Abstract
The pharmacological characterization of a 5-HT receptor-mediated contractile response in the mouse isolated ileum is described.
In the presence of methysergide (1 μM), 5-hydroxytryptamine (5-HT, 0.3–100 μM) produced phasic concentration-dependent contractions of segments of the mouse isolated ileum with a pEC50 value of 5.47±0.09.
The 5-HT3 receptor selective agonists m-chlorophenylbiguanide (0.3–100 μM, pEC50 5.81±0.04), 1-phenylbiguanide (3–100 μM, pEC50 5.05±0.06) and 2-methyl-5-HT (3–100 μM, pEC50 5.00±0.07) acted as full agonists to induce contractile responses. 5-methoxytryptamine (0.1–100 μM), RS 67506 (0.1–100 μM) and α-methyl-5-HT (0.1–100 μM) failed to mimic the 5-HT responses.
The contractile response to 5-HT was not antagonized by either 5-HT2 receptor antagonists ritanserin (0.1 μM) or ketanserin (1 μM) nor the 5-HT4 receptor antagonist SB 204070 (0.1 μM).
The 5-HT3 receptor selective antagonists granisetron (0.3–1 nM), tropisetron (1–10 nM), ondansetron (10 nM–1 μM) and MDL 72222 (10 nM–1 μM) caused rightward displacement of the concentration-response curves to 5-HT. The lower concentrations of the antagonists caused approximate parallel rightward shifts of the concentration-response curves to 5-HT with apparent pKB values for granisetron (9.70±0.39), tropisetron (9.18±0.20), ondansetron (8.84±0.24) and MDL 72222 (8.65±0.35). But higher concentrations of antagonists resulted in a progressive reduction in the maximum responses.
The contractile response to 5-HT was abolished by tetrodotoxin (0.3 μM); atropine (0.1 and 1 μM) decreased the maximum response of the 5-HT concentration-response curve by approximately 65%.
It is concluded that a neuronally located 5-HT3 receptor mediates a contractile response to 5-HT in the mouse ileum. The 5-HT3 receptor in the mouse ileum has a different pharmacological profile to that reported for the guinea-pig ileum.
Keywords: 5-hydroxytryptamine; 5-HT3 receptor; mouse ileum; MDL 72222, m-chlorophenylbiguanide, 2-methyl-5-hydroxytryptamine; granisetron; ondansetron; tropisetron; tetrodotoxin
Introduction
5-Hydroxytryptamine3 receptors are unique among the 5-HT receptors in that they are ligand-gated cation channels, which when stimulated, produce a fast depolarising response (Derkach et al., 1989). The 5-HT3 receptors are almost exclusively located on neurones in both the central nervous system (Kilpatrick et al., 1987; Waeber et al., 1989) and peripheral tissues including the enteric nervous system (Fozard, 1984b). They have been implicated in a range of neuronal functions in both the central nervous system and periphery (Costall & Naylor, 1997; Hoyer et al., 1994). Studies on the 5-HT3 receptor have led to the development of 5-HT3 receptor antagonists which has proved to be a major breakthrough in the control of chemotherapy and radiotherapy induced emesis (Butcher, 1993; Costall & Naylor, 1997). More recently, the 5-HT3 receptor antagonist alosetron has been advanced as a new treatment for the irritable bowel syndrome (Camilleri et al., 1999).
The neuronally located 5-HT receptor-mediating contraction in the guinea-pig ileum through the release of acetylcholine (Gaddum & Picarelli, 1957) was subsequently identified as the first description of a 5-HT3 receptor-mediated response (Bradley et al., 1986). Other functional in vitro preparations which have since been shown to contain 5-HT3 receptors includes the rabbit heart, rat and rabbit vagus nerves (Fozard, 1984b; Ireland & Tyers, 1987; Round & Wallis, 1986), several types of ganglia (Newberry et al., 1991) and neuronally derived cell lines (Lovinger, 1991; Sepulveda et al., 1991).
Much is now known about the 5-HT3 receptor function in these tissues with the development of a number of selective 5-HT3 receptor agonists and antagonists. These selective ligands have revealed differences in agonist and antagonist potencies at the 5-HT3 receptor located in tissues obtained from different species leading to speculation for the existence of 5-HT3 receptor subtypes (Hoyer et al., 1994; Richardson & Engel, 1986). A most notable difference is the 5-HT3 receptor agonist and antagonist profile shown by guinea-pig tissues as compared to the 5-HT3 receptor located in other tissues. Thus, 5-HT3 receptor agonists m-chlorophenylbiguanide and 1-phenylbiguanide are inactive in the guinea-pig ileum whereas they are active on the 5-HT3 receptor present in many tissues including the rat and rabbit afferent neurones (Butler et al., 1990; Fozard, 1990; Newberry et al., 1991). Similarly antagonist potencies at 5-HT3 receptors in the guinea-pig tissues are 1–2 log units lower than that in tissues from other species (Hoyer et al., 1994).
