Abstract
Apical ATP, ATP, UTP and UDP evoked transient increases in short circuit current (ISC, a direct measure of transepithelial ion transport) in confluent Caco-2 cells grown on permeable supports. These responses were mediated by a population of at least three pharmacologically distinct receptors.
Experiments using cells grown on glass coverslips showed that ATP and UTP consistently increased intracellular free calcium ([Ca2+]i) whilst sensitivity to UDP was variable. Cross desensitization experiments suggested that the responses to UTP and ATP were mediated by a common receptor population.
Messenger RNA transcripts corresponding to the P2Y2, P2Y4 and P2Y6 receptors genes were detected in cells grown on Transwell membranes by the reverse transcriptase–polymerase chain reaction. Identical results were obtained for cells grown on glass.
Experiments in which ISC and [Ca2+]i were monitored simultaneously in cells on Transwell membranes, confirmed that apical ATP and UTP increased both parameters and showed that the UDP-evoked increase in ISC was accompanied by a [Ca2+]i-signal.
Ionomycin consistently increased [Ca2+]i in such polarized cells but caused no discernible change in ISC. However, subsequent application of apical ATP or UTP evoked a small rise in ISC but no rise in [Ca2+]i. UDP evoked no such response.
As well as evoking increases in [Ca2+]i, the ATP/UTP-sensitive receptors present in Caco-2 cells thus allow direct control over ion channels in the apical membrane. The UDP-sensitive receptors, however, appear to simply evoke a rise in [Ca2+]i.
Keywords: Purinoceptor, pyrimidinoceptor, apical membrane, epithelial cells, anion secretion, Caco-2 cells
Introduction
Nucleotides in the luminal fluid exert control over epithelial ion transport processes in many polarized epithelia, and these responses are mediated by P2Y receptors in the apical plasma membrane (see e.g. Devor & Pilewski, 1999; Inglis et al., 1999; Kerstan et al., 1998; Wilson et al., 1996; Wong, 1988). Whilst this apical control system's physiological role is unknown, a body of evidence suggests that the apical receptors may be activated by ATP originating from within the epithelial cells themselves, and that they thus form part of an autocrine control system (Schwiebert, 1999; Taylor et al., 1998). Whatever their physiological significance, these receptors have attracted recent interest as they may allow pharmacological correction of the ion transport defects seen in cystic fibrosis, a potentially lethal genetic disease. Epithelial cells' sensitivity to apical nucleotides is often attributed to receptors belonging to the P2Y2 subclass (Knowles et al., 1996; Parr et al., 1994) that are equally sensitive to UTP and ATP but insensitive to nucleotide diphosphates (Nicholas et al., 1996). However, it has recently become clear, that at least two other P2Y receptor subtypes, P2Y4 and P2Y6, allow cells to respond to pyrimidine nucleotides such as UTP (Communi & Boeymans, 1997; Nicholas et al., 1996). Interestingly, these receptors are essentially insensitive to ATP (Communi & Boeymans, 1997; Nicholas et al., 1996) and our earlier work (Ko et al., 1997) suggested that such ‘pyrimidinoceptors' may also allow apical nucleotides to control epithelial transport processes (see also Inoue et al., 1997; Lazarowski et al., 1997). In the present study we have therefore explored the extent to which such receptors allow apical nucleotides to control ion transport processes in a human epithelial cell line cell line. Some of the data have been presented to the Physiological Society (McAlroy et al., 1999).
Methods
Solutions and chemicals
Cell culture medium: Dulbeco's modified Eagle's medium supplemented with 10% foetal calf serum, 2 mM L-glutamine, 1% non-essential amino acids and penicillin/streptomycin (50 u ml−1/50 μg ml−1). Physiological salt solution (mM): NaCl 117, NaHCO3 25, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 2.5 and D-Glucose 11; pH 7.3–7.4 when bubbled with 5% CO2/95% O2. The membrane permeant, acetoxymethyl ester form of Fura-2 (Fura-2-AM) was from Molecular Probes (Eugene, OR, U.S.A.). Trypsin (0.5 g l−1)/ethylene diamine tetraacetic acid (0.2 g l−1) (Trypsin/EDTA solution) and all other cells culture reagents and consumables were from Life Technologies Ltd. (Paisley, Scotland, U.K.). ATP and UTP were obtained from Amersham Pharmacia (Little Chalfont, Bucks, U.K.) as ultra pure solutions (100 mM) whilst UDP and all other general reagents were from Sigma (Poole, Dorset, U.K.). As commercially-available nucleotide diphosphates are almost invariably contaminated by the corresponding nucleotide triphosphates (see e.g. Nicholas et al., 1996), the UDP used in the present study was prepared from a 10 mM stock solution that had been incubated (1 h, 37°C) in 4-(2-hydroxyethyl)-1-piperazine sulphonic acid (HEPES) -buffered saline containing 10 i.u. ml−1 hexokinase (Boehringer, Mannheim, Germany) and 22 mM D-glucose in order to convert contaminating UTP into UDP.
Cell culture
Standard techniques were used to maintain Caco-2 colonic adenocarcinoma cells (passage no. 34–45, split ratio ∼1 : 12) in water-saturated air containing 5% CO2. For experiments, cells were removed from culture flasks using trypsin/EDTA solution were plated (2×105 cells cm−2) onto Transwell 12 mm polycarbonate filter cell culture inserts (Costar, High Wycomb, Bucks, U.K) or small pieces of this membrane attached onto specially-designed O rings that could be mounted in a miniature Ussing chamber. In both instances the apical well was completely filled with medium as previous work has shown that Caco-2 cells maintained under these conditions consistently become integrated into polarized epithelial sheet consisting of a single layer of cells (Riley et al., 1991). Experiments were undertaken after 10–14 days in culture, by which time the cells had developed transepithelial resistances (Rt) ranging from 110–490 Ω cm2. Experiments were also undertaken using cells plated onto glass coverslips or glass Petri dishes that had also been cultured for 10–14 days.
