Abstract
Thrombin causes various cellular events by activating protease-activated receptors (PARs). Here, we showed, for the first time, that thrombin induced myometrial contraction. To determine the mechanism of thrombin-induced myometrial contraction, we simultaneously measured intracellular Ca2+ concentration ([Ca2+]i) and tension of fura-PE3-loaded rat myometrium using front-surface fluorimetry. The expression of thrombin receptor mRNA in the rat myometrium were determined by reverse transcription-polymerase chain reaction analysis (RT–PCR analysis).
Thrombin (0.01–3 u ml−1) caused dose-dependent increase in [Ca2+]i and tension in the rat myometrium, and this effect was greatly enhanced in the pregnant myometrium. PAR1-activating peptide mimicked the effects of thrombin.
In Ca2+-free PSS, thrombin induced no increase in [Ca2+]i and tension in the pregnant myometrium. Both diltiazem (10 μM) and SK-F 96365 (10 μM) significantly inhibited the thrombin-induced elevations of [Ca2+]i and tension, and their effects were additive.
RT–PCR analysis revealed an approximately 10 fold increase in the level of thrombin receptor mRNA in the pregnant myometrium compared to that obtained in the non-pregnant myometrium.
In conclusion, the contractile response to thrombin was greatly enhanced in the pregnant myometrium, mainly due to the up-regulation of thrombin receptor. We propose that initiation of a post-parturitional myometrial contraction is one of the most important physiological roles of thrombin receptor.
Keywords: Thrombin, myometrial contraction, pregnancy, rat, protease-activated receptor
Introduction
Thrombin, a serine protease, converts fibrinogen into fibrin and plays a critical role in haemostasis. It also activates protease-activated receptors (PARs) and induces various cellular responses, including contraction of vascular, tracheal or gastric smooth muscle, endothelium-dependent vasorelaxation and proliferation of smooth muscle (Cicala et al., 1999; Cocks & Moffatt, 2000; Cocks et al., 1999; Coughlin, 1994; Dery et al., 1998; Godin et al., 1995; McNamara et al., 1993; Tesfamariam, 1994). The PAR belongs to a family of G protein-coupled receptor, and four types of PARs, PAR1, PAR2, PAR3 and PAR4, have so far been cloned (Ishihara et al., 1997; Nystedt et al., 1994; Vu et al., 1991; Xu et al., 1998). PAR1, PAR3 and PAR4 are activated by thrombin, while PAR2 and PAR4 are activated by trypsin and tryptase (Cocks & Moffatt, 2000). The different sets of PARs are expressed in a tissue specific manner, and the types of PAR involved in the response to thrombin differs with species and type of tissue (Dery et al., 1998; Ishihara et al., 1998; Kahn et al., 1998). For example, PAR1 and PAR4 mediate thrombin-induced activation of platelet in human, while PAR3 and PAR4 mediate it in the mouse (Ishihara et al., 1998; Kahn et al., 1998). The protease agonists activate PARs by cleaving the NH2-terminal exodomain of receptors to unmask a new NH2-terminus, which in turn serves as a tethered ligand and thus initiates the intracellular signalling to provoke the successive cellular response (Dery et al., 1998; Grand et al., 1996). The synthetic peptides corresponding to the tethered ligand sequences also activate PARs, except for PAR3 (Ishihara et al., 1997; Kahn et al., 1999; Nystedt et al., 1994; Scarborough et al., 1992; Vu et al., 1991; Xu et al., 1998). These peptides are thus referred to as PAR-activating peptides (PAR-APs) in this study. Since the activation of PARs by PAR-APs is independent of proteolysis, PAR-APs are unique tools for investigating the regulation of thrombin receptor activity.
Although PARs are expressed in various tissues and mediate various cellular responses to thrombin and trypsin, activation of thrombin and trypsin is limited to the pathological conditions such as inflammation, tissue damage, and haemorrhage. Therefore, the physiological role of PARs remains still unclear. Haemorrhage and the following activation of thrombin are physiological consequences in the case of delivery and subsequent placental detachment in the myometrium. The myometrial contraction plays a central role in the biological haemostasis in post-parturition. The mechanism of the myometrial contraction of post-parturition and the physiological stimulation to initiate this contraction remain to be clarified. Thrombin, thus, could be a physiological agonist to initiate the post-parturitional contraction, especially at the haemorrhage site. However, so far no report has yet shown the effects of thrombin on myometrium contraction.
In the present study, we examined the effect of thrombin on cytosolic Ca2+ concentration ([Ca2+]i) and contraction of the rat myometrium, by using front-surface fluorimetry of the fura-PE3-loaded strips of myometrium. We thus found thrombin to induce a contraction of the rat myometrial smooth muscle. We investigated the mechanism of thrombin-induced contraction in terms of Ca2+ signalling in the smooth muscle. We also specified the type of PAR involved in the thrombin-induced contraction by examining the effect of PAR-APs on the myometrial contraction. In addition, thrombin-induced contraction was found to be greatly enhanced in the pregnant rat. We thus examined the expression of PARs to elucidate the mechanism of enhancement for thrombin-induced contraction in the pregnant rat myometrium.
Methods
Tissue preparation
The study protocol was approved by the Animal Care and Committee of Research Institute of Angiocardiology, Faculty of Medicine, Kyushu University. The rats were heparinized with 0.3–0.5 mg rat−1 heparin (MW3000) before sacrifice. Strips of the myometrium were prepared from non-pregnant and day 18 pregnant Wister-Kyoto rats as described previously (Shintani et al., 2000). The myometrium of the middle part of the horn was excised in a longitudinal direction, cut into strips measuring 1.5 mm in width and 4.5 mm in length. The wet weight of the strips were 1.08±0.04 mg (non-pregnant myometrium) and 1.30±0.04 mg (pregnant myometrium) (n=7).
Tension measurement of intact myometrial strips
All measurements were performed in physiological salt solution (PSS) at 37°C. The composition of PSS used in the study was (in mM): NaCl 123, KCl 4.7, CaCl2 1.25, MgCl2 1.2, KH2PO4 1.2, NaHCO3 15.5, and D-glucose 11.5, gassed with 95% O2 and 5% CO2. The myometrial strips were mounted vertically in a quartz organ bath and connected to a force transducer (TB-612T, Nihon Koden, Japan). The resting load was adjusted to approximately 50 mg to obtain a maximal response to 40 mM K+ depolarization. The contractile response was analysed by the area under the curve of the tension trace for the first 10 min of contraction, except for Figure 1, where the contractile response was also evaluated by the level of the first peak. The values obtained with 40 mM K+ depolarization were designated to be 100%.
Figure 1.
