Abstract
Intracerebral microdialysis was used to examine the function of the terminal 5-hydroxytryptamine1B (5-HT1B) autoreceptor in the region of the suprachiasmatic nuclei (SCN) of freely moving conscious rats at six time points or zeitgeber times (ZTs) across the light:dark cycle.
Infusion of the 5-HT1A/1B agonist 5-methoxy-3-(1,2,3,6-tetrahydro-4-pyridyl)-1H-indole (RU24969) (1 μM) via the microdialysis probe produced a decrease in 5-HT output when applied at ZTs 3, 6, 15 and 21 (69.8±11.9, 59±11.7, 43.9±17.2 and 45.7±17.0% respectively). At ZTs 9 and 18 RU24969 (1 μm) failed to affect the 5-HT output significantly (28.0±11 and 32.8±24.6% decrease respectively).
The profile of inhibition of 5-HT output following infusion of RU24969 (1 μM) at ZT 6 was unaffected by concurrent infusion of the specific 5-HT1A antagonist N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide trihydrochloride (WAY100635) (1 μM) (52.48±17.5% decrease).
The data demonstrate a circadian rhythm in the activity of the 5-HT1B autoreceptor in the region of the SCN.
Keywords: 5-HT1B autoreceptor, 5-HT release, suprachiasmatic nucleus, diurnal rhythm, microdialysis
Introduction
The suprachiasmatic nuclei (SCN) of the hypothalamus generate, control and express circadian rhythmicity in vertebrates (for review, see Hastings, 1997). In turn afferent fibres collectively entrain the circadian pacemaker to environmental cues. Photic input to the SCN via the retinohypothalamic tract constitutes the principal entrainment pathway to the SCN (Johnson et al., 1988; Cahill & Menaker, 1989) and serotonin (5-hydroxytryptamine, 5-HT) released from the terminals of the raphe-hypothalamic tract modulates the response of the SCN to these photic inputs (Morin & Blanchard, 1991; Bradbury et al., 1997).
In the mid 1950s it was first demonstrated that the concentration of 5-HT in the whole brain and in discrete brain regions varied over 24 h (Albrecht et al., 1956). It is now known that almost all aspects of 5-HT turnover demonstrate a significant circadian rhythmicity (for a comprehensive review see Martin & Redfern, 1997) and the amplitude of the rhythm has been shown to be greatest in the SCN (Martin & Marsden, 1985). The circadian rhythm in 5-HT turnover is thought to be generated by the endogenous pacemaker, but is also influenced by the light-dark cycle (Cagampang & Inouye, 1994). Terminal 5-HT autoreceptors in the rat have been classified as 5-HT1B receptors (Engel et al., 1986; Limberger et al., 1991; Trillat et al., 1997), and binding sites for these receptors have been identified in the rodent SCN (Pickard et al., 1996). Since 5-HT serves to modulate photic inputs, and itself demonstrates a circadian rhythm in the SCN, it is possible that a variation in the function of the 5-HT1B autoreceptor contributes to variation in 5-HT synthesis and release, thereby indirectly influencing clock mechanisms within the SCN.
A variation in function of the 5-HT1B autoreceptor in the anterior hypothalamus of anaesthetized rats at two time points during the light phase has recently been reported (Sayer et al., 1999). It was the aim of the current investigation to examine the extent of circadian rhythmicity in the function of the 5-HT1B autoreceptor in the SCN of freely-moving conscious rats using in vivo microdialysis.
Methods
Animals
Male Wistar rats (University of Bath strain; 260–320 g) were maintained under a 12 : 12 h light:dark (LD) cycle for at least 2 weeks before experimentation (lights on 0700 or 1900 h reverse LD); temperature 22±2°C. The animals were housed in groups of six prior to experimentation, with food and water available ad libitum.
Implantation of dialysis probes
Rats were anaesthetized with equithesin (0.3 ml 100 g−1 body weight i.p.). The animals were placed in a stereotaxic frame (incisor bar set at −3.3 mm) and implanted with a concentric microdialysis probe (Gambro membrane, 290 μm o.d., molecular weight cut-off 6000 Da) directed so that the tip lay immediately adjacent to the SCN (2 mm membrane, co-ordinates with reference to Bregma 6° angle, AP −1.3 mm, ML 1.5 mm, and depth −9.7 mm, according to the atlas of Paxinos & Watson, 1982). If the probe were inserted directly into the SCN the nucleus would be obliterated given its size relative to the probe. On the other hand a major proportion of the serotonergic innervation of the region of the hypothalamus that includes the SCN culminates in the SCN itself (Moore et al., 1978). For this reason we chose to locate the probe immediately adjacent to the SCN rather than risk damage to the nucleus. During the dark phase the animals were anaesthetized under dim red light and their eyes covered with black tape during the implantation procedure. The probe was continuously perfused, at a rate of 1.2 μl min−1 (model 22 Microinjection pump, Harvard Apparatus) with a non-buffered solution of artificial cerebrospinal fluid (aCSF) (composition in mM: NaCl 14.7, KCl 4; CaCl2 4, pH 7.4) containing the selective serotonin reuptake inhibitor citalopram (1 μM). The animals were introduced into the experimental cages (round plastic cage, diameter 30 cm; depth 30 cm) equipped with a liquid swivel switch (Biotech Instruments Ltd.) and allowed to recover from surgery for at least 18 h. At the end of each experiment animals were killed and the brain was removed, frozen onto dry ice and sectioned on a cryostat (30 micron sections) for histological verification. Only dialysate samples collected from animals with correctly-positioned probes were included in the analysis. Approximately 15% of all animals were excluded for this reason. Prior to implantation the recovery of 5-HT from the probe in the dialysis solution was checked in vitro at the flow rate used; probes with recoveries of below 10% were discarded.
