Abstract
Functional human GABAB(1a,2) and GABAB(1b,2) receptors have been stably expressed in mammalian CHO K1 cells.
Detailed characterization of GABAB ligand binding at each of the receptors has been compared using [3H]-CGP54626A. In cell membranes fractions, [3H]-CGP54626A bound to a single site with a KD of 1.51±1.12 nM, Bmax of 2.02±0.17 pmoles mg protein−1 and 0.86±0.20 nM, Bmax of 5.19±0.57 pmoles mg protein−1 for GABAB(1a,2) and GABAB(1b,2) respectively.
In competition binding assays the rank order was identical for both GABAB receptors. For known GABAB agonists the rank order was CGP27492>SKF97541=CGP46381>GABA>Baclofen and for GABAB antagonists the rank order was CGP54262A>CGP55845>CGP52432>SCH 50911>CGP51176>CGP36742=CGP35348 ⩾2-OH Saclofen ⩾ABPA.
The allosteric effect of calcium cations was also investigated. The effect of removal of CaCl2 from the binding assay conditions was ligand dependent to either cause a decrease in ligand affinity or to have no significant effect. However, these effects were similar for both GABAB receptors.
A whole cell, scintillation proximity binding assay was used to determine agonist affinity at exclusively heterodimeric GABAB receptors. In competition assays, the rank order was the same for both GABAB(1a,2) and GABAB(1b,2) and consistent with that seen with cell membrane fractions.
These data suggest that, in terms of ligand binding, the currently identified isoforms of the GABAB receptor are pharmacologically indistinguishable.
Keywords: GABAB receptors, heterodimers, [3H]-CGP54626A, human receptors, stable cell lines, pharmacological characterization, ligand binding, whole cell binding, G protein coupled receptor
Introduction
The metabotropic GABAB receptor, which mediates slow, inhibitory actions of the neurotransmitter γ-aminobutyric acid (GABA), was described pharmacologically almost 20 years ago allowing it to be distinguished from the ionotropic GABAA binding site (Hill & Bowery, 1981). The regulation of synaptic transmission via the GABAB receptor has since been shown to be mediated via the Gi/o family of G proteins (Campbell et al., 1993; Menon-Johansson & Dolphin, 1993). However, the molecular nature of the GABAB receptor has only been revealed very recently. Initially, expression cloning identified a receptor, termed GABAB(1), with homology to other family C G protein coupled receptors (GPCRs), which displayed many of the antagonist binding characteristics of the endogenous rat brain receptor (Kaupmann et al., 1997), and which was expressed in two N terminal isoforms. However, this receptor failed to bind agonists with high affinity and failed to couple efficiently to second messenger pathways when expressed recombinantly. It has since been demonstrated that coexpression of a second homologous 7 transmembrane (7TM) protein, GABAB(2), is required to create a fully functional, high affinity GABAB receptor (for review see Marshall et al., 1999).
The identification of the two main isoforms of GABAB(1a), GABAB(1b) and its partner GABAB(2) (IUPHAR Nomenclature) has allowed the GABAB receptor to be studied in heterologous expression systems and has permitted further pharmacological characterization. For example, it has recently been demonstrated that the binding affinity of antagonist ligands to the large N terminal extracellular domain of GABAB(1) (Galvez et al., 1999; Malitschek et al., 1999) is relatively unaffected by coexpression of GABAB(2) (Kaupmann, et al., 1998; Galvez et al., 2000). In contrast, agonist affinity for GABAB(1) is 10–100 fold lower when expressed alone compared with natively expressed heterodimeric receptors (Kaupmann et al., 1997). There have been a number of reports demonstrating that the GABAB(2) subunit does not respond to GABA agonists and fails to bind antagonist ligands (Jones et al., 1998; White et al., 1998; Kaupmann et al., 1998), however it is worth noting that Martin et al. (1999) showed that Chinese hamster ovary (CHO) cells expressing GABAB(2) alone could be activated weakly by GABA. Furthermore, it appears that generating a heterodimeric receptor, with 1 : 1 stoichiometric expression of each of the subunits, is required to reconstitute a recombinant GABAB receptor with similar agonist affinity relative to its wild-type counterpart in rat brain membrane preparations (White et al., 1998).
In addition to the structural homology to other family C GPCRs, it has been demonstrated that the GABAB receptor, like the Calcium sensing receptor (CaSR) and metabotropic glutamate receptor 1 (mGluR1) is also sensitive to Ca2+ ions. Both Wise et al. (1999) and Galvez et al. (2000) demonstrated that the receptor was allosterically modified by calcium at physiologically relevant concentrations of this cation which lead to an increase of agonist potency. Using site directed mutagenesis Galvez et al. (2000) identified a serine amino acid residue, in the N terminus of GABAB(1) that is critical for the calcium sensitivity of the GABAB receptor and its allosteric effect on ligand binding.
The GABAB receptor complex is widely distributed throughout the central nervous system (CNS) and mRNA for GABAB(1) has also been identified in several peripheral tissues (Isomoto et al., 1998). However, expression of GABAB(2) appears to be restricted to the CNS where its distribution almost entirely parallels that of GABAB(1) (Clark et al., 2000; Jones et al., 1998). A distribution pattern for each of the GABAB(1) receptor isoforms is now emerging and in situ hybridization studies have suggested associations of GABAB(1a) and GABAB(1b) isoforms with pre- and post-synaptic elements, respectively (Billinton et al., 1999).
