Abstract
Immunoreactivity for P2X1, P2X4 and P2X5 receptor subtypes was detected in the smooth muscle cell layer of second and third order rat mesenteric arteries immunoreactivity, for P2X2, P2X3, P2X6 and P2X7 receptors was below the level of detection in the smooth muscle layer.
P2X receptor-mediated currents were recorded in patch clamp studies on acutely dissociated mesenteric artery smooth muscle cells. Purinergic agonists evoked transient inward currents that decayed rapidly in the continued presence of agonist (τ∼200 ms). Standard whole cell responses to repeated applications of agonist at 5 min intervals ran down. Run-down was unaffected by changes in extracellular calcium concentration, intracellular calcium buffering or the inclusion of ATP and GTP in the pipette solution.
Run-down was overcome and reproducible responses to purinergic agonists were recorded using the amphotericin permeabilized patch recording configuration.
The rank order of potency at the P2X receptor was ATP=2 methylthio ATP>α,β-methylene ATP>CTP=l-β,γ-methylene ATP. Only ATP and 2meSATP were full agonists. The P2 receptor antagonists suramin and PPADS inhibited P2X receptor-mediated currents with IC50s of 4 μM and 70 nM respectively.
These results provide further characterization of artery P2X receptors and demonstrate that the properties are dominated by a P2X1-like receptor phenotype. No evidence could be found for a phenotype corresponding to homomeric P2X4 or P2X5 receptors or to heteromeric P2X1/5 receptors and the functional role of these receptors in arteries remains unclear.
Keywords: P2X receptors, P2X1 receptors, immunohistochemistry, artery, patch clamp, suramin
Introduction
P2X receptors for ATP are present on arterial smooth muscle cells and their activation following sympathetic nerve stimulation or agonist application leads to vasoconstriction and an increase in blood pressure (Burnstock, 1997). P2X receptors can contribute a substantial component to sympathetic neurogenic vasoconstriction that is resistant to commonly used antihypertensive drugs (adrenoceptor and calcium channel antagonists) (Evans & Surprenant, 1992; Galligan et al., 1995). Artery P2X receptors may therefore provide a novel target for the modulation of blood pressure and the treatment of cardiovascular disease. Seven P2X receptor isoforms (P2X1–7) have been identified at the molecular level and these constitute a distinct family of ligand gated cation channels with two transmembrane domains, intracellular amino and carboxy termini and a large extracellular loop (Burnstock, 1997). P2X1 receptors were originally isolated from vas deferens smooth muscle, show a widespread distribution in arteries, and are essential for the production of functional P2X receptors in the vas deferens (Valera et al., 1994; Collo et al., 1996; Mulryan et al., 2000). Recent molecular biological studies using RT–PCR have indicated that mRNAs for P2X1–5 and P2X7 receptors can be detected in segments of rat mesenteric arteries (Phillips & Hill, 1999). P2X receptor subunits may form as homomeric or heteromeric channels (Torres et al., 1999). The heteromeric assembly of different P2X receptor isoforms may result in receptors with composite phenotypes derived from the properties of the constituent subunits (e.g. P2X2/3 (Lewis et al., 1995), P2X4/6 (Le et al., 1998), P2X1/5 (Torres et al., 1998) and P2X2/6 (King et al., 2000). At least three P2X receptor subunits are thought to be required to make a functional receptor (Nicke et al., 1998) however the exact stoichiometry of the channel remains unclear (Stoop et al., 1999). The multimeric assembly of P2X receptor channels therefore raises the possibility that native arterial smooth muscle P2X receptors may form as heteromeric channels and/or may express multiple P2X receptor subtypes.
The desensitizing nature and α,β-methylene ATP (α,β-meATP) sensitivity of arterial smooth muscle P2X receptors suggests the native receptor is dominated by a P2X1-like receptor phenotype (Valera et al., 1994; Lewis et al., 1998). The contribution of other P2X receptor subunits expressed by smooth muscle (Nori et al., 1998) to the arterial P2X receptor phenotype however is unclear. Due to problems with agonist breakdown in whole tissue studies and run-down of smooth muscle P2X receptor-mediated responses using standard whole cell recording techniques (Evans & Kennedy, 1994; Khakh et al., 1995) the descriptive pharmacology of P2X receptors in arterial smooth muscle is limited and does not allow a detailed comparison with the properties of recombinant P2X receptors (Evans et al., 1995). In this study we show that reproducible P2X receptor responses can be recorded from acutely dissociated rat mesenteric artery smooth muscle cells using the amphotericin permeabilized patch recording technique. This technique, in contrast to contraction studies, allows activation of the P2X receptor channels to be measured directly in response to drug application under concentration clamp conditions (Evans & Kennedy, 1994). In conjunction with the use of P2X receptor subtype specific antibodies to determine the complement of P2X receptors expressed by these arteries we have characterized the pharmacological properties of rat mesenteric artery smooth muscle P2X receptors. This allows detailed comparisons to be made with the properties of recombinant P2X receptors to determine the molecular nature of native arterial smooth muscle P2X receptors.
Methods
Male wistar rats (250–300 g) were killed by cervical dislocation and femoral exsanguination. The mesentery was removed and second and third order mesenteric arteries were dissected and used for immunohistochemical and electrophysiological studies.
Immunohistochemistry
Transverse sections (12 μm) of mesenteric arteries were processed for immunochemistry as described previously (Lewis et al., 2000) using the following P2X receptor subtype selective antibodies; anti-P2X1, anti-P2X2, anti-P2X4 and anti-P2X7±control antigen blocking peptide (Alomone Lab. Israel); anti-P2X3 (gift from Prof Elde and Dr Vulchanova, University of Minnesota, U.S.A.), anti-P2X5 and anti-P2X6±control antigen blocking peptide (gift from Roche Bioscience, Palo Alto, U.S.A.). Anti-P2X1, anti-P2X2, anti-P2X4 and anti-P2X7 receptor antibodies were used at a dilution of 1 : 200 in 10% donkey serum in PBS. Anti-P2X3, anti-P2X5 and anti-P2X6 were used at a dilution of 1 : 1000 in 10% donkey serum in PBS. Positive control immunoreactivity was recorded in rat dorsal root ganglion sections for P2X2, P2X3 and P2X6 receptors and mouse spinal cord sections for P2X5 receptors.