The guinea-pig ileum has been a reference intestinal in vitro preparation to study 5-HT receptor-mediated changes in contraction/relaxation responses. Such detailed studies of 5-HT3 receptors in the intestine of other species including mice remains to be undertaken, although in the mouse duodenum the 5-HT3 receptor agonist 1-phenylbiguanide has been shown to mimic the contraction response to 5-HT (Drakontides & Gershon, 1968). Such a study and particularly in the mouse, to provide information on responses to murine tissues to 5-HT receptor agonists and antagonists is important in view of the current focus on this species to produce knockout animals lacking the gene responsible for various proteins including 5-HT receptors (ScearceLevie et al., 1999; Stark et al., 1998). Thus, the aim of the present study was to characterize the 5-HT receptor mediating the contractile response in the mouse ileum and to determine the agonist and antagonist profile of various 5-HT receptor ligands in ileal tissue from this species.
Methods
Animals and housing conditions
The experiments were carried out on adult Bankin Kingman White (BKW) mice (Bradford University strain) of either sex weighing between 24–35 g. The mice were housed in single sex groups of 10 in a cage and allowed food and water ad libitum. The cages were floored with sawdust and cleaned on a regular basis. The animals were maintained at humidity of 45–50% at 21°C on a 14/10 h light/dark cycle.
Preparation of tissues
Animals were killed by cervical dislocation and a segment of ileum approximately 15 cm long was removed from a distance of 2 cm from the ileo-caecal junction and kept in Krebs-Heinseleit (KH) (composition mM: NaCl 118, KCl 4.7, KH2 PO4 1.2, MgSO4 1.2, CaCl2 2.5, NaHCO3 25.0, glucose 10.0) solution oxygenated with 95% O2 and 5% CO2. The mesentery and fatty tissue were removed and the lumen carefully flushed of its content with KH solution. Four segments of ileum approximately 2 cm in length were dissected and mounted vertically in 10 ml, water-jacketed organ baths containing KH solution kept at 37°C and oxygenated with 95% O2 and 5% CO2. Changes in tissue tension were measured isometrically using Grass Force Displacement transducers (FT03C, Grass Instrument Co., MA, U.S.A.) and recorded on a multichannel Grass recorder. The tissues were slowly placed under a resting tension of 0.25 g (unless otherwise stated) and allowed to equilibrate for a 1 h period with two washouts every 15 min in the first half of the equilibration period before the construction of the agonist concentration-response curves. The spontaneous activity in the tissues was generally less than 0.2 g and any tissue that exhibited spontaneous activity greater than 0.2 g was discarded.
Construction of concentration-response curves
Non-cumulative concentration-response curves to 5-HT and other agonists were established by adding increasing concentrations of the agonists to the organ baths. The buffer routinely contained methysergide (1 μM) unless stated otherwise. The contact time between tissue and the agonist was 30 s and the preparation was then washed three times at 1 min intervals and allowed to recover for a further 10 min before addition of the next concentration. Each tissue was used to record only one concentration-response curve to 5-HT or other agonist. A comparison of the effects of agonists and antagonists was carried out using paired preparations; one of the tissues from each animal serving as a control. Agonists were added to the organ bath in volumes not exceeding 0.1 ml (1% organ bath volume). The antagonists were added to the buffer reservoir and allowed to equilibrate for 1 h before establishing agonist concentration-response curve ensuring the continuous presence of antagonist during the construction of the concentration-response curves.
A control contraction response to 30 mM KCl was also established after the construction of concentration-response curves to 5-HT or other agonists in each tissue. However, the expression of the responses as a percentage of the KCl response made no discernible difference to the results. Hence, data are expressed in the absolute values.
Analysis of results
All results are expressed as means±s.e.mean. The potencies of the 5-HT receptor agonists were expressed as pEC50 values relative to individual maxima. The apparent pKB values for antagonists were estimated from the equation:
where CR is the concentration ratio derived from the agonist EC50 values in the presence and absence of a concentration of antagonist (B) which resulted in rightward shift of the concentration-response curve with a minimum reduction of the Emax, assuming a competitive interaction with a slope of 1, at this concentration of antagonist. The significance of differences between the values was determined at P<0.05 using Student's unpaired two-tailed t-test.