Experimental protocol
Six 15 min baseline samples were collected into small vials containing 5 μl 0.5 M perchloric acid. Successive 15 min fractions were collected throughout the experiment. For agonist studies the drug was infused via the probe for 15 min, starting immediately after collection of the control samples, and 12 15-min samples were subsequently collected. For antagonist studies, the antagonist was infused alone via the probe for 15 min, starting immediately after collection of the control samples; the agonist and antagonist were then infused together via the probe for a further 15 min.
Dialysate analysis
The analysis of 5-HT was carried out by a reverse phase high performance liquid chromatography coupled to an electrochemical detector (HPLC-ED) using an amperometric detector (Antec Electrochemical detector, Antec Leyden B.V., The Netherlands). 5-HT content was analysed by injecting a 22 μl volume of dialysate via a Hamilton syringe into a Rheodyne Syringe Loading Sample Injector port (model 7125; Rheodyne Incorporated, CA, U.S.A.) holding a 100 μl loop. This off-loads the sample directly into the HPLC-ED equipped with a 10 cm long, 4.6 mm bore, 3 micron 3ODS2 reverse phase column (Spherisorb, Jones Chromatography). The 5-HT was subsequently oxidized on the working electrode at +0.75 volts relative to an Ag/AgCl reference electrode. The current reaching the working electrode resulting from this oxidation of solute was amplified and converted to a voltage (I/E converter). The detector signal was detected on a programmable integrator (Hewlett Packard). Separation at 5.4 min was achieved with a mobile phase consisting of 82.7 mM citric acid monohydrate, 43.4 mM anhydrous di-sodium hydrogen phosphate, 1.02 mM di-sodium EDTA, 214 μM OSA and 20% methanol, pumped around the system using a Gynkotek duel piston pump (model M300, Severn Analytical, Cheshire, U.K.; flow rate 0.7 ml min−1). The mobile phase was filtered prior to use through 0.2 μm filters (Millipore) and continually purged with helium to degas the solution. Concentration of 5-HT was determined by the ratio of sample to standard peak height stored in the memory of the programmed integrator, and the results expressed as femtomol (fmol).
Statistical analysis
5-HT concentration in the dialysate samples (uncorrected for probe recovery) was expressed as a percentage of the mean of three pre-intervention control samples. Values post-drug treatment were analysed by one-way analysis of variance (ANOVA) with repeated measures with Student Newman Keuls post hoc test to detect differences from pre-intervention controls samples. Basal 5-HT concentration was analysed by one way ANOVA with Tukeys post hoc test to detect differences between time points. P<0.05 was considered statistically significant.
Drugs
All drugs infused via the probe were made up in aCSF containing 1 μM citalopram. EDTA, Na2HPO4, NaCl, KCl, CaCl2, were purchased from R & L Slaughter. All other reagents were purchased from Sigma Chemical Company. The following drugs were donated by the companies indicated: RU24969 [5-methoxy-3(1,2,3,6-tetrahydro-4-pyridinyl)-1H-indole] by Roussel-Uclaf and citalopram hydrobromide by Lundbeck (Copenhagen-Valby). WAY100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide trihydrochloride) was donated by P.J. Mitchell, University of Bath.
Results
Experiments were carried out at six zeitgeber times (ZTs) across the LD cycle; ZT 3, 6, 9, 15, 18 and 21 where ZT 12 is the onset of the dark phase.
Basal 5-HT efflux
Basal 5-HT levels in dialysate samples varied significantly across the light : dark cycle. Values in dialysate samples from the region of the SCN were 28.7±2.89, 32.7±7.05, 41.7±5.18, 17.6±3.90, 20.4±4.03 and 15.7±1.56 mean±s.e.mean fmol for ZT 3, 6, 9, 15, 18 and 21 respectively (Figure 1). As a general trend, the 5-HT in the dialysate from around the SCN during the light phase was higher than that collected during the dark phase. Highest levels were measured at ZT 9, and the levels at ZT 15, 18 and 21 were significantly lower than those at ZT 9 (P<0.05). In the absence of drug infusion the 5-HT overflow from the region of the SCN did not vary significantly over the 3 h collection period at any of the six time points.
Figure 1.
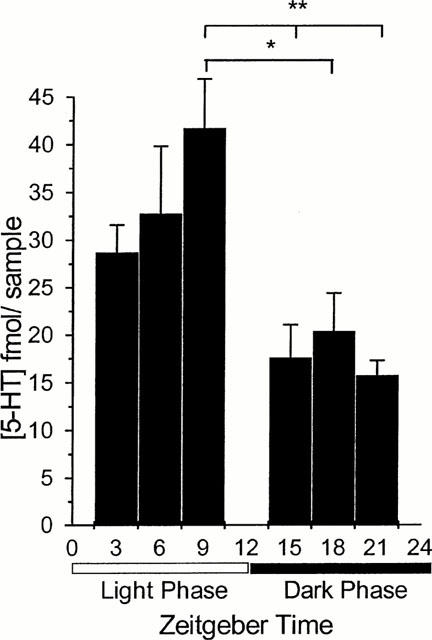
Basal 5-HT concentration as measured in dialysate from the SCN region at six zeitgeber times across the 12 : 12 h light:dark cycle; lights off at ZT 12. Data are expressed as mean±s.e.mean (n=4–8). Basal 5-HT levels at ZT 15, 18 and 21 are significantly different from basal levels measured at ZT 9. *P<0.05; **P<0.01 (one way ANOVA with Tukey's post hoc test).