Electrophysiological recordings
Acutely dissociated smooth muscle cells were prepared from mesenteric arteries using a papain and collagenase/hyaluronidase enzymatic digestion (Quayle et al., 1996) and plated on glass coverslips stored at 4°C and used within 2–36 h. The cells were superfused with a physiological solution of the following composition (mM): NaCl 150, KCl 2.5, HEPES 10, CaCl2 2.5, MgCl2 1, (pH 7.3 with NaOH) at 2 ml min−1. Agonists were applied rapidly using a U-tube perfusion system (Evans & Kennedy, 1994) for 200 or 500 ms at 5 min intervals. Antagonists were superfused for 5–10 min after reproducible agonist responses were obtained before being applied concomitantly with agonist. Whole cell or permeabilized patch recordings were made with an Axopatch 200B amplifier and data was collected using pClamp6 software (Axon Instruments, U.S.A.). Currents were recorded at room temperature at a holding potential of −60 mV: Patch electrodes (2–5 MΩ) were routinely filled with a solution of the following composition for whole cell recordings (mM): potassium gluconate 140, NaCl 5, HEPES 10, EGTA 10, (pH 7.3 with KOH). Permeabilized patch recordings were made with the addition of amphotericin B to the internal solution (3 ng ml−1 with a final dilution of 0.5% DMSO).
Data analysis
Data are reported throughout as mean±s.e.mean, n=number of observations. Peak currents evoked in response to agonists are expressed as a per cent of the response to 10 μM α,β-meATP. It was not always possible to construct a full concentration response curve in a single smooth muscle cell therefore concentration response data was pooled and fitted by the least squares method using Origin software (Microsoft U.S.A.) with the equation; response=(α[A]H)/([A]H+[A50]H) where α and H are the asymptote and Hill coefficient, [A] is the agonist concentration, A50 is the agonist concentration producing 50% of the maximum agonist response (EC50). Antagonist concentration inhibition curves were constructed in a similar way where A50 is equivalent to the IC50 for the antagonist.
Drugs
Papain, dithioerythritol, collagenase, hyaluronidase, α,β-meATP, CTP, ADP, ATP, UTP, GTP, TTP, ITP, Suramin, hexokinase, amphotericin B (Sigma, U.K.). Two methylthio ATP (2meSATP, RBI, U.K.). lβ,γ-methylene ATP (lβ,γ-meATP), iso pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (iso-PPADS, Tocris Cookson, U.K.). HPLC purification of ADP and analysis of CTP and CDP carried out by Dr J. Roberts (University of Leicester).
Results
Immunohistochemical localization of P2X receptor subunits
Immunoreactivity specific for P2X1, P2X4 and P2X5 receptor subunits was detected in the smooth muscle layer of rat mesenteric arteries. P2X7 receptor immunoreactivity was detected in the outer adventitial layer which contains collagen fibres and varicose sympathetic nerves. P2X receptor subtype specific immunoreactivity was abolished or significantly reduced by incubation with the appropriate antibody specific blocking peptide (Figure 1). No immunoreactivity was associated with the endothelial layer. P2X2,3 and 6 receptor immunoreactivity was not detected in mesenteric artery segments. In all of these sections a high level of background autofluorescence was associated with the elastic lamina (see Figure 1).
Figure 1.
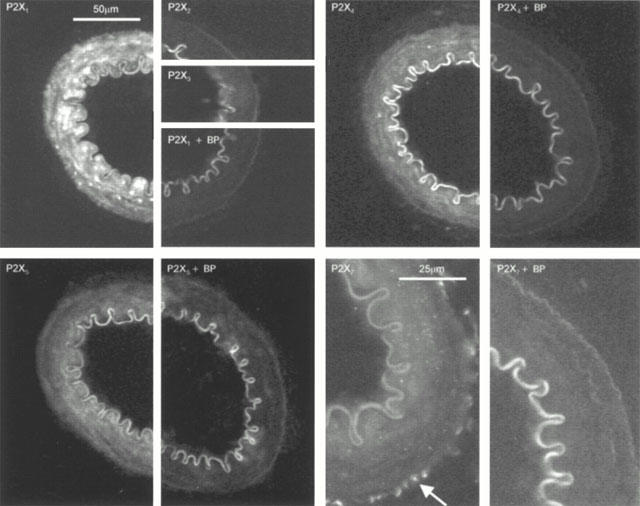
Immunohistochemical detection of P2X receptor isoforms. The figure shows subtype specific staining for P2X1, P2X4, P2X5 and P2X7 receptors, reduction in staining with the appropriate blocking peptide controls and the lack of specific staining for P2X2 and P2X3 receptors. P2X1,4 and P2X5 receptor immunoreactivity was restricted to the smooth muscle layer. P2X7 receptor immunoreactivity was punctate (indicated by arrow) and restricted to the outer adventitial layer.