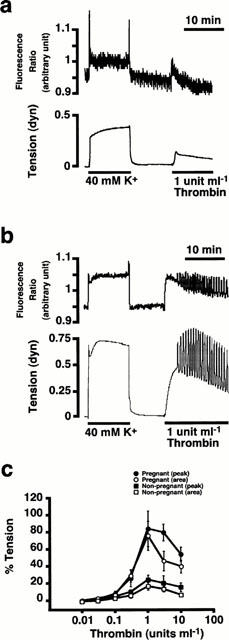
The contractile effects of thrombin on the rat myometrium. (a) and (b) Representative recordings showing the effects of thrombin (1 u ml−1) on the tension of the non-pregnant rat myometrium (a), and on the [Ca2+]i (upper trace) and tension (lower trace) on the day 18 pregnant rat myometrium (b) in the normal PSS. (c) The concentration-response curves of thrombin-induced contraction in the pregnant and non-pregnant myometrium. The contractile response to thrombin was quantitatively evaluated in two ways; the first peak level of tension development and the area under the tension trace for the initial 10 min. The developed tension was expressed as a percentage, assigning the values in normal and 40 nM K+ PSS to be 0% and 100%, respectively. Data are the mean±s.e.mean (n=7).
Front-surface fluorimetry of [Ca2+]i
The myometrial strips of the pregnant rats were loaded with fura-PE3 in the form of acetoxymethyl ester (fura-PE3/AM) as previously described (Kanaide, 1999; Shintani et al., 2000). The changes in the fluorescence ratio and tension development were simultaneously monitored with a front-surface fluorimeter CAM-OF-1 (JASCO, Tokyo, Japan) as previously reported (Niiro et al., 1998; Shintani et al., 2000). The fluorescence (500 nm) intensities at alternating (400 Hz) excitation (340 and 380 nm) and the ratio (F340/F380) were continuously measured. The data were stored in a Macintosh computer using a data acquisition system (MacLab; Analog Digital Instruments, Australia). The fluorescence ratio was expressed as a percentage, assigning the values in normal (5.9 mM K+) and 40 mM K+ PSS to be 0% and 100% respectively. When the fura-PE3-loaded strips of the non-pregnant rat myometrium was stimulated with 40 mM K+, little increase in the fluorescence ratio was observed, although there was an apparent increase in tension (Figure 1a). It was conceivable that the poor fluorescence signal is attributed to the poor loading of fura-PE3 in the non-pregnant myometrium. Therefore, only tension development was evaluated in the non-pregnant myometrium.
Reverse transcription-polymerase chain reaction (RT–PCR) analysis
Total RNAs of rat myometrium and placenta were isolated by the acid guanidinium thiocyanate phenol-chloroform method (Chomczynski & Sacchi, 1987). Total RNAs were digested by RNase-free DNase to rule out the possible contamination by genomic DNA. The primers used in RT–PCR analysis of rat PAR1 (Zhong et al., 1992) were 5′-GAG CAG GTA TCC ATC TTA CT-3′ for RT reaction, 5′-TGA CAG TCA TAA GCA TTG AC-3′ for upper primer in PCR reaction, and 5′-GGC ATA GTA GTA AAT CAA GG-3′ for the lower primer in PCR reaction. The primers used in RT–PCR analysis of rat PAR2 (Saifeddine et al., 1996) were 5′-CAA AGT AGT AGA CAA AGG GG-3′ for the RT reaction, 5′-CCA GGA AGA GGG CCA ACA T-3′ for the upper primer in PCR reaction, and 5′-ACG GTG CGG ACG CTT CGG CA-3′ for the lower primer in PCR reaction. The primers used in RT–PCR analysis of PAR4 (Kahn et al., 1998; 1999) were 5′-GAC ACA TAG TAG TAG ATG AA-3′ for the RT reaction, 5′-TGG TTC AGT GTT GCT GCT GG-3′ for the upper primer in PCR reaction, and 5′-TCC ATA GAG ATT GCC CCA GG-3′ for the lower primer in PCR reaction. One μg of the total RNA was used for the RT reaction using Moloney murine leukaemia virus reverse transcriptase in a total volume of 20 μl. One μl aliquot of RT product was subjected to PCR amplification. The thermal cycle profile used for the amplification of PAR1, PAR2, and PAR4 fragments was composed of the initial denaturation at 94°C, for 2 min and the following 35–40 cycle amplification step of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min. The expression of β-actin was analysed as a control. The primers and condition for the RT–PCR analysis of β-actin was as previously described (Niiro et al., 1998). The RT–PCR products were separated on 3% agarose gel containing 0.05 μg ml−1 ethidium bromide. The density of the bands was analysed by a NIH image (the National Institutues of Health, Bethesola, MD, U.S.A.) after obtaining the fluorescence image with a CCD camera (ATTO, Tokyo, Japan). The nucleotide sequences of the PCR product for PAR1, PAR2 and PAR4 were determined to be identical to the published sequences (Kahn et al., 1998; Saifeddine et al., 1996; Zhong et al., 1992).
Drugs
The composition of the normal physiological salt solution (normal PSS) was described above. High-K+ PSS was made by an equimolar substitution of KCl for NaCl. Diltiazem, heparin (MW3000), thrombin (bovine plasma) and 4-aminidophenylmethane-sulphonyl fluoride (p-APMSF) were purchased from Sigma (St Louis, MO, U.S.A.). SK-F 96365 (1 - {β-[3-(4 - methoxyphenyl)propoxy] - 4 -methoxyphenethyl}-1H-imidazole hydrochloride) was purchased from Calbiochem (La Jolla, CA, U.S.A.). Human PAR1-AP (SFLLRNP) and rat PAR2-AP (SLIGRL) were from BACHEM (Bubendorf, Switzerland). PAR3-AP (SFNGGPQ) and PAR4-AP (GYPGKFC) were synthesized based on the mouse sequence (Kahn et al., 1998) by Sawady Technology (Tokyo, Japan). All primers used in RT–PCR analysis were synthesized by Hokkaido System Science (Hokkaido, Japan). Moloney murine leukaemia virus reverse transcriptase was from BRL (Gaithersburg, MD, U.S.A.). Fura-PE3/AM was from Texas Fluorescence Laboratory (Austin, TX, U.S.A.). Ethyleneglycol-bis(β-aminoethylether)-N′,N′,N′,N′-tetra acetic acid (EGTA) was from Dojindo Laboratories (Kumamoto, Japan). All other chemicals were of the highest grade commercially available.
Statistical analysis
All data are expressed as the mean±s.e.mean. The unpaired Student's t-test was used to determine significant difference and a one way analysis of variance followed by Scheffe's test was used for multiple comparisons. P values <0.05 were considered to be statistically significant.
Results
The effect of thrombin on the rat myometrium
In the non-pregnant myometrium, thrombin induced a small phasic contraction (Figure 1a). The contractile responses to thrombin was quantitatively evaluated in two ways; the first peak level of contraction and the area under the tension trace for the first 10 min. The extent of tension development induced by 1 u ml−1 thrombin was 24.8±5.3% (the first peak, n=7) and 18.0±4.4% (area under the tension trace, n=7), while assigning those obtained with 40 mM K+ to be 100% in the non-pregnant myometrium (Figure 1c).
