Abstract
BAY 12-9566 (4-[4-(chlorophenyl)phenyl]-4-oxo-2S-(phenylthiomethyl) butanoic acid) is a newly developed, synthetic matrix metalloproteinase (MMP) inhibitor (MMPI) that selectively inhibits MMP-2, MMP-3 and MMP-9 isozymes. We study the effect of BAY 12-9566 on inflammation and cartilage destruction in adjuvant-induced arthritis (AA) in rats.
Rats were injected with adjuvant and treated for 21 days with vehicle, Indomethacin or BAY 12-9566. AA was assessed: by measuring arthritic index, paw volume, urinary pyridinoline (Pyr) and deoxypyridinoline (Dpyr); by examining joint inflammation; and by microscopic morphometry of articular cartilages.
Oral treatment of rats for 22 days with 50 mg kg−1 body weight/d BAY 12-9566 showed decreased AA as determined by improvement in body weight gain (P<0.01), arthritic index (P<0.05) and swelling of paws contralateral to the adjuvant injection site (P<0.05). Neutrophil infiltration and collagen degradation were also significantly lower (P<0.01) in this treatment group. Cartilage destruction was successfully suppressed (P<0.01) in rats treated with either 50 mg kg−1 body weight/d BAY 12-9566 or 1 mg kg−1 body weight/d Indomethacin.
These results indicate that BAY 12-9566 successfully suppressed inflammation and cartilage destruction in rats with AA. Moreover, these results also suggested that MMP-2, MMP-3 and MMP-9 are involved in arthritic diseases such as rheumatoid arthritis.
Keywords: Matrix metalloproteinase inhibitor, adjuvant induced-arthritis
Introduction
There is now a considerable body of evidence indicating that matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases, are involved in mediating connective-tissue breakdown in a variety of diseases, including tumour invasion and metastasis, rheumatoid arthritis (RA), osteoarthritis, reactive arthritis, periodontal disease, local and systemic bone destruction, vesicular or ulcerative skin disease, and corneal ulcers (Krane, 1982; Shima et al., 1992; Okada et al., 1987; Aiba et al., 1996).
BAY 12-9566 (4-[4-(chlorophenyl)-phenyl]-4-oxo-2S-(phenylthiomethyl) butanoic acid, Bayer, West Haven, CT, U.S.A.) (Figure 1) is a newly developed MMP inhibitor (MMPI) that potently and selectively inhibits MMP-2, MMP-3 and MMP-9 in vivo (Gatto et al., 1998). In vitro, the inhibition constant of BAY 12-9566 is 11 nM for MMP-2, 301 nM for MMP-9, 134 nM for MMP-3 and greater than 5000 nM for MMP-1. Gelatinase MMP-2 and MMP-9 digest type IV collagen, type V collagen and denatured protein, and act in concert with collagenases to digest type 1 collagen (Okada et al., 1990; 1992). MMP-3 (stromelysin) catalyses degradation of fibronectin, glycoproteins and proteoglycans, cartilage components and collagen type II (Wilhelm et al., 1993). BAY 12-9566 inhibits tumour cell invasion in vitro, and angiogenesis in the in vivo (Gatto et al., 1998). In addition, phase I clinical trials and pharmacological study indicate that oral administration of BAY 12-9566, over a variety of different dosing regimens, is well tolerated (Rowinsky et al., 2000).
Figure 1.
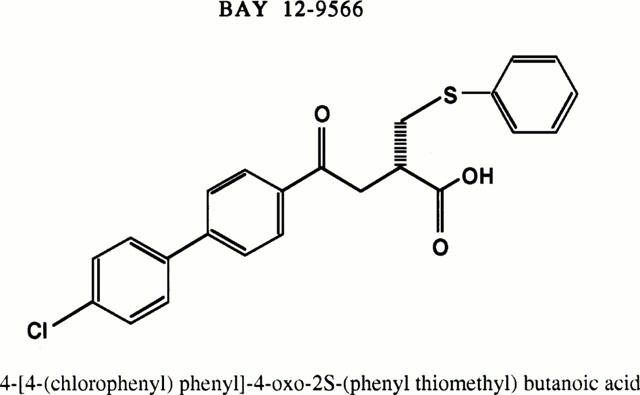
Chemical structure of BAY 12-9566.
Elevated MMP activity is implicated in the pathogenesis of RA in humans and inflammatory joint disease in animal models (Ahrens et al., 1996; Hanemaaijer et al., 1997). Thus, many recent studies have focused on the use of MMPIs to suppress inflammation and cartilage destruction. In this study, we examined the ability of BAY 12-9566 to inhibit adjuvant arthritis (AA) in rats.
Methods
Induction of AA
Seventy inbred male Sprague-Dawley (SD) rats (5 weeks of age, 114–130 g, Charles River Japan, Tokyo, Japan) were given standard rat chow (CE-2, Japan Clea, Tokyo, Japan) and water ad libitum. After observation for 1 week, 60 rats with no remarkable differences in health status and paw volume were selected. Adjuvant solution was prepared by suspending 0.6 mg of heat-killed Mycobacterium butyricum (Lot 84383JA, DIFCO, Detroit, MI, U.S.A.) in 0.05 ml liquid paraffin (Lot DE10, Miyazawa Pharmaceuticals, Tokyo, Japan). Adjuvant solution was injected subcutaneously at the tibiotalar joint of the right hind foot on day 1. AA induction was based on the method of Bonnet et al. (1993).