Intriguingly, some groups have suggested subtle pharmacological differences for pre- and post-synaptic GABAB receptors with both human and rodent preparations (Bonanno et al., 1997; Yu et al., 1999) and Cunningham & Enna (1996) also proposed that two receptor subtypes were capable of regulating cyclic AMP production in rat brain. In contrast, Seabrook et al. (1990) previously concluded that pre- and post-synaptic GABAB receptors were indistinguishable in rat brain slices. These differences may be attributed to the existence of GABAB heterodimers composed of either GABAB(1a/2) or GABAB(1b/2) and such phenomenon can now be addressed at the molecular level following heterologous expression of these proteins.
In view of the effects of heterodimerization on ligand binding, we decided that cell lines stably expressing particular GABAB heterodimeric partners may provide a suitable system from which a thorough and comparable pharmacological characterization of GABAB receptors could be performed. Hence, we report successful stable expression of GABAB(1a,2) and GABAB(1b,2) in CHO K1 cells and detailed characterization of these receptors using the radio-labelled antagonist [3H]-CGP54626A (Bittiger et al., 1992). In addition, the effect of calcium on ligand binding at each of the receptors has also been compared.
Methods
Cloning and expression of GABAB receptor cDNA
GABAB(1a and 1b) and GABAB(2) were cloned into pcDNA 3.1 (−) (Invitrogen) as previously described (White et al., 1998). For the construction of stable cell lines GABAB(1a and 1b) and GABAB(2) inserts were subcloned into the mammalian expression vectors pCIN3 (Neomycin selection) and pCIH6 (Hygromycin selection), respectively, (Rees et al., 1996).
For generation of stable cell lines, CHO K1 cells were maintained in DMEM F12 Ham containing 9% v v−1 heat inactivated foetal calf serum (FCS) and 2 mM glutamine. Cells were seeded in 6 well culture plates and grown to 50% confluency (18–24 h) prior to transfection with vectors containing the relevant cDNA inserts. For transfection, a total of 3 μg DNA/well was mixed with 10 μl of Lipofectamine reagent in 0.2 ml OptiMEM (both Life Technologies Inc.) followed by incubation at room temperature for 30 min prior to the addition of 1.6 ml OptiMEM. Cells were exposed to the transfection mixture for 5 h before 2 ml of 20% v v−1 FCS in DMEM F12 Ham media was added. Cells were transfected with 1 μg each of pCIN3 GABAB(1a or 1b), pCIH6 GABAB(2) and Gal 4 Elk1 Luciferase expression vector (puromycin selection). The latter provides a reporter system for the MAPK pathway but is not employed in the studies reported here. Forty-eight hours post transfection the media was replaced and supplemented with 500 μg ml−1 neomycin, 400 μg ml−1 Hygromycin and 2.5 μg ml−1 puromycin for selection of antibiotic resistant cells. Clonal cell lines were isolated by dilution and tested for functional GABAB receptors. A cyclic AMP accumulation assay was employed to measure GABA mediated inhibition of a 10 μM forskolin evoked cyclic AMP response. Clones in which 1 mM GABA produced a complete inhibition of the forskolin response were selected for further analysis. Stable expression of GABAB(1a and 1b) and GABAB(2) in these clones was also confirmed using Western blotting. Expression was visualized with pan-specific GABAB(1) or specific GABAB(2) receptor antibodies following immunoblotting of P2 membrane fractions (White et al., 1998).
P2 membrane preparation
Plasma membranes containing P2 particulate fractions were prepared from freshly cultured cells. All procedures were carried out at 4°C. Cell pellets were resuspended in 10 mM Tris-HCl and 0.1 mM EDTA, pH 7.4 (buffer A) followed by homogenization for 20 s with an Ultra Turrax and passed five times through a 25-gauge needle. Cell lysates were centrifuged at 1000×g for 10 min in a microcentrifuge to pellet the nuclei and unbroken cells and P2 particulate fractions were recovered by microcentrifugation at 16,000×g for 30 min. P2 particulate fractions were resuspended in buffer A and stored at −80°C until required. Protein concentrations were determined using the bicinchoninic acid (BCA) procedure (Smith et al., 1985) using BSA as a standard.
[3H]-CGP54626A filter binding assays
For competition experiments CHO-GABAB membranes were incubated with ∼0.5 nM [3H]-CGP5426A in 50 mM Tris HCl, 2 mM CaCl2 pH 7.4 in the presence or absence of competitor compound for 30 min at room temperature. P2 membrane protein fractions (10 μg/well) were used for all competition studies in a total volume of 500 μl. Ten mM GABA was used to define non-specific binding. Assays were terminated by vacuum filtration (using a Brandel cell Harvester) over GF/B filters, pre-soaked in assay buffer and the filters were washed four times with 1 ml ice cold buffer. Bound radioactivity (d.p.m.) was counted by liquid scintillation spectrometry.
For saturation studies, specific binding of [3H]-CGP54626A was determined over a range of radioligand concentrations (0.05–30 nM) in the absence or presence of 10 mM GABA using 10 μg protein/well. For Ca2+ free studies, CaCl2 was replaced with 1 mM EGTA. 5′-Guanylyl-imido-diphosphate (Gpp(NH)p) was included at 30 μM where indicated.