Drugs
5-Hydroxytryptamine maleate (Sigma), 5-methoxytryptamine hydrochloride (Sigma), α-methyl-5-hydroxytrptamine maleate (Research Biochemical Inc.), 2-methyl-5-hydroxytryptamine HCl (Tocris), atropine sulphate (Sigma), granisetron (SmithKline Beecham), methysergide hydrogen maleate (Novartis), m-chlorophenylbiguanide (Tocris), 1-phenylbiguanide hydrochloride (Tocris), Ondansetron hydrochloride dihydrate (Glaxo Wellcome), RS 67506 (1-(4-amino-5-chloro-2-methoxyphenyl)-3-[1-2methylsulphonylamino)ethyl-4-piperidinyl]-1-propanone) hydrochloride (Tocris), SB 204070 (8-amino-7-chloro(N-butyl-4-piperidyl) methylbenzo-1,4-di-oxan-5-carboxylate) hydrochloride (SmithKline Beecham), tropisetron hydrochloride (Research Biochemical Inc.), ketanserin tartrate (Research Biochemical Inc.), were dissolved in distilled water. MDL 72222 (tropanyl 3,5-dichlorobenzoate) (Tocris) and ritanserin (Research Biochemical Inc.) were initially dissolved in dimethylsulphoxide and tetrodotoxin (Tocris) was dissolved in citrate buffer of pH 4 before addition to physiological solution.
Results
Preliminary experiments
5-HT (0.3-100 μM) produced a concentration-related contraction of the mouse ileum. During preliminary experiments using 0.5 g of resting tension, the contractions in some tissues were preceded by a small relaxation. No relaxation was observed when the resting tension was reduced to 0.25 g and methysergide (1 μM) was included in the bath. These conditions also produced less spontaneous activity and greater contractile responses in the tissues and were maintained throughout all the subsequent experiments.
The response of mouse ileum to 5-HT and other 5-HT receptor agonists
In the presence of methysergide (1 μM), 5-HT produced concentration related contractile responses in all four segments of the mouse ileum. The concentration-response curves to 5-HT in all four segments were superimposable (data not shown). The individual contractile response to 5-HT (and other 5-HT3 receptor agonists) consisted of a spike-like phasic response with little or no tonic contraction (Figure 1). In contrast, the contraction due to KCl consisted of an initial large phasic response followed by a tonic response which was about 60–80% of the initial phasic response. m-Chlorophenylbiguanide, 2-methyl-5-HT and 1-phenylbiguanide produced qualitatively similar concentration-dependent contractions with maximum responses not significantly different from that of 5-HT. The rank order of potency of these agonists (pEC50±s.e.mean) was m-chlorophenylbiguanide (5.81±0.04, n=4)>5-HT (5.47±0.09, n=12),>1-phenylbiguanide (5.05±0.06, n=8)⩾2-methyl-5-HT (5.00±0.07, n=10). The contractile response to all agonists decreased at higher concentrations once a maximum response had been achieved. This effect was most prominent with m-chlorophenylbiguanide where a contraction response was absent at 100 μM (Figure 2). In contrast, the 5-HT4 receptor agonists 5-methoxytryptamine or RS 67506 (at concentrations up to 100 μM) failed to produce a consistent response, with only a few tissues exhibiting a contraction which did not exceed 0.2 g. The response obtained with the highest concentration of α-methyl-5-HT tested (100 μM) was also less than 25% of the maximum response to 5-HT (Figure 3).
Figure 1.
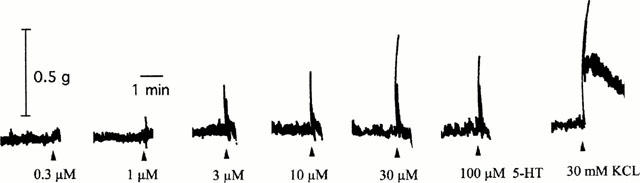
Representative tracing showing the contractile response induced by the non-cumulative addition of increasing concentrations of 5-HT (0.3–100 μM) and finally KCl (30 mM) in the presence of methysergide (1 μM) in the mouse isolated ileum.
Figure 2.