P2X receptor-mediated responses
The metabolically stable ATP analogue α,β-meATP evoked transient inward currents that inactivated during the continued presence of agonist. Following repeated applications at 5 min intervals the peak responses declined in amplitude in the whole cell recording configuration (Figure 2A,B). The extent of recovery from the desensitized state was dependent on the interval between agonist applications and the degree of recovery increased when the interval between applications was increased from 1 to 3 mins (Figure 2B). Increasing the interval between applications from 3 to 10 mins had no further effect on recovery from desensitization indicating that recovery from inactivation was not simply a time dependent process and that during whole cell recordings P2X receptor-mediated responses ‘ran-down' (Figure 2A,B). The normal recording solution contained high EGTA (10 mM), run-down of the currents and recovery from desensitization was essentially the same when the whole cell recording solution contained low EGTA (0.1 mM, n=4). Run-down of whole cell P2X receptor currents evoked by 10 μM α,β-meATP given at 5 min intervals was essentially similar when extracellular calcium was normal (2.5 mM), reduced to 1 mM or nominally free (at 5 mins after first application peak amplitude was 57.2±3.5, 53.5±7.5, 46.2±13.2 of initial response respectively n=4–9). Increasing extracellular calcium to 10 mM appeared to facilitate run-down, at 5 mins after the first application the peak amplitude was 33±7.8% of the initial response (n=4). Similarly, back-filling of the electrodes with ATP and GTP (both 1 mM) had no effect on run-down (at 5 mins after the first application peak amplitude was 49.8±11.8% of initial response, n=4).
Figure 2.
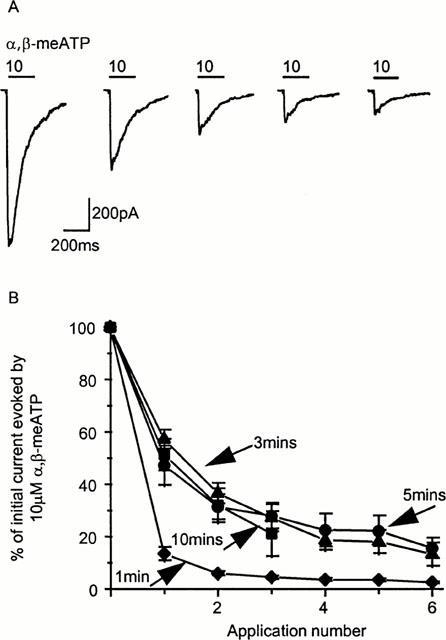
Run-down of P2X receptor currents in the whole cell recording mode. (A) α,β-meATP (10 μM) evokes transient inward currents which desensitize rapidly during agonist application (200 ms, application indicated by bar). The peak amplitude of currents ‘ran-down' during repeated applications of α,β-meATP at 5 min intervals. (B) The timecourse of run-down of P2X currents in the whole cell recording mode. Recovery from desensitization is maximal with a 3–5 min interval between applications.
Run-down may result from the dialysis of the cell with the electrode recording solution leading to the washout/dilution of cellular components that are necessary for the normal regulation of P2X receptor channels. Previously the permeabilized patch recording configuration has been used to maintain the integrity of the cellular environment, regulation of a variety of ion channels and reduce time dependent run-down. In the amphotericin permeabilized patch recording configuration reproducible P2X receptor-mediated inward currents could be recorded when α,β-meATP was applied every 5 min (Figure 3A). The timecourse of recovery from desensitization is similar to that of whole cell currents in that it saturates at ∼4–5 mins (Figure 3B). Previously the run-down of smooth muscle P2X receptors has limited the description of the properties of these native receptors. Recording in the amphotericin permeabilized patch configuration therefore allows detailed physiological and pharmacological measurements to be made.
Figure 3.
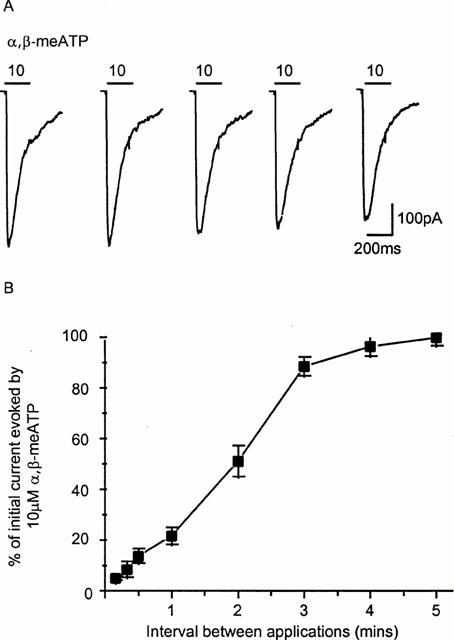
Reproducible P2X receptor currents in response to α,β-meATP (10 μM, 200 ms application indicated by bar) were recorded in the amphotericin permeabilized patch recording configuration when the interval between applications was 5 mins (A). (B) Timecourse of recovery from desensitization of P2X receptor currents, expressed as per cent of the initial response to α,β-meATP (10 μM, n=4–6 for each point).
Current-voltage relations and timecourse of responses
The current-voltage relation for α,β-meATP-evoked P2X receptor currents was inwardly rectifying (Figure 4A,B) and reversed at ∼8 mV (n=4). The decay of the P2X current during the continued presence of the agonist was fitted with a single exponential (at 10 μM the time constant of decay was 216.3±33.8 ms, n=9) (Figure 4C). Reversal potential of currents indicating that the receptor is a non-selective cation channel as reported previously for a variety of native and recombinant P2X receptors (Brake et al., 1994; Haines et al., 1999)
Figure 4.
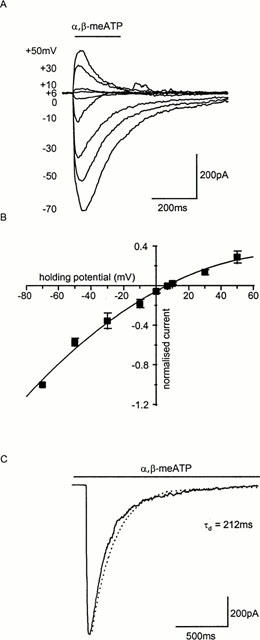
Current-voltage relationship of P2X receptor currents in rat mesenteric artery smooth muscle cells. (A) Currents evoked by α,β-meATP (10 μM, application period indicated by bar) showed inward rectification (B). (C) Mono-exponential fit of the decay of a P2X receptor response to the continued application of α,β-meATP (10 μM).