Quantification of ion transport and [Ca2+]i
Cells on 12 mm Transwell membranes were mounted in standard Ussing chambers, bathed with physiological salt solution and allowed to stabilize under open circuit conditions. The transepithelial potential difference was then clamped to 0 mV using a W.P.I. DVC 1000 voltage/current clamp amplifier (World Precision Instruments. Stevenage, Herts, U.K.). The current required to maintain this potential (short circuit current, ISC) was continuously displayed and recorded to computer disk using a PowerLab-400 interface (AD Instruments, Hastings, East Sussex, U.K.). Positive currents were defined as those carried by anions moving from the basolateral to the apical solution and are shown as upwards deflections of the traces. Rt was monitored by measuring the currents flowing in response to a 1 mV excursion from this holding potential. Cells were stimulated by adding nucleotides directly to the apical solution and the resultant responses quantified by measuring ISC at the peak of a response and subtracting the current measured immediately prior to stimulation.
Cells on coverslips were loaded with the Ca2+-sensitive fluorescent dye Fura-2 by incubation (30–40 min) in culture medium containing 3 μM Fura-2-AM. This loading solution also included Pluronic F127 (0.002%, v v−1), a non ionic detergent, and probenecid (2.5 mM), an inhibitor of organic cation extrusion systems as the cells accumulated very little of the dye unless these compounds were present. Coverslips bearing Fura-2-loaded cells were mounted in a heated chamber attached to the stage of an Nikon Diaphot inverted microscope where the cells were superfused (3–4 ml min−1, 37°C) with physiological saline. A rise in [Ca2+]i causes a corresponding rise in the Fura-2 fluorescence ratio recorded from cells loaded with this dye, which allows changes in [Ca2+]i to be monitored using standard techniques (Grynkiewicz et al., 1985). In the present study, groups of 10–20 Fura-2-loaded cells were viewed using a 40×oil immersion objective (Nikon Fluor 40, 1.3 numerical aperture) and Fura-2 fluorescence ratios (excitation wavelengths 340 and 380 nm) recorded at 4 Hz using a Cairn Research (Faversham, Kent, U.K.) microspectrofluorimetric system. Data were recorded directly to computer disc using a Cairn Research interface and associated software (Version 5.2).
ISC and [Ca2+]i were measured simultaneously using a method that is detailed elsewhere (Ko et al., 1999). Briefly cells grown to confluence on pieces of Transwell membrane attached to specially-designed O rings were loaded with Fura-2 (30–40 min in 3 μM Fura-2-AM) and mounted in a miniature Ussing chamber attached to the stage of a Nikon Diaphot TE300 inverted microscope. The cells were viewed using a 40× extra long working distance objective (Nikon CFI Plan Fluor ELWD, 0.6 numerical aperture) whilst the apical and basolateral sides of the cell layer were independently superfused with physiological salt solution (37°C). Fura-2 fluorescence ratios were recorded at 1 Hz (PTI Ratiomaster Fluorescence System, Photon Technology International, NJ, U.S.A.) from an optical field, containing 20–40 cells whilst ISC was simultaneously recorded using a voltage clamp amplifier (VCC 600: Physiologic Instruments, San Diego, CA, U.S.A.). Both signals were digitized and recorded directly to computer disk.
RNA extraction and reverse transcriptase / polymerase chain reaction (RT–PCR) assay
These experiments were undertaken using strictly paired protocols in which cells at the same passage were cultured for identical lengths of time on Transwell membranes or glass Petri dishes. Cells were then harvested by scraping and lysed using Tri-Zol reagent (∼ 1 ml 10−6 cells). After standing on ice for 15 min, 20% (v v−1) of ice cold chloroform was added to the lysate and the tube vigorously shaken before being centrifuged (12,000×g, 15 min, 4°C). The aqueous phase was carefully transferred to a separate tube to which isopropanol (0.5 ml) was added in order to precipitate the RNA. This was then pelletted by centrifugation (12,000×g, 10 min), re-suspended in 75% (v v−1) ethanol and stored at −70°C pending analysis. Aliquots (50 μg) of extracted RNA were treated (15 min, 37°C) with 1 unit per μg of RNAase-free RQ1 DNAase (Promega, Southampton, Hants, U.K.) in a reaction buffer composed of (mM): Tris / HCl (pH 7.9) 40, NaCl 10, MgCl2 6 and CaCl2 10. RNA was then isolated from the reaction mixture by phenol / chloroform extraction / isopropanol precipitation, and the mRNA present in this extract reverse-transcribed into cDNA using oligo dT primers and MMLV reverse transcriptase (RT, Clontech, Palo Alto, CA, U.S.A.). Aliquots of the resultant cDNA corresponding to 0.8 μg of extracted RNA then served as templates in the polymerase chain reaction (PCR).
The primers used were designed to amplify cDNA sequences specific to the human P2Y2 (sense: 5′-CTTCAACGAGGACTTCAAGTACGTGC-3′, antisense: 5′-CATGTTGATGCGTTGAG-3′, annealing temperature (Ta) 60°C, predicted product size 850 base pairs), P2Y4 (sense: 5′-CCACCTGGCATTGTCAGACACC-3′, antisense: 5′-GAGTGACCAGGCAGGGCACGC-3′, Ta 60°C, predicted product size 431 base pairs) and P2Y6 (sense: 5′-cgcttcctcttctatgccaacc-3′, antisense: 5′-CCATCCTGGCGGCACAGGCGGC-3′, Ta 58°C, predicted product size 380 base pairs) receptor genes. All samples were first denatured at 94°C (5 min) and the PCR reaction then allowed to proceed for 40 denaturing (1 min, 94°C) / annealing (1 min at Ta) / polymerization (1 min, 72°C) cycles, followed by a final polymerization period of 7 min at 72°C. The resultant products were fractionated on 1% (w v−1) agarose / ethidium bromide gels and visualized under ultraviolet light. The size of the generated products was determined by comparing their electrophoretic mobilities with those of standard cDNA fragments of known size. All products were extracted from the gels (Qiaex II agarose Gel Extraction Kit, Qiagen, Crawley, West Sussex, U.K.) and their origin verified by sequencing (ABI Prism automated DNA sequencer). PCR reactions were also undertaken using aliquots of extracted RNA that had not been treated with MMLV-RT, but which had otherwise been handled identically. The reason for this is that genomic DNA can contaminate extracted RNA and, as all known P2Y receptor subtypes are encoded by genes that do not include introns, this DNA will contain sequences identical to those generated in the reverse transcription reaction. Genomic DNA will thus give a false positive result under these conditions and so the inclusion of such a control is vital if the formation of a PCR product is to unequivocally demonstrate the presence of a defined mRNA transcript. Negative controls were also undertaken by running PCR reactions in which neither RNA nor cDNA had been added to the reaction tubes in order to verify that there had been no contamination of the reagents used.