Administration of MMPI
BAY (Bayer, West Haven, CT, U.S.A.) solutions (0.03, 0.1 and 0.5% w v−1) were prepared by dissolving the drug in carboxymethylcellulose-sodium (CMC, Lot PDJ0188, Wako chemicals, Tokyo, Japan). Six groups of rats in total (10 per group) were injected with either liquid paraffin or adjuvant and treated daily for 21 days with either CMC solution, Indomethacin (IND; lot 26H0807, Sigma, St. Louis, MO, U.S.A.) at a dose of 1 mg kg−1 body weight, or BAY 12-9566 at doses of either 3, 10 or 50 mg kg−1 body weight. Treatments were administered orally at 10 ml kg−1 body weight.
Assessment of AA
Body weight and paw volume were measured 1 h after BAY 12-9566 treatment on day 0 (the day before adjuvant injection), and on days 1, 3, 5, 8, 11, 14 and 18, and immediately after urine sampling on day 22. Changes in hindpaw volume were determined by plethysmography. In brief, each hind paw was immersed vertically to the level of the lateral malleolus into pre-weighed, detergent-filled plastic tubes. The displacement volume was determined by calculating the difference in tube weight before and after immersing the paw, and the newly measured volume was shown as a percentage of the day 0 volume. The difference between day 0 values and the new volume was defined as the changes in hindpaw volume.
Score ranging from 0 (no observable erythema or swelling) to 4 (severe swelling and erythema) was given for the left hindpaw, both forepaws, the ear and the tail of each animal, yielding a maximum possible score of 20 on days 14 and 22 after treatment with adjuvant. Total score was defined as the secondary inflammation index (arthritic index). Urine was collected for 24 h with a metabolic cage on day 21. Concentrations of Pyr and Dpyr were estimated by high-performance liquid chromatography (Tordjman et al., 1994) and expressed as pM μM−1 creatinine.
Pathological evaluation of AA
Animals were sacrificed on day 22 after general anaesthesia by inhalation of ethyl ether, and both adrenal glands, the thymus and the spleen were dissected and weighed. Both knees and both hindpaws were fixed with 10% formalin solution and decalcified with 5% (v v−1) formic acid and 5% (v v−1) formalin solution. Paraffin sections (3 μM) of the tissues were prepared and observed with hematoxylin and eosin (H&E), and Alcian blue (AB; 8GX, C. I. 74240, Nakarai Chemicals Ltd, Tokyo, Japan) stainings (Lillie, 1977). Sections stained with H&E were evaluated histopathologically for AA and scored from 0 (no detectable change), 1 (slight change), 2 (mild change), 3 (moderate change), 4 (remarkable change) to 5 (severe change) for each of the following features: bone erosion, infiltration of osteoclasts (OCs), bone remodelling, destruction of cartilage, pannus formation, oedema, synovial proliferation, synovial necrosis, infiltration of polymorphonuclear leukocytes (PMNs), infiltration of lymphocytes and joint-capsule fibrosis. Histopathological parameters were scored by two surgical pathologists (TH and NA).
Microscopic morphometry of cartilage destruction
Areas of damaged articular cartilage were determined by computer-aided image analysis of micrographs (IBAS, Kontron Electronics, Munich, Germany) of AB sections taken at a magnification of 1.25× with a yellow filter. Input and analysis of cartilage images were performed with software designed to determine automatically or manually the AB-positive area and whole area of the articular plate and to exclude the areas of soft tissue outside the head of the bone. Data are expressed as per cent cartilage area (%CA), which is defined as the positive area divided by the total area of the cartilage cap and multiplied by 100.
Data analysis
Differences between histopathological evaluation scores, %CA and urinary Pyr and Dpyr values were statistically evaluated by one-way analysis of variance and Dunnett's multiple comparisons test. Distribution equality of all other data was determined by Bartlett's test. If the data were distributed equally, and if analysis of variance indicated significant differences between treatment groups, each group was compared to the untreated adjuvant group by one-paired Dunnett's test. If the data were unequally distributed, Dunnett's analysis was done after a Kruskal–Wallis test (Armitage, 1977).
Results
Assessment of AA
Secondary (immunological) inflammation
The arthritis indices of the adjuvant-injected rats receiving either no treatment, 3 mg, or 10 mg BAY 12-9566 kg−1 body weight increased from 6.8 on treatment day 14 to 13 on treatment day 22 and were not significantly different. In all treatment groups, the indices of rats treated with 50 mg BAY 12-9566 kg−1 body weight of IND were significantly lower than the indices in untreated adjuvant-injected rats (P<0.05). Between these three groups, no differences in arthritis indices were detected at day 14 (Figure 2).
Figure 2.