Effects of purinergic agonists
α,β-meATP evoked concentration dependent inward currents with an EC50 of 1.16 μM (Hill slope 1.4) (Figures 5A and 6) ATP and 2meSATP were more potent than α,β-meATP in evoking inward currents (EC50S 0.6 and 0.4 μM, Hill slopes 0.8 and 1.1 respectively) and peak responses were larger indicating that α,β-meATP is an apparent partial agonist at the receptor (evoking 73% of the maximum ATP response) (Figure 6). The P2X1 receptor selective agonist 1-β,γ-meATP had an EC50 of 10.5 μM (Hill slope 1.7) and was a partial agonist at the receptor (maximum was 42% of the response to ATP) (Figures 5B and 6). Recovery of P2X receptor currents evoked by α,β-meATP (10 μM) following brief application and desensitization with high concentrations of ATP and 2meSATP (10–30 μM) required >10 min. CTP was also a partial agonist evoking 70% of maximal ATP response with an EC50 of 27 μM (Figures 5C and 6). The rank order of potency of agonists was ATP=2meSATP>α,β-meATP⩽CTP=1-β,γ-meATP. UTP, GTP, TTP, ITP and CDP (up to 1 mM) were ineffective as agonists at rat mesenteric artery P2X receptors (Figure 6). In cross-desensitization studies following superfusion of α,β-meATP (10 μM), responses to ATP, 2meSATP, CTP and 1β-γ-meATP (30–100 μM) were abolished (n=3 for each) confirming that the agonists all acted at the same receptor.
Figure 5.
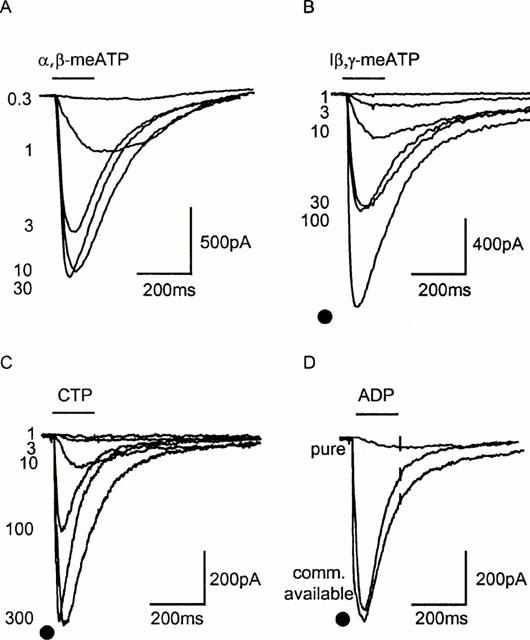
Effects of nucleotides at rat mesenteric artery P2X receptors. Sample traces recorded using the permeabilized patch configuration of responses to α,β-meATP (A), l-β,γ-meATP (B) and CTP (C). Commercially available ADP (100 μM) appeared an effective agonist but when purified activity was reduced by >95% (responses to 10 μM α,β-meATP indicated by • in B–D). Agonist application indicated by bar.
Figure 6.
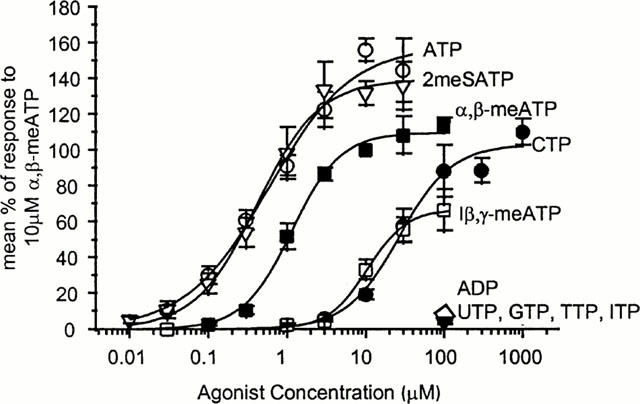
Concentration response relationships for a range of nucleotides at rat mesenteric artery P2X receptors showing ATP, 2meSATP, α,β-meATP, l-β,γ-meATP, UTP, GTP, TTP, and ITP. All data are expressed as per cent of response to 10 μM α,β-meATP, n=4–11 for each point.
ADP has been reported to be an agonist at P2X1 and P2X1/5 receptors (Evans et al., 1995; Haines et al., 1999). Initial studies indicated that ADP was an agonist at mesenteric artery smooth muscle P2X receptors (Figure 5D) however the purity of nucleotides, particularly those with low potency, needs to be considered when characterizing receptors. For example commercially available ADP is an agonist at P2Y2 and P2X1 receptors (∼100 times less potent than ATP) but when trace contaminations of ATP are removed with hexokinase treatment, ADP is ineffective as an agonist (Nicholas et al., 1996; Mahaut-Smith et al., 2000). At rat mesenteric P2X receptors commercially available ADP (100 μM) evoked responses that were 89.6±11.3% of those to 10 μM α,β-meATP (n=4) (Figure 5A). HPLC analysis of commercially available ADP indicated a low level (<1%) contamination with ATP that may account for these results. When purified, ADP (100 μM) was a very weak partial agonist at smooth muscle P2X receptors (7.2±1.8% of response to 10 μM α,β-meATP, n=5) (Figure 5D).
Effects of purinergic antagonists
The effects of antagonists were tested against an EC90 concentration of α,β-meATP. The relatively non-selective P2 receptor antagonist suramin (1–30 μM) inhibited α,β-meATP (3 μM) evoked inward currents with an IC50 of 4.2 μM (Hill slope 1.63) (Figure 7A,C). The effects of suramin were reversed within the 5 min washout cycle. Low concentrations (300 nM) of suramin potentiated the response to 3 μM α,β-meATP by 17.3±16.1 (n=4) and this effect was reversed on washout. These effects of low concentrations of suramin may explain the increased current amplitude occasionally seen following washout of higher suramin concentrations. The P2X receptor selective antagonist iso-PPADS inhibited responses to α,β-meATP (3 μM) in a concentration dependent manner with an IC50 of 70 nM (Hill slope 1.06) (Figure 7B,C). The effects of PPADS were reversed within a 5–10 min washout period.