[3H]-CGP54626A whole cell scintillation proximity binding assays
Scintillation proximity assays were carried out under identical buffer conditions to those used in filtration assays. Conditions were optimized with respect to bead and cell density prior to performing characterization studies. Freshly cultured CHO GABAB cells were harvested with phosphate buffered saline, 5 mM EDTA, pH 7.4 and washed twice in 50 mM Tris Buffer, 2.5 mM CaCl2 pH 7.4. For competition studies, 105 cells/well were incubated in white, clear bottom, 96 well plates (Wallac) with 0.5 nM [3H]-CGP54626A in the absence or presence of competitor compound together with 2 mg/well Wheatgerm Agglutinin SPA beads (Amersham) in a total of 200 μl. Binding was allowed to proceed at room temperature for 30 min on an orbital shaker. Plates were then spun at 1500 r.p.m. for 5 min and cell bound radioactivity (corrected counts per min, CCPM) was determined on a Wallac Trilux Microbeta Counter. Specific binding was determined using 10 mM GABA. For saturation studies, specific binding of [3H]-CGP54626A was determined over a range of radioligand concentrations (0.05–30 nM) in the absence or presence of 10 mM GABA.
Data analysis
All experiments were performed a minimum of three times. All saturation studies were performed in duplicate. Single data points were obtained for competition binding with membranes and in duplicate for whole cells. Results are given as means±s.e.mean.
[3H]-CGP54626A saturation binding data was analysed by a computer based non-linear curve fitting program to obtain KD and Bmax values. Competition curves were analysed by use of the ALLFIT model (DeLean et al., 1977). IC50 values were derived from this analysis and converted to Ki values by use of the Cheng & Prusoff (1973) equation.
Materials
([S-(R*,R)]-[3-[[1-(3,4-Dichlorophenyl)ethyl]amino]-2-hydroxypropyl]([3,4-3H]-cyclohexylmethyl)phosphinic acid ([3H]-CGP54626A) (40 Ci/mmol); GABA, (RS)Baclofen, (+)-2S-5,5-Dimethyl-20-morpholineacetic acid (SCH 50911); (3-Aminopropyl)(methyl)phosphinic acid) (SKF 97541); (RS)-3-Amino-2-(4-chlorophenyl)-2-hydroxypropylsulphonic acid) (2-Hydroxysaclofen); 4-Aminobutylphosphonic acid (ABPA) were all purchased from Tocris Cookson (Bristol, U.K.).
3-Aminopropylphosphinic acid (CGP27492); ((3-Aminopropyl)(diethoxymethyl)phosphinic acid) (CGP35348); [3-[[(3,4-Dichlorophenyl)methyl]amino]propyl](diethoxymethyl)phosphinic acid (CGP 52432); [(2S)-3-[[(1S)-1-(3,4-Dichlorophenyl)ethyl]amino]-2-hydroxypropyl](phenylmethyl)phosphinic acid (CGP55845); [S-(R*,R)]-[3-[[1-(3,4-Dichlorophenyl)ethyl]amino]-2-hydroxypropyl](cyclohexylmethyl)phosphinic acid (CGP54626A); ((3-Amino-propyl)(cyclohexylmethyl)phosphinic acid) (CGP46381); [(2R)-3-Amino-2-hydroxypropyl](cyclohexylmethyl)phosphinic acid (CGP51176) and (3-aminopropyl)butylphosphinic acid (CGP36742) are Novartis compounds but were synthesized in house. Other reagents listed were from the stated suppliers unless from Sigma–Aldrich or Fisher Scientific.
Results
Stable expression of GABAB receptors in CHO cells
Stable expression of functional GABAB(1a,2) and GABAB(1b,2) receptors was achieved with a CHO K1 host cell line. Employing a cyclic AMP assay, GABA produced an inhibition of forskolin evoked cyclic AMP accumulation in>75% of the clones tested (data not shown). Six clones each representing GABAB(1a,2) and GABAB(1b,2) expression, in which complete inhibition of forskolin was measured, were selected for immunoblotting of GABAB receptor proteins. GABAB(2) protein was detected in all of the P2 membrane preparations, appearing as a distinct single band of the expected size with relative molecular mass ∼120 K (Mr 120 K). The GABAB(1) subunits were also visualized and appeared as bands of the expected sizes of Mr ∼120 K and ∼100 K for GABAB(1a) and GABA B(1b), respectively (Figure 1). Unlike the GABAB(2) both of the GABAB(1) proteins appeared to run as doublets. The immunoblotting also revealed small differences in the relative amount of GABAB protein expression between the clones tested. However, a direct comparison of expression of GABAB(1) relative to GABAB(2) cannot be made due to the different antigenic specificities of antibodies used. In order to provide membrane preparations for binding studies, those clones expressing the highest amounts of GABAB protein were selected for detailed characterization. Hence, all subsequent data was obtained using GABAB(1a,2) #33 and GABAB(1b,2) #74.
Figure 1.
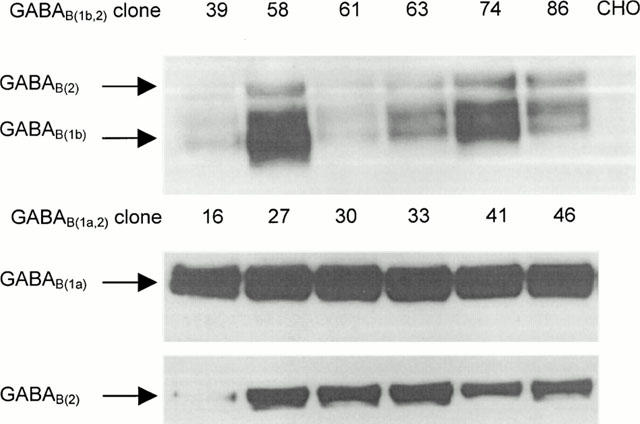
Visualization of GABAB receptors stably expressed in CHO cell clones by immunoblotting with pan-specific GABAB(1) and specific GABAB(2) antibodies to C terminal epitopes. Western blots reveal relative expression levels of selected clones compared with untransfected CHO K1 cells.