The effects of thrombin on myometrial contraction were examined using front-surface fluorimetry (Kanaide, 1999) in the fura-PE3-loaded strips of the pregnant rat myometrium, and the changes in [Ca2+]i and tension of smooth muscle were simultaneously monitored. In an 18-day pregnant rat myometrium, thrombin induced much greater contractions than that seen in the non-pregnant rat myometrium. Thrombin (1 u ml−1) caused a rapid increased in [Ca2+]i and tension, followed by a slowly declining phase (Figure 1b). Spontaneous oscillatory contractions usually overrode the basal thrombin-induced contractions (Figure 1b). The level of [Ca2+]i elevation at the peak was 82.3±8.2% (n=8) of that obtained during the sustained phase of the contraction induced by 40 mM K+-depolarization. Thrombin induced contractions at 0.3 u ml−1 and higher concentrations in the pregnant myometrium (Figure 1c). The maximum tension development (84.9±20.7% by the first peak; 77.6±17.1% by the area under the tension trace, n=7) was observed at 1 u ml−1. The level of [Ca2+]i elevation (82.3±8.2%) and tension development (77.6±17.1% by the area) obtained with 1 u ml−1 thrombin was similar to the level of [Ca2+]i (91.14±9.1%, n=5) and tension (89.3±19.7%, n=5, by the area under the tension trace) obtained with 30 mM K+-depolarization. Within the concentration range between 0.01 and 1 u ml−1, the concentration-response curve of the first peak overlapped with that of the area under the tension trace (Figure 1c). A further increase in the thrombin concentration caused slightly less contraction, thus resulting in a bell-shaped concentration-response curve. The decrease in the contractile response at higher concentrations of thrombin was considered to be due to the rapid desensitization of thrombin receptor. This was supported by the observation that increasing thrombin concentration to 3 and 10 u ml−1 progressively accelerated the decline in the contraction phase (data not shown), and that the concentration-response curve for the area under tension trace located lower than that for the first peak at concentrations higher than 1 u ml−1 (The area under the tension trace is considered to reflect not only the peak tension development but also the duration of tension generation.) (Figure 1c).
Gestational change of the contractile response to thrombin in the rat myometrium
As shown in Figure 2, the contractile response to 1 u ml−1 thrombin was enhanced as the day of pregnancy increased (n=3–7, P<0.05), and the enhancement of the contraction reached a maximum in the middle to late period of pregnancy. The maximum tension development induced by 1 u ml−1 thrombin in the 18-day pregnant rat was significantly greater than those obtained in the non-pregnant rat (P<0.01), while assigning those obtained with 40 mM K+ to be 100% (Figure 2).
Figure 2.
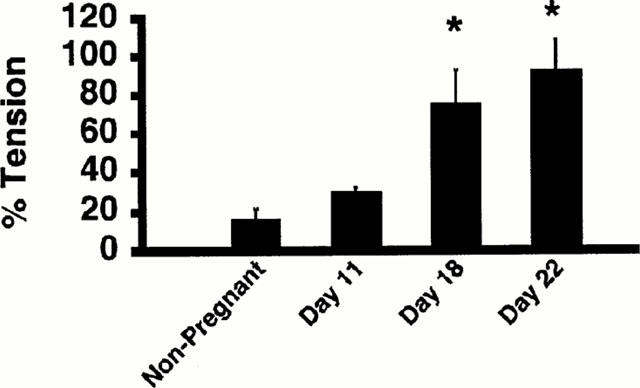
Gestational change of the contractile response to thrombin in the rat myometrium. (a) Comparison of the contractile response to thrombin based on the day of pregnancy. Contractile response was evaluated by the area under the tension trace, assuming those obtained with 40 mM K+ to be 100%. The data are the mean±s.e.mean (n=3–7). *P<0.05 as compared with the non-pregnant rats.
When the developed tension was evaluated as an absolute value, 40 mM K+-depolarization produced 401.2±25.9 dyne g−1 of wet tissue tension in the non-pregnant myometrium (n=7) and 623.1±62.1 dyne g−1 of wet tissue tension in the pregnant myometrium (n=7, P<0.05). Thrombin (1 u ml−1) produced 102.9±20.1 dyne g−1 of wet tissue tension in the non-pregnant myometrium, and 604.0±126.8 dyne g−1 of wet tissue tension in the pregnant myometrium (n=7, P<0.01). The thrombin-induced contractile response was thus more potently enhanced (approximately 6 fold) in the pregnant myometrium than the depolarization-induced contraction (almost 1.5 fold). Even when normalized by the 40 mM K+-induced contraction, the thrombin-induced contraction was still greatly enhanced (about 4 fold).
Effect of PARs-AP on the contraction in the rat myometrium
PAR1 (Vu et al., 1991) PAR3 (Ishihara et al., 1997) and PAR4 (Xu et al., 1998) are candidate receptors mediating the thrombin-induced contractile response in the rat myometrium. We thus examined the effects of the synthetic peptide corresponding to the region of the tethered ligand (Vu et al., 1991; Walz et al., 1986) on the contraction in the rat myometrium. As shown in Figure 3, a human PAR1-AP (SFLLRNP) (Vu et al., 1991) induced a contraction in both the pregnant and non-pregnant rat myometrium at concentrations higher than 0.1 μM. As is the case with thrombin, the contractile response to PAR1-AP was enhanced in the pregnant myometrium (Figure 3c). When evaluated by the area under the tension trace, the level of contraction induced by 100 μM PAR1-AP was 23.8±4.1% (n=5) in the non-pregnant and 102.0±3.7% (n=7, P<0.01) in the pregnant myometrium (about 4 fold increase). Previous reports have shown that PAR1-AP was able to activate both PAR1 and PAR2 (Blackhart et al., 1996), but not PAR3 (Ishihara et al., 1997) or PAR4 (Kahn et al., 1999). However, a rat PAR2-AP (SLIGRL) (Saifeddine et al., 1996) failed to induce contraction in both the pregnant and non-pregnant myometrium (data not shown). The PAR3-AP failed to induce any contraction in the rat myometrium (data not shown). A similar peptide mimicking a putative tethered ligand of the human PAR3 was not able to activate the aggregation of platelets which express PAR3 (Ishihara et al., 1997), and this finding was consistent with that of another report. The PAR4-AP also failed to induce contraction in the rat myometrium (data not shown).
Figure 3.
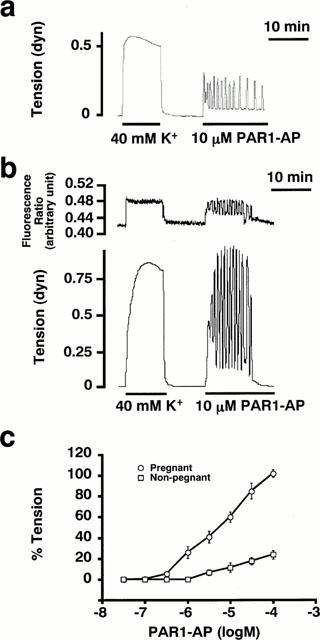
The contractile effects of thrombin receptor activating peptide on the rat myometrium. (a) and (b) Representative traces showing the effects of PAR1-AP (10 μM) on the tension of the non-pregnant rat myometrium (a) and on the [Ca2+]i (upper trace) and tension (lower trace) of the pregnant rat myometrium (b) in the normal PSS. (c) The concentration-response curves of PAR1-AP-induced contraction. The tension development was evaluated by the area under the tension trace for the initial 10 min, assigning the values obtained with 40 mM K+ PSS to be 100%. The data are the mean±s.e.mean (n=5–7).