Figure 7.
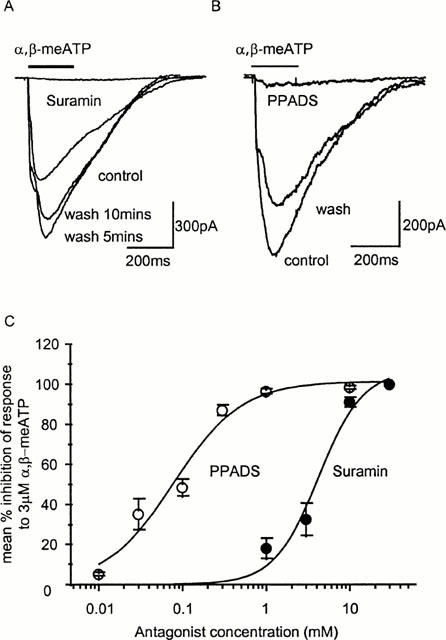
Antagonism of P2X receptor-mediated currents in rat mesenteric artery smooth muscle cells. (A) The response to α,β-meATP (3 μM) is abolished by 30 μM suramin, note the potentiation of the response on washout. (B) iso-PPADS (1 μM) abolished the response to α,β-meATP (3 μM), this effect was partially reversed after 5 mins washout. (C) Summary of the concentration dependence of inhibition of α,β-meATP (3 μM) evoked responses by the antagonists suramin and iso-PPADS. (n=4–6 for each point).
Discussion
In this study we have used the amphotericin permeabilized patch clamp technique to overcome the inherent run-down of artery smooth muscle P2X receptor currents previously reported using standard whole cell recording techniques. This has allowed us to provide a direct and in depth characterization of native rat mesenteric artery P2X receptors to allow comparison with the complement of P2X receptors expressed on these arteries and the properties of recombinant P2X receptors.
P2X receptor currents recorded from rat mesenteric artery smooth muscle cells desensitized during continued agonist application as has been reported previously for rat mesenteric arteries (Lewis et al., 1998) and other smooth muscle P2X receptors and recombinant P2X1 receptors (Inoue & Brading, 1990; Evans & Kennedy, 1994; Evans et al., 1995; Khakh et al., 1995). The mono-exponential decay of the response suggests that this process constitutes a single step to an inactivated state similar to that proposed for nicotinic acetylcholine (Katz & Thesleff, 1957) and AMPA receptors (Ambros-Ingerson & Lynch, 1993). The degree of recovery from the desensitized state was dependent on the recording conditions; it was incomplete and responses ran-down in the whole cell configuration. Run-down in the whole cell recording mode was unaffected by changes in intracellular calcium or inclusion of ATP or GTP in the recording pipette. However in the amphotericin permeabilized patch mode reproducible responses could be evoked at 5 min intervals. These results suggest that an intracellular component necessary for recovery is dialysed out of the cell in the whole cell configuration and/or that disruption of the cytoskeleton may interfere with the resensitization process. Thus recovery from desensitization of rat mesenteric artery P2X receptors may be a biochemically regulated process and could provide a mechanism for the modulation of the postjunctional actions of sympathetic nerve released ATP. The run-down of whole cell P2X receptor currents reported here is in contrast to those of recombinant P2X1 receptors expressed in HEK cells where reproducible responses could be evoked in the whole cell recording mode (Evans et al., 1995; Haines et al., 1999). This may reflect differences in the intracellular environment of smooth muscle cells and HEK cells. Alternatively it may indicate that rat mesenteric artery P2X receptors are not homomeric P2X1 receptors but form as heteromeric channels and/or express an extra modulatory subunit.
Recovery of mesenteric artery P2X receptors from desensitization did not appear to be regulated by changes in extracellular calcium concentration. This is in contrast to studies on other native desensitizing P2X receptors. For example (1) the recovery of P2X3-like receptors expressed in dorsal root ganglion neurons is speeded by increases in extracellular calcium (Cook et al., 1998), and reproducible responses can be recorded in the whole cell configuration using the same minimal electrode filling solution used in the present study (Grubb & Evans, unpublished observations) and (2) run-down of desensitizing megakaryocyte P2X receptor currents observed in the whole cell recording mode is reversed when cells are bathed in low calcium solutions (Kawa, 1996). These observations suggest that recovery from desensitization of P2X receptors does not have a common mechanism and may be receptor subtype and/or tissue specific.