Pharmacological controls
When calcium ions were removed from the perfusing aCSF there was an immediate and significant decrease in 5-HT overflow, with a maximal decrease of 93.0±3.67% (P<0.001). Upon reperfusion with aCSF containing calcium ions, 5-HT overflow began to increase, but remained significantly lower than pre-intervention controls 45 min after reperfusion (Figure 2a). Modified aCSF containing 100 mM KCl was perfused for 60 min, causing an immediate and significant increase in 5-HT overflow. Maximal increase was 349±80.8% (P<0.01). After reperfusion with normal aCSF, 5-HT levels returned to basal (Figure 2b).
Figure 2.
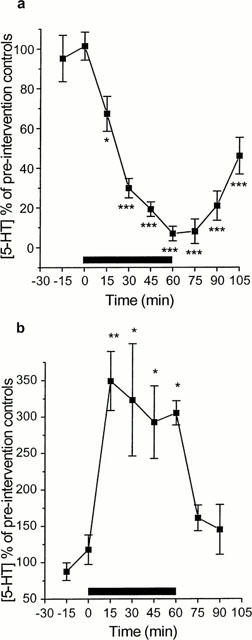
Effect of removal of calcium ions from (A) and increasing potassium ion concentration (100 mM) in (B) the perfusing aCSF on the 5-HT overflow from dialysate from the SCN region at ZT 6. Calcium ions were removed from, or potassium ions were added to, the aCSF for a period of 60 min, starting at time 0. n=3; *P<0.05; **P<0.01, ***P<0.001 one way ANOVA with repeated measures with Student Newman Keuls post hoc test).
Characterization of the 5-HT autoreceptor
RU24969 is not completely selective for the 5-HT1B receptor, having almost equal affinity for the 5-HT1A receptor (van Wijngaarden et al., 1990). Therefore to exclude the involvement of the 5-HT1A receptor in the observed response to RU24969, WAY100635, an antagonist specific for the 5-HT1A receptor site, was administered at ZT 6. WAY100635 (1 μM) was infused for 15 min prior to and 15 min during RU24969 (1 μM) infusion. The ability of RU24969 to decrease dialysate 5-HT levels was not affected by the presence of WAY100635 (5-HT output decreased to 47.52±17.5% of baseline; data not shown). WAY100635 infusion alone at ZT 6 did not significantly affect 5-HT levels in dialysate from the region of the SCN (data not shown). Therefore it can be assumed that under these circumstances RU24969 is acting solely by stimulation of the 5-HT1B receptor.
Diurnal variation in 5-HT autoreceptor function in the region of the SCN
The effect of stimulation of the 5-HT1B autoreceptor with RU24969 (1 μM; 15 min infusion) was dependent upon the zeitgeber time at which the drug was infused (Figure 3). During the light phase, infusion of RU24969 at ZT 3 and 6 produced a significant and prolonged decrease in 5-HT overflow, reaching a maximal decrease of 30.6±11.9 and 41.0±11.7% mean±s.e.mean of baseline, respectively (P<0.01; Figure 3a,b). At ZT 9 infusion of RU24969 failed to produce any significant change in dialysate 5-HT concentration (28.0±11.1% decrease, Figure 3c). During the dark phase, infusion of RU24969 at ZT 15 produced a significant and prolonged decrease in 5-HT from the SCN region, reaching a nadir of 56.1±17.2% of baseline (P<0.05; Figure 3d). At ZT 18, RU24969 infusion failed to significantly change dialysate 5-HT concentration (32.8±24.6% decrease, Figure 3e). At ZT 21, RU24969 infusion produced a significant, but short lived, decrease in 5-HT overflow from the region of the SCN, reaching a low of 54.3±17.0% of baseline (P<0.05; Figure 3f). These findings are summarized in Figure 4 in which the maximal percentage decrease in dialysate 5-HT post-RU24969 infusion is plotted versus the zeitgeber time, demonstrating the circadian variation in the functional activity of the 5-HT1B autoreceptor.
Figure 3.
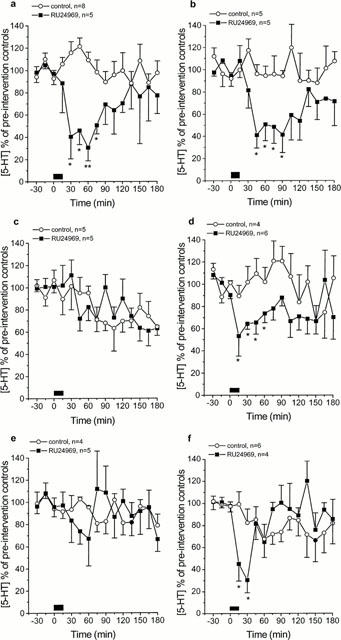
(a–f) Effect of RU24969 infusion (1 μM) on 5-HT overflow in the region of the SCN at ZT 3 (a), 6 (b), 9 (c), 15 (d), 18 (e) and 21 (f). Values are mean±s.e.mean expressed as a percentage of three pre-intervention control samples. RU24969 was infused for 15 min starting at time 0. *P<0.05; **P<0.01 (one way ANOVA with repeated measures with Student Newman Keuls post hoc test).