Effects of protease inhibitor on the myometrial contractions induced by thrombin and PAR1-AP
The requirement of the proteolytic activity of thrombin to induce myometrial contraction was examined as shown in Figure 4. When thrombin was pretreated with 10 μM p-APMSF, a serine protease inhibitor, for 10 min and then applied to the strip, it failed to induce any contractions both in the pregnant (Figure 4a) and non-pregnant myometrium (data not shown). The subsequent application of untreated thrombin induced the contraction in the absence of p-APMSF (Figure 4a). However, the pretreatment with p-APMSF had no effect on the PAR1-AP-induced contraction (Figure 4b). The contraction induced by PAR1-AP treated with 10 μM p-APMSF elicited as much myometrial contraction as that obtained with the untreated PAR1-AP (Figure 3b). These findings indicated that the contractile response to thrombin but not to PAR1-AP required the proteolytic activity, thus suggesting that the thrombin-induced contraction is mediated by PARs (Dery et al., 1998).
Figure 4.
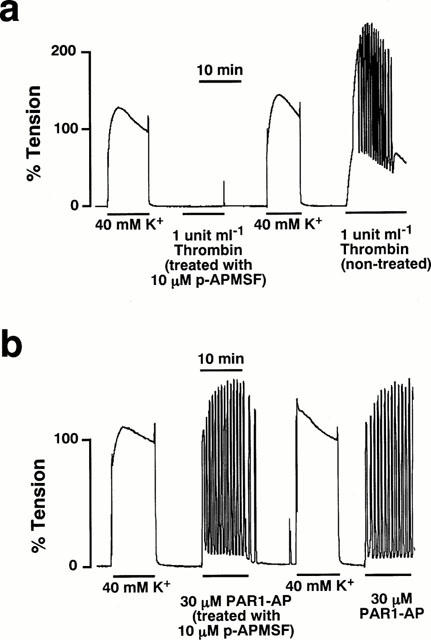
The effect of the serine protease inhibitor on the myometrial contractions induced by thrombin and PAR1-AP. (a) Representative trace showing the inhibitory effect of the serine protease inhibitor, 4-aminidophenylmethane-sulphonyl fluoride (p-APMSF, 10 μM) on the 1 u ml−1 thrombin-induced contraction of the pregnant rat myometrium. After observing the effect of p-APMSF-treated thrombin and stimulation once with 40 mM K+, the contracting effect of the untreated thrombin was examined. (b) Representative trace showing the effect of 10 μM p-APMSF on the 30 μM PAR1-AP-induced contraction of the pregnant rat myometrium. After observing the effect of p-APMSF-treated PAR1-AP and stimulation once with 40 mM K+, the contracting effect of the untreated PAR1-AP was examined.
Mechanism of the thrombin-induced contraction
Using front-surface fluorimetry (Kanaide, 1999), the intracellular Ca2+ signalling involved in the thrombin-induced contraction was investigated. In the PSS containing 1.25 mM Ca2+, thrombin induced a sustained elevation of [Ca2+]i in accordance with the tension development (Figure 1). On the other hand, the exposure to the Ca2+-free PSS caused the gradual decline of the resting [Ca2+]i level, and the subsequent stimulation with thrombin did not induce any [Ca2+]i elevation or contraction (Figure 5a). NiCl2, an inorganic Ca2+ channel blocker (Chaib et al., 1998), also completely inhibited the elevation of [Ca2+]i and contraction induced by thrombin (Figure 5b). However, the [Ca2+]i elevation and contraction induced by thrombin was partially inhibited by 10 μM diltiazem (Figure 5c). The extent of [Ca2+]i elevation and contraction obtained with 10 μM diltiazem was 44.7±9.9 and 14.4±4.7%, respectively (Figure 5e). Higher concentrations of diltiazem (30 μM) did not cause any further inhibition (Figure 5e). The remaining [Ca2+]i elevation and force was completely inhibited by 10 μM SK-F96365 (Waldron et al., 1997) (Figure 5e). Although 10 μM SK-F96365 alone partially inhibited the thrombin-induced [Ca2+]i elevation and force development, a further increment of the concentrations to 30 and 100 μM almost completely inhibited the contraction (Figure 5d,e).
Figure 5.
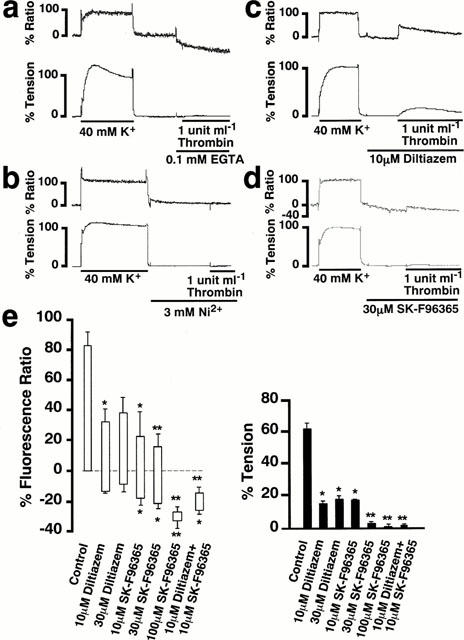
Mobilization of Ca2+ in the pregnant rat myometrium during the thrombin-induced contraction. (a) (b) (c) and (d) Representative traces of the thrombin-induced changes in [Ca2+]i (upper trace) and tension (lower trace) in the pregnant rat myometrium observed in the Ca2+-free PSS containing 0.1 mM EGTA (a), and in the normal PSS containing 3 mM Ni2+ (b), 10 μM diltiazem (c), and 30 μM SK-F96365 (d) in the normal PSS. (e) Summary of the inhibitory effects of diltiazem and SK-F96365 on the thrombin-induced increase in [Ca2+]i and tension. Control; the 1 u ml−1 thrombin-induced [Ca2+]i elevation and tension development in the normal PSS with no inhibitors. The bottom and top levels of columns for [Ca2+]i indicate the values obtained just before the initiation of contraction by thrombin and at the peak contractile response, respectively. The developed tension was evaluated by the area under the tension trace. The [Ca2+]i and developed tension were expressed as a percentage, assigning the values obtained with 40 mM K+ PSS to be 100%. The data are the mean±s.e.mean (n=5–8). *P<0.05, **P<0.01 as compared with the control.