These two clones have also been employed for the functional characterization of GABAB receptors (Martin et al., 2000).
Saturation binding
Saturation analysis of [3H]-CGP54626A binding to both GABAB receptors was saturable and non linear curve fitting of this data indicated that this binding was to a single site. Affinity estimates, slope and Bmax values for [3H]-CGP54626A binding are summarized in Table 1. Total binding did not exceed 10% of the amount of [3H]-CGP54626A added and specific binding was>80% at the KD value (Figure 2).
Table 1.
Summary of saturation studies of [3H]-CGP54626A binding to GABAB(1a,2) and GABAB(1b,2) receptors

Figure 2.

Representative saturation curve data for [3H]-CGP54626A binding to GABAB(1a,2) (A) and GABAB(1b,2) (B). Each point represents mean of duplicate determinations from a single experiment.
Effect of Gpp(NH)p and Ca2+ removal on saturation binding
Saturation binding was repeated in the absence of calcium cations and in the presence of Gpp(NH)p. Their effects on the binding of the antagonist ligand to GABAB receptors are summarized in Table 1. For both GABAB(1a,2) and GABAB(1b,2) the removal of calcium from the assay caused an increase in KD value of [3H]-CGP54626A without affecting slope values but produced a reduction in calculated Bmax values. The inclusion of 1 mM EGTA, to ensure complete chelation of Ca2+ ions from the assay, caused a decrease in both total and non-specific binding in all assays when included and may be attributable to non receptor mediated effects. The addition of 30 μM Gpp(NH)p had no significant effect on the estimated affinity of [3H]-CGP54626A for both GABAB(1a,2) and GABAB(1b,2). However, for both receptors Bmax values were slightly increased relative to control conditions.
Competition studies
A panel of GABAB ligands was used to displace [3H]-CGP54626A binding to both GABAB(1a,2) and GABAB(1b,2) receptors. All of the compounds tested produced complete inhibition of specific binding with a range of calculated affinity estimates that are summarized in Table 2. Inhibition curves, under normal conditions with CaCl2 included in the assay buffer, are shown in Figures 3 and 4 for displacement of binding to GABAB(1a,2) and GABAB(1b,2) receptors, respectively. The rank order for ligand displacement was identical for binding at both GABAB(1a,2) and GABAB(1b,2). For GABAB agonists tested the rank order was CGP27492>SKF97541=CGP46381>GABA>Baclofen. For GABAB antagonists the rank order was CGP54262A>CGP 55845>CGP52432>SCH50911>CGP51176>CGP36742=CGP35348⩾2-OH Saclofen⩾ABPA.
Table 2.
Summary of affinity values for the displacement of [3H]-CGP54626A binding to GABAB(1a,2) and GABAB(1b,2) receptors

Figure 3.
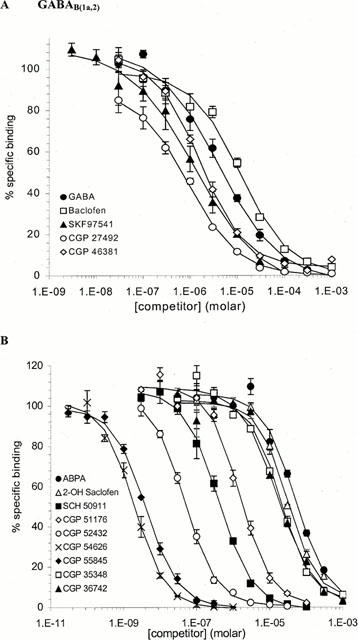
Competition studies of [3H]-CGP54626A binding to GABAB(1a,2) receptors. Displacement of [3H]-CGP546262A binding by GABAB agonists (A) and antagonists (B) is represented as per cent specific binding. Each curve represents mean±s.e.mean from n=3 experiments.
Figure 4.

Competition studies of [3H]-CGP54626A binding to GABAB(1b,2) receptors. Displacement of [3H]-CGP546262A binding by GABAB agonists (A) and antagonists (B) is represented as per cent specific binding. Each curve represents mean±s.e.mean from n=3 experiments.
In the absence of CaCl2, a complete displacement of [3H]-CGP54626A binding was observed with all of the GABAB ligands. However, the effect of removal of CaCl2 from such assays was ligand dependent and resulted in either no marked effect or a decrease in ligand affinity. These effects are also summarized in Table 2. We also derived a relative molar ratio for ligand affinity from the Ki values obtained in the absence and presence of calcium and indicate that similar effects were observed for both of the GABAB receptors. GABA, SKF 97541, CGP27492, ABPA and CGP52432 were most sensitive to the removal of calcium which lead to at least a 10 fold decrease in affinity for both receptors. In contrast, the affinity of 2-OH Saclofen, CGP51176 and CGP35348 was only decreased by a maximum of 3 fold at both GABAB(1a,2) and GABAB(1b,2).