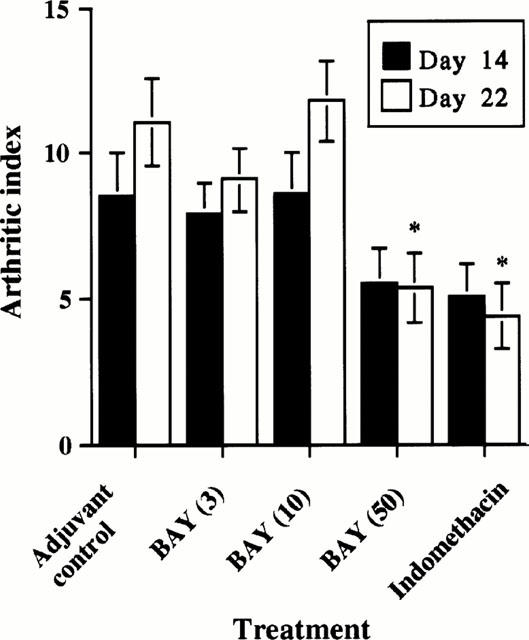
Arthritic indices of control, adjuvant control, BAY 12-9566-treated and Indomethacin-treated rats. The arthritic index in rats with adjuvant arthritis was significantly lowered to about 50% on day 22 by BAY 12-9566 (50 mg kg−1 d−1) treatment, compared with that in adjuvant controls. This effect was similar to that of Indomethacin. (Error bar: standard error).
Paw volume
Plethysmographic estimation demonstrated that the right hindpaws (adjuvant-injected sites) in groups receiving no treatment, 3 mg, or 10 mg BAY 12-9566 kg−1 body weight, swelled markedly 1 day after injection and remained swollen through day 22 (Figure 3). In adjuvant-injected rats, paw swelling was biphasic; acutely increasing from day 1 to 8, and gradually increasing thereafter. Although paws of rats treated with BAY at 50 mg kg−1 body weight were less swollen than those of rats receiving either no treatment, 3 mg, or 10 mg BAY 12-9566 kg−1 body weight, swelling between these treatment groups did not differ significantly. Paw swelling in rats treated with IND was significantly less than that in untreated, adjuvant-injected rats from day 5 to 22 (P<0.01).
Figure 3.
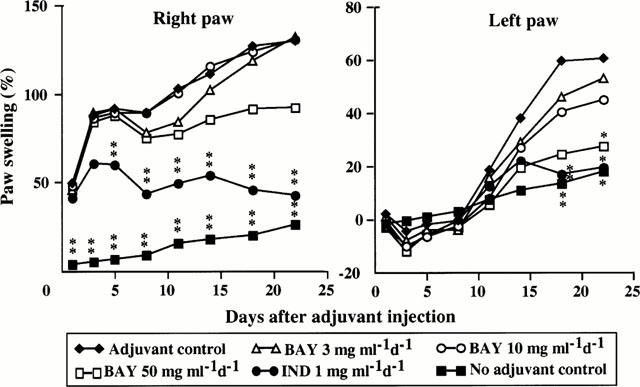
Changes of right and left hindpaw volumes in control, adjuvant control, BAY 12-9566-treated and Indomethacin-treated rats. BAY 12-9566 (50 mg kg−1 d−1) treatment tended to reduce the right hindpaw volume, but the effect was not significant compared with that in the adjuvant controls. Indomethacin significantly lowered the right hindpaw volume from day 5 to day 22. The left hindpaw volumes in rats that received 50 mg kg−1 d−1 of BAY 12-9566 significantly decreased to about 50%, as did those in Indomethacin-treated rats, compared with those of the adjuvant controls.
No swelling of the left contralateral paw was detected in any treatment group through day 14 (Figure 3). Swelling of the left hind paw was first detectable in all adjuvant-injected rats on treatment day 11, and significantly less swelling was detected in rats treated with 50 mg BAY 12-9566 kg−1 body weight on day 22 (P<0.05), and in rats treated with IND on days 18 (P<0.01) and 22 (P<0.05). No significant differences in paw volumes was detected between rats receiving either no treatment, 3 mg, 10 mg BAY 12-9566 kg−1 body weight. However, paw swelling tended to be less in rats receiving BAY 12-9566 after day 11.
Body and organ weights
Body weights of rats treated with BAY 12-9566 (50 mg kg−1 body weight) and IND were significantly greater than weights of adjuvant-injected rats receiving no treatment on days 18 (P<0.05) and 22 (P<0.01). Moreover, growth rates of rats treated with either 50 mg BAY 12-9566 per kg body weight or IND were better than growth rates of untreated adjuvant-injected rats. Body weight of rats that were untreated and of those treated with BAY 12-9566 at either 3 mg or 10 mg kg−1 body weight were not significantly different. No significant differences in ratios of thymus weight and body weight were detected between any groups. The ratio of spleen to body weight of adjuvant-injected rats receiving either no treatment or BAY 12-9566 treatments was significantly greater than vehicle-injected rats at day 22 (P<0.01), and spleen-to-body weight ratios of adjuvant-injected rats treated with IND were lower than those of untreated, adjuvant-injected rats (data not shown). The ratio of adrenal gland weight to body weight was slightly but not significantly greater in rats treated with BAY 12-9566 at 3, 10 or 50 mg kg−1 body weight than in adjuvant-injected untreated rats. The ratio of adrenal gland-to-body weight ratio of rats treated with IND was significantly less (P<0.01) than that of untreated adjuvant-injected rats and nearly equal to that of CMC-injected, untreated rats (data not shown). The rats treated by BAY 12-9566 showed neither gastrointestinal symptoms nor remarkable macroscopic changes in the digestive tract.
