Abstract
The mechanism of vasorelaxation induced by SR33805 was investigated by simultaneously monitoring the cytosolic Ca2+ concentration ([Ca2+]i) and force, and by determining level of myosin light chain (MLC) phosphorylation in the medial strip of the porcine coronary artery.
SR33805 inhibited the sustained increases in [Ca2+]i and force (IC50; 3.2±1.0 and 49.4±27.5 nM, respectively) induced by 118 mM K+-depolarization. There was about a 10 fold difference in the inhibitory potency between [Ca2+]i and force.
SR33805 completely inhibited the [Ca2+]i elevation induced by a thromboxane A2 analogue, U46619 and histamine, at concentrations (1 μM) higher than those required for the complete inhibition of K+-depolarization induced [Ca2+]i elevation.
SR33805 had no effect on the [Ca2+]i elevation induced by histamine or caffeine in the absence of extracellular Ca2+.
SR33805 caused a leftward shift of the [Ca2+]i-force relationship of the contraction induced by cumulative application of extracellular Ca2+ during 118 mM K+-depolarization. The relationship between [Ca2+]i and MLC phosphorylation also shifted to the left by SR33805, while the relationship between MLC phosphorylation and force remained unaffected.
In conclusion, SR33805 caused an apparent leftward shift of the [Ca2+]i-force relationship, accompanied by a greater degree of MLC phosphorylation for a given level of [Ca2+]i. The mechanism of this leftward shift, however, still remains to be elucidated.
Keywords: SR33805, Ca2+ channel blocker, vasorelaxation, Ca2+-sensitivity
Introduction
The intracellular Ca2+ signal is a primary determinant of the smooth muscle contraction. The molecular mechanisms for the Ca2+-dependent regulation of smooth muscle contraction have been well investigated (Hartshorne, 1987). The elevation of cytosolic Ca2+ concentration ([Ca2+]i) causes the calmodulin-mediated activation of myosin light chain kinase (MLCK), which phosphorylates 20 kDa MLC and thereby induce contraction. The contractile stimulations cause [Ca2+]i elevation by releasing Ca2+ from the intracellular store sites and by activating an influx of the extracellular Ca2+ (Karaki et al., 1997). The Ca2+ influx plays a major role in maintaining the sustained [Ca2+]i elevation during contractions (Somlyo & Somlyo, 1994). The voltage-operated Ca2+ channel (VOC) is one of the major pathways of the Ca2+ influx not only in smooth muscle but also in other excitable cells, including cardiac muscle and neuronal cells. Among the five different types of VOCs, the L-type channel is a dominant type in smooth muscle cells (Watson & Girdlestone, 1994). Many varieties of L-type Ca2+ channel blocker have been developed and successfully used for the treatment of cardiovascular diseases such as hypertension and ischaemic heart disease (Fleckenstein, 1983). Most of the Ca2+ channel blockers belong to three chemically different subgroups; dihydropyridines, phenylalkylamines and benzodiazepines. The classical Ca2+ channel blockers were shown to bind to the α1 subunit of the L-type Ca2+ channels and inhibit the channel activity (Hockerman et al., 1997; Striessnig et al., 1998).
Using front-surface fluorometry of fura-2 and porcine coronary arterial strips, we elucidated the mechanism of vasorelaxation induced by diltiazem, one of benzodiazepines (Hirano et al., 1990). We found that diltiazem, at therapeutic concentrations, causes vasorelaxation by inhibiting [Ca2+]i elevation due to Ca2+ influx, with no effect on the Ca2+-sensitivity of the contractile apparatus of smooth muscle. Dihydropyridines and phenylalkylamines were also shown to induce vasorelaxation in a similar manner (Karaki et al., 1997). It is thus conceivable that the classical Ca2+ channel blockers cause vasorelaxation mainly by inhibiting Ca2+ influx and thereby decreasing [Ca2+]i, while having no effect on the Ca2+ sensitivity of the contractile apparatus. On the other hand, bepridil, a Ca2+ channel blocker, was reported to antagonize the effect of calmodulin as well as to inhibit Ca2+ (Itoh et al., 1984). It is possible that bepridil may decrease in the Ca2+-sensitivity of the smooth muscle contractile apparatus.
SR33805 ([[N-[dimetoxy-3,4-phenethyl]-N-methyl-amino-propoxyl]-4-benzenesulphonyl]-2-isopropyl-3-methyl-1-indole) is a recently developed Ca2+ channel blocker (Chatelain et al., 1993). SR33805 has a different chemical structure from that of classical Ca2+ channel blockers. This compound binds to the α1 subunit of the L-type Ca2+ channels, but at a site different from the binding site of the classical Ca2+ channel blockers (Melliti et al., 1996; Romey & Lazdunski, 1994). It binds with a high affinity (KD value=20 pM) to a unique site of the rat cardiac plasma membrane (Chatelain et al., 1994), and potently inhibits the Ca2+ channel in the mouse cardiac myocytes in primary culture with IC50 being 4.1–33 nM (Romey et al., 1994). Another study demonstrated that SR33805 totally blocked the L-type Ca2+ channel with IC50 being 26 nM in chick dorsal root ganglion neurones (Romey & Lazdunski, 1994). SR33805 was shown to induce a significant relaxation in the rabbit basilar artery strips contracted with K+, with IC50 value being 3.03 nM (Chatelain et al., 1993). A comparison of IC50 values for cardiac and smooth muscle indicated that SR33805 is highly selectivity to vascular smooth muscle, compared to other Ca2+ channel blockers, such as verapamil, diltiazem and nifedipine (Chatelain et al., 1993). In addition to the inhibition of the L-type Ca2+ channel, SR33805 has also been shown to inhibit a Ca2+ influx and smooth muscle cell proliferation induced by platelet-derived growth factor (Dol et al., 1995; Magnier Gaubil et al., 1996). However, the mechanism of the SR33805-induced vasorelaxation is yet to be elucidated.
In the present study, we simultaneously determined the effects of SR33805 on smooth muscle [Ca2+]i and force development by using front-surface fluorometry and fura-2-loaded arterial strips of the porcine coronary artery. In addition to an inhibition of the Ca2+ influx, SR33805 was found to induce an apparent leftward shift of the [Ca2+]i-force relationship. We therefore further investigated the effect of SR33805 on MLC phosphorylation in intact muscle.
Methods
Tissue preparation for the simultaneous measurement of [Ca2+]i and force of coronary arterial medial strips
Fresh pig hearts were obtained at a local slaughterhouse. The left circumflex branches of the coronary arteries (2–3 cm from the origin) were immediately isolated and brought to the laboratory in ice-cold normal physiological salt solution (normal PSS). After the segment was opened longitudinally, the adventitia was removed under a binocular microscope and the internal surface of the artery was gently rubbed off with a cotton swab to remove the endothelium. The medial preparation was cut into strips of equal size (1 mm width×5 mm length×0.2 mm thickness).