[3H]-CGP54626A whole cell scintillation proximity binding assays
A whole cell binding assay was established to determine ligand binding at GABAB receptors expressed at the cell surface. This characterization was performed under normal GABAB binding conditions with calcium included in the buffer. Saturation studies were performed on both cell lines and analysis of [3H]-CGP54626A binding to both GABAB receptors was saturable and non-linear curve fitting of this data indicated that this binding was to a single site. Affinity estimates and slope values for [3H]-CGP54626A binding are summarized in Table 3. Typical saturation data from whole cell binding is shown in Figure 5. As scintillation proximity assays do not permit accurate determination of DPM values, Bmax values cannot be quoted. However, specific whole cell binding was higher with the GABAB(1b,2) cells relative to GABAB(1a,2) cells, which was consistent with the expression levels calculated in the P2 membrane filter binding assays.
Table 3.
Summary of whole cell binding saturation studies of [3H]-CGP54626A binding to GABAB(1a,2) and GABAB(1b,2) receptors

Figure 5.

Representative saturation curve data for [3H]-CGP54626A binding to GABAB(1a,2) Each point represents the mean of duplicate determinations from a single experiment.
In competition studies all of the GABAB agonists tested fully displaced the [3H]-CGP54626A whole cell binding. Displacement of binding to both GABAB(1a,2) and GABAB(1b,2) are summarized in Figure 6. The rank order for displacement of whole cell binding was identical for both receptors: CGP27492>SKF97541⩾CGP46381⩾GABA>Baclofen which was in good agreement with that obtained from filtration binding to P2 membrane fractions.
Figure 6.

Competition studies of [3H]-CGP54626A binding to GABAB receptors. Displacement of [3H]-CGP546262A whole cell binding at GABAB(1a,2) (A) and GABAB(1b,2) (B) is represented as per cent specific binding. Each curve represents mean±s.e.mean from n=3 experiments.
Discussion
There have been several reports describing the pharmacology of the GABAB receptor since it was first cloned (for review see Bowery & Enna, 2000). These studies have relied primarily on the use of transiently expressed GABAB subunits individually or as pairs to reconstitute a heterodimeric receptor. This approach has permitted the functional characterization of receptor behaviour and provided further insight into the molecular nature of the receptor such as allosteric modulation of the receptor by calcium cations (Wise et al., 1999; Galvez et al., 2000). Such experiments have also highlighted the importance of heterodimerization of GABAB receptor subunits with respect to ligand affinity at the receptor.
In this paper we have described the assembly and characterization of GABAB receptor cell lines stably expressing particular pairs of proteins that represent the two major isoforms of the GABAB receptor complex that have been identified in distribution studies, that is GABAB(1a,2) and GABAB(1b,2).
Clones to be used for detailed characterization were initially selected by their ability to respond to GABAB agonists in a functional assay, such as the inhibition of cyclic AMP accumulation. Expression of GABAB(1) and GABAB(2) proteins in these functional clones was then confirmed by immunoblotting with specific protein antibodies (Figure 1). Whilst this information alone cannot determine the stoichiometry of subunit expression in these cell lines, we have extended our ligand binding studies to include a whole cell (SPA) binding assay to assess agonist affinity at the cell surface and therefore at exclusively heterodimeric receptors. As agonist affinity has been shown to be lower at GABAB(1) expressed alone, if the pharmacology of agonists in the whole cell binding assay is comparable with that using membranes preparations containing total expressed protein, it may suggest that the clones are expressing amounts of GABAB(2) proteins that are in excess of GABAB(1). Therefore, any characterization of these cell lines may be expected to be representative of the native receptor pharmacology.
We have compared the pharmacology of GABAB receptors using the tritiated, high affinity antagonist ligand CGP54626A. In saturation studies with membrane preparations, under standard buffer conditions, that included calcium cations, we obtained KD values that were consistent with previously published data using rat brain membranes. These were 1.51 nM and 0.86 nM for GABAB(1a,2) and GABAB(1b,2) respectively, compared with 1.48 nM from rat brain membranes that contain a heterologous population of GABAB receptors (Bittiger et al., 1992). Similar values were also obtained in saturation studies using the whole cell binding assay (Tables 1 and 3).
To further investigate the G protein coupling of these receptors we next examined the effects of the guanylylnucleotide analogue Gpp(NH)p. In paired, saturation studies, the KD was unaffected by the presence of Gpp(NH)p whilst we observed a trend towards an increase in the calculated Bmax value. However, statistical analysis indicated that this effect was not significant due to experimental variation (Table 1). For most G protein coupled receptors the addition of such analogues results in a shift in the equilibrium from high to low affinity states. Where certain ligands can discriminate between high and low affinity receptor states this would alter the number of sites labelled. For example, Marshall et al. (1997) demonstrated that an agonist ligand for prostaglandin EP4 receptors only labelled high affinity state receptors and a similar observation was obtained by Clark & Hill (1995) for [3H]-Nα-methyl-histamine binding to histamine H3 receptors. Our studies may indicate that this ligand discriminates for low affinity state receptors only. Although further studies are required to investigate this suggestion in greater detail, our observations with Gpp(NH)p combined with the functional data to select clones, indicate that the stably expressed receptors are G protein coupled.