Figure 4.
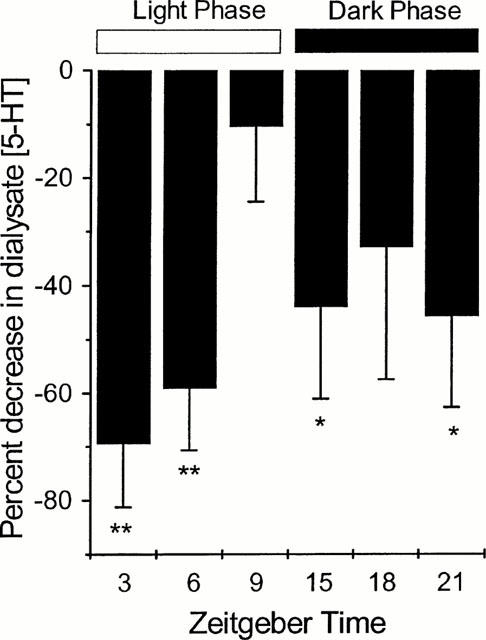
Percentage maximal decrease in 5-HT overflow following infusion of RU24969 (1 μM; 15 min) at ZTs 3, 6, 9, 15, 18 and 21. *P<0.05; **P<0.01 (one way ANOVA with repeated measures with Student Newman Keuls post hoc test). Maximal decrease in dialysate 5-HT concentration post-RU24969 infusion is plotted versus the zeitgeber time and represented as a negative percentage.
Discussion
This study, in which microdialysis was used to measure extracellular 5HT in the region of the SCN of conscious, freely-moving rats, confirmed previous reports that basal 5-HT levels in the SCN region varied over the LD cycle (Martin & Marsden, 1984; Ferraro & Steger, 1990; Cagampang & Inouye, 1994).
It was important first to establish the origin of the 5-HT in the dialysate. Exocytotic release of neurotransmitter is a calcium-dependent process and removal of calcium ions from the perfusion fluid resulted in an immediate and significant decrease in dialysate 5-HT. Conversely, infusion of 100 mM KCl led to an immediate and significant increase in 5-HT overflow, presumably because of local depolarization of serotonergic terminals. Two essential criteria for establishing the neuronal origin of the 5-HT present in the dialysate were thus fulfilled. This is in agreement with other authors who have measured 5-HT levels in dialysate samples from the hypothalamus (Auerbach et al., 1989).
Extensive studies have confirmed that RU24969 is an agonist at 5-HT1B autoreceptors regulating 5-HT release in the hippocampus (Sharp et al., 1989; Martin et al., 1992; Sayer et al., 1999), the frontal cortex (Middlemiss, 1985) and the amygdala (Bosker et al., 1997). RU24969 has also been demonstrated to block 5-HT reuptake in the absence of a reuptake blocker in the perfusing aCSF (Auerbach et al., 1991; Hjorth & Tao, 1991). The routine inclusion of citalopram in the dialysing aCSF in our experiments removed this mechanism as a possible confounding factor, and it may be assumed that RU24969 is acting as a receptor agonist. Although RU24969 is an agonist for the 5-HT1B receptor (Sills et al., 1984) it also has affinity for the 5-HT1A receptor. It was thus important to establish whether RU24969 was acting via 5-HT1A or 5-HT1B receptors. Simultaneous infusion of RU24969 and the 5-HT1A receptor antagonist WAY100635 at ZT 6 did not alter the profile of inhibition by RU24969 alone (data not shown), and it may therefore be assumed that under these experimental conditions RU24969 was acting solely by activation of 5-HT1B receptors. Infusion of WAY100635 alone was also without effect. Additionally, in a recent report, Sayer et al. (1999) failed to demonstrate a significant effect on 5-HT overflow in the SCN following infusion of the 5-HT1A receptor agonist 8-OH-DPAT into the SCN at mid-light in anaesthetized animals, confirming that 5HT1A receptors are not involved in local control of 5-HT release from serotonergic terminals in the SCN.
The effect of 5-HT1B receptor stimulation by RU24969 on 5-HT overflow from the region of the SCN was strikingly dependent upon the zeitgeber time at which the drug was applied. During the early- and mid-light phase (ZTs 3 and 6) and the early-dark phase (ZT 15) RU24969 produced a significant and prolonged decrease in dialysate 5-HT levels. Towards the end of the dark period (ZT 21) the response to RU24969 was to produce a significant, but short acting reduction in dialysate 5-HT levels. By contrast RU24969 produced no significant effect on dialysate 5-HT levels towards the end of the light period (ZT 9) or at the mid-dark phase (ZT 18). In general the response to RU24969 was significantly smaller during the dark phase (Figure 3). A similar phenomenon has recently been reported in anaesthetized rats at two time points, mid- and end-light phase (Sayer et al., 1999). In that study RU24969 (5 μM) infused via the probe at mid-light produced a significant decrease in dialysate 5-HT content to 35% of baseline, which is comparable to the effect of 1 μM RU24969 administered at the same time point reported here (41%).