Fura-2 loading
The medial strips were loaded with the Ca2+ indicator dye, fura-2, by incubation in oxygenated (a mixture of 95% O2 and 5% CO2) Dulbecco's modified Eagle medium containing 25 μM fura-2 acetoxymethyl ester and 5% foetal bovine serum for 4 h at 37°C. After loading with fura-2, the strips were rinsed in normal PSS to remove the dye remaining in the extracellular space and equilibrated in normal PSS for about 1 h before starting the experimental protocols. Loading the medial strips with fura-2, per se, did not affect the contractility, as previously described (Hirano et al., 1990).
Front-surface fluorometry
The changes in fluorescence intensity of Ca2+-fura-2 complex of the arterial strips were monitored using a front-surface fluorometer specifically designed for fura-2 fluorometry (CAM-OF3, Japan Spectroscopic Co., Tokyo, Japan) (Kanaide, 1999). The quartz optic fibres were used to transmit alternation (400 Hz) 340 and 380 nm excitation light from a xenon lamp. The surface fluorescence of the strips was collected by the glass optic fibres and introduced through a 500 nm band-pass filter into a photomultiplier. At one end of the optic fibres facing the strips, the quartz and glass optic fibres were arranged in a concentric inner circle (3 mm diameter) and an outer circle (7 mm diameter), respectively. The fluorescence intensities at 340 nm (F340) and 380 nm (F380) excitation were monitored and their ratio (F340/F380) was recorded as an indicator of [Ca2+]i. Before starting each experimental protocol, the response to 118 mM K+ depolarization was recorded as a reference response. The changes in the fluorescence ratio were expressed as a percentage, assigning a value in normal PSS (5.9 mM K+) and that obtained at the steady state contraction induced by 118 mM K+ depolarization to be 0 and 100%, respectively, which were determined prior to the experimental protocol for each arterial strip.
Measurement of force development
The fura-2 loaded strip was mounted vertically to a strain gauge (TB-612-T, Nihon Koden, Japan) in a quartz organ bath. One end of the strip was connected to a force transducer, while the other end was connected to a fixed hook. During the 1-h fura-2 equilibration period, the strip was stimulated with 118 mM K+-depolarization every 15 min. The resting tension was increased in a stepwise manner and was then finally adjusted to 250–300 mg to obtain a maximum force development with 118 mM K+-depolarization. The developed tension was expressed as a percentage, assuming the values obtained at rest in normal PSS (5.9 mM K+) and that at the steady state of contraction induced by 118 mM K+-depolarization to be 0 and 100%, respectively.
Measurement of MLC phosphorylation
The extent of MLC phosphorylation in the arterial strips of the left circumflex branch were determined using the urea-glycerol gel electrophoresis technique (Persechini et al., 1986), followed by immunoblot detection with a specific mouse monoclonal anti-MLC antibody (Zhou et al., 1999). In brief, strips were prepared as described in tissue preparation for the simultaneous measurement of [Ca2+]i and force. The strips were pulled to 1.2 fold the resting length and pinned onto a rubber block to keep the resting load similar to that given in the force measurement of intact strips. At the indicated times after stimulation, the strips were transferred to a solution consisting of 90% acetone, 10% trichloroacetic acid and 10 mM dithiothreitol pre-chilled at −80°C. The strips were then washed extensively and stored in acetone containing 10 mM dithiothreitol at −80°C. After the strips were dried to remove the acetone, they were extracted in the sample buffer (8 M urea, Tris (hydroxymethyl) aminomethare 20 mM, glycine 23 mM, 0.004% bromophenol blue and dithiothreitol 10 mM) at room temperature for 2 h. The supernatant was subjected to electrophoresis on 10% polyacrylamide gel containing 40% glycerol, followed by transfer onto a polyvinylidene difluoride membrane (BioRad, Hercules, CA, U.S.A.) in 10 mM Na2HPO4 (pH 7.6). The 20 kDa MLC, both unphosphorylated and phosphorylated, was detected by a specific antibody (×200 dilution), and a horse radish peroxidase-conjugated secondary antibody (×1000 dilution). An immune complex was detected using an enhanced chemiluminescence technique (ECL plus kit; Amersham, Buckinghamshire, U.K.). X-OMAT AR Film (Kodak, Rochester, NY, U.S.A.) was used to detect light emission. After scanning the X-ray film on an ATTO ImageSaver AE-6905C, the density of unphosphorylated and phosphorylated MLCs were determined by Gel Plotting Macros of the NIH image ver. 1.61 (National Institute of Health, U.S.A.). The percentage of the phosphorylated form in total MLC (sum of unphosphorylated and phosphorylated forms) was calculated to indicate the extent of MLC phosphorylation.
Solutions and chemicals
The composition of the normal (5.9 mM K+) PSS was as follows (in mM): NaCl 123, KCl 4.7, NaHCO3 15.5, KH2PO4 1.2, MgCl2 1.2, CaCl2 1.25, and D-glucose 11.5. High K+ PSS was prepared by replacing an equimolar substitution of KCl for NaCl. The Ca2+-free PSS was prepared by adding 2 mM ethyleneglycol-bis (β-aminoethylether)-N,N,N′,N′,-tetraacetic acid (EGTA) in place of CaCl2, unless otherwise specified. PSS was gassed with a mixture of 5% CO2 and 95% O2, with the resulting pH being 7.4. SR33805 was donated by Dr J.M. Herbert (Sanofi Reserche, Toulouse, France). Fura-2 acetoxymethyl ester was purchased from Dojindo Laboratories (Kumamoto, Japan). U46619 (9, 11-dideoxy-9α, 11α-methanoepoxy prostaglandin F2) was purchased from Funakoshi (Osaka, Japan). Histamine dihydrochloride was purchased from Wako (Osaka, Japan). Anti-MLC antibody and anti-mouse IgM conjugated with horseradish peroxidase were purchased from Sigma (St. Louis, MO, U.S.A.).
Data analysis
All data from the simultaneous measurements of [Ca2+]i and tension were collected by a computerized data acquisition system (MacLab; Analog Digital instruments, Castle Hill, Australia: Macintosh, Apple Computer, Cupertino, CA, U.S.A.). The representative traces shown were directly printed from the data obtained with this system. All data are the mean±s.e.mean (n=number of experiments). A strip obtained from one animal was used for each experiment, therefore n value indicates the number of animal. The statistical analyses were performed using the unpaired Student's t-test, and P values of less than 0.05 were considered to be statistically significant.