Several groups have suggested the existence of GABAB receptor subtypes on the basis of subtle pharmacological differences in functional studies using in vitro and in vivo preparations. In contrast, binding studies have not revealed significant pharmacological differences of antagonist affinity between GABAB(1a) and GABAB(1b) receptors expressed alone (Kaupmann et al., 1997) compared with native receptors or in combination with GABAB(2) (Kaupmann et al., 1998). Similarly, functional assays using transiently transfected mammalian cells to express heterodimeric receptors, did not suggest pharmacological differences using a limited number of ligands (Brauner-Osborne & Krogskard-Larsen, 1999). Clearly, it is not possible to confirm the existence of GABAB receptor subtypes with these current data.
In these competition studies, under normal conditions, the rank order for displacement of [3H]-CGP546262A binding by agonists and antagonists was identical for both GABAB(1a,2) and GABAB(1b,2) receptors (Figures 3 and 4). The rank order for GABAB agonists correlates well with the literature in which the phosphinic acids are shown to be the most potent GABAB agonists. The highest affinity agonists were CGP27492 (3-aminopropyl) phosphinic acid and its methyl analogue, SKF97541. CGP46381 ((3-Amino-propyl)(cyclohexylmethyl)phosphinic acid), which we have described as a partial agonist (unpublished observation), also produced complete displacement of binding. The rank order for GABAB antagonists is also in accordance with published data with the phosphinic analogues showing the highest affinity. Interestingly, the rank order generated with these heterodimeric receptors is in good agreement with rank orders obtained with binding studies at the GABAB(1) subunit expressed alone. This further supports the growing evidence in favour of GABAB ligands exclusively binding to the GABAB(1) subunit. Additionally, in all studies calculated Hill coefficients were close to unity suggestive of ligand binding to a single site. Clearly, the GABAB(2) contributes to formation of the heterodimer and ligand affinity but there is a lack of evidence to date in support of GABAB(2) binding ligands directly.
We have established a whole cell binding assay to further study the affinity of GABAB agonists at the GABAB receptor. This robust assay format uses a solid bead-based scintillation counting method for detecting receptor-ligand binding interactions. SPA beads only emit a light response when ligand is in close proximity to receptor and since they cannot penetrate the plasma membrane of intact cells, this technique permits examination of ligand binding to exclusively cell surface receptors. In the case of GABAB, this permits the study of a pure population of heterodimeric receptors without any interference from non-dimerized GABAB receptor proteins that could exist in membrane preparations. This approach is particularly pertinent to the characterization of GABAB receptor agonists since their true affinity (or that which is comparable to native receptors) is only revealed when the receptor is heterodimeric with 1 : 1 stoichiometry of receptor subunit proteins (White et al., 1998).
In saturation studies with whole cells, the affinity of [3H]-CGP54626A was marginally lower, but still comparable to that observed with membrane fractions. Furthermore, in competition studies the rank order for agonist displacement also closely resembled that previously determined in the filter binding assay with membrane fractions. This rank order was once again identical for both GABAB receptor isoforms. As the rank order is the same, an interpretation of this is that these stable cell lines are expressing GABAB(2) proteins in excess of GABAB(1) hence the latter will be binding ligand in its heterodimeric form.
It was revealed early in the initial characterization of GABAB receptors that physiological concentrations of calcium are required to promote [3H]-GABA binding (Hill and Bowery, 1981; Bowery et al., 1983). More recent studies have revealed that this phenomenon may be attributed to the allosteric modulation of the GABAB receptor by calcium. Regulation of receptor function has been shown to modulate ligand potency and affinity (Wise et al., 1999; Galvez et al., 2000; Martin et al., 2000).
We have also compared the effects of calcium cations on ligand binding to a range of agonists and antagonists at both of the GABAB receptor subtypes with competition binding assays. These data also indicate that the two, heterodimeric isoform pairs behave identically to one another in the absence of calcium cations. The rank order for ligands is altered from that determined in the presence of calcium but still remains equal for each of the receptor isoforms examined. We have calculated relative molar ratios derived from the Ki values obtained in the presence and absence of calcium. This helps to illustrate which ligands are affected by the allosteric regulation of the receptor and a good correlation is apparent between the values obtained at each of the receptor isoforms (Table 2).
The molecular mode of action of calcium cations on receptor-ligand binding is not yet fully understood. However, structural similarity exists between GABAB receptors and other members of the family C GPCRs such as the Ca sensing receptor and mGluR1, which are also influenced by calcium cations (Hammerland et al., 1999; Kubo et al., 1998). A comparison of these receptors at amino acid sequence level prompted subsequent site directed mutation studies and led to Galvez et al. (2000) elucidating key serine residues that are critical for the allosteric modulation of the GABAB receptor. These residues are thought to be in close proximity to other amino acids associated with GABAB ligand binding (Galvez et al., 1999), hence, the allosteric modulation of the GABAB receptor by calcium is likely to exert significant effects on ligand affinity and potency. The data presented herein exemplify that numerous ligands are sensitive to this allosterism, further supporting the probable overlap in the ligand and calcium binding domains of the receptor. It has previously been illustrated that baclofen is less sensitive to this allosterism and it has been suggested that its molecular structure may sterically hinder the Ca2+ allosterism. We have obtained similar observations in these studies and have also shown that other agonist and antagonist ligands, in particular 2-OH Saclofen and CGP35348, are less susceptible to the effects of calcium. In light of this data, the calcium sensitivity of potential therapeutic agents at the GABAB receptor should be an important consideration. We have also attempted to use the calcium sensitivity data in parallel with three-dimensional modelling of such ligand structures to predict which pharmacophores are most likely to be influenced by calcium. However, at present we still have insufficient data or enough chemical diversity to draw any conclusions.