We cannot at this stage be sure of the precise location of the 5-HT1B receptors responsible for this phenomenon. 5-HT1B receptors are known to be located on presynaptic terminals of glutamatergic neurones of the retinohypothalamic tract (Pickard et al., 1999). Glutamate has been shown to affect 5-HT release in the SCN (Prosser, 2000), possibly indirectly through GABA interneurons, so that inhibition of glutamate release mediated by an action on 5-HT1B receptors could indirectly result in the changed overflow of 5-HT observed in our experiments. A simpler explanation is that RU24969 acts on presynaptic 5-HT1B autoreceptors on serotonergic terminals in the SCN, producing a more direct inhibition of 5-HT overflow.
The circadian variation in function of the 5-HT1B autoreceptor in response to RU24969 stimulation has not been reported before in freely-moving conscious rats. However, the results of a study by Martin et al. (1987) suggested that the behavioural response to RU24969 in the mouse exhibited a diurnal variation. The effect of RU24969 on locomotor activity was examined at different time points during the light : dark cycle. The increase in locomotor activity induced by RU24969 did not vary across the light : dark cycle, but the ability of the antagonist metergoline to block the response varied at ZT 5 versus ZT 17. The antagonist was 3–5 fold more effective at blocking the response to RU24969 at ZT 8 than at ZT 17 (i.e. just after mid-light versus mid-dark). These authors were unable at that time not only to distinguish between blockade of pre- and post-synaptic receptors but also to determine whether the phase shift resulted from stimulation of 5-HT1A or 5-HT1B receptors.
Several in vitro studies have failed to detect circadian variation in the function of the terminal 5-HT1B receptor. Singh & Redfern (1994) failed to demonstrate a circadian variation in the function of rat terminal 5-HT1B autoreceptors sampled at four equally spaced time points across the light : dark cycle. These authors sampled from hippocampus and cortex. Blier et al. (1989) investigated the ability of RU24969 to decrease stimulation-induced release of tritiated 5-HT from preloaded rat hypothalamic slices. Their experiments were carried out at only two time points. The release of tritium was not significantly different in slices obtained from the light phase and the dark phase.
At this stage we can only speculate as to the mechanism behind the observed variation in 5-HT1B receptor function. Possibilities include differential expression of receptor message over the light:dark cycle, 5-HT1B receptor occupation by endogenous ligands, changes in the density of the 5-HT1B autoreceptors in the region of the SCN, differences in second messenger systems and receptor phosphorylation or internalization. Roca et al. (1993) failed to detect any difference in mRNA for the 5-HT1B receptor at two equally spaced time points 12 h apart in SCN tissue from rats held in constant darkness. One must bear in mind that mRNA expression within the SCN will measure receptor expression in the cell body relating to postsynaptic receptors, and will not measure the expression of the presynaptic autoreceptor examined in these microdialysis experiments. More recently Redfern et al. (1999) failed to detect any circadian variation in 5-HT1B receptor mRNA measured in raphe nuclei at 3-h intervals across the 24 h light-dark cycle.
A simple explanation of the observed circadian variation in response to stimulation of the 5-HT1B autoreceptor would be to suggest that the exogenous ligand is competing with different concentrations of endogenously released 5-HT. Unfortunately the experimental observations do not support this hypothesis. In general during the dark phase when basal concentrations of 5-HT were low, autoreceptor activity was also low.
Another possibility is that the number of available receptors varies over 24 h. 5-HT1B receptor binding has been shown in one study to be significantly higher during the dark phase than during the light phase (Prosser et al., 1993). On the other hand there is no evidence of diurnal variation in receptor expression (Redfern et al., 1999).
5-HT1B receptors are negatively coupled to adenylate cyclase (Bouhelal et al., 1988), stimulation of which will lead to changes in intracellular levels of cyclic AMP. The functional activity of the 5-HT1B autoreceptors is related to availability of the second messengers and the substrate cyclic AMP. Circadian variation in the levels of endogenous cyclic AMP have been detected in rat SCN micropunches (Prosser & Gillette, 1991; Yamazaki et al., 1994) with peak levels during the late day and late night, but low during early night. Again, this pattern does not follow the rhythm in function of the 5-HT1B receptor reported here, but the experiments examining the levels of cyclic AMP were carried out in vitro in tissue taken from animals housed under constant darkness. If in animals maintained under a 12 : 12 h light : dark cycle, cyclic AMP levels were low towards the end of the light period and throughout the dark period, one might speculate that the low availability of substrate for the enzyme adenylate cyclase could account for the variation in receptor function. However, since the 5-HT1B receptor is negatively coupled to adenylate cyclase, activation of the receptor would reduce rather than promote cyclic AMP conversion. It would therefore be more useful to measure the activity of the enzyme adenylate cyclase itself. Prosser & Gillette (1991) measured adenylate cyclase activity in addition to cyclic AMP levels, but found no significant variation in activity over the four different sampling times.
Another possible explanation for the observed variation in autoreceptor function is circadian fluctuation in endogenous ligands or modulators. It has been proposed (Massot et al., 1996) that an endogenous ligand may be present in cerebral tissue capable of interacting with 5-HT1 receptor function and more specifically the 5-HT1B receptor. This simple 4 amino acid peptide (Leu-Ser-Ala-Leu) was purified and named 5-HT moduline. This molecule specifically inhibited the binding of tritiated 5-HT to 5-HT1B receptors, thus acting as an allosteric modulator at the 5-HT1B site, and was shown in behavioural experiments to have an antagonistic effect on 5-HT1B receptor activity. Since that time, 5-HT moduline has been localized immunocytochemically in rodent brain after an antibody was raised against the peptide (Grimaldi et al., 1997). Localization of 5-HT moduline appeared to overlap binding at 5-HT1B receptors, giving strength to the suggestion that it specifically interacts with 5-HT1B receptors. Indeed in mutant mice lacking the gene encoding the 5-HT1B receptor there was no binding of 5-HT moduline (Cloez-Tayarani et al., 1997). 5-HT moduline is present in the hypothalamus, (Grimaldi et al., 1997), but to our knowledge there is as yet no evidence of whether 5-HT moduline levels fluctuate over 24 h and whether the amplitude of any such variation is sufficient to account for the changes in receptor activity seen in our experiments.