Concentration-response curves to 5-hydroxytryptamine (5-HT, n=12), m-chlorophenylbiguanide (MCPBG, n=4), 1-phenylbiguanide (PBG, n=8) and 2-methyl-5-HT (2-Me-5-HT, n=10) in the mouse isolated ileum. Buffer routinely contained methysergide (1 μM). Each point represents the mean±s.e.mean.
Figure 3.
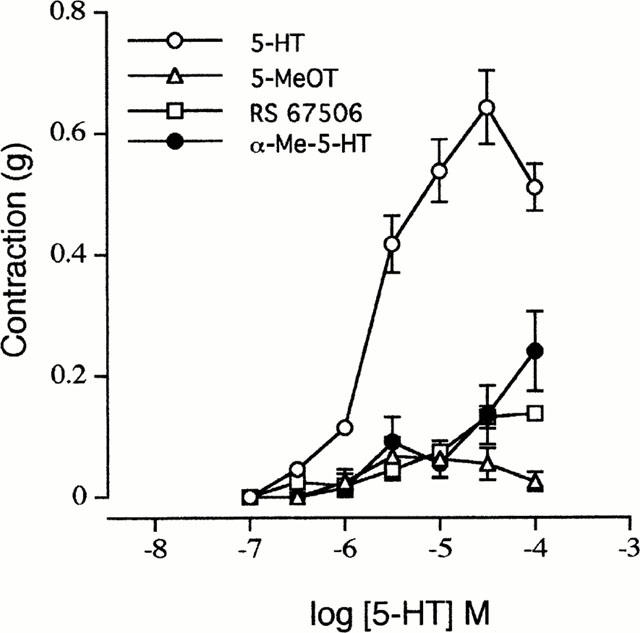
Concentration-response curves to 5-hydroxytryptamine (5-HT, n=6), 5-methoxytryptamine (5-MeOT, n=6), RS 67506 (RS 67506, n=4) and α-methyl-5-HT (α-Me-5-HT, n=5) in the mouse isolated ileum. The buffer routinely contained methysergide (1 μM). Each point represents the mean±s.e.mean.
The effect of ritanserin, ketanserin and SB 204070 on the contractile response to 5-HT
The inclusion of ritanserin (0.1 μM), ketanserin (1 μM) or SB 204070 (0.1 μM) had no significant effect on the concentration-response curve to 5-HT (data not shown).
The effect of granisetron, tropisetron, ondansetron and MDL 72222 on the contractile response mediated by 5-HT agonists
In addition to methysergide, SB 204070 (0.1 μM) was also included in these experiments to block any potential interference from the 5-HT4 receptor-mediated response. All four selective 5-HT3 receptor antagonists granisetron (0.3–1 nM), tropisetron (1–10 nM), ondansetron (10 nM–1 μM) and MDL 72222 (10 nM–1 μM) caused a right-ward shift of the concentration-response curve to 5-HT. Although the lowest effective concentration caused a more or less parallel shift of the concentration-response curve to 5-HT without a significant decrease in the maximum response, the higher concentrations of all four antagonists caused a progressive decrease in the maximum response, resulting in a complete loss of response at the highest concentration of antagonist tested (Figure 4a–d). The apparent pKB values estimated with the lowest effective concentration of the 5-HT3 receptor antagonists (which exhibited approximately parallel shifts) are shown in Table 1. The rank order of potency of the four 5-HT3 receptor antagonists was granisetron>tropisetron>ondansetron>MDL 72222. Tropisetron (2 nM), ondansetron (5 nM) and MDL 72222 (20 nM) also exhibited non-surmountable antagonism of m-chlorophenylbiguanide, 1-phenybiguanide and 2-methyl-5-HT concentration-response curves (data not shown).
Figure 4.