Results
Nucleotide-evoked increases in ISC
Studies of cultured epithelia mounted in standard Ussing chambers showed that apical ATP and UTP (100 μM) increased ISC. These responses occurred with no discernible latency, rising to clear peaks reached after ∼1 min (Figure 1). Thereafter, ISC fell rapidly despite the continued presence of agonist until, after 5–10 min, it had returned to its basal level (Figure 1). There was no statistically significant difference between the responses to UTP and ATP (Table 1). Apical UDP (100 μM) also increased ISC (Table 1) but the response to this nucleotide was smaller than the responses evoked by ATP and UTP (P<0.001 for both, Students t-test).
Figure 1.
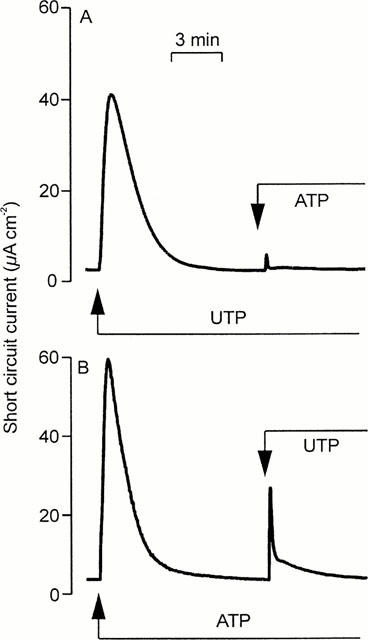
Nucleotide-evoked increases in ISC. Cells grown to confluence on Transwell membranes were mounted in standard Ussing chambers to explore the effects of apical nucleotides upon ISC. (a) Addition of 100 μM UTP followed by 100 μM ATP. Essentially identical records obtained in four instances. (b) Addition of 100 μM ATP followed by 100 μM UTP. Essentially identical records obtained in nine instances.
Table 1.
Nucleotide-evoked autologous and cross-desensitization
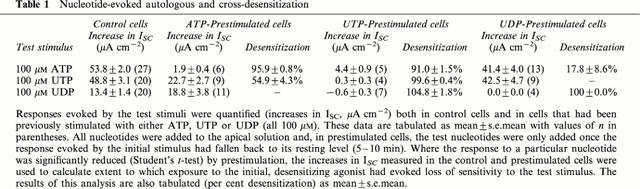
Experiments in which the cells were challenged with two separate aliquots (both 100 μM) of ATP, UTP or UDP showed that all three nucleotides evoked essentially complete (>90%) autologous desensitization (Table 1). UTP also caused essentially complete loss of sensitivity to ATP (Figure 1A) and UDP (Table 1) whereas ATP-prestimulated epithelia retained ∼ 50% of their sensitivity to UTP (Figure 1B, Table 1) and analysis of these data confirmed that UTP desensitized the cells to ATP more effectively (P<0.001, Students t-test) than ATP desensitized the cells to UTP. ATP-prestimulated cells continued to respond to UDP; indeed, the response to UDP seen in such cells appeared larger than normal although the effect was not statistically significant (Table 1). Prestimulation with UDP had no effect upon the cell's sensitivity to UTP, and exerted minimal loss of sensitivity to ATP (Table 1). In further experiments (n=4) ATP-prestimulated (100 μM) cells were stimulated with 100 μM UDP followed by 100 μM UTP (Figure 2). The initial application of ATP increased ISC by 62.2±2.5 μA cm−2 and, as anticipated, these cells subsequently responded to UDP (18.7±2.9 μA cm−2). However, the final addition of UTP also elicited a clear response (18.2±2.2 μA cm−2). Similar increases in ISC were observed in epithelia (n=5) that were exposed to 100 μM apical UDP (14.5±3.3 μA cm−2) followed by 100 μM ATP (39.7±4.3 μA cm−2) and then 100 μM UTP (41.2±3.2 μA cm−2). These data indicate that apical nucleotides can activate at least three pharmacologically distinct receptor populations. One of these appears to be sensitive to ATP and UTP whilst the others are much less sensitive to ATP but can be activated by UTP and UDP respectively.
Figure 2.
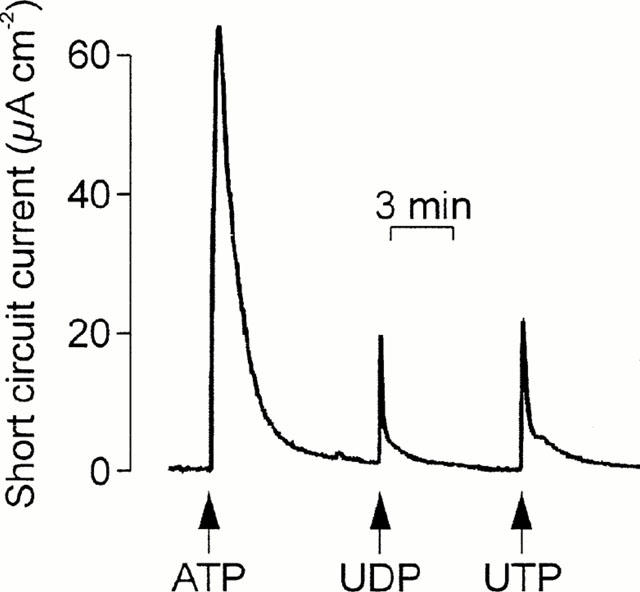
Evidence for multiple receptor subtypes in the apical membrane. ISC was recorded whilst cells were exposed to apical ATP followed by UDP and then UTP. Arrows denote the addition of the appropriate nucleotides (100 μM); these were present throughout the remainder of the experiment. Essentially identical records were obtained in four instances.