Histopathological evaluation of AA
Histopathological findings
No inflammation or tissue destruction was detected in H&E sections from untreated CMC-injected rats (Figure 4A). In contrast, H&E sections of the right tibiotalar joints of untreated adjuvant-injected rats revealed prominent destructive inflammation of articular bone tissue and extra-articular tissue (Figure 4B). Thinning of the cartilage plate the talus head was evident in the H&E sections (Figure 4B). However, in rats treated with either 50 mg BAY 12-9566 kg−1 body weight of IND, articular inflammation was successfully suppressed (Figure 4C,D). Alcian blue staining revealed that treatment of rats with BAY 12-9566 (Figure 5C) at 50 mg kg−1 body weight or IND (Figure 5D) slowed cartilagenous cap matrix loss due to adjuvant injection (Figure 5B). Although treatment of adjuvant-injected rats with BAY 12-9566 at 10 mg kg−1 body weight slightly slowed matrix loss in cartilaginous cap, the loss was not significantly different than matrix loss in the untreated adjuvant-injected rats.
Figure 4.
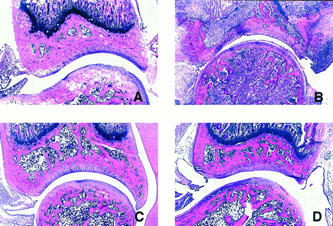
Histopathologic changes of left tibiotalar joints of non-adjuvant control (A), adjuvant control (B), BAY 12-9566-treated (C), and Indomethacin-treated (D) rats. Destructive inflammation was remarkable in the tibiotalar joints of the adjuvant controls (B). This section shows marked inflammatory infiltrate in both synovial and bone tissues, destruction of bone trabeculae, thinning of the articular plate and new bone formation. The inflammation was suppressed in the joints of BAY 12-9566-treated rats (C) as well as in the joints of Indomethacin-treated rats (D), compared with the controls (A). (Hematoxylin and eosin staining, ×14.1).
Figure 5.
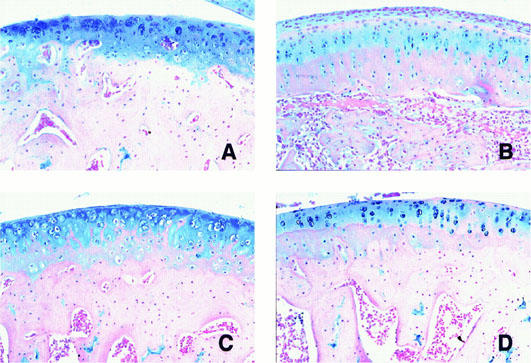
Histopathologic changes of cartilagenous cap of left tibial bone head. Alcian blue staining demonstrated that cartilagenous cap of non-adjuvant control (A) had abundant matrix positively stained with the dye. In adjuvant controls (B), decrease of Alcian blue positive areas was markedly reduced associated with pannus formation around the cartilagenous tissue. The destruction of the cartilagenous cap was improved in BAY 12-9566 treated (C) and IND treated rats (D).
Histological scores
In the right tibiotalar joint, no statistical differences were detected in histological scores for bone erosion, pannus formation, oedema, synovial proliferation, or infiltration of lymphocytes between adjuvant-injected rats that received either no treatment or BAY 12-9566. Synovial necrosis (P<0.05), infiltration of PMNs (P<0.01) and capsular fibrosis (P<0.05) were significantly less in rats treated with 50 mg BAY 12-9566 kg−1 body weight than in untreated, adjuvant-injected rats. Bone remodelling tended to be suppressed by BAY 12-9566 at 50 mg kg−1 body weight. Bone erosion, osteoclast infiltration (P<0.01), bone remodelling (P<0.01), cartilage destruction (P<0.01), pannus formation (P<0.01), oedema (P<0.05) and synovial necrosis (P<0.01) were significantly less in IND-treated rats than in untreated adjuvant-injected rats. In contrast to rats treated with 50 mg BAY 12-9566 kg−1 body weight, PMN infiltration and capsular fibrosis in IND-treated rats were not significantly different than that in untreated, adjuvant-injected rats. In the left tibiotalar joint of rats treated with 50 mg BAY 12-9566 kg−1 body weight and IND, only PMN infiltration was significantly less than that of untreated adjuvant-injected rats (P<0.05) (Figure 6).
Figure 6.
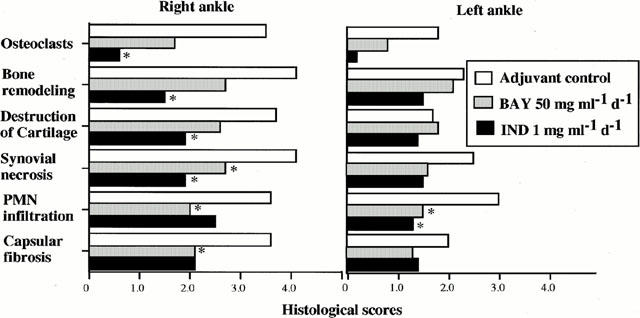
Histological scores of bilateral tibiotalar joints of control, adjuvant control, BAY 12-9566-treated and Indomethacin-treated rats. In the right tibiotalar joint (the adjuvant-injected site), BAY 12-9566 significantly inhibited synovial necrosis and PMN infiltration, whereas Indomethacin suppressed osteoclast infiltration, bone remodelling, cartilage destruction synovial necrosis and capsular fibrosis. In the left tibiotalar joint (contralateral to the site of adjuvant injection), PMN infiltration was significantly lowered by both BAY 12-9566 and Indomethacin treatment.