Pregnancy increased the expression level of PAR1 mRNA
The changes in the expression level of PAR1, PAR2, and PAR4 mRNA in the rat myometrium during pregnancy was examined by a RT–PCR analysis (Figure 6). In the non-pregnant myometrium, PAR1 mRNA was slightly detected (Figure 6a). On the other hand, the level of PAR1 mRNA increased approximately 10 fold (n=5, P<0.05) in the myometrium during both pregnancy and the post-parturition period compared to that in the non-pregnant myometrium (Figure 6a,b). The level of PAR1 mRNA did not significantly differ with the day of pregnancy. The level of β-actin mRNA slightly increased (1.5 fold, n=5, P<0.05) in the pregnant myometrium (Figure 6b).
Figure 6.
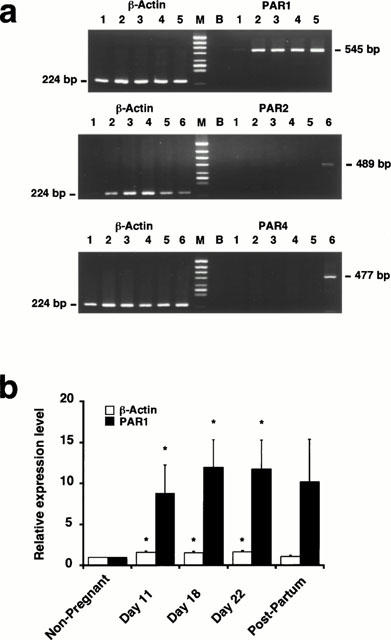
Expression of PAR1, PAR2 and PAR4 mRNA in the rat myometrium during the pregnancy. (a) Representative photographs showing the expression of PAR1, PAR2, PAR4 and β-actin mRNA in the myometrium of non-pregnant (lane 1), pregnant day 11 (lane 2), pregnant day 18 (lane 3), pregnant day 22 (lane 4) and postpartum rats (lane 5), as well as in the rat placenta (lane 6). Lane M, HincII digest of ΦX174 as the DNA size marker. Lane B, PCR product obtained with no addition of RT products. The predicted size of the products is indicated on the side of the photographs. (b) The changes in the expression of β-actin and PAR1 in the rat myometrium during pregnancy. The expression level was expressed as the value relative to that seen in the non-pregnant myometrium. The data are the mean±s.e.mean (n=5–6). *P<0.05 as compared with the non-pregnant rats.
The expression of PAR2 mRNA was hardly detected in both the non-pregnant and the pregnant rat myometrium by a RT–PCR analysis using the set of primers based on the sequence of rat PAR2 (Saifeddine et al., 1996), while it detected the expression of PAR2 in the rat placenta (Figure 6a). Furthermore, PAR4 mRNA also was scarcely detected in both the non-pregnant and pregnant rat myometrium by a RT–PCR analysis using the set of primers based on the sequence of mouse PAR4 (Kahn et al., 1998), while the expression of PAR4 mRNA was detected in the rat placenta (Figure 6a).
Discussion
We herein demonstrated that thrombin induced a contraction in the myometrium of the rat, and that this contractile response was greatly enhanced in the pregnant rat. Moreover the expression level of PAR1 mRNA was significantly increased in the pregnant myometrium. It is thus likely that the enhanced contractile response in the pregnant rat myometrium was mainly due to the up-regulation of the PAR1. Previous studies reported that thrombin induced contraction of several types of smooth muscle, such as vascular (Bretschneider et al., 1995; deBlois et al., 1992; Godin et al., 1995; Tay-Uyboco et al., 1995), gastric (Yang et al., 1992), and tracheal (Cicala et al., 1999) smooth muscle. However, there has been no report on the thrombin-induced myometrial contraction. This is thus the first report on the myometrial contraction induced by thrombin.
Thrombin was shown to activate PAR1, PAR3 and PAR4 (Coughlin, 1999). In the present study, PAR1 was considered to be the major receptor for thrombin to initiate myometrium contraction for the following reasons: (i) PAR1-AP induced a contraction in the rat myometrium, and this contraction was greatly enhanced in the pregnant myometrium as in the case of thrombin. Although PAR1-AP was reported to activate PAR2 as well as PAR1 (Blackhart et al., 1996), the expression of PAR2 mRNA was not detected in the rat myometrium. As a result, PAR1-AP was considered to induce the contraction by activating PAR1 in the rat myometrium; (ii) the expression of PAR4 mRNA was not detected in the rat myometrium and (iii) the involvement of PAR3 could not be completely ruled out even though a synthetic PAR3-AP had no effect on the myometrial contraction. A similar peptide was reported to have no effect on aggregation of the mouse platelets which express PAR3 (Ishihara et al., 1997; Kahn et al., 1999). Moreover, PAR3 has recently been suggested to act as a cofactor for thrombin to activate PAR4 (Nakanishi-Matsui et al., 2000).
Our results showed that thrombin caused the large sustained contraction of the rat myometrium, accompanied with the increase of [Ca2+]i level. As shown in Figure 3, the [Ca2+]i elevation due to the Ca2+ influx was the most important intracellular signal for thrombin to induce contraction. The contribution of the Ca2+ release from the intracellular stores to the thrombin-induced contraction was, if any, negligible. The voltage-operated Ca2+ channels as well as the other Ca2+ entry pathways were suggested to be involved in the thrombin-induced Ca2+ influx. Oxytocin and prostaglandins (PGs) have been reported to induce the rat myometrial contraction by increasing [Ca2+]i partly due to the Ca2+ release, which is induced by inositol 1,4,5-trisphosphae (IP3) generated by phospholipase Cβ (PLCβ) (Izumi et al., 1995; 1996; Wray, 1993), especially during pregnancy (Taggart & Wray, 1998). In addition, these uterotonic agents have been reported to increase Ca2+ sensitivity of the contractile apparatus of smooth muscle through GTP-binding protein, which is linked to PLCβ (Izumi et al., 1995; 1996). However, in the present study, the tension-development for a given change in [Ca2+]i in the thrombin-induced contraction was similar to that obtained with high K+-depolarization. It is thus unlikely that thrombin enhanced the Ca2+ sensitivity of a contractile apparatus in the rat myometrium. As a result, thrombin was considered to induce myometrial contraction by elevating [Ca2+]i mainly due to [Ca2+]i influx, while having negligible effect on the Ca2+ sensitivity of the contractile apparatus. PAR1 was shown to couple to several different G proteins including Gαq, Gαi, Gαo, Gα12 and Gα13 (Cocks & Moffatt, 2000; Swift et al., 2000). Gα12 was shown to activate a GDP/GTP exchange factor, which in turn specifically activate a small GTP-binding protein, RhoA (Buhl et al., 1995; Collins et al., 1996). RhoA and RhoA-associated kinase were suggested to be involved in potentiation of the Ca2+-sensitivity of the contractile apparatus (Hirata et al., 1992; Kureishi et al., 1997). The GTP-binding proteins linked to PAR1 in the rat myometrium thus remain to be elucidated.