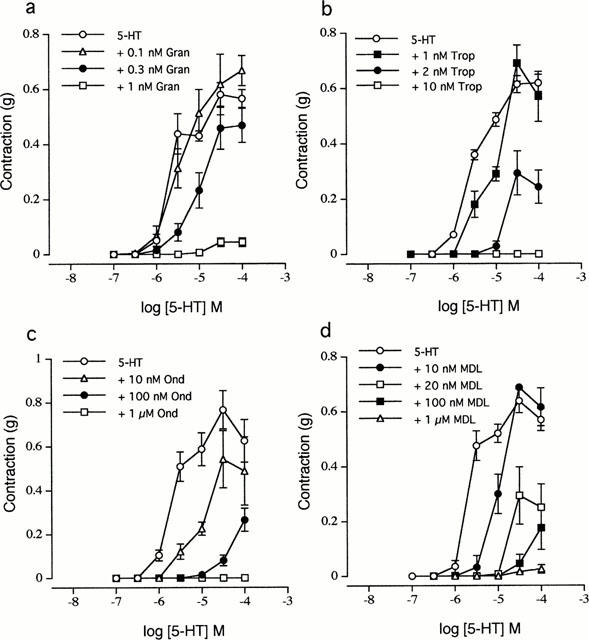
Antagonism of the contractile response to 5-HT in the mouse ileum by granisetron, tropisetron, ondansetron and MDL 72222. Concentration-response curves to 5-HT (a) in the absence (5-HT, n=10) and presence of 0.1 nM (+0.1 nM Gran, n=6), 0.3 nM (+0.3 nM Gran, n=6) and 1 nM (+1 nM Gran, n=6) granisetron (b) in the absence (5-HT, n=13) and presence of 1 nM (+1 nM Trop, n=5), 2 nM (+2 nM Trop, n=7) and 10 nM (+10 nM Trop, n=6) tropisetron, (c) in the absence (5-HT, n=6) and presence of 10 nM (+10 nM Ond, n=6), 100 nM (+100 nM Ond, n=5) and 1 μM (+1 μM Ond, n=5) ondansetron, (d) in the absence (5-HT, n=12) and presence of 10 nM (+10 nM MDL, n=5), 20 nM (+20 nM MDL, n=6), 100 nM (+100 nM MDL, n=5), and 1 μM (+1 μM MDL, n=5) MDL 72222. The buffer routinely contained methysergide (1 μM) and SB 204070 (0.1 μM). Each point represents the mean±s.e.mean.
Table 1.
Apparent pKB estimations with 5-HT3 receptor antagonists to antagonize the contractile effects of 5-HT in the mouse ileum

Effects of atropine and tetrodotoxin on the contractile responses to 5-HT
The inclusion of atropine (0.1 and 1 μM) reduced the mean contractile response mediated by 5-HT to 36% of the control value (Figure 5). The concentration-response curves to 5-HT in the presence of 0.1 and 1 μM atropine were almost identical. The addition of tetrodotoxin (0.3 μM) to the buffer abolished the contractile response to 5-HT but had no significant effect on the contractile response to KCl (added at the end of the construction of the 5-HT response curves). Tetrodotoxin also caused an approximate 2-fold increase in the resting length of the tissues.
Figure 5.

Concentration-response curves to 5-HT in the absence (5-HT, n=11) and presence of 0.1 μM (+0.1 μM Atrop, n=7) and 1 μM (+1 μM Atrop, n=5) atropine. Buffer routinely contained methysergide (1 μM). Each point represents the mean±s.e.mean.
Discussion
The ability of the four structurally different but selective 5-HT3 receptor antagonists granisetron, tropisetron, ondansetron and MDL 72222 to antagonize the 5-HT mediated contraction in the mouse ileum at nanomolar concentrations indicates that the contractile response is mediated by a 5-HT3 receptor (Hoyer et al., 1994). This conclusion is further supported by the ability of selective 5-HT3 receptor agonists m-chlorophenylbiguanide, 1-phenylbiguanide and 2-methyl-5-HT to mimic the 5-HT responses and where the contractile responses are again antagonized by the 5-HT3 receptor antagonists.
An involvement of the 5-HT1/2 or the 5-HT7 receptor in the contractile response to 5-HT is unlikely as contractile responses to 5-HT were obtained in the presence of methysergide. A lack of involvement of 5-HT2 receptors is also indicated by the failure of α-methyl-5-HT to mimic and ritanserin and ketanserin to antagonize the 5-HT induced contraction. Similarly, the failure of the selective 5-HT4 receptor antagonist SB 204070 to antagonize the effects of 5-HT and the inability of the 5-HT4 receptor agonists 5-methoxytrptamine and RS 67506 to induce contraction excludes the possibility of a 5-HT4 receptor involvement.
The ability of tetrodotoxin to abolish the 5-HT induced contractile response indicates a neuronal location for the 5-HT3 receptors. As in other tissues and species (Hoyer et al., 1994; Zifa & Fillion, 1992), the 5-HT3 receptor in the mouse ileum appears to be involved in the modulation of neuronal activity within the enteric nerves leading to the contraction of the smooth muscle. This probably involves the release of acetylcholine since atropine (0.1 and 1 μM) reduced by about 65% the contraction induced by 5-HT. The failure of the higher concentration of atropine to reduce further the residual contraction indicates an additional non-cholinergic component of the contractile response to 5-HT in the mouse ileum. It is possible that this involves the release of neurokinins since neurokinin-mediated contractile responses to 5-HT have been demonstrated both in the guinea-pig (Chahl, 1983) and rat ileum (McLean & Coupar, 1996).