In the right knee joint of rats treated with 50 mg BAY 12-9566 kg−1 body weight and IND, only cartilage destruction was significantly (P<0.01) less than that of untreated adjuvant-injected rats (data not shown). In the left knee joint, infiltration of osteoclasts (P<0.05) in rats treated with BAY 12-9566 at 50 mg kg−1 body weight, and cartilage destruction (P<0.05), pannus formation (P<0.05), oedema (P<0.05), synovial necrosis (P<0.05) and lymphocytic infiltration (P<0.01) in rats treated with IND were significantly less than such in untreated adjuvant-injected rats (P<0.05) (data not shown). Thus, daily administration of BAY 12-9566 at 50 mg kg−1 body weight showed characteristic suppression of PMN infiltration and capsular fibrosis at the injection site in AA; these effects were not seen in the IND-treated rats. In contrast, daily administration of IND at 1.0 mg−1 kg body weight had other broad and potent effects at the injection site.
Microscopic morphometry of cartilage destruction
Microscopic morphometry of cartilage revealed that the %CA in the distal head of the right tibia (at the adjuvant injected site), the distal head of the right femur, the proximal head of the right tibia and the distal head of the left femur of rats treated with either IND or 50 mg BAY 12-9566 kg−1 body weight was significantly higher than that of untreated adjuvant-injected rats. The inhibitory effect of BAY at 50 mg kg−1 body weight on destruction of cartilagenous caps in AA was similar to that of IND at 1.0 mg kg−1 body weight (Figure 7). No significant differences in articular cartilage were detected between the treatment groups.
Figure 7.
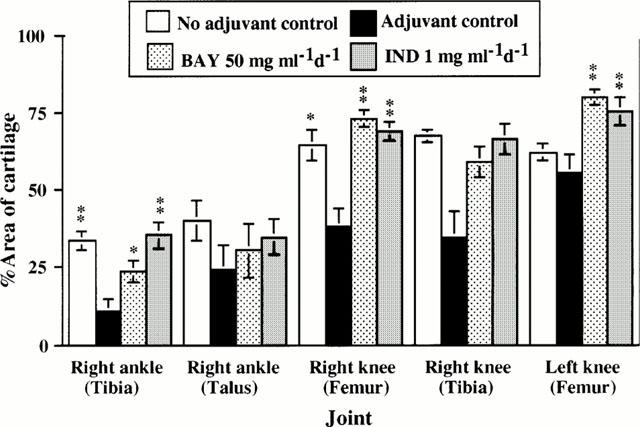
Histological morphometric analysis of articular cartilages of joints of non-adjuvant control, adjuvant control, BAY 12-9566-treated, and Indomethacin-treated rats. The per cent area of articular cartilage in adjuvant controls was significantly reduced in the right peripheral tibia, right peripheral femur and left peripheral femur compared with that in the control joints. BAY 12-9566 (50 mg kg−1 d−1) treatment suppressed the destruction of these articular cartilages, as did Indomethacin treatment. The per cent areas of these cartilages were as well preserved as those of the control. (Error bar: standard error).
Urinary pyridinoline and deoxypyridinoline levels
Levels of urinary Pyr and Dpyr in untreated adjuvant-injected rats steadily increased during the 21-day treatment period to twice that of the untreated CMC-injected (control) rats. Urinary Pyr and Dpyr levels were significantly (P<0.01) less in rats treated with either IND or BAY 12-9566 50 mg kg−1 body weight than in untreated adjuvant-injected rats (Figure 8). The Pyr Dpyr−1 ratio was 1.12 in untreated CMC-injected rats, 1.31 in untreated adjuvant-injected rats, 1.32 in rats treated with BAY 50 mg kg−1 body weight, and 1.22 in rats treated with IND. None of these ratios differed significantly.
Figure 8.
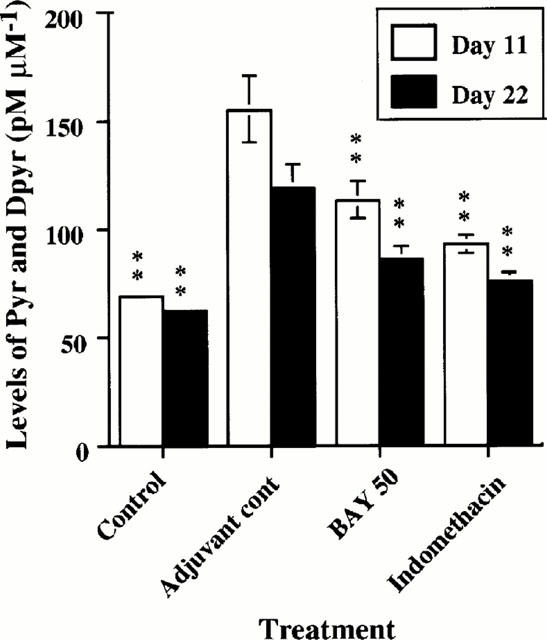
Levels of urinary pyridinoline (Pyr) and deoxypyridinolin (Dpyr) in control, adjuvant control, BAY 12-9566-treated and Indomethacin-treated rats. Levels of urinary Pyr and Dpyr were significantly decreased in groups treated with BAY 12-9566 (50 mg kg−1 d−1) and Indomethacin compared with adjuvant controls. (Error bar: standard error).