Previous studies on P2X receptors in arteries have generally focused on contractile studies where some purinergic agonists are subject to metabolism. Patch clamp recordings had been frustrated by the run-down of P2X receptor currents. In the present study the use of the amphotericin recording technique has allowed the properties of P2X receptor channels in artery smooth muscle to be examined in detail where agonists can be applied under concentration clamp conditions. The potency and rank order of purinergic agonists at rat mesenteric artery P2X receptor ion channels; ATP=2meSATP>α,β-meATP>CTP=1-β,γ-meATP is essentially the same as that reported previously for recombinant P2X1 receptors (Evans et al., 1995). In previous studies on whole mesenteric arteries a potency order of α,β-meATP>2meSATP>ATP was reported (Ralevic & Burnstock, 1988). It is likely that the relative low potency of ATP and 2meSATP in these studies results from the breakdown of these agonists by ectonucleotidases (Evans & Kennedy, 1994). Of interest diagnostically is the sensitivity to l-β,γ-meATP that appears to be a hallmark for smooth muscle P2X receptors and the contribution of P2X1 receptor subunits (Evans et al., 1995; Trezise et al., 1995; Grubb & Evans, 1999) and can be useful in determining the subunit composition of native P2X receptor channels. The assertion that artery P2X receptors correspond to the P2X1 receptor phenotype was previously based on the desensitizing nature of P2X receptor-mediated currents and contractions and the sensitivity to α,β-meATP, TNP-ATP and l-β,γmeATP (e.g. Valera et al., 1994; Lewis et al., 1998). We have extended this work to characterize in detail the pharmacological properties of P2X receptor channels in mesenteric arteries. The sensitivity of mesenteric artery P2X receptors to the antagonists suramin IC50 ∼4 μM (this study) and 2′,3′-O-(2,4,6-trinitrophenyl) ATP (TNP-ATP) IC50 ∼2 nM (Lewis et al., 1998) also shows a close correlation with the properties of recombinant P2X1 receptors (Virginio et al., 1998). Our data for the P2X receptor antagonist PPADS IC50 ∼70 nM is similar to that reported for recombinant P2X1 receptors in Xenopus oocytes and smooth muscle P2X receptors (Jacobson et al., 1998; Lambrecht et al., 2000). However in other studies on rat mesenteric artery contractions and recombinant P2X1 receptors, PPADS was less effective as an antagonist (Windscheif et al., 1994; Evans et al., 1995). The reasons for these discrepancies are at present unclear. The combination of agonist and antagonist sensitivity and timecourse of responses and demonstration of P2X1 receptor immunoreactivity on artery smooth muscle demonstrates that the mesenteric artery P2X receptor is dominated by a P2X1 receptor phenotype.
The use of receptor specific antibodies in this study has allowed the distribution of P2X receptor subtypes within mesenteric arteries to be determined and allows direct comparisons to be made with the properties of P2X receptor channels in these arteries. We have shown that in addition to P2X1 receptors, P2X4 and P2X5 receptors are also expressed on artery smooth muscle cells using subtype selective antibodies directed against the intracellular carboxy terminus of the receptor. P2X7 receptor staining was punctate and restricted to the outer adventitial layer that contains collagen fibres and varicose sympathetic nerves. Molecular analysis had previously indicated that messenger RNAs for P2X1–5 and P2X7 receptors are expressed in mesenteric artery segments (Phillips & Hill, 1999). These segments constitute a heterogeneous population of cell types including smooth muscle cells, endothelial cells, neurons coursing the surface of the vessels and blood cells. In the present study P2X2 and P2X3 receptor immunoreactivity was below the level of detection (in positive control studies these antibodies produced a high level of receptor specific immunoreactivity on dorsal root ganglion sections). In a previous study RNA transcripts for P2X2 and P2X3 receptors were only weakly amplified in mesenteric artery segments (Phillips & Hill, 1999). P2X2 receptor immunoreactivity has been associated with sympathetic nerves on the surface of arterioles (Vulchanova et al., 1996) and P2X3 receptors are thought to be expressed predominantly by sensory neurons (Chen et al., 1995), thus it seems likely that these P2X2 and P2X3 receptor subunits were amplified from the neurons present on the artery segment and not directly from the smooth muscle cells. The lack of immunoreactivity and weak amplification of RNAs for P2X2 receptors suggests that in contrast to guinea-pig submucosal arterioles (Vulchanova et al., 1996) P2X2 receptors are expressed at low levels by neurons associated with the mesenteric artery.
The immunohistochemical localization of P2X1, P2X4 and P2X5 receptor subunits on arterial smooth muscle documents the P2X receptor ‘building-blocks' present in the artery and indicates the possibility for the expression of multiple types of either homomeric or heteromeric P2X receptors in this preparation. The question is how these subunits assemble to give rise to the P2X receptor in mesenteric artery cells. The mono-exponential decay of P2X receptor currents in arterial muscle cells coupled with the cross-desensitization studies with α,β-meATP (this study and Ralevic & Burnstock, 1988) and other properties indicates that mesenteric artery smooth muscle cells express a homogeneous population of P2X receptors dominated by a P2X1 receptor phenotype. As no residual sustained response was recorded in the present study there is no evidence to suggest that rat mesenteric artery smooth muscle cells express homomeric P2X4 or P2X5 receptors (Bo et al., 1995; Buell et al., 1996; Collo et al., 1996). The lack of evidence for the contribution of P2X4 or P2X5 subunits to the mesenteric artery P2X receptor-mediated response suggests that these isoforms are either (1) expressed at low levels, (2) unable to form functional channels in smooth muscle cells and/or (3) that they may produce heteromeric complexes which are dominated by the properties of constituent P2X1 receptor subunits.
Demonstration of the formation of heteromeric P2X receptors in recombinant systems has relied on either immunoprecipitation studies (Torres et al., 1999) or more indirect methods dependent on the production of a new phenotype e.g. P2X2/3 receptors (Lewis et al., 1995). The expression of P2X1,4 and 5 receptor subunits in the present study suggests that heteromerization could occur in artery smooth muscle. The native receptor is considered unlikely to result from the heteromerization of P2X1/4 receptor subunits as these do not readily associate when expressed in HEK293 cells (Torres et al., 1999). However whether an auxiliary protein or differential processing in smooth muscle cells results in the production of P2X1/4 heteromeric channels remains to be determined. A contribution of P2X1/5 receptors can be discounted, as P2X receptor-mediated responses in the arteries do not show a secondary sustained component or the pharmacology of P2X1/5 heteromeric channels (Torres et al., 1998). Recombinant studies have shown that P2X4/5 and P2X1/5 receptors readily assemble (Torres et al., 1999). Given the P2X receptors are thought to form from at least three P2X receptor subunits (Stoop et al., 1999) the existence of a heteromeric channel incorporating P2X1/4/5 subunits in smooth muscle with a phenotype dominated by the properties of P2X1 receptors is possible. In many other cell types there is evidence for the apparent functional redundancy of P2X receptor subtypes and domination of the P2X receptor phenotype by a particular subunit. For example sensory neurons are thought to express P2X1–6 receptors (Collo et al., 1996) and yet the properties of the majority of dorsal root ganglion neurons appear to correspond to homomeric P2X3 receptors (Robertson et al., 1996; Grubb & Evans, 1999). Thus certain P2X receptor subunits may contribute to a functional heteromeric channel but by present phenotypic markers make no apparent contribution to the phenotype and thus exist as ‘silent subunits'–this may be the case for P2X4 and P2X5 receptor subunits in arterial smooth muscle. However, future studies may demonstrate physiological or pharmacological properties that reveal a functional contribution of these apparently ‘silent subunits' in artery smooth muscle.