In the mouse duodenum and in the presence of methysergide, the contractile responses to a higher concentration (10 μg ml−1) of both 5-HT and 1-phenylbiguanide are reversed to relaxation following blockade of muscarinic receptors (Drakontides & Gershon, 1968). This would indicate that 5-HT3 receptors in the mouse duodenum are present on both excitatory and inhibitory neurones. In the present study, no relaxation was observed at any concentration of 5-HT in the mouse ileum in the presence of methysergide and atropine. Thus unlike the duodenum, the present study provided no indication of the presence of 5-HT3 receptors to mediate an inhibitory role. In contrast, preliminary results showed that contractile responses to 5-HT were enhanced and any small relaxation due to 5-HT (which preceded contraction) was abolished in the presence of methysergide, indicating the possibility of a 5-HT1/2 receptor-mediated relaxation in the mouse ileum. However, it is emphasized that the experimental design of the present study focused on examining the contractile responses with an attempt to minimize any relaxation responses to 5-HT. Thus, the lack of relaxation to 5-HT in the presence of methysergide and atropine cannot definitely exclude an inhibitory role for the 5-HT3 receptor in ileal sections of the mouse intestine.
The antagonism exhibited by higher concentrations of the 5-HT3 receptor antagonists in the present study were unsurmountable with a rightward shift accompanied by a progressive decrease in the maximum response. Such an unsurmountable antagonism makes the potency estimation of the antagonist difficult. However, the unsurmountable antagonism was expected as similar observations have been observed in most 5-HT3 receptor-mediated responses including chronotopic response in rabbit isolated heart (Fozard, 1984a; Sanger & Nelson, 1989), von Bezold-Jarish response (Fozard, 1984a), depolarization of primary afferent and sympathetic neurones (Azami et al., 1985), depolarization of rat vagus nerves (Ireland & Tyers, 1987) and the contractile responses in the guinea-pig ileum (Butler et al., 1988). The reason for the unsurmountable blockade of 5-HT3 receptor-mediated responses with such highly selective 5-HT3 receptor antagonists is not fully understood and awaits further understanding of the structural and functional properties of 5-HT3 receptors. Nevertheless, in agreement with the previous findings (Hoyer et al., 1994), the lower concentration of 5-HT3 receptor antagonists produced an approximate parallel shift of the contraction with no significant reduction in the maximum response; apparent pKB values were estimated using these concentrations to compare antagonist potency.
The potency of all four 5-HT3 receptor antagonists in the mouse ileum were found to be substantially (>1 to 2 log units) higher than that on guinea-pig tissues including the ileum, colon and vagus (Butler et al., 1990; Hoyer et al., 1994) but broadly in line with potencies reported in the rabbit and rat isolated vagus nerves, rabbit heart and nodose ganglion and mouse NIE-115 cell lines (Hoyer et al., 1994). m-Chlorophenylbiguanide and 1-phenylbiguanide were observed to be full agonists in the mouse ileum. These agonists are ineffective in the guinea-pig tissues but have been shown to have agonist activity at other 5-HT3 receptor preparations (Bradley et al., 1986; Hoyer et al., 1994). These data indicate that the agonist and antagonist profile of the 5-HT3 receptor ligands in the mouse ileum is different from that in guinea-pig tissues, including the ileum. Thus, the results further support the existence of species differences in the properties of 5-HT3 receptor and possibility of different 5-HT3 receptor subtypes. Molecular biological studies should help clarify the existence of 5-HT3 receptor subtypes. A difference in the potency of the 5-HT3 receptor ligands between the 5-HT3 receptors in cortex and ileum of the CD-1 strain of mouse has also been previously reported in radioligand binding studies, suggesting a possibility of tissue differences in 5-HT3 receptors within species (Bonhaus et al., 1993). The rank order of agonist potency and antagonist pKB values obtained in the present study in the mouse ileum generally matched (more closely) with the reported affinity values for the cortex of the CD-1 mouse rather than the values for the ileum (Bonhaus et al., 1993). Although the quantitative differences observed in the affinities of 5-HT3 receptor ligands in the CD-1 mouse ileum and the present study could be a result of many factors including the strain differences (Bonhaus et al., 1993), nevertheless, our data does not provide support to possible tissue differences in 5-HT3 receptor in this species.