Discussion
MMPIs prevent matrix degradation in vitro. These MMPIs include Ro 32-3555 (Wood et al., 1998), TIMP, TIMP-2, BB87 (Ellis et al., 1994) and U24522 (Doughty et al., 1993), and possess varying anti-degradation activity for proteoglycans and collagens. MMPIs were recently tested for anti-arthritic activity in a unique osteoarthritis model in rats, which involved measuring degradation of subcutaneously-implanted femoral head cartilage from a donor rat (Karran et al., 1995). MMPI MI-1 reproducibly reduced proteoglycan and collagen degradation of implanted, donor femoral head cartilage. Conway et al. (1985) reported that continuous infusion into rats of the synthetic MMPI GI168 via an implanted mini-pump significantly decreased AA. Orally administered MMPI CMT-1 acts synergistically with other drugs to improve AA (Leung et al., 1995). However, in a phase I clinical trial, oral administration of high doses of the synthetic, broad spectrum MMPI Marimastat caused polyarthritis in patients with advanced lung cancer (Wojtowicz-Paraga et al., 1998). Thus, it seems likely that an imbalance in activities of MMPI isoenzymes can result in destruction of cartilage, which may lead to arthritis. Moreover, these findings suggest that articular tissue metabolism is tightly regulated by MMPs and naturally occurring MMPIs (Dean et al., 1989).
The matrix in articular cartilage comprises 68–78% water, 13.5–18% type II collagen, 7–10% proteoglycan, and 1–5% types IX, X and XI collagens. Between the larger collagen fibres that form the arcades (Benninghoff arcades) is a meshwork of smaller collagen and proteoglycan molecules. The earliest recognizable histological change in osteoarthritis is fraying of the superficial cartilagenous matrix zone along the lines of the Benninghoff arcades (Poole et al., 1984). Alcian blue, which stains articular cartilage, is a dye that stains acid mucopolysaccharides sulfomucin and carboxylmucin at pH 2.5 (Hronowski & Anastassiades, 1988). Acid mucopolysaccharides are major components of proteoglycans and the second most abundant constituent of cartilagenous matrix. Therefore, decrease in Alcian blue positive areas directly indicates degradation of proteoglycan, which is one component of the meshwork in the cartilage matrix (Bjornsson, 1993). Urinary Pyr and Dpyr levels were significantly lower in BAY- and IND-treated rats than untreated adjuvant-injected rats. Pyr and Dpyr are degradation products associated with cross-linkage of collagen macromolecules and, therefore, biochemical markers for destruction of cartilage and bone (Uebelhart et al., 1990). Our results indicate that BAY indirectly inhibits degradation of collagen, which is a major component of the meshwork of bone and cartilage matrix. BAY inhibits only MMP-2, MMP-3 and MMP-9. MMP-1 and MMP-8 directly degrade collagen types 1 and type II, respectively. Collagen degradation may be associated in part with the metabolism of proteoglycan macromolecules.
In addition to quantitative findings, our histological observations revealed that BAY (50 mg kg−1 d−1) inhibited PMN infiltration in both the adjuvant-injected paw and the contralateral paw. BAY was originally developed as an inhibitor of MMP-2, MMP-3 and MMP-9. Although BAY is likely to protect cartilage matrix by inhibiting these MMPIs, it may also directly or indirectly inhibit PMN infiltration of cartilage. PMNs may activate pro-MMPs MMP-1 and MMP-3 by secreting proteinases, and PMN may secrete MMP-8 (Santos et al., 1997). Moreover, anti-neutrophil monoclonal antibody therapy inhibits the development of AA in rats by depleting circular neutrophils. Therefore, inhibition of PMN and neutrophil infiltration by BAY 12-9566 is likely to block cartilage destruction by MMPs that are not directly inhibited by BAY. In contrast to the inhibitory effect of BAY 12-9566 on PMN infiltration, inspection of tissue sections stained with H&E revealed a slight but insignificant decrease in cartilage destruction in rats treated daily with BAY 12-9566 at 50 mg kg−1 body weight. In contrast, staining with AB revealed significant inhibition of cartilage destruction by BAY treatment. This discrepancy is likely to be due to false negative findings with semi-quantitative H&E staining method and the more sensitive, quantitative, and less arbitrary character of the AB staining.
Paw swelling at the injection site was significantly suppressed by IND but not by BAY. This indicates that BAY has a mild effect on acute AA in the injected paw (Walz et al., 1971). In the contralateral paw, swelling was significantly suppressed by BAY 12-9566 (50 mg kg−1 d−1) and was similar to that of IND treated rats. These results indicate that BAY 12-9566 is effective for treatment of immunologic and chronic inflammation that occurs in joints without adjuvant injection. Arthritic index was markedly improved in rats treated daily with BAY 12-9566 at 50 mg kg−1 body weight or with IND, and decreased to about 60% of the arthritic index of untreated adjuvant-injected rats on day 22. Thus, BAY inhibits chronic and immunologic inflammation associated with arthritis. Moreover, the general condition of adjuvant-injected rats was generally better when treated daily with BAY 50 mg kg−1 body weight or IND, as evidenced by increased body weight. Immunologic or inflammatory reaction produced by injection of rats with adjuvant as evidenced by greater spleen and adrenal gland weight was greater than in BAY 12-9566 treated rats than in IND-treated rats.
Many anti-inflammatory agents are available clinically for controlling pain and swelling due to osteoarthritis. However, in chronic osteoarthritis, for example, rheumatoid arthritis, it is difficult to prevent destruction of cartilage and bone of the inflamed joint and deformation and ankylosis can result. In our study, morphometric analysis and histologic observation revealed that BAY 12-9566 treatment potently inhibits destruction of cartilage in secondary arthritis. Our morphological data are supported by biochemical data quantitating the effects of BAY 12-9566 on cartilage destruction with urinary Pyr and Dpyr measurement. BAY 12-9566 is a MMPI that is solely effective for treatment of adjuvant-induced arthritis and can be administered orally. Thus, drugs like BAY are likely to be used clinically to prevent cartilage destruction and attendant functional disorders that result from this destruction.