Nucleotide-evoked [Ca2+]i signals in cells on glass coverslips
ATP and UTP (both 100 μM) elicited essentially identical [Ca2+]i signals in cells on glass coverslips, each response of a rapid rise to a peak value followed by a slow fall. Experiments in which cells were repeatedly stimulated with pulses (30 s) of increasing concentrations (0.3–300 μM) of ATP or UTP (see e.g. Wilson et al., 1996) showed that the EC50 values for these nucleotides were 6.7±1.9 μM (n=4) and 3.4±0.4 μM (n=3) respectively. To explore the possibility that the responses to ATP and UTP were mediated by a common receptor population, cells stimulated with 100 μM UTP were subsequently exposed to 100 μM ATP in the continued presence of UTP. The responses to ATP seen under these conditions were then compared with those of age-matched control cells at identical passage. UTP caused the cells to lose 93.8±1.3% (n=6) of their sensitivity to ATP. Experiments in which the nucleotides were delivered in the reverse sequence showed that prestimulation with ATP caused 84±3.4% (n=5) desensitization to UTP; the desensitizing actions of ATP and UTP did not differ significantly. Moreover, comparison with the results of the electrometric studies (Table 1) showed that pre-stimulation with ATP desensitized UTP-evoked [Ca2+]i signals more effectively (P<0.01, Students t-test) than the increases in ISC.
UDP (100 μM) failed to elicit a discernible response in five experiments although each preparation subsequently showed a clear response to 100 μM ATP or UTP. However, responses to UDP were seen in some instances and studies of these cells (n=5) suggested that the response to 100 μM UDP was similar to that evoked by 100 μM ATP or UTP, with an EC50 value of 12.0±2.2 μM.
Simultaneous measurement of [Ca2+]i and ISC
Experiments in which ISC and [Ca2+]i were measured simultaneously confirmed that 100 μM ATP (n=13), UTP (n=8), and UDP (n=7) consistently increased both parameters. Analysis of these data (Figure 4) showed that the ATP- and UTP-evoked increases in ISC (ATP: 49.6±4.7 μA cm−2, UTP: 49.2±5.2 μA cm−2) and [Ca2+]i (0.40±0.17 ratio units, 0.37±0.06 ratio units, P<0.005) were essentially identical. However, both components of the response to 100 μM UDP (ISC: 30.9±3.5 μA cm−2; [Ca2+]i signals: 0.13±0.02 ratio units) were smaller than the corresponding responses to 100 μM ATP or UTP (P<0.05). Experiments in which cells were exposed to a series of 30 s pulses of increasing concentrations (0.3–500 μM) of ATP, UTP and UDP gave EC50 values for the Ca2+ signals of 4.3±0.7, 1.3±0.4 and 4.6±0.4 μM respectively. The corresponding EC50 values for the increases in ISC were 9.2±1.1, 5.0±0.7 and 6.1±0.8 μM (Figure 3).
Figure 4.
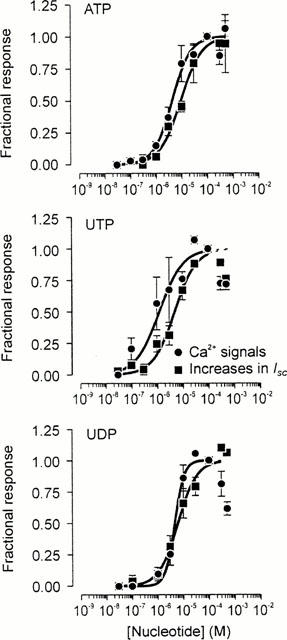
Pharmacological properties of the apical receptor. Cells grown to confluence on Transwell membranes were repeatedly stimulated with a series of pulses (30 s) of increasing concentrations (0.1 − 500 μM) of ATP (n=13), UTP (n=8) or UDP (n=6) whilst [Ca2+]i and ISC were monitored simultaneously (see Methods). The resultant responses were quantified and have each been normalized to the responses evoked by a maximally-effective concentration (100 μM) of the appropriate nucleotide. These data are plotted against the concentration of nucleotide used. As every concentration of the appropriate nucleotide could not be tested in each experiment, each data point is the mean±standard error of between four and 13 observations. The solid curves in each figure are sigmoid curves fitted to the appropriate data sets using a least squares regression procedure implemented in a commercially-available software package (Grafit 4, Erithacus Software, Staines, U.K.).
Figure 3.
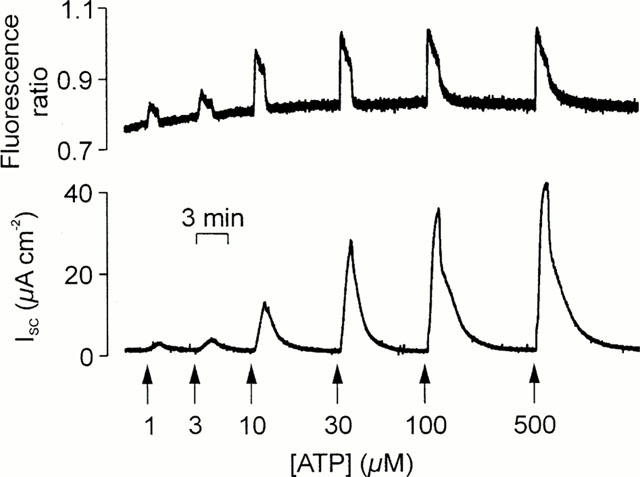
Simultaneously measured changes in [Ca2+]i in ISC. Cells grown to confluence on culture membranes were loaded with Fura-2 and membranes bearing these cells and mounted in a miniature Ussing chamber attached to the stage of an inverted microscope so that changes in [Ca2+]i (upper panel) and ISC (lower panel) could be measured simultaneously. The figure shows the responses evoked by a series of 30 s pulses of increasing concentrations (1–500 μM) of ATP that were delivered to the cells' apical membrane as denoted by the arrows.
Cross desensitization experiments confirmed that UTP (100 μM) increased both [Ca2+]i and ISC and showed that subsequent application of apical ATP (100 μM) evoked no discernible Ca2+ signal and only a minimal rise in ISC (Figure 5a). However, experiments in which these nucleotides were delivered in the reverse sequence showed that ATP-prestimulated cells retained sensitivity to UTP (Figure 5b). Analysis of these data confirmed that, for the increases in both Ca2+ and ISC, UTP was a more effective desensitizing agonist than ATP (Figure 5c,d). Analogous experiments showed that UDP-prestimulated cells retained sensitivity to ATP, and vice versa, and formal analysis of these data showed no statistically significant difference between the desensitizing actions of these nucleotides (Figure 5c,d). The results of this entire series of experiments showed clearly that the Ca2+ signals were always subject to more extensive desensitization (P<0.02 for each protocol) than the increases in ISC. We therefore undertook experiments to explore the relationship between the Ca2+ signals and the electrometric responses.