In summary, using ligand binding studies we have performed an extensive characterization of the two main isoforms of the GABAB receptor. Our data strongly support other published observations suggesting that in terms of ligand binding, using currently available ligand tools, these heterodimeric proteins do not represent any pharmacological subtypes. However, the existence of receptor subtypes cannot be disregarded. Further characterization should give consideration to the distribution of particular GABAB receptor proteins and their relative expression levels as this could modify receptor-ligand interactions. The functionality of these and other, as yet unidentified GABAB receptor proteins, in respect to G protein coupling, their second messenger and effector systems could also be responsible for possible existence of novel receptor subtypes.
Abbreviations
- CaSR
Calcium sensing receptor
- CHO
Chinese hamster ovary
- GABA
γ-amino butyric acid
- GPCR
G protein coupled receptor
- mGluR
metabotropic glutamate receptor
- SPA
scintillation proximity assay
References
- BILLINTON A., UPTON N., BOWERY N.G. GABAB receptor isoforms GBR1a and GBR1b, appear to be associated with pre- and post-synaptic elements respectively in rat and human cerebellum. Br. J. Pharmacol. 1999;126:1387–1392. doi: 10.1038/sj.bjp.0702460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTIGER H., REYMANN N., FROESTL W., MICKEL S.J. [3H]CGP54626A: a potent antagonist radioligand for GABAB receptors. Pharmacol. Comm. 1992;2:23. [Google Scholar]
- BONANNO G., FASSIO A., SCHMID G., SEVERI P., SALA R., RAITERI M. Pharmacologically distinct GABAB receptors that mediate inhibition of GABA and glutamate release in human neocortex. Br. J. Pharmacol. 1997;120:60–64. doi: 10.1038/sj.bjp.0700852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWERY N.G., ENNA S.J. γ-aminobutyric acid B Receptors: First of the functional heterodimers. J.P.E.T. 2000;292:2–7. [PubMed] [Google Scholar]
- BOWERY N.G., HILL D.R., HUDSON A.L. Characterisation of GABAB receptor binding sites on rat whole brain synaptic membranes. Br. J. Pharmacol. 1983;78:191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUNER-OSBOURNE H., KROGSKARD-LARSEN P. Functional pharmacology of cloned heterodimeric GABAB receptors expressed in mammalian cells. Br. J. Pharmacol. 1999;128:1370–1374. doi: 10.1038/sj.bjp.0702914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL V., BERROW N., DOLPHIN A.C. GABAB receptor modulation of Ca2+ currents in rat sensory neurones by the G protein Go: Antisense oligonucleotide studies. J. Physiol. 1993;470:1–11. doi: 10.1113/jphysiol.1993.sp019842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 59 percent inhibition (IC50) of an enzyme reaction. Biochem. Pharmacol. Sci. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CLARK E.A., HILL S.J. Differential effect of sodium ions and guanylnucleotides on the binding of thioperamide and clobenpropit to histamine H3 receptors in rat cerebral cortical membranes. Br. J. Pharmacol. 1995;114:357–362. doi: 10.1111/j.1476-5381.1995.tb13234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK J.A., MEZEY E., LAM A., BONNER T.I. Distribution of the GABAB receptor subunit gb2 in the rat CNS. Brain Res. 2000;86:41–52. doi: 10.1016/s0006-8993(00)01958-2. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM M.D., ENNA S.J. Evidence for pharmacologically distinct GABAB receptors associated with cAMP production in rat brain. Brain Research. 1996;720:220–224. doi: 10.1016/0006-8993(96)00120-5. [DOI] [PubMed] [Google Scholar]
- DE LEAN A., MUNSON P.J., RODBARD D. Simultaneous analysis of families of sigmoidal curves: applications to bioassay, radio-ligand assay, and physiological dose-response curves. Am. J. Physiol. 1977;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- GALVEZ T., PARMENTIER M.L., JOLY C., MALITSCHEK B., KAUPMANN K., KUHN R., BITTIGER H., FROESTL W., BETTLER B., PIN J.P. Mutagenesis and modelling of the GABAB receptor extracellular domain support a venus flytrap mechanism for ligand binding. J. Biol. Chem. 1999;274:13362–13369. doi: 10.1074/jbc.274.19.13362. [DOI] [PubMed] [Google Scholar]
- GALVEZ T., URWYLER S., PREZEAU L., MOSBACHER J., JOLY C., MALITSCHEK B., HEID J., BRABET I., FROESTL W., BETTLER B., KAUPMANN K., PIN J.P. Ca2+ requirement for high-affinity gamma-aminobutyric acid (GABA) binding at GABAB receptors: involvement of serine 269 of the GABA(B)R1 subunit. Molecular Pharmacology. 2000;57:419–426. doi: 10.1124/mol.57.3.419. [DOI] [PubMed] [Google Scholar]
- HAMMERLAND L., GARRET J., HUNG B., LEVINTHAL C., NEMETH E. Domains determining ligand specificity for Ca2+ receptors. Mol. Pharmacol. 1999;55:642–648. [PubMed] [Google Scholar]
