our results that the TnC isoform also established the Sr2+ reactivity of slow and fast fibres, in agreement with numerous studies (Babu et al., 1987; Moss et al., 1986; Hoar et al., 1988).
To conclude, the use of different recombinant TnC isoforms has defined more clearly the intrinsic role of the TnC isoform (and even the regulatory sites of TnC) in establishing the different activation characteristics in the presence of the Ca2+ sensitizer BPD. It appears unlikely that the regulatory cascade of events downstream of the TnC could exert an influence in setting the differential effect of bepridil in slow and fast fibres, although this possibility cannot be ruled out.
In vivo, the functional behaviour of a skinned muscle fibre in response to bepridil revealed the TnC isoform predominantly expressed. More practically, the interesting properties of the bepridil (i.e. a reinforcement of the Ca2+-affinity whichever the TnC isoform targeted, with an optimal efficiency in the most ‘pathological' conditions) constitute a strong basis to design specific compounds in the treatment of heart failure.
Acknowledgments
The authors thank Dr J.M. François (University of Liège, Belgium) for helpful advice throughout the study and Dr D. Pette (University of Konstanz, Germany) for kindly providing us with TnC antibodies. This work was supported by the Centre National d'Etudes Spatiales (CNES, grant no. 993027), the Fonds Européen de Développement Régional (F007), the Nord Pas-de-Calais Regional Council and the National Institute of Health ((J.D. Potter) AR45391 and HL42325) .
Abbreviations
- BPD
bepridil
- EGTA
ethylene glycol bis (β-aminoethyl ether) N,N,N′,N′ tetraacetic acid
- K Prop
potassium propionate
- MgAc
magnesium acetate
- MLC
myosin light chain
- MOPS
3-(N-morpholino) propanesulphonic acid
- SL
Sarcomere length
References
- ARMSTRONG R.B., PHELPS R.O. Muscle fiber type composition of the rat hindlimb. Am. J. Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- BABU A., SCORDILIS S.P., SONNENBLICK E.H., GULATI J. The control of myocardial contraction with skeletal fast muscle troponin C. J. Biol. Chem. 1987;262:5815–5822. [PubMed] [Google Scholar]
- BRANDT P.W., COX R.N., KAWAI M., ROBINSON T. Effect of cross-bridge kinetics on apparent Ca2+ sensitivity. J. Gen. Physiol. 1982;79:997–1016. doi: 10.1085/jgp.79.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRANDT P.W., DIAMOND M.S., SCHACHAT F.H. The thin filament of vertebrate skeletal muscle co-operatively activates as a unit. J. Mol. Biol. 1984;180:379–384. doi: 10.1016/s0022-2836(84)80010-8. [DOI] [PubMed] [Google Scholar]
- BURTNICK L.D., KAY C.M. The calcium-binding properties of bovine cardiac troponin C. FEBS Lett. 1977;75:105–110. doi: 10.1016/0014-5793(77)80063-x. [DOI] [PubMed] [Google Scholar]
- FABIATO A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- HERZIG J.W., QUAST U. On the role of Ca2+ binding proteins as possible targets for Ca2+ sensitizing agents. Z. Kardiol. 1992;81 Suppl. 4:49–55. [PubMed] [Google Scholar]
- HOAR P.E., POTTER J.D., KERRICK W.G. Skinned ventricular fibres: troponin C extraction is species-dependent and its replacement with skeletal troponin C changes Sr2+ activation properties. J. Muscle Res. Cell. Motil. 1988;9:165–173. doi: 10.1007/BF01773738. [DOI] [PubMed] [Google Scholar]
- KERRICK W.G., MALENCIK D.A., HOAR P.E., POTTER J.D., COBY R.L., POCINWONG S., FISCHER E.H. Ca2+ and Sr2+ activation: comparison of cardiac and skeletal muscle contraction models. Pflügers Arch. 1980;386:207–213. doi: 10.1007/BF00587470. [DOI] [PubMed] [Google Scholar]
- KISCHEL P., STEVENS L., MOUNIER Y. Differential effects of bepridil on functional properties of troponin C in slow and fast skeletal muscles. Br. J. Pharmacol. 1999;128:767–773. doi: 10.1038/sj.bjp.0702820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEEUW T., KAPP M., PETTE D. Role of innervation for development and maintenance of troponin subunit isoform patterns in fast- and slow-twitch muscles of the rabbit. Differentiation. 1994;55:193–201. doi: 10.1046/j.1432-0436.1994.5530193.x. [DOI] [PubMed] [Google Scholar]