The enhancement of contractile response in the pregnant myometrium was not specific to thrombin or PAR1 but also observed with other contractile stimuli including K+ depolarization, oxytocin, PGF2α, endothelin, carbachol, and neurokinin A (Osada et al., 1997; Riemer & Heymann, 1998; Shintani et al., 2000; Taggart & Wray, 1998; Tuross et al., 1987). Oxytocin induced approximately 3.6 and 6.4 fold greater contraction in the pregnant rat and human myometrium, respectively, than in the non-pregnant myometrium (Izumi et al., 1995, 1990). In the present study, 40 mM K+ depolarization induced about 1.5 fold greater contraction in the pregnant myometrium than in the non-pregnant myometrium. The myometrium hypertrophy due to pregnancy may thus partly contribute to the enhancement of myometrium contraction (Riemer & Heymann, 1998). However, the enhancement of thrombin-induced contraction was about 6 fold, and even after normalization by the contraction induced by 40 mM K+, thrombin-induced contraction in the pregnant myometrium was about 4 fold greater than that observed in the non-pregnant myometrium, It is thus conceivable that there is some mechanism specific to the enhancement of the thrombin-induced contraction. In case of oxytocin-induced contraction, the expression of oxytocin receptor mRNA markedly (nearly 300 fold) increased just before labour in the human myometrium (Kimura et al., 1996), and thus this increase in the expression of oxytocin receptor was considered to be linked to the enhancement of the oxytocin-induced contraction. In the present study, the expression of PAR1 was up-regulated about 10 fold in the pregnant myometrium, while the expression of PAR1 was scarcely detected in the non-pregnant myometrium. As a result, these findings strongly suggested that the enhancement of the thrombin-induced contraction was mainly due to the up-regulation of PAR1. However, the up-regulation of PAR1 was observed as early as day 11, while the enhancement of the thrombin-induced contraction was observed on days 18 and 22. It is thus possible that other mechanisms such as post-transcriptional or post-translational regulation, contributed to the enhancement of the contraction.
The mechanism of the up-regulation of PAR1 in the pregnant rat myometrium remains to be elucidated. However, it is conceivable that the expression of PAR1 is regulated by hormones which are involved in pregnancy. In fact, the expression of the oxytocin receptor was shown to be up-regulated by oestrogen and corticosterone as well as cyclic adenosine monophosphate during pregnancy (Fang et al., 1996; Fuchs et al., 1983; Hinko & Soloff, 1993; Soloff et al., 1983; Uenoyama et al., 1997). The expression of haemeoxygenase-1 was up-regulated by progesterone during gestation (Acevedo & Ahmed, 1998). In addition to such hormonal regulation, other mechanisms related to the myometrium hypertrophy could contribute to the up-regulation of PAR1 expression. The stretch-induced gene expression is one of such mechanisms, and this was in fact reported in rat cardiac hypertrophy (Komuro et al., 1990). It is thus possible that the up-regulation of PAR1 is mediated by either hormonal regulation or the mechanical stress in the pregnant myometrium. However, these possibilities still remain to be proved.
It has now been established that thrombin exerts its various cellular effect by activating PARs. However, since the activation of thrombin is limited to the pathological conditions such as haemorrhage, inflammation or tissue damage, the physiological role of PARs remains unclear. Haemorrhage and subsequent activation of thrombin is a physiological consequence of delivery. Since the activation of thrombin is temporally and spatially specific to the placental detachment site in post-parturition, the contraction induced by thrombin is suggested to contribute to the biological haemostasis in post-parturition, especially at the haemorrhage site of the uterus. The concentrations of thrombin required to induce myometrial contraction have been reported to be achievable in the haemorrhagic lesion, assuming 1 u ml−1 to be equivalent to 10 nM (Walz et al., 1986). We propose that initiation of the post-parturitional contraction of myometrium is one of the physiological functions of PAR1. However, the contractile effects of thrombin on the human myometrium remained to be examined. Furthermore, the PAR1 gene knock-out mice have not yet been reported to undergo post-delivery intractable haemorrhaging, although it was reported that about half of the tr−/− embryos died at embryonic days 9–10 and the other half survived to become grossly normal adult mice with no bleeding diathesis (Connolly et al., 1996). Further study is thus needed to establish the physiological role of PAR1 in the post-partum myometrial haemostasis in human, and to extend our discovery to the development of a new therapeutic strategy for the post-partum uterine bleeding.
In conclusion, we demonstrated, for the first time, that thrombin induced a contraction both in the pregnant and non-pregnant rat myometrium. In the pregnant rat myometrium, thrombin-induced contraction is mainly due to Ca2+ influx, while having no effect on the Ca2+-sensitivity of the contractile apparatus. Since the fluorimetry was not feasible in the non-pregnant rat myometrium, the role of the Ca2+ signal during the thrombin-induced contraction remains to be determined. The most important finding of the present study is that the thrombin-induced contraction was greatly enhanced during pregnancy. The up-regulation of PAR1 was considered to be linked to this enhancement of the contractile response. The mechanism of such up-regulation of PAR1, however, remains to be elucidated.
Acknowledgments
We thank Mr Brian Quinn for comments and help with the manuscript. This study was supported in part by Grants-in-Aid for Scientific Research (No. 10557072, 11838013, 11670687) and for Scientific Research on Priority Area (No. 12213103) from the Ministry of Education, Science, Sports and Culture, Japan, by the Research Grant for Cardiovascular Diseases (11C-1) from the Ministry of Health and Welfare, Japan, and by grants from the Foundation for the Promotion of Clinical Medicine, the Suzuken Memorial Foundation and KANZAWA Medical Research Foundation.