In summary by using the amphotericin permeabilized patch recording technique we have extended the characterization of native smooth muscle P2X receptors and shown that the properties of mesenteric artery P2X receptors are indistinguishable from homomeric P2X1 receptors. No obvious role for the P2X4 and P2X5 receptor subunits also expressed by the muscle cells could be found in the present study.
Acknowledgments
We would like to thank Prof M. Boarder and Dr J. Roberts for analysis and purification of nucleotides. This work was supported by the Wellcome Trust.
Abbreviations
- α,β-meATP
α,β-methylene ATP
- iso-PPADS
iso-pyridoxalphosphate-6-azophenyl-2′-5′-disulphonate
References
- AMBROS-INGERSON J., LYNCH G. Channel gating kinetics and synaptic efficacy: A hypothesis for expression of long-term potentiation. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7903–7907. doi: 10.1073/pnas.90.16.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENHAM C.D. ATP-activated channels gate calcium entry in single smooth muscle cells from rabbit ear artery. J. Physiol. 1989;419:689–701. doi: 10.1113/jphysiol.1989.sp017893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BO X., ZHANG Y., NASSAR M., BURNSTOCK G., SCHOEPFER R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- BRAKE A.J., WAGENBACH M.J., JULIUS D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- BUELL G., LEWIS C., COLLO G., NORTH R.A., SURPRENANT A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacol. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- CHEN C., AKOPIAN A.N., SIVILOTTI L., COLQUHOUN D., BURNSTOCK G., WOOD J.N. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- COLLO G., NORTH R.A., KAWASHIMA E., MERLO-PICH E., NEIDHART S., SURPRENANT A., BUELL G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK S.P., RODLAND K.D., MCCLESKEY E.W. A memory for extracellular Ca2+ by speeding recovery of P2X receptors from desensitisation. J. Neurosci. 1998;18:9238–9244. doi: 10.1523/JNEUROSCI.18-22-09238.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS R.J., KENNEDY C. Characterization of P2-purinoceptors in the smooth muscle of the rat tail artery: a comparison between contractile and electrophysiological responses. Br. J. Pharmacol. 1994;113:853–860. doi: 10.1111/j.1476-5381.1994.tb17071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS R.J., LEWIS C., BUELL G., VALERA S., NORTH R.A., SURPRENANT A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2X purinoceptors) Mol. Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- EVANS R.J., SURPRENANT A. Vasoconstriction of guinea-pig submucosal arterioles following sympathetic nerve stimulation is mediated by the release of ATP. Br. J. Pharmacol. 1992;106:242–249. doi: 10.1111/j.1476-5381.1992.tb14323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLIGAN J.J., HERRING A., HARPSTEAD T. Pharmacological characterization of purinoceptor-mediated constriction of submucosal arterioles in guinea pig ileum. J. Pharmacol. Exp. Ther. 1995;274:1425–1430. [PubMed] [Google Scholar]
- GRUBB B.D., EVANS R.J. Characterization of cultured dorsal root ganglion neuron P2X receptors. Eur. J. Neurosci. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- HAINES W.R., TORRES G.E., VOIGT M.M., EGAN T.M. Properties of the novel ATP-gated ionotropic receptor composed of the P2X1 and P2X5 isoforms. Mol. Pharmacol. 1999;56:720–727. [PubMed] [Google Scholar]
- INOUE R., BRADING A.F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br. J. Pharmacol. 1990;100:619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON K.A., KIM Y.C., WILDMAN S.S., MOHANRAM A., HARDEN T.K., BOYER J.L., KING B.G., BURNSTOCK G. A pyridoxine cyclic phosphate and its 6-azoaryl derivative selectively potentiate and antagonize activation of P2X1 receptors. J. Med. Chem. 1998;18:2201–2206. doi: 10.1021/jm980183o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J. Physiol. 1957;138:63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWA K. ADP-induced rapid inward currents through Ca2+-permeable cation channels in mouse, rat and guinea-pig megakaryocytes: a patch clamp study. J. Physiol. 1996;495:339–352. doi: 10.1113/jphysiol.1996.sp021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAKH B.S., SURPRENANT A., HUMPHREY P.P.A. A study on P2X purinoceptors mediating the electrophysiological and contractile effects of purine nucleotides in rat vas deferens. Br. J. Pharmacol. 1995;115:177–185. doi: 10.1111/j.1476-5381.1995.tb16336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING B.F., TOWNSEND-NICHOLSON A., WILDMANN S.S., THOMAS T., SPYER K.M., BURNSTOCK G. Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. J. Neurosci. 2000;20:4817–4877. doi: 10.1523/JNEUROSCI.20-13-04871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMBRECHT G., RETTINGER J., BAUMERT H.G., CZECHE S., DAMER S., GANSO M., HILDEBRAN N.B., SPATZ-KUMBEL G., SCHMALZING G., MUTSCHLER E. The novel pyridoxal-5′-phosphate derivative PPNDS potently antagonizes activation of P2X1 receptors. Eur. J. Pharmacol. 2000;387:R19–R21. doi: 10.1016/s0014-2999(99)00834-1. [DOI] [PubMed] [Google Scholar]