Figure 5.
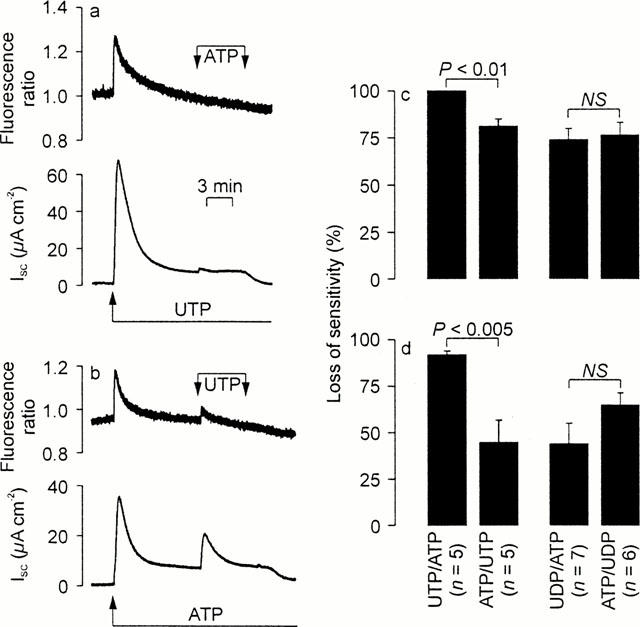
Cross desensitization experiments. In each experiment, a nucleotide was first added to the apical solution (100 μM) and a second, test nucleotide (100 μM) then added in the continued presence of the first stimulus. The resultant changes in [Ca2+]i (upper panels) and ISC (lower panels) were monitored simultaneously. The responses evoked by the second stimuli were then compared with control responses measured in age-matched cells at identical passage in order to calculate the extent to which the initial stimuli had evoked loss of sensitivity. Student's t-test was then used to test the nul hypothesis that the sequence in which the nucleotides were delivered had no effect upon the extent to which they evoked desensitization. This protocol was used to explore the desensitizing interactions between ATP / UTP and ADP / UDP (a) Typical traces showing the effects of ATP upon UTP-prestimulated cells (b). Results of an experiment in which these nucleotides were delivered in the reverse sequence. (c) The loss of sensitivity calculated (mean±s.e.mean) for the Ca2+ signals. (d) The results of a directly analogous analysis of the changes in ISC.
Nucleotide-evoked changes in ISC can occur without a change in [Ca2+]i
Figure 6 shows that exposing the cultured epithelial cells to ionomycin evoked a sustained rise in [Ca2+]i (0.18±0.03 ratio units, n=22) but this response was never accompanied by an significant rise in ISC (0.05±0.05 μA cm−2). Exposing ionomycin-stimulated cells to apical ATP, however, failed to evoke a further rise in [Ca2+]i. Indeed apical ATP evoked a fall in [Ca2+]i in many such experiments. However, ATP consistently caused a rise in ISC (17.1±1.6 μA cm−2) under these conditions. Further experiments showed that UTP evoked clear electrometric responses in such ionomycin-stimulated cells showed to (18.0±2.5 μA cm−2) in the absence of any further rise in [Ca2+]i. Apical UDP, however, had no discernible effect upon either parameter (n=3).
Figure 6.
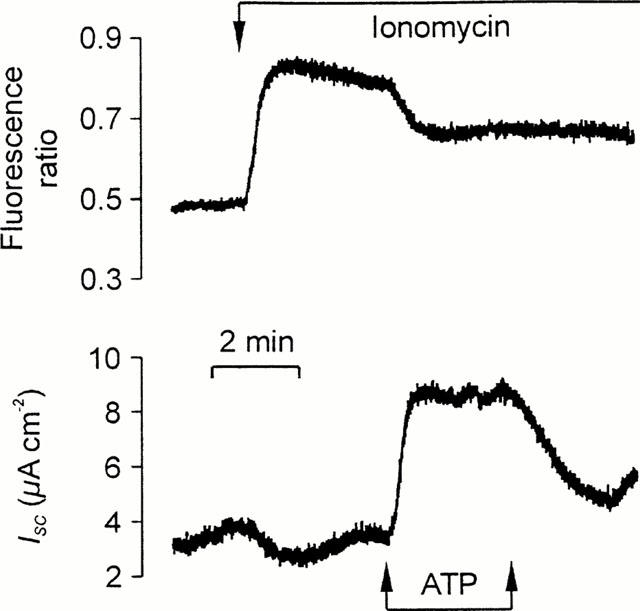
[Ca2+]i-independent responses to ATP. Fura-2-loaded cells on culture membranes mounted in the miniature Ussing chamber that changes in [Ca2+]i (upper panel) and ISC (lower panel) could be measured simultaneously. The cells were first exposed to 1 μM ionomycin and then stimulated with a pulse of 100 μM in the continued presence of this ionophore. Essentially identical responses were obtained in 22 experiments.
Expression of P2Y receptor mRNA transcripts
Analysis of RNA isolated from Caco-2 cells showed that mRNA transcripts specific for the P2Y2, P2Y4 and P2Y6 receptor genes were present in cells grown for 11 days on Transwell membranes or glass Petri dishes (Figure 7).
Figure 7.
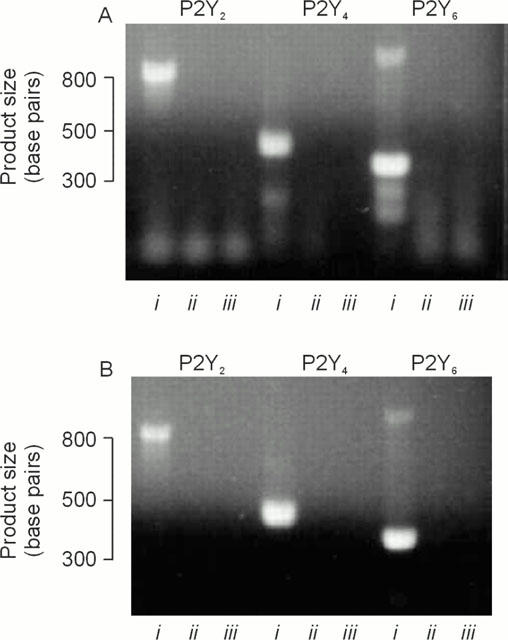
Expression of P2Y receptor mRNA. (a) PCR products obtained using RNA isolated from cells grown on glass Petri dishes. (b) Products obtained RNA isolated from age-matched cells at identical passage grown on Transwell membranes. For each set of primers, products obtained under standard conditions are in lane i, whilst the results of the ‘no RT' and negative (i.e. water) controls are in lanes ii and iii respectively. Essentially identical data obtained in three independent analyses.