It will be important to determine whether the circadian variation we have observed in the region of the SCN is unique to the circadian pacemaker or whether it is repeated in other areas receiving serotonergic innervation. Many studies have centred on the effect of 5-HT1B autoreceptor stimulation in the hippocampus (Sharp et al., 1989; Hjorth & Tao, 1991; Martin et al., 1992; Bolanos-Jimenez et al., 1995; Bosker et al., 1995). In all these studies, experiments were performed during the light phase and application of an agonist for the 5-HT1B receptor produced comparable reduction in 5-HT overflow ranging from 33 to 67% of basal. In preliminary experiments (unpublished observations) we found that in the hippocampus RU24969 produced a prolonged and statistically significant decrease in 5-HT overflow at both mid-light and mid-dark. A more comprehensive study encompassing time points across the 24-h cycle needs to be done before any definitive conclusions can be reached, but there is at least a suggestion that the ‘on-off' phenomenon of 5-HT1B autoreceptor function may be specific to the SCN.
In conclusion, this study has demonstrated that the activity of 5-HT1B receptors in the region of the suprachiasmatic nucleus of the conscious Wistar rat exhibits a diurnal variation. This may be significant in terms of control and release of 5-HT with subsequent influence on clock functioning in the SCN. The fact that a number of previous in vitro studies have failed to show any circadian variation in 5-HT1B receptor activity might suggest that the mechanism responsible is a dynamic one and might point to fluctuation in endogenous modulators or in the dynamic state of the receptor. The mechanism(s) by which variation in receptor function is mediated remains to be elucidated.
Acknowledgments
This work was supported by a BBSRC CASE studentship in collaboration with Knoll Ltd, Nottingham.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- HPLC-ED
high performance liquid chromatography with electrochemical detection
- 5-HT
5-hydroxytryptamine
- LD
light:dark
- RU24969
5-methoxy-3(1,2,3,6-tetrahydro-4-pyridyl)-1H-indole
- SCN
suprachiasmatic nuclei
- WAY100635
N-[2-[4-(2-methoxyphenol)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide trihydrochloride
- ZT
zeitgeber time
References
- ALBRECHT P., VISSCHER M.B., BITTNER J.J., HALBERG F. Daily changes in 5-hydroxytryptamine concentration in mouse brain. P.S.E.B.M. 1956;92:703–706. doi: 10.3181/00379727-92-22586. [DOI] [PubMed] [Google Scholar]
- AUERBACH S.B., MINZENBERG M.J., WILKINSON L.O. Extracellular serotonin and 5-hydroxyindoleacetic acid in hypothalamus of the anaesthetised rat measured by in vivo dialysis coupled to high-performance liquid chromatography with electrochemical detection: dialysate serotonin reflects neuronal release. Brain Res. 1989;499:281–290. doi: 10.1016/0006-8993(89)90776-2. [DOI] [PubMed] [Google Scholar]
- AUERBACH S.B., RUTTER J.J., JULIANO P.J. Substituted piperazine and indole compounds increase extracellular serotonin in rat diencephalon as determined by in vivo microdialysis. Neuropharmacology. 1991;30:307–311. doi: 10.1016/0028-3908(91)90054-f. [DOI] [PubMed] [Google Scholar]
- BLIER P., GALZIN A.-M., LANGER S.Z. Diurnal variation in the function of serotonin terminals in the rat hypothalamus. J. Neurochem. 1989;52:453–459. doi: 10.1111/j.1471-4159.1989.tb09142.x. [DOI] [PubMed] [Google Scholar]
- BOLANOS-JIMENEZ F., DECASTRO R.M., SEGUIN L., CLOEZ-TAYARANI I., MONNERET V., DRIEU K., FILLION G. Effects of stress on the functional properties of presynaptic and postsynaptic 5-HT1B receptors in the rat brain. Eur. J. Pharmacol. 1995;294:531–540. doi: 10.1016/0014-2999(95)00590-0. [DOI] [PubMed] [Google Scholar]
- BOSKER F.J., VANESSEVELDT K.E., KLOMPMAKERS A.A., WESTENBERG H.G.M. Chronic treatment with fluvoxamine by osmotic minipumps fails to induce persistent functional changes in central 5-HT1A and 5-HT1B receptors, as measured by in vivo microdialysis in dorsal hippocampus of conscious rats. Psychopharmacol. 1995;117:358–363. doi: 10.1007/BF02246110. [DOI] [PubMed] [Google Scholar]
- BOSKER F., VRITEN D., KLOMPMAKERS A., WESTENBERG H. The effects of a 5-HT1A receptor agonist and antagonist on the 5-hydroxytryptamine release in the central nucleus of the amygdala: a microdialysis study with flesinoxan and WAY 100635. N. S. Arch. Pharmacol. 1997;355:347–353. doi: 10.1007/pl00004953. [DOI] [PubMed] [Google Scholar]