- HILL D.R., BOWERY N.G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABAB sites in rat brain. Nature. 1981;290:149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- ISOMOTO S., KAIBARA M., SAKURAI-YAMASHITA Y., NAGAYAMA Y., UEZONO Y., YANO K., TANIYAMA K. Cloning and tissue distribution of novel splice variants of the rat GABAB receptor. Biochem. Biophys. Res. Comm. 1998;253:10–15. doi: 10.1006/bbrc.1998.9706. [DOI] [PubMed] [Google Scholar]
- JONES K.A., BOROWSKY B., TAMM J.A., CRAIG D.A., DURKIN M.M., DAI M., YAO W.J., JOHNSON M., GUNWALDSEN C., HUANG L.Y., TANG C., SHEN Q., SALON J.A., MORSE K., LAZ T., SMITH K., NAGARATHNAM D., NOBLE S.A., BRANCHEK T.A., GERALD C. GABAB receptors function as a heteromeric assembly of the subunits GABAB -R1 and GABAB -R1. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- KAUPMANN K., HUGGEL K., HEID J., FLOR P.J., BISCHOFF S., MICKEL S.J., MCMASTER G., ANGST C., BITTIGER H., FROESTL W., BETTLER B. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- KAUPMANN K., MALITSCHEK B., SCHULER V., HEID J., FROESTL W., BECK P., MOSBACHER J., BISCHOFF S., KULIK A., SHIGEMOTO R., KARSCHIN A., BETTLER B. GABAB receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- KUBO Y., MIYASHITA T., MURATA Y. Structural basis for a Ca2+-sensing function of the metabotropic glutamate receptors. Science. 1998;279:1722–1725. doi: 10.1126/science.279.5357.1722. [DOI] [PubMed] [Google Scholar]
- MALITSCHEK B., SCHWEIZER C., KEIR M., HEID J., FROESTL W., MOSBACHER J., KUHN R., HENLEY J., JOLY C., PIN J.P., KAUPMANN K., BETTLER B. The N-terminal domain of gamma-aminobutyric Acid(B) receptors is sufficient to specify agonist and antagonist binding. Mol. Pharmacol. 1999;56:448–454. doi: 10.1124/mol.56.2.448. [DOI] [PubMed] [Google Scholar]
- MARSHALL F.H., JONES K.A., KAUPMANN K., BETTLER B. GABAB Receptors–the first of the heterodimers. Trends in Pharmacol. Sci. 1999;20:396–399. doi: 10.1016/s0165-6147(99)01383-8. [DOI] [PubMed] [Google Scholar]
- MARSHALL F.H., PATEL K., LUNDSTROM K., CAMACHO J., FOORD S., LEE M.G. Characterisation of [3H]-PGE2 binding to prostanoid EP4 receptor expressed with Semliki Forest Virus. Br. J. Pharmacol. 1997;121:1673–1678. doi: 10.1038/sj.bjp.0701332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A., PARSONS S., WILSON R., GREEN A., WALLS S., GILES H., MARSHALL F.H. Pharmacological analysis of human GABAB(1a,2) and GABAB(1b,2) heterodimers expressed in CHO cells: allosteric modulation of agonists and antagonists by Ca2+ Br. J. Pharmacol. 2000;129:82P. [Google Scholar]
- MARTIN S.C., RUSSEK S.J., FARB D.H. Molecular identification of the human GABABR2: cell surface expression and coupling to adenylyl cyclase in the absence of GABABR1. Mol. Cell. Neurosci. 1999;13:180–191. doi: 10.1006/mcne.1999.0741. [DOI] [PubMed] [Google Scholar]
- MENON-JOHANSSON A., DOLPHIN A.C. Go transduces GABA B receptor modulation of N-type calcium channels in cultured dorsal root ganglion neurons. Pflügers Arch. 1993;425:335–343. doi: 10.1007/BF00374184. [DOI] [PubMed] [Google Scholar]
- REES E.S., COOTE J., STABLES J., GOODSON S., HARRIS S., LEE M.G. Bicistronic vector for the creation of stable cell lines that predisposes all antibiotic-resistant cells to express recombinant protein. Biotechniques. 1996;20:102–110. doi: 10.2144/96201st05. [DOI] [PubMed] [Google Scholar]
- SEABROOK G.R., HOWSON W., LACEY M.G. Electrophysiological characterisation of potent agonists and antagonists at pre- and post-synaptic GABAB receptors on neurones in rat brain slices. Br. J. Pharmacol. 1990;101:949–957. doi: 10.1111/j.1476-5381.1990.tb14186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P.K., KROHN R.I., HERMANSON G.T., MALLIA A.K., GARTNER F.H., PROVENZANO M.D., FUJIMOTO E.K., GOEKE N.M., OLSON B.J., KLENK D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- WHITE J.H., WISE A., MAIN M.J., GREEN A., FRASER N.J., DISNEY G.H., BARNES A.A., EMSON P., FOORD S.M., MARSHALL F.H. Heterodimerization is required for the formation of a functional GABAB receptor. Nature. 1998;396:679–683. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- WISE A., GREEN A., MAIN M.J., WILSON R., FRASER N., MARSHALL F.H. Calcium sensing properties of the GABAB receptor. Neuropharmacology. 1999;38:1647–1656. doi: 10.1016/s0028-3908(99)00119-7. [DOI] [PubMed] [Google Scholar]
- YU B., YAMADA K., GALLAGHER J.P. GABAB auto versus hetero-receptor sensitivity: implications for novel pharmacotherapy. Neuropharmacology. 1999;38:1805–1809. doi: 10.1016/s0028-3908(99)00120-3. [DOI] [PubMed] [Google Scholar]