Abbreviations
- [Ca2+]i
the intracellular Ca2+ concentration
- cyclic AMP
adenosine 3′,5′-cyclic phosphate
- EGTA
ethyleneglycol-bis(β-aminoethylether)-N′,N′,N′,N′-tetra acetic acid
- G protein
GTP-binding protein
- IP3
inositol 1,4,5-trisphosphate
- p-APMSF
4-aminidophenylmethane-sulphonyl fluoride
- PAR
protease-activated receptor
- PAR-AP
PAR-activating peptide
- PGs
prostaglandins
- PLCβ
β isoform of phospholipase C
- PSS
physiological saline solution
- RT–PCR
reverse transcription-polymerase chain reaction
- VOC
voltage-operated Ca2+ channel
References
- ACEVEDO C.H., AHMED A. Hemeoxygenase-1 inhibits human myometrial contractility via carbon monoxide and is upregulated by progesterone during pregnancy. J. Clin. Invest. 1998;101:949–955. doi: 10.1172/JCI927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKHART B.D., EMILSSON K., NGUYEN D., TENG W., MARTELLI A.J., NYSTEDT S., SUNDELIN J., SCARBOROUGH R.M. Ligand cross-reactivity within the protease-activated receptor family. J. Biol. Chem. 1996;271:16466–16471. doi: 10.1074/jbc.271.28.16466. [DOI] [PubMed] [Google Scholar]
- BRETSCHNEIDER E., PAINTZ M., GLUSA E. Inositol, 1,4,5-triphosphate and protein kinase C are involved in thrombin- and trap-induced vascular smooth muscle contraction. Agents Actions (Suppl.) 1995;45:309–313. doi: 10.1007/978-3-0348-7346-8_42. [DOI] [PubMed] [Google Scholar]
- BUHL A.M., JOHNSON N.L., DHANASEKARAN N., JOHNSON G.L. G alpha 12 and G alpha 13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J. Biol. Chem. 1995;270:24631–24634. doi: 10.1074/jbc.270.42.24631. [DOI] [PubMed] [Google Scholar]
- CHAIB N., KABRE E., METIOUI M., ALZOLA E., DANTINNE C., MARINO A., DEHAYE J.P. Differential sensitivity to nickel and SK&F96365 of second messenger-operated and receptor-operated calcium channels in rat submandibular ductal cells. Cell Calcium. 1998;23:395–404. doi: 10.1016/s0143-4160(98)90096-3. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CICALA C., BUCCI M., DE DOMINICIS G., HARRIOT P., SORRENTINO L., CIRINO G. Bronchoconstrictor effect of thrombin and thrombin receptor activating peptide in guinea-pigs in vivo. Br. J. Pharmacol. 1999;126:478–484. doi: 10.1038/sj.bjp.0702303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCKS T.M., MOFFATT J.D. Protease-activated receptors: sentries for inflammation. Trends Pharmacol. Sci. 2000;21:103–108. doi: 10.1016/s0165-6147(99)01440-6. [DOI] [PubMed] [Google Scholar]
- COCKS T.M., SOZZI V., MOFFATT J.D., SELEMIDIS S. Protease-activated receptors mediate apamin-sensitive relaxation of mouse and guinea-pig gastrointestinal smooth muscle. Gastroenterology. 1999;116:586–592. doi: 10.1016/s0016-5085(99)70180-0. [DOI] [PubMed] [Google Scholar]
- COLLINS L.R., MINDEN A., KARIN M., BROWN J.H. Galpha12 stimulates c-Jun NH2-terminal kinase through the small G proteins Ras and Rac. J. Biol. Chem. 1996;271:17349–17353. doi: 10.1074/jbc.271.29.17349. [DOI] [PubMed] [Google Scholar]
- CONNOLLY A.J., ISHIHARA H., KAHN M.L., FARESE R.V., JR, COUGHLIN S.R. Role of the thrombin receptor in development and evidence for a second receptor. Nature. 1996;381:516–519. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- COUGHLIN S.R. How the protease thrombin talks to cells. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11023–11027. doi: 10.1073/pnas.96.20.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUGHLIN S.R. Thrombin receptor function and cardiovascular disease. Trends Cardiovasc. Med. 1994;4:77–83. doi: 10.1016/1050-1738(94)90013-2. [DOI] [PubMed] [Google Scholar]
- DEBLOIS D., DRAPEAU G., PETITCLERC E., MARCEAU F. Synergism between the contractile effect of epidermal growth factor and that of des-Arg9-bradykinin or of alpha-thrombin in rabbit aortic rings. Br. J. Pharmacol. 1992;105:959–967. doi: 10.1111/j.1476-5381.1992.tb09085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERY O., CORVERA C.U., STEINHOFF M., BUNNETT N.W. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- FANG X., WONG S., MITCHELL B.F. Relationships among sex steroids, oxytocin, and their receptors in the rat uterus during late gestation and at parturition. Endocrinology. 1996;137:3213–3219. doi: 10.1210/endo.137.8.8754742. [DOI] [PubMed] [Google Scholar]
- FUCHS A.R., PERIYASAMY S., ALEXANDROVA M., SOLOFF M.S. Correlation between oxytocin receptor concentration and responsiveness to oxytocin in pregnant rat myometrium: effects of ovarian steroids. Endocrinology. 1983;113:742–749. doi: 10.1210/endo-113-2-742. [DOI] [PubMed] [Google Scholar]
- GODIN D., RIOUX F., MARCEAU F., DRAPEAU G. Mode of action of thrombin in the rabbit aorta. Br. J. Pharmacol. 1995;115:903–908. doi: 10.1111/j.1476-5381.1995.tb15895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAND R.J., TURNELL A.S., GRABHAM P.W. Cellular consequences of thrombin-receptor activation. Biochem. J. 1996;313:353–368. doi: 10.1042/bj3130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINKO A., SOLOFF M.S. Up-regulation of oxytocin receptors in rabbit amnion by glucocorticoids: potentiation by cyclic adenosine 3′,5′-monophosphate. Endocrinology. 1993;133:1511–1519. doi: 10.1210/endo.133.4.8404589. [DOI] [PubMed] [Google Scholar]
- HIRATA K., KIKUCHI A., SASAKI T., KURODA S., KAIBUCHI K., MATSUURA Y., SEKI H., SAIDA K., TAKAI Y. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. J. Biol. Chem. 1992;267:8719–8722. [PubMed] [Google Scholar]
- ISHIHARA H., CONNOLLY A.J., ZENG D., KAHN M.L., ZHENG Y.W., TIMMONS C., TRAM T., COUGHLIN S.R. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- ISHIHARA H., ZENG D., CONNOLLY A.J., TAM C., COUGHLIN S.R. Antibodies to protease-activated receptor 3 inhibit activation of mouse platelets by thrombin. Blood. 1998;91:4152–4157. [PubMed] [Google Scholar]
- IZUMI H., BIAN K., BUKOSKI R.D., GARFIELD R.E. Agonists increase the sensitivity of contractile elements for Ca2+ in pregnant rat myometrium. Am. J. Obstet. Gynecol. 1996;175:199–206. doi: 10.1016/s0002-9378(96)70275-2. [DOI] [PubMed] [Google Scholar]
- IZUMI H., BYAM-SMITH M., GARFIELD R.E. Gestational changes in oxytocin- and endothelin-1-induced contractility of pregnant rat myometrium. Eur. J. Pharmacol. 1995;278:187–194. doi: 10.1016/0014-2999(95)00089-4. [DOI] [PubMed] [Google Scholar]
- IZUMI H., ICHIHARA J., UCHIUMI Y., SHIRAKAWA K. Gestational changes in mechanical properties of skinned muscle tissues of human myometrium. Am. J. Obstet. Gynecol. 1990;163:638–647. doi: 10.1016/0002-9378(90)91216-y. [DOI] [PubMed] [Google Scholar]