- BOUHELAL R., SMOUNYA L., BOCKAERT J. 5-HT1B receptors are negatively coupled with adenylate cyclase in rat substancia nigra. Eur. J. Pharmacol. 1988;151:189–196. doi: 10.1016/0014-2999(88)90799-6. [DOI] [PubMed] [Google Scholar]
- BRADBURY M.J., DEMENT W.C., EDGAR D.M. Serotonin-containing fibres in the suprachiasmatic hypothalamus attenuate light-induced phase delays in mice. Brain Res. 1997;768:125–134. doi: 10.1016/s0006-8993(97)00629-x. [DOI] [PubMed] [Google Scholar]
- CAGAMPANG F.R.A., INOUYE S.I.T. Diurnal and circadian changes of serotonin in the suprachiasmatic nuclei: regulation by light and an endogenous pacemaker. Brain Res. 1994;639:175–179. doi: 10.1016/0006-8993(94)91780-9. [DOI] [PubMed] [Google Scholar]
- CAHILL G.M., MENAKER M. Effects of excitatory amino acid receptor antagonists and agonists on suprachiasmatic nucleus responses to retinohypothalamic tract volleys. Brain Res. 1989;479:76–82. doi: 10.1016/0006-8993(89)91337-1. [DOI] [PubMed] [Google Scholar]
- CLOEZ-TAYARANI I., CARDONA A., ROUSSELLE J.-C., MASSOT O., EDELMAN L., FILLION G. Autoradiographic characterization of [3H]-5-HT-moduline binding sites in rodent brain and their relationship to 5-HT1B receptors. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9899–9904. doi: 10.1073/pnas.94.18.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGEL G., GOTHERT M., HOYER D., SCHUCKER E., HILLENBRAND K. Identity of inhibitory presynaptic 5-hydoxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. N.S. Arch. Pharmacol. 1986;332:1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- FERRARO J.S.K., STEGER R.W. Diurnal variations in brain serotonin are driven by the photic cycle and are not circadian in nature. Brain Res. 1990;512:121–124. doi: 10.1016/0006-8993(90)91179-k. [DOI] [PubMed] [Google Scholar]
- GRIMALDI B., FILLION M.-P., BONNIN A., ROUSSELLE J.C., MASSOT O., FILLION G. Immunological localization of neurons expressing 5-HT-moduline in the mouse brain. Neuropharmacology. 1997;36:1079–1087. doi: 10.1016/s0028-3908(97)00099-3. [DOI] [PubMed] [Google Scholar]
- HASTINGS M.H.The vertebrate clock: localisation, connection and entrainment Handbook of Experimental Pharmacology, Vol 125, Physiology and Pharmacology of Biological Rhythms 1997Springer-Verlag: Berlin; 1–28.eds. Redfern, P.H. & Lemmer, B [Google Scholar]
- HJORTH S., TAO R. The putative 5-HT1B agonist CP-93,129 suppresses rat hippocampal 5-HT release in vivo: comparison with RU 24969. Eur. J. Pharmacol. 1991;209:249–252. doi: 10.1016/0014-2999(91)90177-r. [DOI] [PubMed] [Google Scholar]
- JOHNSON R., MOORE R., MORIN L. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- LIMBERGER N., DEICHER R., STARKE K. Species differences in presynaptic serotonin autoreceptors: mainly 5-HT1B but possibly in addition 5-HT1D in the rat, 5-HT1D in the rabbit and guinea-pig brain cortex. N.S. Arch. Pharmacol. 1991;343:353–364. doi: 10.1007/BF00179039. [DOI] [PubMed] [Google Scholar]
- MARTIN K.F., HANNON S., PHILLIPS I., HEAL D.J. Opposing roles for 5HT1B and 5HT3 receptors in the control of 5HT release in rat hippocampus in vivo. Br. J. Pharmacol. 1992;106:139–142. doi: 10.1111/j.1476-5381.1992.tb14306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN K.F., MARSDEN C.A.In vivo diurnal variations of 5HT release in hypothalamic nuclei Circadian rhythms in the central nervous system 1985London: Macmillan; 81–92.ed. Redfern, P.H., Campbell, I.C., Davies, J.A., & Martin, K.F. [Google Scholar]
- MARTIN K.F., REDFERN P.H.5-hydroxytryptamine and noradrenaline synthesis, release and metabolism in the central nervous system: circadian rhythms and control mechanisms Handbook of Experimental Pharmacology 1997Vol 125157–176.Physiology and Pharmacology of Biological Rhythms. ed. Redfern, P.H > Lemmer B. pp [Google Scholar]
- MARTIN K.F., WEBB A.R., MARSDEN C.A. The behavioural response to the 5-hydroxytryptamine1B (5-HT1B) receptor agonist–RU24969 may exhibit a circadian variation in the mouse. Chronobiol. Int. 1987;4:493–498. doi: 10.3109/07420528709078540. [DOI] [PubMed] [Google Scholar]
- MASSOT O., ROUSSELLE J.-C., FILLION M.-P., GRIMALDI B., CLOEZTAYARANI I., FUGELLI A., PRUDHOMME N., SEGUIN L., ROUSSEAU B., PLANTEFOL M., HEN R., FILLION G. 5-hydroxytryptamine-moduline, a new endogenous cerebral peptide, controls the serotonergic activity via its specific interaction with 5-hydroxytryptamine1B/1D receptors. Mol. Pharmacol. 1996;50:752–762. [PubMed] [Google Scholar]