Results
Effect of SR33805 on the increases in [Ca2+]i and force induced by 118 mM K+-depolarization
Figure 1a shows the representative recordings of the changes in the intensity of 500 nm fluorescence at 340 nm (F340) and 380 nm (F380) excitation, their ratio (F340/F380) and force induced by 118 mM K+-depolarization in the porcine coronary arterial medial strips. Depolarization with 118 mM K+ induced a rapid increase in F340 and a decrease in F380, thus resulting in a rapid increase in the fluorescence ratio. After reaching its peak, the ratio, namely [Ca2+]i declined to reach a plateau within 5 min. The force also rapidly elevated to reach a plateau level. When the levels of [Ca2+]i and force at 5 min after stimulation with 118 mM K+ were designated to be 100%, those obtained at 15 min were 84.5±4.4 and 97.9±2.9% (n=6), respectively.
Figure 1.
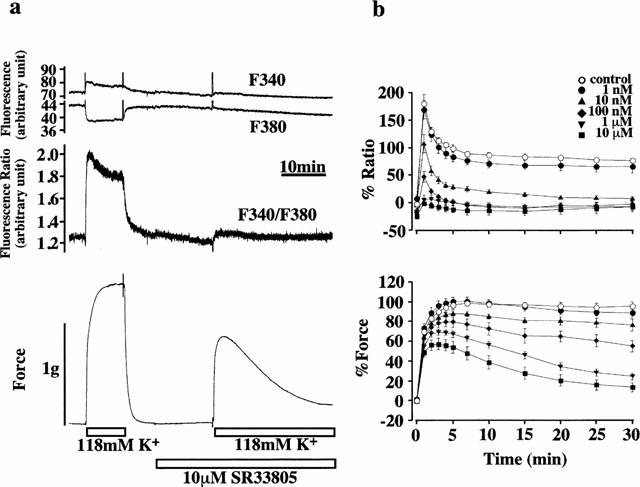
Effects of SR33805 on the increases in [Ca2+]i concentration and force induced by 118 mM K+-depolarization. (a) Representative recordings showing the changes in the fluorescence and force development induced by 118 mM K+ depolarization both in the presence and absence of 10 μM SR33805 in the normal PSS. The first and second traces from the top show the changes in the 500 nm fluorescence intensities obtained at 340 nm (F340) and 380 nm (F380) excitations, respectively. The third trace shows the changes in the fluorescence ratio of F340 to F380. The fourth trace shows the force development. (b) The effects of various concentrations of SR33805 on changes in [Ca2+]i and force development. The strips were treated with 0 M, 1 nM, 10 nM, 100 nM, 1 μM and 10 μM SR33805 15 min before and during the 118 mM K+-depolarization. The level of [Ca2+]i and the developed force were expressed in percentages, assigning the values at rest (5.9 mM K+-PSS) and those at the steady state of contraction induced by 118 mM K+-depolarization-induced contraction to be 0 and 100%, respectively. The data are the means±s.e.mean (n=5–8).
To investigate the effects of SR33805 on this contraction, the strips were treated with various concentrations of SR33805 for 15 min before and during the stimulation with 118 mM K+-depolarization. When SR33805 was applied during the resting state, SR33805, at the concentration higher than 0.1 μM, slightly but significantly decreased the resting level of [Ca2+]i. The resting [Ca2+]i levels obtained with 0.1, 1 and 10 μM SR33805 were −17.1±7.8, −14.6±3.9 and −24.1±4.7% (n=6–8), respectively. However, the decrease in the resting [Ca2+]i was not accompanied by a decrease in the resting level of force.
The treatment with SR33805 inhibited the sustained elevation of [Ca2+]i and force induced by subsequent stimulation with 118 mM K+-depolarization in a concentration-dependent manner (Figure 1b). Inhibition of [Ca2+]i elevation was observed at 1 nM and higher concentrations. In the presence of SR33805, high K+-depolarization caused an initial rapid elevation of [Ca2+]i, which thereafter declined to a steady state within 15 min. At a concentration of 10 nM, SR33805 inhibited 118 mM K+-induced increases in [Ca2+]i and force to 15.2±3.6 and 81.2±2.9% (n=8) at 15 min after stimulation, respectively. At 100 nM and higher concentrations, the 118 mM K+-induced sustained [Ca2+]i elevation decreased to below the resting level (0%). On the other hand, the inhibition of force development required a higher concentration of SR33805. A significant inhibition in the force was observed at 10 nM and higher concentrations. Even at 10 μM, SR33805 did not completely inhibit the 118 mM K+-induced force development. In the presence of 10 μM SR33805, 118 mM K+-induced a rapid development of force, which reached its peak at 3 min and thereafter declined to a steady state within 30 min (Figure 1b). The level of force obtained at 15 min after the stimulation with 118 mM K+ in the presence of 10 μM SR33805 were 27.6±6.0% (n=6), and the [Ca2+]i was −14.7±7.7% (n=6). The concentration of SR33805 required to decrease [Ca2+]i elevation and force to 50% of the control level was 3.2±1.0 and 49.4±27.5 nM (Figure 1b). The force development of the 118 mM K+-induced contraction was about 10 fold more resistant to the inhibition by SR33805 than the [Ca2+]i elevation.
Effects of SR33805 on the increases in [Ca2+]i and force induced by U46619 and histamine in the presence of extracellular Ca2+
In order to examine the effect of SR33805 on the Ca2+ influx pathways other than those activated by K+-depolarization, U46619, a thromboxane A2 analogue, and histamine were used to induce contraction. Figure 2a shows representative recordings of the changes in [Ca2+]i and force induced by 100 nM U46619 in normal PSS. The application of 100 nM U46619 induced considerably rapid increases in both [Ca2+]i and force, which reached the steady state within 10–15 min. The level of force (112.3±5.8%, n=8) at 15 min after stimulation by 100 nM U46619 was similar to that obtained with 118 mM K+-depolarization, while the level of [Ca2+]i (78.6±4.4%, n=8) was significantly lower. Figure 2b summarizes the effects of various concentrations of SR33805 on [Ca2+]i and force induced by 100 nM U46619. SR33805 was applied 15 min prior and during the U46619-induced contractions. As observed with K+-induced contraction, SR33805 inhibited [Ca2+]i elevation more potently than force development. The inhibition of [Ca2+]i elevation was observed with 1 nM and higher contractions. SR33805 (100 nM) inhibited the elevation of [Ca2+]i and force seen at 15 min after the initiation of the precontraction to 12.7±3.7 and 86.3±6.1% (n=5, respectively). The complete inhibition of the sustained [Ca2+]i elevation required 1 μM SR33805. This concentration was higher than that required to cause a complete inhibition of the sustained [Ca2+]i elevation induced by K+-depolarization. SR33805 thus inhibited the contraction induced by 118 mM K+ depolarization more potently than that induced by 100 nM U46619.
Figure 2.