Abbreviations
- AA
adjuvant-induced arthritis
- AB
alcian blue
- MMP
matrix metalloproteinase
- CMC
carboxymethylcellulose
- %CA
per cent cartilage area
- Dpyr
deoxypyridinoline
- H&E
hematoxylin and eosin
- IND
Indomethacin
- MMPI
matrix metalloproteinase inhibitor
- Pyr
pyridinoline
- RA
rheumatoid arthritis
References
- AHRENS D., KOCH A.E., POPE R.M., STEIN P.M., NIEDBALA M.J. Expression of matrix metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid arthritis. Arthritis. Rheum. 1996;39:1576–1587. doi: 10.1002/art.1780390919. [DOI] [PubMed] [Google Scholar]
- AIBA T., AKENO N., KAWANE T., OKAMOTO H., HORIUCHI N. Matrix metalloproteinases-1 and -8 and TIMP-1 mRNA levels in normal and diseased human gingivae. Eur. J. Oral Sci. 1996;104:562–569. doi: 10.1111/j.1600-0722.1996.tb00142.x. [DOI] [PubMed] [Google Scholar]
- ARMITAGE P.Comparison of several groups Statistical methods in medical research 1977Oxford: Blackwell Scientific Publications; 189–216.ed. Armitage, P. pp [Google Scholar]
- BJORNSSON S. Simultaneous preparation and quantitation of proteoglycans by precipitation with Alcian blue. Anal. Biochem. 1993;210:282–291. doi: 10.1006/abio.1993.1197. [DOI] [PubMed] [Google Scholar]
- BONNET J., ZERATH E., PICAUD N., LESUR C., MATTIO A., TORDJMAN C., HOTT M., MARIE P.J. Bone morphometric changes in adjuvant-induced polyarthritic osteopenia in rats: evidence for an early bone formation defect. J. Bone Mineral Res. 1993;8:659–668. doi: 10.1002/jbmr.5650080603. [DOI] [PubMed] [Google Scholar]
- CONWAY J.G., WAKEFIELD J.A., BROWN R.H., MARRON B.E., SEKUT L., STIMPSON S.A., MCELRY A., MENIUS J.A., JEFFREYS J.J., CLARK R.L. Inhibition of cartilage and bone destruction in adjuvant arthritis in the rat by a matrix metalloproteinase inhibitor. J. Exp. Med. 1995;182:449–457. doi: 10.1084/jem.182.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEAN D.D., MARTEL P.J., PELLETIER J.P., HOWELL D.S., WOESSNER J.J. Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J. Clin. Invest. 1989;84:678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGHTY J.R., GOLDBERG R.L., GANU V., MELTON R.A., HU S.I., DI P.G. A stromelysin assay for the assessment of metalloproteinase inhibitors on human aggregated proteoglycan. Agents Actions. 1993;39:C151–C153. doi: 10.1007/BF01972750. [DOI] [PubMed] [Google Scholar]
- ELLIS A.J., CURRY V.A., POWELL E.K., CAWSTON T.E. The prevention of collagen breakdown in bovine nasal cartilage by TIMP, TIMP-2 and a low molecular weight synthetic inhibitor. Biochem. Biophys. Res. Commun. 1994;201:94–101. doi: 10.1006/bbrc.1994.1673. [DOI] [PubMed] [Google Scholar]
- GATTO C., RIEPPI M., BORSOTTI P., INNOCENTI S., CERUTI R., DRUDIS T., SCANZIANI E., CASAZZA A.M., TARABOLETTI G., GIAVAZZI R. BAY 12-9566, a novel inhibitor of matrix metalloproteinases with antiangiogenic activity. Clin. Cancer Res. 1999;5:3603–3607. [PubMed] [Google Scholar]
- HANEMAAIJER R., SORSA T., KONTTINEN Y.T., DING Y., SUTINEN M., VISSER H., VAN HINSBERGH V.W., HELAAKOSKI T., KAINULAINEN T., RONKA H., TSCHESCHE H., SALO T. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J. Biol. Chem. 1997;272:31504–31509. doi: 10.1074/jbc.272.50.31504. [DOI] [PubMed] [Google Scholar]
- HRONOWSKI L.J., ANASTASSIADES T.P. Detection and quantitation of proteoglycans extracted from cell culture medium and cultured cartilage slices. Anal. Biochem. 1988;174:501–511. doi: 10.1016/0003-2697(88)90050-4. [DOI] [PubMed] [Google Scholar]
- KARRAN E.H., YOUNG T.J., MARKWELL R.E., HARPER G.P. In vivo model of cartilage degradation – effects of a matrix metalloproteinase inhibitor. Ann. Rheum. Dis. 1995;54:662–669. doi: 10.1136/ard.54.8.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRANE S.M. Collagenases and collagen degradation. J. Invest. Dermatol. 1982;79:S83–S86. doi: 10.1111/1523-1747.ep12545849. [DOI] [PubMed] [Google Scholar]
- LEUNG M.K., GREENWALD R.A., RAMAMURTHY N.S., MOAK S.A., KOSZULINSKI R., DIEUDONNE D., GOLUB L.M. Tenidap and flurbiprofen enhance uptake of matrix metalloproteinase inhibitor 4-dedimethylaminotetracycline in inflamed joints of adjuvant arthritic rats. J. Rheumatol. 1995;22:1726–1731. [PubMed] [Google Scholar]