In conclusion, the present study demonstrates the presence of a neuronally located 5-HT3 receptor-mediating contractile response in the mouse ileum and provides a potency estimate for major 5-HT receptor agonists and antagonists. The study also supports the existence of species differences in 5-HT3 receptors between the mouse and guinea-pig.
Acknowledgments
The authors wish to thank Glaxo-Wellcome, Novartis and SmithKline Beecham for the gifts of drugs.
Abbreviations
- KH solution
Krebs-Heinseleit solution
- RS 67506
1-(4-amino-5-chloro-2-methoxyphenyl)-3-[1-2methylsulphonylamino)ethyl-4-piperidinyl]-1-propanone hydrochloride
- SB 204070
8-amino-7-chloro(N-butyl-4-piperidyl) methylbenzo-1,4-di-oxan-5-carboxylate hydrochloride
- MDL 72222
tropanyl 3,5-dichlorobenzoate
References
- AZAMI J., FOZARD J.R., ROUND A.A., WALLIS D.I. The depolarizing action of 5-hydroxytryptamine on rabbit vagal primary afferent and sympathetic neurons and its selective blockade by MDL-72222. Naunyn-Schmiedebergs Arch. Pharmacol. 1985;328:423–429. doi: 10.1007/BF00692911. [DOI] [PubMed] [Google Scholar]
- BONHAUS D.W., WONG E.H.F., STEFANICH E., KUNYSZ E.A., EGLEN R.M. Pharmacological characterization of 5-hydroxytryptamine3 receptors in murine brain and ileum using the novel radioligand [3H]RS-42358- 197: Evidence for receptor heterogeneity. J. Neurochem. 1993;61:1927–1932. doi: 10.1111/j.1471-4159.1993.tb09835.x. [DOI] [PubMed] [Google Scholar]
- BRADLEY P.B., ENGEL G., FENIUK W., FOZARD J.R., HUMPHREY P.P.A., MIDDLEMISS D.N., MYLECHARANE E.J., RICHARDSON B.P., SAXENA P.R. Proposals for the classification and nomenculture of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986;25:563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- BUTCHER M.E. Global experience with ondansetron and future potential. Oncology. 1993;50:191–197. doi: 10.1159/000227176. [DOI] [PubMed] [Google Scholar]
- BUTLER A., ELSWOOD C.J., BURRIDGE J., IRELAND S.J., BUNCE K.T., KILPATRICK G.J., TYRES M.B. The pharmacological characterisation of 5-HT3 receptors in three isolated preparations derived from guinea-pig tissues. Br. J. Pharmacol. 1990;101:591–598. doi: 10.1111/j.1476-5381.1990.tb14126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTLER A., HILL J.M., IRELAND S.J., JORDAN C.C., TYERS M.B. Pharmacological properties of GR38032F, a novel antagonist at 5-HT3 receptors. Br. J. Pharmacol. 1988;94:397–412. doi: 10.1111/j.1476-5381.1988.tb11542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMILLERI M., MAYER E.A., DROSSMAN D.A., HEATH A., DUKES G.E., MCSORLEY D., KONG S., MANGEL A.W., NORTHCUTT A.R. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment. Pharmacol. Ther. 1999;13:1149–1159. doi: 10.1046/j.1365-2036.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- CHAHL L.A. Substance P mediates atropine-sensitive response of guinea-pig ileum to serotonin. Eur. J. Pharmacol. 1983;87:485–489. doi: 10.1016/0014-2999(83)90090-0. [DOI] [PubMed] [Google Scholar]
- COSTALL B., NAYLOR R.J. Neuropharmacology of 5-HT3 receptor ligands. Handbook of Experimental Pharmacology 1997Berlin: Springer; 409–438.ed. Baumgarten, H.G. & Gothert, M. pp [Google Scholar]
- DERKACH V., SURPRENANT A., NORTH R.A. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- DRAKONTIDES A.B., GERSHON M.D. 5-Hydroxytryptamine receptors in the mouse duodenum. Br. J. Pharmacol. 1968;33:480–492. doi: 10.1111/j.1476-5381.1968.tb00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOZARD J.R. MDL 72222: a potent and highly selective antagonist at neuronal 5-hydroxytryptamine receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1984a;326:36–44. doi: 10.1007/BF00518776. [DOI] [PubMed] [Google Scholar]