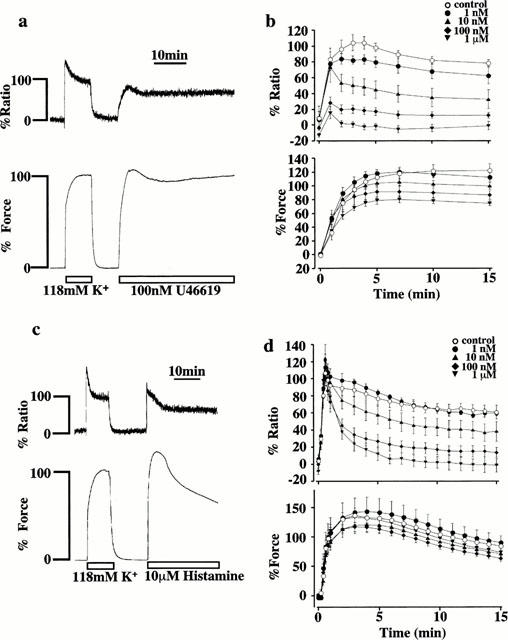
The effects of SR33805 on the increases in [Ca2+]i and force induced by U46619 and histamine. (a, c) Representative recordings showing changes in the fluorescence ratio and force development induced by 100 nM U46619 (a) and 10 μM histamine (c) in the normal PSS. The level of [Ca2+]i and force at rest (5.9 mM K+-PSS) and those at the steady state of contraction induced by 118 mM K+-depolarization were assigned to be 0 and 100%, respectively. (b, d) Concentration-dependent effects of SR33805 on [Ca2+]i elevation and force development induced by 100 nM U46619 (b) and 10 μM histamine (d). The strips were pretreated with 0 M, 1 nM, 10 nM, 100 nM, 1 μM and 10 μM SR33805, 15 min before and during the application of 100 nM U46619 and 10 μM histamine. The data are the mean±s.e.mean (n=5–7).
Figure 2c shows representative recordings of the changes in [Ca2+]i and force induced by 10 μM histamine in normal PSS. The fluorescence ratio abruptly increased and reached the first peak (107.9±14.0%, n=5) at about 30 s, and then declined. The force also developed rapidly, and reached the maximum (137.6±11.6%, n=5) at about 3 min, and thereafter gradually declined. The levels of [Ca2+]i and force at 5 min after the stimulation with 10 μM histamine were 76.8±7.3 and 131.6±10.0% (n=5) (Figure 2c,d), respectively. Figure 2d shows the effects of various concentrations of SR33805 on a time course of change in [Ca2+]i and force induced by 10 μM histamine. The strips were pretreated with SR33805 15 min prior and during histamine-induced contraction. SR33805 inhibited the declining phase of [Ca2+]i elevation and force development in a concentration-dependent manner. The inhibition of sustained [Ca2+]i elevation was observed at 10 nM and higher concentration. In the concentration range between 1 nM and 1 μM, SR33805 had no effect on the first peak of [Ca2+]i elevation. On the other hand, force development was inhibited much less than the [Ca2+]i elevation. Again, there was a discrepancy in the inhibition between [Ca2+]i and force during the sustained phase.
Effects of SR33805 on the Ca2+ release induced by histamine and caffeine in the absence of extracellular Ca2+
The first peak of [Ca2+]i elevation induced by histamine is due to the Ca2+ release from the intracellular store (Hirano et al., 1990). Since SR33805 did not inhibit the first peak of [Ca2+]i elevation induced by histamine in normal PSS (Figure 2d), the effects of SR33805 on the Ca2+ release were further examined. Figure 3a shows the representative recordings of the histamine-induced [Ca2+]i and force in the Ca2+-free PSS. Changing the bathing solution (normal PSS) to the Ca2+-free PSS containing 2 mM EGTA decreased the basal [Ca2+]i level to −39.0±7.5% (n=8) by 15 min, whereas the resting tension remained unchanged. Stimulation with 10 μM histamine in the Ca2+-free PSS containing 2 mM EGTA induced only transient increases in [Ca2+]i and force, with the peak levels being 3.6±8.3 and 42.9±5.8% (n=8), respectively (Figure 3b). The strips were treated with SR33805 15 min prior to and during the stimulation by histamine. The treatment with SR33805 had no effect on the decrease in the resting [Ca2+]i level induced by the exposure to the Ca2+-free PSS (Figure 3b). The pretreatment with SR33805 (1 nM–10 μM) had no significant effect on the histamine-induced increases in [Ca2+]i and force (Figure 3b).
Figure 3.
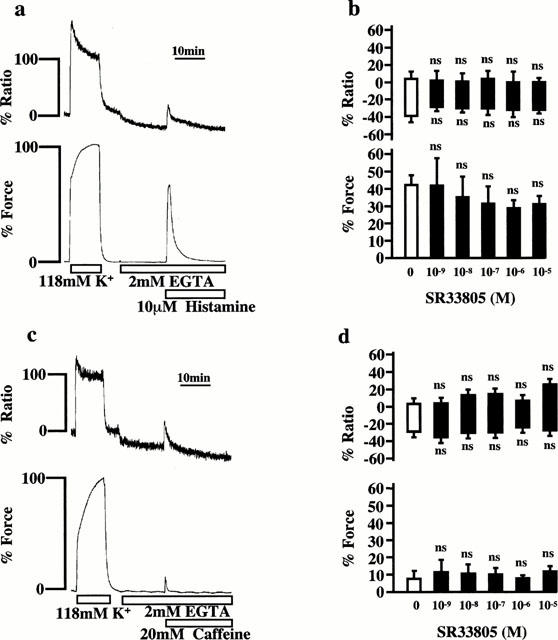
The effects of SR33805 on transient increases in [Ca2+]i and force induced by histamine and caffeine in the absence of extracellular Ca2+. (a, c), Representative recordings showing change in the fluorescence ratio and force development induced by 10 μM histamine (a) and 20 mM caffeine (c) in the Ca2+-free PSS containing 2 mM EGTA. The strips were exposed to the Ca2+-free PSS for 15 min and then were stimulated with 10 μM histamine and 20 mM caffeine. The levels of [Ca2+]i and force at rest (5.9 mM K+-PSS) and those at the steady state of contraction induced by 118 mM K+ depolarization were assigned a value of 0 and 100%, respectively. (b, d), The concentration-dependent effects of SR33805 on [Ca2+]i elevation and force development induced by 10 μM histamine (b) and 20 mM caffeine (d). The strips were pretreated with SR33805 15 min before and during the application of 10 μM histamine and 20 mM caffeine. The bottom and top levels of the column indicate the levels of [Ca2+]i and force obtained before the stimulation and those at the peak response, respectively. The data are the mean±s.e.mean (n=4–8). ns, not significantly different from the control value obtained in the absence of SR33805.
Similarly, the effects of SR33805 on the Ca2+ release induced by caffeine were examined. Stimulation with 20 mM caffeine in the Ca2+-free PSS containing 2 mM EGTA induced transient increases in [Ca2+]i and force, with the peak levels being 7.2±9.6 and 5.5±3.1% (n=5), respectively (Figure 3d). The caffeine-induced increases in [Ca2+]i and force in the presence of SR33805 (1 nM–10 μM) did not significantly differ from those observed in the absence of SR33805 (Figure 3d).