- MIDDLEMISS D.N. The putative 5-HT1 receptor agonist, RU24969, inhibits the efflux of 5-hydroxytryptamine from rat frontal cortex slices by stimulation of the 5-HT autoreceptor. J. Pharm. Pharmacol. 1985;37:434–437. doi: 10.1111/j.2042-7158.1985.tb03032.x. [DOI] [PubMed] [Google Scholar]
- MOORE R.Y., HALARIS A.E., JONES B.E. Serotonin neurons of the midbrain raphe: Ascending projections. J. Comp. Neurol. 1978;180:417–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- MORIN L.P., BLANCHARD J. Depletion of brain serotonin by 5,7-DHT modifies hamster circadian rhythm response to light. Brain Res. 1991;566:173–185. doi: 10.1016/0006-8993(91)91696-x. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The rat brain in stereotaxic coordinates 1982Academic Press: San Diego, CA; 2nd edn [Google Scholar]
- PICKARD G.E., SMITH B.N., BELENKY M., REA M.A., DUDEK F.E., SOLLARS P.J. 5-HT1B receptor-mediated presynaptic inhibition of retinal input to the suprachiasmatic nucleus. J. Neurosci. 1999;9:4034–4045. doi: 10.1523/JNEUROSCI.19-10-04034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICKARD G.E., WEBER E.T., SCOTT P.A., RIBERD A.F., REA M.A. 5-HT1B receptor agonists inhibit light-induced phase shifts of behavioural circadian rhythms and expression of the immediate early gene c-fos in the suprachiasmatic nucleus. J. Neurosci. 1996;16:8208–8220. doi: 10.1523/JNEUROSCI.16-24-08208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROSSER R.A. Serotonergic actions and interactions on the SCN circadian pacemaker: in vitro investigations. Biol. Rhythms. Res. 2000;31:315–339. [Google Scholar]
- PROSSER R.A., DEAN R.R., EDGAR D.M., HELLER H.C., MILLER J.D. Serotonin and the mammalian circadian system: I. in vitro phase shifts by serotonergic agonists and antagonists. J. Biol. Rhythms. 1993;8:1–16. doi: 10.1177/074873049300800101. [DOI] [PubMed] [Google Scholar]
- PROSSER R.A., GILLETTE M.U. Cyclic changes in cAMP concentration and phosphodiesterase activity in mammalian circadian clock studies in vitro. Brain Res. 1991;568:185–192. doi: 10.1016/0006-8993(91)91396-i. [DOI] [PubMed] [Google Scholar]
- REDFERN P.H., GARABETTE M., MARTIN K.F. 5-HT1B receptor mRNA in the rat SCN does not display diurnal variation in expression as measured by RT-PCR. Chronobiol. Int. 1999;16:89. [Google Scholar]
- ROCA A.L., WEAVER D.R., REPPERT S.M. Serotonin receptor gene expression in the rat suprachiasmatic nuclei. Brain Res. 1993;608:159–165. doi: 10.1016/0006-8993(93)90789-p. [DOI] [PubMed] [Google Scholar]
- SAYER T.J.O., HANNON S.D., REDFERN P.H., MARTIN K.F. Diurnal variation in 5-HT1B autoreceptor function in the anterior hypothalamus in vivo: effect of chronic antidepressant drug treatment. Br. J. Pharmacol. 1999;126:1777–1784. doi: 10.1038/sj.bjp.0702535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP T., BRAMWELL S.R., GRAHAME-SMITH D.G. 5-HT1 agonists reduce 5-hydroxytryptamine release in rat hippocampus in vivo as determined by brain microdialysis. Br. J. Pharmacol. 1989;96:283–290. doi: 10.1111/j.1476-5381.1989.tb11815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILLS M.A., WOLFE B.B., FRAZER A. Determination of selective and non-selective compounds for the 5-HT1A and 5-HT1B receptor subtypes in rat frontal cortex. J. Pharm. Exp. Ther. 1984;231:480–487. [PubMed] [Google Scholar]
- SINGH A., REDFERN P.H. Lack of circadian variation in the sensitivity of rat terminal 5-HT1B autoreceptors. J. Pharm. Pharmacol. 1994;46:366–370. doi: 10.1111/j.2042-7158.1994.tb03814.x. [DOI] [PubMed] [Google Scholar]
- TRILLAT A.C., MALAGIE I., SCEARSE K., PONS D., ANMELLA M.C., JACQUOT C., HEN R., GARDIER A.M. Regulation of serotonin release in the frontal cortex and ventral hippocampus of homozygous mice lacking 5-HT1B receptors: In vivo microdialysis studies. J. Neurochem. 1997;69:2019–2025. doi: 10.1046/j.1471-4159.1997.69052019.x. [DOI] [PubMed] [Google Scholar]
- VAN WIJNGAARDEN I., TULP M.T.M., SOUDIJN W. The concept of selectivity in 5-HT receptor research. Eur. J. Pharmacol. 1990;188:301–312. doi: 10.1016/0922-4106(90)90190-9. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI S., MARUYAMA M., CAGAMPANG F.R.A., INOUYE S.-I.T. Circadian fluctuations of cAMP content in the suprachiasmatic nucleus and the anterior hypothalamus of the rat. Brain Res. 1994;651:329–331. doi: 10.1016/0006-8993(94)90713-7. [DOI] [PubMed] [Google Scholar]