- LILLIE R.D.Phthtalocyanin dyes Biological stains 1977Philadelphia: Williams & Wilkins; 452–457.ed. Lillie, R.D. pp [Google Scholar]
- OKADA Y., GONOJI Y., NAKA K., TOMITA K., NAKANISHI I., IWATA K, YAMASHITA K., HAYAKAWA T. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells. Purification and activation of the precursor and enzymic properties. J. Biol. Chem. 1992;267:21712–21719. [PubMed] [Google Scholar]
- OKADA Y., MORODOMI T., ENGHILD J.J., SUZUKI K., YASUI A., NAKANISHI I., SALVESEN G., NAGASE H. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymic properties. Eur. J. Biochem. 1990;194:721–730. doi: 10.1111/j.1432-1033.1990.tb19462.x. [DOI] [PubMed] [Google Scholar]
- OKADA Y., NAGASE H., HARRIS E.D.J. Matrix metalloproteinases 1, 2, and 3 from rheumatoid synovial cells are sufficient to destroy joints. J. Rheumatol. 1987;14:41–42. [PubMed] [Google Scholar]
- POOLE C.A., FLINT M.H., BEAUMONT B.W. Morphological and function interrelationships of articular cartilage matrices. J. Anat. 1984;1:113–138. [PMC free article] [PubMed] [Google Scholar]
- ROWINSKY E.K., HUMPHREY R., HAMMOND L.A., AYLESWORTH C., SMETZER L., HIDALGO M., MORROW M., SMITH L., GARNER A., SORENSEN J.M., VON HOFF D.D., ECKHARDT S.G. Phase I and pharmacologic study of the specific matrix metalloproteinase inhibitor BAY 12-9566 on a protracted oral daily dosing schedule in patients with solid malignancies. J. Clin. Oncol. 2000;18:178–185. doi: 10.1200/JCO.2000.18.1.178. [DOI] [PubMed] [Google Scholar]
- SANTOS L.L., MORAND E.F., HUTCHINSON P., BOYCE N.W., HOLDSWORTH S.R. Anti-neutrophil monoclonal antibody therapy inhibits the development of adjuvant arthritis. Clin. Exp. Immunol. 1997;107:248–253. doi: 10.1111/j.1365-2249.1997.263-ce1154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMA I., SASAGURI Y., KUSUKAWA J., YAMANA H., FUJITA H., KAKEGAWA T., MORIMATSU M. Production of matrix metalloproteinase-2 and metalloproteinase-3 related to malignant behavior of esophageal carcinoma. A clinicopathologic study. Cancer. 1992;70:2747–2753. doi: 10.1002/1097-0142(19921215)70:12<2747::aid-cncr2820701204>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- TORDJMAN C., LHUMEAU A., PASTOUREAU P., MEUNIER F., SERKIZ B., VOLLAND J.P., BONNET J. Evaluation and comparison of urinary pyridinium crosslinks in two rat models of bone loss–ovariectomy and adjuvant polyarthritis–using a new automated HPLC method. Bone Miner. 1994;26:155–167. doi: 10.1016/s0169-6009(08)80060-8. [DOI] [PubMed] [Google Scholar]
- UEBELHART D., GINEYTS E., CHAPUY M.C., DELMAS P.D. Urinary excretion of pyridinium crosslinks: a new marker of bone resorption in metabolic bone disease. Bone Miner. 1990;8:87–96. doi: 10.1016/0169-6009(91)90143-n. [DOI] [PubMed] [Google Scholar]
- WALZ D.T., DIMARTINO M.J., MISHER A. Adjuvant-induced arthritis in rats. II. Drug effects on physiologic, biochemical and immunologic parameters. J. Pharmacol. Exp. Ther. 1971;178:223–231. [PubMed] [Google Scholar]
- WILHELM S.M., SHAO Z.H., HOUSLEY T.J., SEPERACK P.K., BAUMANN A.P., GUNJA-SMITH Z., WOESSNER J.F.J. Matrix metalloproteinase-3 (stromelysin-1). Identification as the cartilage acid metalloprotease and effect of pH on catalytic properties and calcium affinity. J. Biol. Chem. 1993;268:21906–21913. [PubMed] [Google Scholar]
- WOJTOWICZ-PARAGA S., TORRI J., JOHNSON M., STEEN V., MARSHALL J., NESS E., DICKSON R., SALE M., RASMUSSEN H.S., CHIODO T.A., HAWKINS M.J. Phase I trial of Marimastat, a novel matrix metalloproteinase inhibitor, administered orally to patients with advanced lung cancer. J. Clin. Oncol. 1998;16:2150–2156. doi: 10.1200/JCO.1998.16.6.2150. [DOI] [PubMed] [Google Scholar]
- WOOD N.D., AITKEN M., DURSTON S., HARRIS S., MCCLELLAND G.R., SHARP S. Cartilage protective agent (CPA) Ro 32-3555, a new matrix metalloproteinase inhibitor for the treatment of rheumatoid arthritis. Agents Actions. 1998;49:49–55. doi: 10.1007/978-3-0348-8857-8_8. [DOI] [PubMed] [Google Scholar]