Effects of SR33805 on the [Ca2+]i-force relationships
To determine the effect of SR33805 on the Ca2+-sensitivity of the contractile apparatus of the coronary arterial smooth muscle, we examined the [Ca2+]i-force relationship of the contractions induced by the cumulative applications of extracellular Ca2+ during 118 mM K+-depolarization. Figure 4a shows representative recording of the changes in [Ca2+]i and force in the absence of SR33805. Strips were first exposed to the Ca2+-free PSS containing 2 mM EGTA for 10 min and then to the Ca2+-free PSS without EGTA for 5 min before the stimulations with 118 mM K+-depolarization. When extracellular Ca2+ was cumulatively applied from 0 to 1.25 mM in a stepwise manner, the graded elevations of [Ca2+]i and force were observed. During 118 mM K+ depolarization, [Ca2+]i and tension increased to 92.1±4.3 and 108.9±3.5% (n=5), respectively, at the extracellular Ca2+ concentration of 1.25 mM. When the effects of SR33805 on these contractions were examined, SR33805 was applied 20 min prior to the induction of contraction by the readdition of extracellular Ca2+. The effect of SR33805 on the Ca2+-sensitivity of the contractile apparatus was evaluated by examining the [Ca2+]i-force relationship (Figure 4b). SR33805 caused a leftward shift of the [Ca2+]i-force relation curves in a concentration-dependent manner (Figure 4b).
Figure 4.
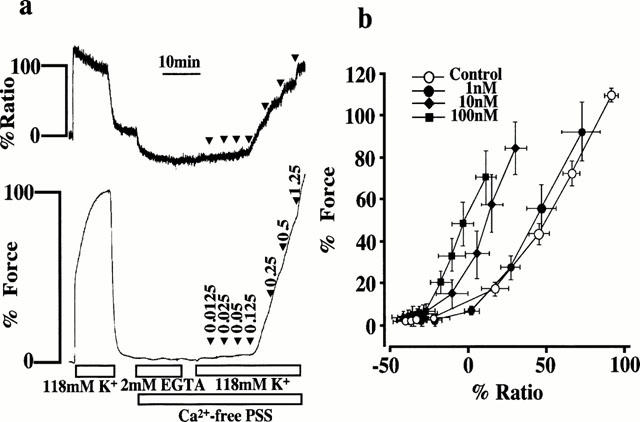
The effects of SR33805 on the [Ca2+]i-force relationships of the contraction induced by the cumulative application of extracellular Ca2+ during 118 mM K+-depolarization. (a) Representative recordings showing the changes in fluorescence ratio and force induced by the cumulative applications of extracellular Ca2+ during the 118 mM K+ depolarization. After exposure to 2 mM EGTA-containing Ca2+-free medium for 10 min and to the Ca2+-free media without EGTA for 5 min, the strips were stimulated with 118 mM K+-depolarization in the Ca2+-free media, and then the extracellular Ca2+ was increased in a stepwise manner from 0 to 1.25 mM. The number shown by an arrow head indicates the final concentration of extracellular Ca2+ at each step. The levels of [Ca2+]i and force at rest (5.9 mM K+-PSS) and those at the steady state of contraction induced by 118 mM K+-depolarization were assigned value of 0 and 100%, respectively. (b) The [Ca2+]i-force relation of the Ca2+-induced contraction obtained in the absence and presence of 1, 10 and 100 nM SR33805. The data are the mean±s.e.mean (n=5).
Effects of SR33805 on the [Ca2+]i-MLC phosphorylation relationship and the MLC phosphorylation-force relationship
We examined whether or not an apparent potentiation of the Ca2+-sensitivity of the contractile apparatus during the SR33805-induced relaxation was associated with an increase in the level of MLC phosphorylation. For this purpose, we evaluated the levels of [Ca2+]i, MLC phosphorylation and force at 10 min after the stimulation with high K+-depolarization (Figure 5a). Under the resting condition (0 [Ca2+]i and 0 force) in the 5.9 mM K+-PSS, 12.6±2.5% of MLC was phosphorylated (n=8). Elevation of K+ concentrations increase the levels of [Ca2+]i, MLC phosphorylation and force in a concentration-dependent manner. The [Ca2+]i elevation reached the maximal level at 40 mM K+, while MLC phosphorylation and force development reached the maximal level at 60 mM K+. The levels of [Ca2+]i, MLC phosphorylation and force of the contraction induced by 118 mM K+ were 97.8±4.6, 34.8±1.9 and 101.6±2.9% (n=6–8), respectively (Figure 5a). When SR33805 was applied 15 min prior and during the stimulation with 118 mM K+, the levels of [Ca2+]i, MLC phosphorylation and force were inhibited to 49.1±7.7, 27.8±6.6 and 91.6±2.1% (n=6–8) by 3 nM SR33805, and to −5.3±4.7, 22.0±7.6 and 54.7±3.4% (n=8) by 1 μM SR33805, respectively (Figure 5a). We evaluated the effect of SR33805 on the relationship between [Ca2+]i and force, [Ca2+]i and MLC phosphorylation, and MLC phosphorylation and force (Figure 5b). The [Ca2+]i-force relationship obtained with SR33805 at 10 min after the stimulation with 118 mM K+ shifted to the left of the control [Ca2+]i-force relation curve obtained in the absence of SR33805. Those at 30 min after stimulation remained shifted to the left of the control (Figure 5b). The [Ca2+]i-MLC phosphorylation relationship obtained with SR33805 also shifted to the left of the control [Ca2+]i-MLC phosphorylation relation curve obtained in the absence of SR33805. However, the MLC phosphorylation-force relationship obtained in the presence of SR33805 overlapped with the control MLC phosphorylation-force relation curve. As a result, SR33805 had no effect on the relationship between MLC phosphorylation and force development, while it apparently potentiated the MLC phosphorylation for a given change in [Ca2+]i.
Figure 5.