- KAHN M.L., NAKANISHI-MATSUI M., SHAPIRO M.J., ISHIHARA H., COUGHLIN S.R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAHN M.L., ZHENG Y.W., HUANG W., BIGORNIA V., ZENG D., MOFF S., FARESE R.V., JR, TAM C., COUGHLIN S.R. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- KANAIDE H.Measurement of [Ca2+]i in Smooth Muscle Strips Using Front-Surface Fluorimetry Methods in Molecular Biology, Vol. 114: Calcium Signaling Protocols 1999Humana Press Inc., Totowa, New Jersey; 269–277.ed. Lambert DG. pp [DOI] [PubMed] [Google Scholar]
- KIMURA T., TAKEMURA M., NOMURA S., NOBUNAGA T., KUBOTA Y., INOUE T., HASHIMOTO K., KUMAZAWA I., ITO Y., OHASHI K., KOYAMA M., AZUMA C., KITAMURA Y., SAJI F. Expression of oxytocin receptor in human pregnant myometrium. Endocrinology. 1996;137:780–785. doi: 10.1210/endo.137.2.8593830. [DOI] [PubMed] [Google Scholar]
- KOMURO I., KAIDA T., SHIBAZAKI Y., KURABAYASHI M., KATOH Y., HOH E., TAKAKU F., YAZAKI Y. Stretching cardiac myocytes stimulates protooncogene expression. J. Biol. Chem. 1990;265:3595–3598. [PubMed] [Google Scholar]
- KUREISHI Y., KOBAYASHI S., AMANO M., KIMURA K., KANAIDE H., NAKANO T., KAIBUCHI K., ITO M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J. Biol. Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- MCNAMARA C.A., SAREMBOCK I.J., GIMPLE L.W., FENTON J.W.D., COUGHLIN S.R., OWENS G.K. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J. Clin. Invest. 1993;91:94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKANISHI-MATSUI M., ZHENG Y.W., SULCINER D.J., WEISS E.J., LUDEMAN M.J., COUGHLIN S.R. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 20000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- NIIRO N., NISHIMURA J., KANAIDE H. Mechanisms of galanin-induced contraction in the rat myometrium. Br. J. Pharmacol. 1998;124:1623–1632. doi: 10.1038/sj.bjp.0702004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSADA K., TSUNODA H., MIYAUCHI T., SUGISHITA Y., KUBO T., GOTO K. Pregnancy increases ET-1-induced contraction and changes receptor subtypes in uterine smooth muscle in humans. Am. J. Physiol. 1997;272:R541–R548. doi: 10.1152/ajpregu.1997.272.2.R541. [DOI] [PubMed] [Google Scholar]
- RIEMER R.K., HEYMANN M.A. Regulation of uterine smooth muscle function during gestation. Pediatr. Res. 1998;44:615–627. doi: 10.1203/00006450-199811000-00001. [DOI] [PubMed] [Google Scholar]
- SAIFEDDINE M., AL-ANI B., CHENG C.H., WANG L., HOLLENBERG M.D. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br. J. Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCARBOROUGH R.M., NAUGHTON M.A., TENG W., HUNG D.T., ROSE J., VU T.K., WHEATON V.I., TURCK C.W., COUGHLIN S.R. Tethered ligand agonist peptides. Structural requirements for thrombin receptor activation reveal mechanism of proteolytic unmasking of agonist function. J. Biol. Chem. 1992;267:13146–13149. [PubMed] [Google Scholar]
- SHINTANI Y., NISHIMURA J., NIIRO N., HIRANO K., NAKANO H., KANAIDE H. Mechanisms underlying neurokinin A-induced contraction of the pregnant rat myometrium. Br. J. Pharmacol. 2000;130:1165–1173. doi: 10.1038/sj.bjp.0703410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLOFF M.S., FERNSTROM M.A., PERIYASAMY S., SOLOFF S., BALDWIN S., WIEDER M. Regulation of oxytocin receptor concentration in rat uterine explants by estrogen and progesterone. Can. J. Biochem. Cell Biol. 1983;61:625–630. doi: 10.1139/o83-078. [DOI] [PubMed] [Google Scholar]
- SWIFT S., SHERIDAN P.J., COVIC L., KULIOPULOS A. PAR1 thrombin receptor-G protein interactions. J. Biol. Chem. 2000;275:2627–2635. doi: 10.1074/jbc.275.4.2627. [DOI] [PubMed] [Google Scholar]
- TAGGART M.J., WRAY S. Contribution of sarcoplasmic reticular calcium to smooth muscle contractile activation: gestational dependence in isolated rat uterus. J. Physiol. 1998;511:133–144. doi: 10.1111/j.1469-7793.1998.133bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAY-UYBOCO J., POON M.C., AHMAD S., HOLLENBERG M.D. Contractile actions of thrombin receptor-derived polypeptides in human umbilical and placental vasculature: evidence for distinct receptor systems. Br. J. Pharmacol. 1995;115:569–578. doi: 10.1111/j.1476-5381.1995.tb14970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESFAMARIAM B. Thrombin receptor-mediated vascular relaxation differentiated by a receptor antagonist and desensitization. Am. J. Physiol. 1994;267:H1962–H1967. doi: 10.1152/ajpheart.1994.267.5.H1962. [DOI] [PubMed] [Google Scholar]
- TUROSS N., MAHTANI M., MARSHALL J.M. Comparison of effects of oxytocin and prostaglandin F2-alpha on circular and longitudinal myometrium from the pregnant rat. Biol. Reprod. 1987;37:348–355. doi: 10.1095/biolreprod37.2.348. [DOI] [PubMed] [Google Scholar]
- UENOYAMA Y., HATTORI S., MIYAKE M., OKUDA K. Up-regulation of oxytocin receptors in porcine endometrium by adenosine 3′,5′-monophosphate. Biol. Reprod. 1997;57:723–728. doi: 10.1095/biolreprod57.4.723. [DOI] [PubMed] [Google Scholar]
- VU T.K., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- WALDRON R.T., SHORT A.D., GILL D.L. Store-operated Ca2+ entry and coupling to Ca2+ pool depletion in thapsigargin-resistant cells. J. Biol. Chem. 1997;272:6440–6447. doi: 10.1074/jbc.272.10.6440. [DOI] [PubMed] [Google Scholar]
- WALZ D.A., ANDERSON G.F., FENTON J.W. Responses of aortic smooth muscle to thrombin and thrombin analogues. Ann. NY Acad. Sci. 1986;485:323–334. doi: 10.1111/j.1749-6632.1986.tb34594.x. [DOI] [PubMed] [Google Scholar]
- WRAY S. Uterine contraction and physiological mechanisms of modulation. Am. J. Physiol. 1993;264:C1–C18. doi: 10.1152/ajpcell.1993.264.1.C1. [DOI] [PubMed] [Google Scholar]
- XU W.F., ANDERSEN H., WHITMORE T.E., PRESNELL S.R., YEE D.P., CHING A., GILBERT T., DAVIE E.W., FOSTER D.C. Cloning and characterization of human protease-activated receptor 4. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG S.G., LANIYONU A., SAIFEDDINE M., MOORE G.J., HOLLENBERG M.D. Actions of thrombin and thrombin receptor peptide analogues in gastric and aortic smooth muscle: development of bioassays for structure-activity studies. Life Sci. 1992;51:1325–1332. doi: 10.1016/0024-3205(92)90631-x. [DOI] [PubMed] [Google Scholar]
- ZHONG C., HAYZER D.J., CORSON M.A., RUNGE M.S. Molecular cloning of the rat vascular smooth muscle thrombin receptor. Evidence for in vitro regulation by basic fibroblast growth factor. J. Biol. Chem. 1992;267:16975–16979. [PubMed] [Google Scholar]