- LI Y., LOVE M.L., PUTKEY J.A., COHEN C. Bepridil opens the regulatory N-terminal lobe of cardiac troponin C. Proc. Natl. Acad. Sci. 2000;97:5140–5145. doi: 10.1073/pnas.090098997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAC LACHLAN L.K., REID D.G., MITCHELL R.C., SALTER C.J., SMITH S.J. Binding of a calcium sensitizer, bepridil, to cardiac troponin C. A fluorescence stopped-flow kinetic, circular dichroism, and proton nuclear magnetic resonance study. J. Biol. Chem. 1990;265:9764–9770. [PubMed] [Google Scholar]
- MOISESCU D.G., THIELECZEK R. Sarcomere length effects on the Sr2+- and Ca2+-activation curves in skinned frog muscle fibres. Biochem. Biophys. Acta. 1979;546:64–76. doi: 10.1016/0005-2728(79)90170-1. [DOI] [PubMed] [Google Scholar]
- MOSS R.L., GIULIAN G.G., GREASER M.L. Physiological effects accompanying the removal of myosin LC2 from skinned skeletal muscle fibers. J. Biol. Chem. 1982;257:8588–8591. [PubMed] [Google Scholar]
- MOSS R.L., LAUER M.R., GIULIAN G.G., GREASER M.L. Altered Ca2+ dependence of tension development in skinned skeletal muscle fibers following modifications of troponin by partial substitution with cardiac troponin C. J. Biol. Chem. 1986;261:6096–6099. [PubMed] [Google Scholar]
- ORENTLICHER M., BRANDT P.W., REUBEN J.P. Regulation of tension in skinned muscle fibers: effect of high concentrations of Mg-ATP. Am. J. Physiol. 1977;233:C127–C134. doi: 10.1152/ajpcell.1977.233.5.C127. [DOI] [PubMed] [Google Scholar]
- POTTER J.D., GERGELY J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J. Biol. Chem. 1975;261:6096–6099. [PubMed] [Google Scholar]
- PUTKEY J.A., LIU W., SWEENEY H.L. Function of the N-terminal calcium-binding sites in cardiac/slow troponin C assessed in fast skeletal muscle fibers. J. Biol. Chem. 1991;266:14881–14884. [PubMed] [Google Scholar]
- REISER P.J., KASPER C.E., GREASER M.L., MOSS R.L. Functional significance of myosin transitions in single fibers of developing soleus muscle. Am. J. Physiol. 1988;254:C605–C613. doi: 10.1152/ajpcell.1988.254.5.C605. [DOI] [PubMed] [Google Scholar]
- SHENG Z., STRAUSS W.L., FRANCOIS J.M., POTTER J.D. Evidence that both Ca2+-specific sites of skeletal muscle TnC are required for full activity. J. Biol. Chem. 1990;265:21554–21560. [PubMed] [Google Scholar]
- SIA S.K., LI M.X., SPYRACOPOULOS L., GAGNÉ S.M., LIU W., PUTKEY J.A., SYKES B.D. Structure of cardiac muscle troponin C unexpectedly reveals a closed regulatory domain. J. Biol. Chem. 1997;272:18216–18221. doi: 10.1074/jbc.272.29.18216. [DOI] [PubMed] [Google Scholar]
- SOLARO R.J., BOUSQUET P., JOHNSON D. Stimulation of cardiac myofilament force, ATPase activity and troponin C Ca2+ binding by bepridil. J. Pharmacol. Exp. Ther. 1986;238:502–507. [PubMed] [Google Scholar]
- SPYRACOPOULOS L., LI M.X., SIA S.K., GAGNÉ S.M., CHANDRA M., SOLARO R.J., SYKES B.D. Calcium-induced structural transition in the regulatory domain of human cardiac troponin C. Biochemistry. 1997;36:12138–12146. doi: 10.1021/bi971223d. [DOI] [PubMed] [Google Scholar]
- STEVENS L., MOUNIER Y., HOLY X. Functional adaptation of different rat skeletal muscles to weightlessness. Am. J. Physiol. 1993;264:R770–R776. doi: 10.1152/ajpregu.1993.264.4.R770. [DOI] [PubMed] [Google Scholar]
- TOURSEL T., BASTIDE B., STEVENS L., RIEGER F., MOUNIER Y. Alterations in contractile properties and expression of myofibrillar proteins in Wobbler mouse muscles. Exp. Neurol. 2000;162:311–320. doi: 10.1006/exnr.1999.7349. [DOI] [PubMed] [Google Scholar]
- WOOD D.S., ZOLLMAN J., REUBEN J.P., BRANDT P.W. Human skeletal muscle; properties of the ≈lt;chemically skinned≈gt; fiber. Science. 1975;187:1075–1076. doi: 10.1126/science.187.4181.1075. [DOI] [PubMed] [Google Scholar]
- ZOT H.G., POTTER J.D. A structural role for the Ca2+-Mg2+ sites on troponin C in the regulation of muscle contraction. Preparation and properties of troponin C depleted myofibrils. J. Biol. Chem. 1982;257:7678–7683. [PubMed] [Google Scholar]