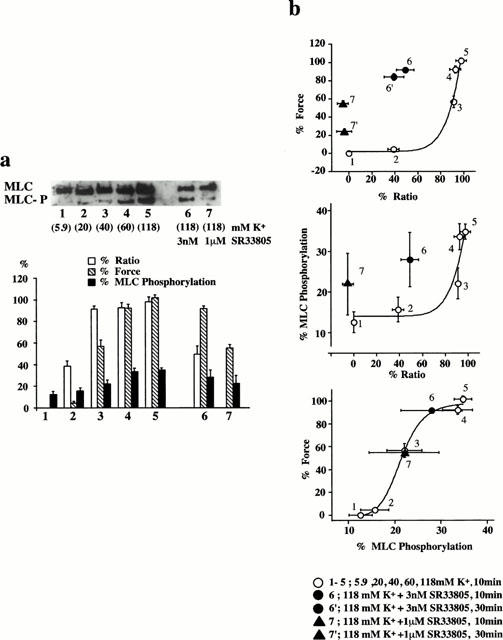
The effects of SR33805 on [Ca2+]i, MLC phosphorylation and force of the contraction induced by 118 mM K+ depolarization. (a) A representative photo of immunoblot detection of unphosphorylated and phosphorylated MLC, and a summary of the [Ca2+]i, MLC phosphorylation and force obtained with 5.9 mM K+, 20 mM K+, 40 mM K+, 60 mM K+, 118 mM K+, 118 mM K+ plus 3 nM SR33805 and 118 mM K+ plus 1 μM SR33805. SR33805 was applied 15 min before and during the 118 mM K+-induced contraction. The levels of [Ca2+]i and force at rest (5.9 mM K+-PSS) and those at a steady state of contraction induced by 118 mM K+ depolarization was expressed as a percentage of the phosphorylated MLC in total MLC (sum of unphosphorylated and phosphorylated forms). The data are the means±s.e.mean (n=6–8). (b) The [Ca2+]i-force relationship, the [Ca2+]i-MLC phosphorylation relationship and the MLC phosphorylation-force relationship obtained at 10 min after the initiation of contraction by 5.9 mM K+, 20 mM K+, 40 mM K+, 60 mM K+, 118 mM K+, 118 mM K+ plus 3 nM SR33805, 118 mM K+ plus 1 μM SR33805. The [Ca2+]i-force relation includes data obtained at 30 min after the initiation of contraction by 118 mM K+ plus 3 nM SR33805 and 118 mM K+ plus 1 μM SR33805. The data are the means±s.e.mean (n=6–8).
Discussion
We herein demonstrated SR33805 to inhibit the sustained phase of [Ca2+]i elevation and force development induced by not only high K+ depolarization but also receptor agonists such as U46619 and histamine in the porcine coronary artery. On the other hand, SR33805 did not inhibit the Ca2+ release from the intracellular store sites, induced by histamine or caffeine. It is thus conceivable that SR33805 specifically inhibits the [Ca2+]i elevation and force development due to Ca2+ influx. The IC50 values for the inhibition of 118 mM K+-depolarization-induced [Ca2+]i elevation was 3.2±1.0 nM. The inhibition of the [Ca2+]i elevation induced by high K+ was observed at concentrations lower than those required to inhibit the [Ca2+]i elevation induced by U46619 or histamine, thus suggesting that SR33805 preferentially inhibits VOCs to those activated by receptor stimulations. The concentrations of SR33805 required to inhibit high K+-induced [Ca2+]i elevation in the present study are similar to those reported in previous studies (Chatelain et al., 1993). These observations are consistent with previous reports which demonstrated SR33805 to be a L-type Ca2+ channel blocker (Romey & Lazdunski, 1994).
In the present study, SR33805 showed a potent inhibition of the Ca2+ influx induced by not only high K+-depolarization but also by receptor stimulations such as U46619 or histamine. At least four mechanisms should be considered for Ca2+ influx induced by agonist in vascular smooth muscle cells; (1) activation of VOCs by the agonist-induced membrane depolarization (Casteels & Suzuki, 1980; Miyoshi & Nakaya, 1991; Pacaud et al., 1991; Scornik & Toro, 1992), (2) activation of VOCs by intracellular second messengers or by trimeric G proteins activated by agonist stimulation (Karaki et al., 1997; Nelson et al., 1988), (3) so called receptor-operated Ca2+ channel (Bolton, 1979), and (4) the Ca2+ release-activated Ca2+ channel or capacitative Ca2+ influx pathway (Putney & McKay, 1999). U46619 was thus shown to depolarize the membrane potential of the porcine coronary artery (Scornik & Toro, 1992). Histamine was shown to depolarize the membrane potential in the rabbit ear artery and guinea-pig main pulmonary artery (Casteels & Suzuki, 1980; Suzuki & Kou, 1983). It is thus possible that VOCs contributed, at least in part, to the Ca2+ influx induced by U46619 and histamine in the porcine coronary artery, and that the inhibition by SR33805 of such Ca2+ influxes was partly due to the inhibition of VOCs. Since SR33805 inhibited K+-depolarization-induced Ca2+ influx more potently than that induced by either U46691 or histamine, SR33805 was thus suggested to inhibit mainly VOCs in both contractions induced by depolarization and by agonists. However, the detailed mechanisms of Ca2+ influx induced by U46619 and histamine in porcine coronary artery are still unclear. The possibility that SR33805 inhibits Ca2+ influx pathways other than VOCs therefore cannot be ruled out.
In the present study, SR33805 had no effect on the [Ca2+]i elevation induced by histamine or caffeine in the Ca2+ free PSS. In addition, SR33805 had no effect on the first peak of [Ca2+]i elevation induced by histamine in normal PSS. It was therefore suggested that SR33805 had no effect on the Ca2+ release from the intracellular store site induced by inositol 1,4,5-trisphosphate or caffeine. We reported that diltiazem, one of the Ca2+ entry blockers, did not inhibit the histamine-induced Ca2+ release in the porcine coronary artery, except for an extremely high concentration (100 nM) (Hirano et al., 1990).
The important findings of the present study is that the force development was more resistant to the inhibition by SR33805 than [Ca2+]i elevation. The concentration of SR33805 required to inhibit contraction was approximately 10 times higher than that required to inhibit [Ca2+]i elevation. Furthermore, the inhibition of force development required a longer time to reach the steady state than the inhibition of the [Ca2+]i elevation. As a result, SR33805 caused a leftward shift of the [Ca2+]i-force relationship (Figure 4), especially at the early phase of the contraction (Figure 5b), thus suggesting apparent potentiation of the Ca2+-sensitivity of the contractile apparatus. By using the same fluorometry system and the same arterial sample, we previously reported that diltiazem caused vasorelaxation by decreasing the [Ca2+]i level, while the Ca2+-sensitivity of the contractile apparatus remained unaffected (Hirano et al., 1990). Other Ca2+ channel blockers such as verapamil, dihydropyridines were also shown to have no effect on the Ca2+-sensitivity (Karaki et al., 1997; Kim et al., 1992). SR33805 was thus suggested to be unique as a Ca2+ channel blocker in that it apparently increased the Ca2+-sensitivity of the contractile apparatus.
Regarding the effect of Ca2+ channel blocker on the Ca2+-sensitivity of the contractile apparatus, bepridil (Advenier et al., 1984; Flaim et al., 1985; Gill et al., 1992) was reported to inhibit the effect of calmodulin (Itoh et al., 1984). It is thus possible that bepridil induces vasorelaxation due to not only inhibition of the Ca2+ influx but also decrease in the Ca2+-sensitivity of the contractile apparatus. Bepridil was indeed shown to induce potent vasorelaxation (Winslow et al., 1986). In contrast, SR33805 did not induce relaxation as much as expected due to the reduction of [Ca2+]i.