Discussion
The present study, in common with data presented by Inoue et al. (1997), shows that apical nucleotides increase ISC in Caco-2 cells grown to confluence on permeable supports. Cross desensitization experiments (present study, Inoue et al., 1997) indicate that these responses are mediated by a complex receptor population that appears to include receptors with properties similar to the recently characterized P2Y2, P2Y4 and P2Y6 subclasses (see Nicholas et al., 1996). This observation predicts that mRNA transcripts corresponding to these genes should be present in Caco-2 cells, and this was verified by analysis of RNA extracted from cells grown on transwell membranes. As all known P2Y receptors allow nucleotides to increase [Ca2+]i by activating phospholipase C (see e.g. Nicholas et al., 1996), we explored the properties of this receptor population further by studying nucleotide-evoked [Ca2+]i-signals in cells plated onto glass cover slips. As anticipated, this widely-used approach confirmed that ATP and UTP increased [Ca2+]i and showed that these nucleotides acted with similar potencies. This is consistent with the expression of P2Y2 receptors (Nicholas et al., 1996). Although UDP, which is not a P2Y2 receptor agonist, did increase [Ca2+]i in some experiments, this was not a consistent response and cross desensitization experiments indicated that ATP and UTP increased [Ca2+]i by acting upon a common receptor population in this experimental system. These experiments, in contrast to the electrometric studies, thus failed to provide convincing evidence for the presence of multiple receptor subtypes.
Studies of other cell types have shown that the functional expression of P2Y receptor subtypes can be influenced by the conditions under which cells are maintained in vitro (Clunes et al., 1998; Wilson et al., 1998). We therefore explored the possibility that the apparent discrepancy between cells maintained on glass and on Transwell membranes was due to differential expression of such genes. However, RT–PCR-based analysis showed that mRNA transcripts specific for the P2Y2, P2Y4 and P2Y6 receptor subtypes were present under both culture conditions. The differences between the two experimental systems may, therefore, reflect events downstream of gene expression, such as the insertion of receptors into the plasma membrane. Alternatively, there may be differences between the mechanisms that allow nucleotides to increase [Ca2+]i and ISC. Whatever its basis, it was clear that this contradiction would not be resolved using standard techniques in which ISC and [Ca2+]i are monitored separately in cells grown on different substrates. We therefore expanded our initial experiments using a technique (Ko et al., 1999) that allows changes in [Ca2+]i and ISC to be measured simultaneously in cells grown to confluence on Transwell membranes.
In these experiments ATP, UTP and UDP evoked electrometric responses similar to those recorded using standard techniques. However, the new approach allowed us to expand upon earlier work (Inoue et al., 1997) by establishing the EC50 values for these nucleotides, which lay within the range 1–10 μM. The rank order of potency was UTP>UDP>ATP which does not correspond to any known P2Y receptor subtype (Nicholas et al., 1996). Because we could also measure [Ca2+]i-signals during these experiments, we could establish that each such response was accompanied by a rise in [Ca2+]i. Although the EC50 values for these signals also lay within the 1–10 μM range, the nucleotide concentrations required to raise [Ca2+]i were slightly lower than those needed to increase ISC. Moreover, for the [Ca2+]i-signals, the rank order of potency was UTP>ATP≈amp;UDP which is slightly different to that associated with the electrometric responses.
We also undertook cross desensitization experiments which clearly confirmed that apical nucleotides exert control over epithelial ion transport processes by activating selective pyrimidinoceptors as well as P2Y2 receptors. However, these experiments showed that the [Ca2+]i-signals were more susceptible to desensitization than were the corresponding increases in ISC. This rather surprising observation may, at least in part, unify the seemingly disparate results of the experiments in which ISC and [Ca2+]i were measured separately and could explain why the studies of cells on glass coverslips did not provide convincing evidence for multiple receptor subtypes. The discrepancy also indicates that receptor-mediated changes in [Ca2+]i may not be the only means by which apical nucleotides exert control over ion transport processes, and this possibility prompted us to explore the effects of nucleotides upon ionomycin-treated cells. This substance is an ionophore that prevents the cytoplasmic sequestration of this cation by rendering cell membranes permeable to Ca2+ (see e.g. Lui & Hermann, 1978; Morgan & Jacob, 1993). As anticipated, ionomycin consistently evoked a sustained rise in [Ca2+]i but this was never accompanied by a discernible increase in ISC. This was surprising in view of the central role of [Ca2+]i in stimulus-secretion coupling, but similar discrepancies have been noted in other epithelial tissues. In cultured human sweat gland epithelial cells, for example, thapsigargin causes a large rise in [Ca2+]i but no change in ion secretion even though muscarinic agonists evoke robust secretory responses in these cells, apparently by activating a Ca2+-dependent signal transduction pathway (Pickles & Cuthbert, 1992). Similarly, in polarized equine epithelial cells, maximally effective concentrations of ATP evoke similar Ca2+-signals when applied to the apical or the basolateral plasma membrane. In these cells, however, apical ATP evokes a secretory response whilst the basolaterally-mediated Ca2+-signals are not associated with any change in the rate of transepithelial ion transport (Ko & Wong, 1999). It is therefore clear that changes in [Ca2+]i do not necessarily evoke corresponding changes in transepithelial ion transport.