- LE K., BABINSKI K., SEGUELA P. Central P2X4 and P2X6 channel subunits co-assemble into a novel heteromeric ATP receptor. J. Neurosci. 1998;18:7152–7159. doi: 10.1523/JNEUROSCI.18-18-07152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS C.J., GITTERMAN D.P., SCHLUTER H., EVANS R.J. Effects of diadenosine polyphosphates (ApnAs) and adenosine polyphospho guanosines (ApnGs) on rat mesenteric artery P2X receptor ion channels. Br. J. Pharmacol. 2000;129:124–130. doi: 10.1038/sj.bjp.0702993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS C., NEIDHART S., HOLY C., NORTH R.A., BUELL G., SURPRENANT A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- LEWIS C., SURPRENANT A., EVANS R.J. 2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate (TNP-ATP)–a nanomolar affinity antagonist at rat mesenteric artery P2X receptor ion channels. Br. J. Pharmacol. 1998;124:1463–1466. doi: 10.1038/sj.bjp.0702001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHAUT-SMITH M.P., ENNION S.J., ROLF M., EVANS R.J. ADP is not an agonist at P2X1 receptors: evidence for separate receptors stimulated by ATP and ADP on human platelets. Br. J. Pharmacol. 2000;131:108–114. doi: 10.1038/sj.bjp.0703517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULRYAN K., GITTERMAN D.P., LEWIS C.J., VIAL C., LECKIE B.J., COBB A.L., BROWN J.E., CONLEY E.C., BUELL G., PRITCHARD C.A., EVANS R.J. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- NICHOLAS R.A., WATT W.C., LAZAROWSKI E.R., LI Q., HARDEN T.K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol. Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- NICKE A., BAUMERT H., RETTINGER J., EICHELE A., LAMBRECHT G., MUTSCHLER E., SCHMALZING G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORI S.L., FUMAGALLI L., BO X., BOGDANOV Y., BURNSTOCK G. Coexpression of mRNAs for P2X1, P2X2 and P2X4 receptors in rat vascular smooth muscle: an in situ hybridization and RT-PCR study. J. Vasc. Res. 1998;35:179–185. doi: 10.1159/000025582. [DOI] [PubMed] [Google Scholar]
- PHILLIPS J.K., HILL C.E. Neuroreceptor mRNA expression in the rat mesenteric artery develops independently of innervation. Int. J. Devel. Neurosci. 1999;17:377–386. doi: 10.1016/s0736-5748(99)00032-5. [DOI] [PubMed] [Google Scholar]
- QUAYLE J.M., DART C., STANDEN N.B. The properties and distribution of inward rectifier potassium currents in pig coronary arterial smooth muscle. J. Physiol. 1996;494:715–726. doi: 10.1113/jphysiol.1996.sp021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Actions mediated by P2-purinoceptor subtypes in the isolated perfused mesenteric bed of the rat. Br. J. Pharmacol. 1988;95:637–645. doi: 10.1111/j.1476-5381.1988.tb11686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON S.J., RAE M.G., ROWAN E.G., KENNEDY C. Characterization of a P2X-purinoceptor in cultured neurones of the rat dorsal root ganglia. Br. J. Pharmacol. 1996;118:951–956. doi: 10.1111/j.1476-5381.1996.tb15491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOOP R., THOMAS S., RASSENDREN F., KAWASHIMA E., BUELL G., SURPRENANT A., NORTH R.A. Contribution of individual subunits to the multimeric P2X2 receptor: estimates based on methanethiosulfonate block at T336C. Mol. Pharmacol. 1999;56:973–981. doi: 10.1124/mol.56.5.973. [DOI] [PubMed] [Google Scholar]
- TORRES G.E., EGAN T.M., VOIGT M.M. Hetero-oligomeric assembly of P2X receptor subunits. J. Biol. Chem. 1999;274:6653–6659. doi: 10.1074/jbc.274.10.6653. [DOI] [PubMed] [Google Scholar]
- TORRES G.E., HAINES W.R., EGAN T.M., VOIGT M.M. Co-expression of P2X1 and P2X5 receptor subunits reveals a novel ATP-gated ion channel. Mol. Pharmacol. 1998;54:989–993. doi: 10.1124/mol.54.6.989. [DOI] [PubMed] [Google Scholar]
- TREZISE D.J., MICHEL A.D., GRAHAMES C.B.A., KHAKH B.S., SURPRENANT A., HUMPHREY P.P.A. The selective P2X purinoceptor agonist, beta,gamma,-methylene-L-adenosine 5′-triphosphate, discriminates between smooth muscle and neuronal P2X purinoceptors. Naunyn Schmiedeberg's Arch. Pharmacol. 1995;351:603–609. doi: 10.1007/BF00170159. [DOI] [PubMed] [Google Scholar]
- VALERA S., HUSSY N., EVANS R.J., ADAMI N., NORTH R.A., SURPRENANT A., BUELL G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- VIRGINIO C., ROBERTSON G., SURPRENANT A., NORTH R.A. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3 and heteromeric P2X2/3 receptors. Mol. Pharmacol. 1998;53:969–973. [PubMed] [Google Scholar]
- VULCHANOVA L., ARVIDSSON U., RIEDL M., BUELL G., SURPRENANT A., NORTH R.A., ELDE R.P. Differential distribution of two ATP-gated ion channels (P2X receptors) determined by immunohistochemistry. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDSCHEIF U., RALEVIC V., BAUMERT H.G., MUTSCHLER E., LAMBRECHT G., BURNSTOCK G. Vasoconstrictor and vasodilator responses to various agonists in the rat perfused mesenteric arterial bed: selective inhibition by PPADS of contractions mediated via P2X-purinoceptors. Br. J. Pharmacol. 1994;113:1015–1021. doi: 10.1111/j.1476-5381.1994.tb17094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