The potentiation of the Ca2+-sensitivity was associated with an increase in MLC phosphorylation. The relationship between MLC phosphorylation and force development remained unchanged, thus supporting the hypothesis that the increase in the myofilament Ca2+-sensitivity was due to an increase in MLC phosphorylation for a given [Ca2+]i. The level of MLC phosphorylation is determined by the balance between phosphorylation and dephosphorylation activity, and therefore the increase in MLC phosphorylation could be achieved either by the Ca2+-independent activation of kinase or the inhibition of phosphatase (Hartshorne et al., 1998; Somlyo & Somlyo, 1994). Rho-associated kinase, which is activated by a small G protein Rho, has recently been shown to increase MLC phosphorylation in a Ca2+-independent manner either by direct phosphorylation of MLC or by inhibition of MLC phosphatase in smooth muscle cells (Amano et al., 1996; Kureishi et al., 1997). The inhibition of MLC phosphatase was shown to cause a Ca2+-independent contraction in intact arterial strips and potentiate the Ca2+-sensitivity in permeabilized smooth muscle preparations (Hartshorne et al., 1998; Somlyo & Somlyo, 1994; Hirano et al., 1989). However, the effect of SR33805 either on Rho-Rho kinase system or the phosphatase is yet to be examined.
The underestimation of [Ca2+]i due to optical artifact and hindrance of fura-2 fluorometry does not appear to be based on the following observations. (1) As shown in Figure 1, the application of SR33805 to the normal PSS did not cause gross shift of fluorescence intensities at either 340 or 380 nm excitation lights. (2) SR33805 had no effect on the increases in fluorescence ratio induced by histamine and caffeine in the absence of extracellular Ca2+. (3) A spectrophotometric analysis showed that SR33805 had no specific absorbance at 340 and 380 nm (data not shown). (4) An analysis using a fluorescence spectrophotometer revealed that SR33805 had no effect on the fura-2 fluorescence in the Ca2+-EGTA buffer containing free Ca2+ at concentrations of 100 nM, 1 μM and 10 μM (data not shown).
In conclusion, SR33805 was shown to be a potent inhibitor of the Ca2+-influx induced by high K+-depolarization, U46619 and histamine. A preferential inhibition of the depolarizatoin-induced Ca2+ influx to the agonist-induced Ca2+ influx suggests that SR33805 mainly inhibits VOCs. In contrast to the potent inhibition of [Ca2+]i elevation, SR33805 exhibited a much weaker inhibition of force development in all types of contractions tested in the present study. This difference in the inhibitory effect on [Ca2+]i and force resulted in a leftward shift of the [Ca2+]i-force relationship during the SR33805-induced relaxation, thus indicating an apparent potentiation of the Ca2+-sensitivity of contractile apparatus. However, the extent of the inhibition of force was accompanied by a comparable extent of the change in MLC phosphorylation, namely, the MLC phosphorylation-force relation was not changed. SR33805 is thus the first example of a Ca2+ channel blocker which has an effect on the [Ca2+]i-force relationship in addition to an inhibitory effect on Ca2+ influx. The mechanism of this apparent Ca2+-sensitization, however, remains to be elucidated.
Acknowledgments
We thank Mr Brian Quinn for comments and help with the manuscript. This study was supported in part by Grants-in-Aid for Scientific Research (No. 10557072, 11838013, 11670687) and for Scientific Research on Priority Area (No. 12213103) from the Ministry of Education, Science, Sports and Culture, Japan, by the Research Grant for Cardiovascular Diseases (11C-1) from the Ministry of Health and Welfare, Japan, and by grants from the Foundation for the Promotion of Clinical Medicine, the Suzuken Memorial Foundation and KANZAWA Medical Research Foundation.
Abbreviations
- [Ca2+]i
intracellular calcium
- CSS
cytoplasmic substitution solution
- EGTA
ethyleneglycol-bis (β-aminoethylether)-N,N,N′,N′,-tetraacetic acid
- MLC
myosin light chain
- MLCK
MLC kinase
- PSS
physiological salt solution
- VOC
voltage-operated Ca2+ channel
References
- ADVENIER C., CERRINA J., DUROUX P., FLOCH A., RENIER A. Effects of five different organic calcium antagonists on guinea-pig isolated trachea. Br. J. Pharmacol. 1984;82:727–733. doi: 10.1111/j.1476-5381.1984.tb10812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMANO M., ITO M., KIMURA K., FUKATA Y., CHIHARA K., NAKANO T., MATSUURA Y., KAIBUCHI K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- BOLTON T.B. Mechanisms of action of transmitters and other substances on smooth muscle. Pharmacol Rev. 1979;59:606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- CASTEELS R., SUZUKI H. The effect of histamine on the smooth muscle cells of the ear artery of the rabbit. Eur. J. Pharmacol. 1980;387:17–25. doi: 10.1007/BF00580839. [DOI] [PubMed] [Google Scholar]
- CHATELAIN P., CLINET M., POLSTER P., CHRISTOPHE B., MANNING A.S. In vitro characterization of a novel Ca2+ entry blocker: SR 33805. Eur. J. Pharmacol. 1993;246:181–193. doi: 10.1016/0922-4106(93)90030-d. [DOI] [PubMed] [Google Scholar]
- CHATELAIN P., DEWINKELEER P., BEAUFORT P., MEYSMANS L., CLINET M. Characterization of the binding of [3H]SR 33805 to the slow Ca2+ channel in rat heart sarcolemmal membrane. Eur. J. Pharmacol. 1994;267:151–160. doi: 10.1016/0922-4106(94)90166-x. [DOI] [PubMed] [Google Scholar]
- DOL F., SCHAEFFER P., LAMARCHE I., MARES A.M., CHATELAIN P., HERBERT J.M. Effect of SR 33805 on arterial smooth muscle cell proliferation and neointima formation following vascular injury. Eur. J. Pharmacol. 1995;280:135–142. doi: 10.1016/0014-2999(95)00196-r. [DOI] [PubMed] [Google Scholar]
- FLAIM S.F., RATZ P.H., SWIGART S.C., GLEASON M.M. Bepridil hydrochloride alters potential-dependent and receptor-operated calcium channels in vascular smooth muscle of rabbit aorta. J. Pharmacol. Exp. Ther. 1985;234:63–71. [PubMed] [Google Scholar]