The present experiments also show that apical UTP and ATP can increase ISC in ionomycin-treated cells and that these responses are not accompanied by increases in [Ca2+]i. Indeed ATP caused [Ca2+]i to fall in many such experiments and this phenomenon, which has been described previously, may reflect P2Y-receptor-mediated control over the plasma membrane Ca2+-extrusion pump (Wolff et al., 1993). However, these experiments clearly show that Caco-2 cells express apical P2Y receptors that allow [Ca2+]i-independent control over ion channels. Although P2Y receptors that allow nucleotides to evoke cyclic AMP accumulation have been described, these are unlikely to underlie this part of the response as they are characteristically insensitive to UTP (Communi et al., 1999). Moreover, work from this laboratory, and elsewhere, suggests that extracellular UTP actually lowers cellular cyclic AMP levels (Schulze-Lohoff et al., 1995; Sipma et al., 1994; Wilson et al., 1996; 1999). A number of previous studies have shown, however, that epithelial P2Y receptors can exert control over anion channel activity by activating a signaling pathway that does not involve a cytoplasmic second messenger (Guo et al., 1995; 1997; Ko et al., 1999; Stutts et al., 1994). Although the present data suggest that such direct control also occurs in Caco-2 cells, the increases in ISC seen in ionomycin treated cells exposed to ATP or UTP were only ∼20% of those seen under control conditions. It is therefore possible that the control response involves both a local rise [Ca2+]i and direct control over channels in the apical membrane. Interestingly, UDP, in contrast to ATP and UTP, had no effect upon ionomycin-treated cells suggesting that the receptors sensitive to this nucleotide may not allow direct control over ion channels. This may explain why the electrometric response to UDP is smaller that evoked by ATP or UTP. Similarly, previous studies of equine epithelial cells showed that loading cells with BAPTA, and so increasing the intracellular buffering capacity, essentially (>90% inhibition) abolishes the response to apical UDP whilst causing only ∼70% inhibition of the response to ATP (Wilson et al., 1998).
Responses to nucleotides present in the luminal fluid have generally been attributed to the activation of P2Y2 receptors (Knowles et al., 1996; Parr et al., 1994). The present data show clearly that the apical membrane also contains [Ca2+]i-mobilizing receptors that are insensitive to ATP and these appear to belong to the P2Y4 and P2Y6 subclasses (see e.g. Communi & Boeymans, 1997; Nicholas et al., 1996). The apical expression of such pyrimidinoceptors would allow nucleotides such as UTP and UDP to contribute to the autocrine control of epithelial transport processes (Taylor et al., 1998). Moreover, it is possible that drugs which activate these receptors may permit correction of ion transport defects such as those seen in the genetic disease cystic fibrosis.
Acknowledgments
The authors thank R.E. Olver, A. Jovanović and S.K. Inglis for their helpful comments and suggestion. This study was made possible by project grants from the Wellcome Trust (S.M. Wilson) and Tenovus (A. Collett, H.L. McAlroy) and by travel grants from the Physiological Society and the Lawrence Bequest.
Abbreviations
- [Ca2+]i
intracellular free calcium concentration
- EDTA
ethylene diamine tetraacetic acid
- Fura-2-AM
acetoxymethyl ester form of Fura-2
- HEPES
4-(2-hydroxyethyl)-1-piperazine sulphonic acid
- ISC
short circuit current
- PCR
polymerase chain reaction
- RT
MMLV reverse transcriptase
- Rt
transepithelial resistance
- Ta
annealing temperature
References
- CLUNES M.T., COLLETT A., BAINES D.L., BOVELL D.L., MURPHIE H., INGLIS S.K., MCALROY H.L., OLVER R.E., WILSON S.M. Culture substrate-specific expression of P2Y2 receptors in distal lung epithelial cells isolated from foetal rats. Br. J. Pharmacol. 1998;124:845–847. doi: 10.1038/sj.bjp.0701942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMUNI D., BOEYMANS J.M. Receptors responsive to extracellular pyrimidine nucleotides. Trends Pharmacol. Sci. 1997;18:83–86. doi: 10.1016/s0165-6147(96)01035-8. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., ROBAYE B., BOEYMANS J.-M. Pharmacological characterization of the human P2Y11 receptor. Br. J. Pharmacol. 1999;128:1199–1206. doi: 10.1038/sj.bjp.0702909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVOR D.C., PILEWSKI J.M. UTP inhibits Na+ absorption in wild type and ΔF508 CFTR-expressing human bronchial epithelia. Am. J. Physiol. 1999;276:C827–C837. doi: 10.1152/ajpcell.1999.276.4.C827. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+-indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;265:3440–3450. [PubMed] [Google Scholar]
- GUO X., MERLIN D., HARVEY R.D., LABOISE C., HOPFER U. Pharmacological evidence that calcium is not required for P2–receptor–stimulated Cl- secretion in HT29–Cl.16E. J. Memb. Biol. 1997;155:239–246. doi: 10.1007/s002329900176. [DOI] [PubMed] [Google Scholar]
- GUO X., MERLIN D., HARVEY R.D., LABOISSE C., HOPFER U. Stimulation of Cl− secretion by extracellular ATP does not depend upon increased cytosolic Ca2+ in HT-29.cl16E. Am. J. Physiol. 1995;269:Cl457–Cl463. doi: 10.1152/ajpcell.1995.269.6.C1457. [DOI] [PubMed] [Google Scholar]
- INGLIS S.K., COLLETT A., MCALROY H.L., WILSON S.M., OLVER R.E. Effect of luminal nucleotides on Cl− secretion and Na+ absorption in distal bronchi. Pflügers Arch. 1999;438:621–627. doi: 10.1007/s004249900096. [DOI] [PubMed] [Google Scholar]
- INOUE C.N., WOO J.S., SCHWEIBERT E.K., MORITA T., HANAOKA K., GUGGINO S.E., GUGGINO W.B. Role of purinergic receptors in chloride secretion in Caco–2 cells. Am. J. Physiol. 1997;272:Cl862–Cl870. doi: 10.1152/ajpcell.1997.272.6.C1862. [DOI] [PubMed] [Google Scholar]