- FOZARD J.R. Neuronal 5-HT receptors in the periphery. Neuropharmacology. 1984b;23:1473–1486. doi: 10.1016/0028-3908(84)90091-1. [DOI] [PubMed] [Google Scholar]
- FOZARD J.R.Agonists and antagonists of 5-HT3 receptors Cardiovascular Pharmacology of 5-Hydroxytryptamine 1990Kluwer: Dordrecht, The Netherlands; 101–115.ed. Saxena, P.R., Wallis, D.I., Wouters, W. & Bevan, P. pp [Google Scholar]
- GADDUM J.H., PICARELLI Z.P. Two kinds of tryptamine receptor. Br. J. Pharmacol. 1957;12:323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P.A. VII. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- IRELAND S.J., TYERS M.B. Pharmacological characterization of 5-hydroxytryptamine-induced depolarization of the rat isolated vagus nerve. Br. J. Pharmacol. 1987;90:229–238. doi: 10.1111/j.1476-5381.1987.tb16844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILPATRICK G.J., JONES B.J., TYERS M.B. Identification and distribution of 5-HT3 receptor in rat brain using radioligand binding. Nature. 1987;330:746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- LOVINGER D.M. Inhibition of 5-HT3 receptor-mediated ion current by divalent metal-cations in NCB-20 neuroblastoma-cells. J. Neurophysiol. 1991;66:1329–1337. doi: 10.1152/jn.1991.66.4.1329. [DOI] [PubMed] [Google Scholar]
- MCLEAN P.G., COUPAR I.M. Characterisation of a postjunctional 5-ht7-like and a prejunctional 5-HT3 receptor mediating contraction of rat isolated jejunum. Eur. J. Pharmacol. 1996;312:215–225. doi: 10.1016/0014-2999(96)00456-6. [DOI] [PubMed] [Google Scholar]
- NEWBERRY N.R., CHESHIRE S.H., GILBERT M.J. Evidence that the 5-HT3 receptors of the rat, mouse and guinea-pig superior cervical-ganglion may be different. Br. J. Pharmacol. 1991;102:615–620. doi: 10.1111/j.1476-5381.1991.tb12221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON B.P., ENGEL G. The pharmacology and function of 5HT3 receptors. Trends. Neurosci. 1986;9:424–428. [Google Scholar]
- ROUND A., WALLIS D.I. The depolarizing action of 5-hydroxytryptamine on rabbit vagal afferent and sympathetic neurons in vitro and its selective blockade by ICS 205-930. Br. J. Pharmacol. 1986;88:485–494. doi: 10.1111/j.1476-5381.1986.tb10227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER G.J., NELSON D.R. Selective and functional 5-hydroxytryptamine3 receptor antagonism by BRL 43694 (granisetron) Eur. J. Pharmacol. 1989;159:113–124. doi: 10.1016/0014-2999(89)90695-x. [DOI] [PubMed] [Google Scholar]
- SCEARCELEVIE K., CHEN J.P., GARDNER E., HEN R. 5-HT receptor knockout mice: Pharmacological tools or models of psychiatric disorders. Ann. NY Acad. Sci. 1999;868:701–715. doi: 10.1111/j.1749-6632.1999.tb11350.x. [DOI] [PubMed] [Google Scholar]
- SEPULVEDA M.I., LUMMIS S.C.R., MARTIN I.L. The agonist properties of m-chlorophenylbiguanide and 2-methyl-5-hydroxytryptamine on 5-HT3 receptors in N1E-115 neuroblastoma-cells. Br. J. Pharmacol. 1991;104:536–540. doi: 10.1111/j.1476-5381.1991.tb12464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARK K.L., OOSTING R.S., HEN R. Inducible knockout strategies to probe the functions of 5-HT receptors. Ann. NY Acad. Sci. 1998;861:57–66. doi: 10.1111/j.1749-6632.1998.tb10173.x. [DOI] [PubMed] [Google Scholar]
- WAEBER C., HOYER D., PALACIOS J.M. 5-Hydroxytryptamine3 receptors in the human-brain–Autoradiographic visualization using [3H] ICS 205-930. Neuroscience. 1989;31:393–400. doi: 10.1016/0306-4522(89)90382-5. [DOI] [PubMed] [Google Scholar]
- ZIFA E., FILLION G. 5-Hydroxytryptamine receptors. Pharmacol. Rev. 1992;44:401–458. [PubMed] [Google Scholar]