- FLECKENSTEIN A. History of calcium antagonists. Circ. Res. 1983;52:13–16. [PubMed] [Google Scholar]
- GILL A., FLAIM S.F., DAMIANO B.P., SIT S.P., BRANNAN M.D. Pharmacology of bepridil. Am. J. Cardiol. 1992;69:11D–16D. doi: 10.1016/0002-9149(92)90953-v. [DOI] [PubMed] [Google Scholar]
- HARTSHORNE D.J.Biochemistry of the contractile process in smooth muscle Physiology of the Gastrointestinal tract 1987New York: Raven Press; 423–482.ed. Johnson, L.R. pp [Google Scholar]
- HARTSHORNE D.J., ITO M., ERDÖDI F. Myosin light chain phosphatase: subunitcomposition, interactions and regulation. J. Muscle Res. Cell Motil. 1998;19:325–341. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- HIRANO K., KANAIDE H., ABE S., NAKAMURA M. Effects of diltiazem on calcium concentrations in the cytosol and on force of contractions in porcine coronary arterial strips. Br. J. Pharmacol. 1990;101:273–280. doi: 10.1111/j.1476-5381.1990.tb12700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRANO K., KANAIDE H., NAKAMURA M. Effects of okadaic acid on cytosolic calcium concentrations and on contractions of the porcine coronary artery. Br. J. Pharmacol. 1989;98:1261–1266. doi: 10.1111/j.1476-5381.1989.tb12672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOCFERMAN G.H., PETERSON B.Z., JOHNSON B.D., CATTERLL W.A. Molecular determinants of drug binding and action on L-type calcium channels. Ann. Rev. Pharmacol. Toxicol. 1997;37:361–369. doi: 10.1146/annurev.pharmtox.37.1.361. [DOI] [PubMed] [Google Scholar]
- ITOH H., ISHIKAWA T., HIDAKA H. Effects on calmodulin of bepridil, an antianginal agent. J. Pharmacol. Exp. Ther. 1984;230:737–741. [PubMed] [Google Scholar]
- KANAIDE H.Measurement of [Ca2+]i in smooth muscle strips using front-surface fluorimetry Methods in Molecular Biology 1999Totowa, NJ: Humana Press Inc; 269–277.ed. Lambert, D.G. pp [DOI] [PubMed] [Google Scholar]
- KARAKI H., OZAKI H., HORI M., MITSUI-SAITO M., AMANO K., HARADA K., MIYAMOTO S., NAKAZAWA H., WON K.J., SATO K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- KIM B.K., MITSUI M., KARAKI H. The long-term inhibitory effect of a Ca2+ channel blocker, nisoldipine, on cytosolic Ca2+ and contraction in vascular smooth muscle. Eur. J. Pharmacol. 1992;223:157–162. doi: 10.1016/0014-2999(92)94834-i. [DOI] [PubMed] [Google Scholar]
- KUREISHI Y., KOBAYASHI S., AMANO M., KIMURA K., KINAIDE H., NAKANO T., KAIBUCHI K., ITO M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J. Biol. Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- MAGNIER GAUBIL C., HERBERT J.M., QUACK R., PAPP B., CORVAZIER E., WUYTACK F., LÈVY TOLÉDANO S., ENOUF J. Smooth muscle cell cycle and proliferation. Relationship between calcium influx and sarco-endoplasmic reticulum Ca2+ATPase regulation. J. Biol. Chem. 1996;271:27788–27794. doi: 10.1074/jbc.271.44.27788. [DOI] [PubMed] [Google Scholar]
- MELLITI K., BOURNAUD R., BASTIDE B., HIDALGO J., SHIMAHARA T. Effect of SR33805 on barium current and asymmetric intramembrane charge movement in freshly dissociated mouse cerebellar Purkinje neurons. Neurosci. Lett. 1996;216:167–170. doi: 10.1016/0304-3940(96)13038-x. [DOI] [PubMed] [Google Scholar]
- MIYOSHI Y., NAKAYA Y. Angiotensin II blocks ATP-sensitive K+ channels in porcine coronary artery smooth muscle cells. Biochem. Biophys. Res. Commun. 1991;181:700–706. doi: 10.1016/0006-291x(91)91247-a. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., STANDEN N.B., BRAYDEN J.E., WORLEY J.F.D. Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988;336:382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- PACAUD P., LOIRAND G., BARON A., MIRONNEAU C., MIRONNEAU J. Ca2+ channel activation and membrane depolarization mediated by Cl− channels in response to noradrenaline in vascular mycoytes. Br. J. Pharmacol. 1991;104:1000–1006. doi: 10.1111/j.1476-5381.1991.tb12540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERSECHINI A., KAMM K.E., STULL J.T. Different phosphorylated forms of myosin in contracting tracheal smooth muscle. J. Biol. Chem. 1986;261:6293–6299. [PubMed] [Google Scholar]
- PUTNEY J.W., JR, MCKAY R.R. Capacitative calcium entry channels. BioEssays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- ROMEY G., BOIS P., LAZDUNSKI M. Effects of two chemically related new Ca2+ channel antagonists, SR33557 (fantofarone) and SR33805, on the L-type cardiac channel. Eur. J. Pharmacol. 1994;263:101–105. doi: 10.1016/0014-2999(94)90529-0. [DOI] [PubMed] [Google Scholar]
- ROMEY G., LAZDUNSKI M. Effects of a new class of calcium antagonists, SR33557 (fantofarone) and SR33805, on neuronal voltage-activated Ca2+ channels. J. Pharmacol. Exp. Ther. 1994;271:1348–1352. [PubMed] [Google Scholar]
- SCORNIK F.S., TORO L. U46619, a thromboxane A2 agonist, inhibits KCa channel activity from pig coronary artery. Am. J. Physiol. 1992;262:C708–C713. doi: 10.1152/ajpcell.1992.262.3.C708. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- STRIESSNIG J., GRABNER M., MITTERDORFER J., HERING S., SINNEGGER M.J., GLOSSMANN H. Structural basis of drug binding to L Ca2+ channels. Trends. Pharmacol. Sci. 1998;19:108–115. doi: 10.1016/s0165-6147(98)01171-7. [DOI] [PubMed] [Google Scholar]
- SUZUKI H., KOU K. Direct and indirect effects of histamine on the smooth muscle cells of the guinea-pig main pulmonary artery. Eur. J. Pharmacol. 1983;339:46–53. doi: 10.1007/BF00652521. [DOI] [PubMed] [Google Scholar]
- WATSON S., GIRDLESTONE D.TiPS receptor and ion channel nomenclature Trends. Pharmacol. Sci. 1994Suppl 435th ed
- WINSLOW E., FARMER S., MARTORANA M., MARSHALL R.J. The effects of bepridil compared with calcium antagonists on rat and rabbit aorta. Eur. J. Pharmacol. 1986;131:219–228. doi: 10.1016/0014-2999(86)90575-3. [DOI] [PubMed] [Google Scholar]
- ZHOU Y., HIRANO K., SAKIHARA C., NISHIMURA J., KANAIDE H. NH2-terminal fragments of the 130 kDa subunit of myosin phosphatase increase the Ca2+ sensitivity of porcine renal artery. J. Physiol. 1999;516:55–65. doi: 10.1111/j.1469-7793.1999.055aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