- KERSTAN D., GORDJANI N., NITSCKE R., GREGER R., LEIPZIGER J. Luminal ATP induces K+ secretion via a P2Y2 receptor in rat distal colonic mucosa. Pflügers Arch. 1998;436:712–716. doi: 10.1007/s004240050693. [DOI] [PubMed] [Google Scholar]
- KNOWLES M.R., OLIVIER K., NOONE P., BOUCHER R.C. Pharmacological modulation of salt and water transport in the airway epithelium in cystic fibrosis. Am. J. Resp. Crit. Care Med. 1996;151:S65–S69. doi: 10.1164/ajrccm/151.3_Pt_2.S65. [DOI] [PubMed] [Google Scholar]
- KO W.H., LAW W.Y.K., WONG H.Y., WILSON S.M. The simultaneous measurement of ISC and intracellular free Ca2+ in equine cultured equine sweat gland secretory epithelia. J. Memb. Biol. 1999;170:205–211. doi: 10.1007/s002329900550. [DOI] [PubMed] [Google Scholar]
- KO W.H., WILSON S.M., WONG P.Y.D. Purine and pyrimidine nucleotide receptors in the apical membranes of equine cultured epithelia. Br. J. Pharmacol. 1997;121:150–156. doi: 10.1038/sj.bjp.0701093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KO W.H., WONG C.H.Y. Simultaneous measurements of ATP-activated calcium entry and short circuit current (ISC in polarized cultured equine sweat gland epithelia. J. Physiol. Lond. 1999;517:20P–21P. [Google Scholar]
- LAZAROWSKI E.R., PARADISO A.M., WATT W.C., HARDEN T.K., BOUCHER R.C. UDP activates a mucosal-restricted receptor on human nasal epithelial cells that is distinct from the P2Y2 receptor. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2599–2603. doi: 10.1073/pnas.94.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUI C., HERMANN T.E. Characterization of ionomycin as a calcium ionophore. J. Biol. Chem. 1978;253:5892–5894. [PubMed] [Google Scholar]
- MCALROY H.L., COLLETT A., AHMED S., KO W.H., BAINES D.L., WILSON S.M. Multiple P2Y receptor subtypes in the apical membranes of Caco-2 colonic adenocarcinoma cells. J. Physiol. Lond. 1999;517:97P. doi: 10.1038/sj.bjp.0703743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN A.J., JACOB R. Ionomycin-induced Ca2+ influx in cultured human endothelial cells. J. Physiol. Lond. 1993;473:93P. [Google Scholar]
- NICHOLAS R.A., WATT W.C., LAZAROWSKI E.R., QING L., HARDEN T.K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective and an ATP- and UTP-specific receptor. Mol. Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- PARR C.E., SULLIVAN D.M., PARADISO A.M., LAZAROWSKI E.R., BIRCH L.H., OLSEN J.C., ERB L., WEISMAN G.A., BOUCHER R.C., TURNER J.T. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICKLES R.J., CUTHBERT A.W. Failure of thapsigarin to alter ion transport in human sweat gland epithelia while intracellular Ca2+ concentration is raised. J. Biol. Chem. 1992;267:14818–14825. [PubMed] [Google Scholar]
- RILEY S.A., WARHURST G., CROWE P.T., TURNBERG L.A. Active hexose transport across cultured human Caco-2 cells – Characterization and influence of culture conditions. Biochim. Biophys. Acta. 1991;1066:175–182. doi: 10.1016/0005-2736(91)90184-a. [DOI] [PubMed] [Google Scholar]
- SCHULZE-LOHOFF E., BITZER M., OGILVIE A., STERZEL R.B. P2U-purinergic receptor activation mediates inhibition of cAMP accumulation in cultured renal mesangial cells. Renal Physiol. Biochem. 1995;18:219–230. doi: 10.1159/000173919. [DOI] [PubMed] [Google Scholar]
- SCHWIEBERT E.M. ABC transporter-facilitated ATP conductive transport. Am. J. Physiol. 1999;276:C1–C8. doi: 10.1152/ajpcell.1999.276.1.C1. [DOI] [PubMed] [Google Scholar]
- SIPMA H., DEN HERTOG A., NELEMANS A. The phospholipase C activating P2U purinoceptor also inhibits cyclic AMP formation in DDT1 MF-2 smooth muscle cells. Eur. J. Pharmacol. 1994;268:431–437. doi: 10.1016/0922-4106(94)90069-8. [DOI] [PubMed] [Google Scholar]
- STUTTS M.J., FITZ J.G., PARADISO A.M., BOUCHER R.C. Multiple modes of regulation of airway epithelial chloride secretion by extracellular ATP. Am. J. Physiol. 1994;267:C1442–C1451. doi: 10.1152/ajpcell.1994.267.5.C1442. [DOI] [PubMed] [Google Scholar]
- TAYLOR A.L., KUDLOW B.A., MARS K.L., GRUENERT D.C., GUGGINO W.B., SCHWIEBERT E.K. Bioluminescence detection of ATP release mechanisms in epithelia. Am. J. Physiol. 1998;275:C1391–C1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- WILSON S.M., GALLACHER M., RAKHIT S., REMSBURY A., KO W.H. Pyrimidine-nucleotide-evoked inhibition of cyclic AMP accumulation in equine epithelial cells. Exp. Physiol. 1999;84:639–649. doi: 10.1111/j.1469-445x.1999.01869.x. [DOI] [PubMed] [Google Scholar]
- WILSON S.M., LAW V.W.Y., PEDIANI J.D., ALLEN E.A., WILSON G., KHAN Z., KO W.H. Nucleotide-evoked calcium signals and anion secretion in equine cultured epithelia that express apical P2Y2 receptors and pyrimidine nucleotide receptors. Br. J. Pharm. 1998;124:832–838. doi: 10.1038/sj.bjp.0701888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON S.M., RAKHIT S., MURDOCH R., PEDIANI J.D., ELDER H.Y., BAINES D.L., KO W.H., WONG P.Y.D. Activation of apical P2U purine receptors permits inhibition of adrenaline-evoked cyclic AMP accumulation in cultured equine sweat gland epithelial cells. J. Exp. Biol. 1996;199:2153–2160. doi: 10.1242/jeb.199.10.2153. [DOI] [PubMed] [Google Scholar]
- WOLFF T., LEIPZIGER J., FISHER K.-G., KLAR B., NITSCHKE R., GREGER R. Evidence for agonist-induced export of extracellular Ca2+ in epithelial cells. Pflügers Arch. 1993;424:423–430. doi: 10.1007/BF00374904. [DOI] [PubMed] [Google Scholar]
- WONG P.Y.D. Control of anion and fluid secretion by apical P2-purinoceptors in the rat epididymis. Br. J. Pharmacol. 1988;95:1315–1321. doi: 10.1111/j.1476-5381.1988.tb11770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


