Abstract
The effects of the long lasting and potent PAF receptor antagonist UK74505 were assessed on the local and remote injuries following ischaemia and reperfusion (I/R) of the superior mesenteric artery (SMA) in rats.
In a severe model of ischaemia (120 min) and reperfusion (120) injury, in addition to the local and remote increases in vascular permeability and neutrophil accumulation, there was significant tissue haemorrhage, blood neutropenia, systemic hypotension and elevated local and systemic TNF-α levels.
Post-ischaemic treatment with the selectin blocker fucoidin (10 mg kg−1) prevented neutrophil accumulation in tissue and, in consequence, all the local and systemic injuries following severe I/R.
Treatment with an optimal dose of UK74505 (1 mg kg−1) also reversed local and remote neutrophil accumulation, increases in vascular permeability and intestinal haemorrhage. UK74505 partially inhibited blood neutropenia and reperfusion-induced hypotension.
Interestingly, both fucoidin and UK74505 prevented the local, but not systemic, increases of TNF-α levels following severe I/R injury, demonstrating an important role of migrating cells for the local production of TNF-α. However, the results do not support a role for PAF as an intermediate molecule in the production of systemic TNF-α.
The beneficial effects of UK74505 and other PAF receptor antagonists in models of I/R injury in animals and the safety of UK74505 use in man warrant further investigations of the use of this drug as preventive measure for I/R injury in humans.
Keywords: Ischaemia-reperfusion, neutrophils, oedema, PAF antagonist, TNF-α, mesenteric artery, UK74505
Introduction
The reduction in blood flow, ischaemia, to an organ or a vascular bed can lead to significant tissue injury and cell death if it is prolonged. Thus, the main goal following ischaemia is the restoration of blood flow, i.e. the ‘reperfusion' of the ischaemic vascular bed (Lefer & Lefer, 1996; Willerson, 1997). However, reperfusion may lead to recruitment and activation of leukocytes, release of mediators of the inflammatory process and further injury to the affected vascular bed (Lefer & Lefer, 1996; Willerson, 1997). Neutrophils and TNF-α are among the cell types and inflammatory mediators, respectively, thought to be of major relevance in the pathophysiology of ischaemia and reperfusion (I/R) injury (e.g. Kubes et al., 1995; Gilmont et al., 1996; Cornejo et al., 1997). Thus, strategies which limit neutrophil accumulation and TNF-α production induced by the reperfusion process may be useful in the treatment of ischaemic disorders in various organs.
The administration of the membrane-derived phospholipid PAF in vivo mimics several aspects of the injury occurring after reperfusion of ischaemic vascular beds, including the local increase of vascular permeability, remote pulmonary oedema and hypotension (see Carter et al., 1996 and references therein). In addition, PAF is a potent and effective neutrophil chemoattractant and activator (e.g. Kim et al., 1995; Werr et al., 1998) and may also play a role in the transduction pathways leading to TNF-α production (Yamada et al., 1999). Pharmacological strategies which block the PAF receptor or inhibit the production of this lipid mediator may prevent neutrophil accumulation and TNF-α production and, thus, be useful in the prevention of injuries following myocardial I/R (Loucks et al., 1997). Fewer studies have evaluated the potential usefulness of PAF receptor antagonists for the treatment of the reperfusion injuries following ischaemia of other arterial beds (e.g. Canale et al., 1994; Carter et al., 1996). This is clinically relevant as I/R injuries occur in several situations other than just after myocardial infarction. In addition, most studies evaluate the effects of pharmacological strategies on the local (specially macromolecular leakage) and, less frequently, the systemic changes and lethality following I/R injuries (e.g. Kubes et al., 1990; Canale et al., 1994). Moreover, little emphasis has been placed on remote injury to the lung (e.g. Carter et al., 1996) or the control of systemic versus local TNF-α levels.
Here, we describe the effects of the PAF receptor antagonist UK74505 (Parry et al., 1994) on the local and systemic injuries following I/R of the superior mesenteric artery (SMA) in rats. UK74505 is a long-acting and selective PAF receptor antagonist in animals and humans (Alabaster et al., 1991; Parry et al., 1994; Jezequel et al., 1996) and has been tested clinically for asthma (Kuitert et al., 1995). Thus, this compound could potentially be tested for other human inflammatory diseases. Initial dose-response experiments were conducted in a mild model of neutrophil-dependent I/R injury previously described by our group (Souza et al., 2000). We then tested the effectiveness of our strategy in a more severe I/R model in which severe local and systemic changes were observed. In this more severe model, the role of neutrophils was assessed by pretreating animals with the selectin blocker fucoidin (Teixeira & Hellewell, 1997 and references therein) at doses previously shown to inhibit neutrophil rolling and accumulation in vivo (Ley et al., 1993; Souza et al., 2000). Of interest, the PAF receptor antagonist and fucoidin were administered just prior to the reperfusion at the end of the ischaemic period. This is an important point because it bears the closest resemblance to what happens in the real clinical situation (Loucks et al., 2000) when patients are usually seen during the period of ischaemia and prior to the strategy which will lead to reperfusion.
Methods
Animals
Male Wistar rats (200–200 g) obtained from the Bioscience unit of our Institution were housed in standard conditions and had free access to commercial chow and water. All procedures described here had previous approval from our Institution animal ethics committee.
Ischaemia and reperfusion injury
Rats were anaesthetized with urethane (140 mg kg−1, i.p.) and laparotomy was performed. The superior mesenteric artery (SMA) was isolated and ischaemia was induced by totally occluding the SMA for 30 or 120 min. After ischaemia, reperfusion was initiated by removal of the occlusion. Animals made ischaemic for 30 or 120 min were allowed to reperfuse for 30 (mild I/R) or 120 (severe I/R) min, respectively. The times of I/R were chosen based on previous experiments (Souza et al., 2000 and data not shown) demonstrating these to be optimal for mild and severe reperfusion injuries. Sham-operated animals or animals only made ischaemic were used as controls for the reperfusion-induced injury.
Initial dose-response experiments were carried out in the mild reperfusion injury model to determine the optimal dose of the PAF receptor antagonist, UK74505, to be used in subsequent experiments. In these experiments, UK74505 was administered i.v. 5 min prior to reperfusion of the superior mesenteric artery at the doses of 0.01 to 1.0 mg kg−1. For comparison, we also tested the effects of the PAF receptor antagonist WEB2170 (10 mg kg−1) administered i.v. 5 min prior to reperfusion. We then tested the effects of the post-ischaemic administration of UK74505 (1.0 m kg−1) in the more severe I/R model. Previous studies from our laboratory (Souza et al., 2000) have shown that the reperfusion injuries in our mild model are neutrophil dependent, as assessed by using the selectin blocker fucoidin or an anti-CD18 monoclonal antibody. Here, a role for neutrophils in the severe reperfusion injury model was assessed by using fucoidin (10 mg kg−1 in saline) administered i.v. 5 min prior to reperfusion. Control animals received drug vehicles. None of the drugs used in the present study had any significant effects on basal parameters (data not shown) and to simplify the graphs presented, basal data obtained in vehicle or drug treated animals have been pooled for presentation.
Evaluation of changes in vascular permeability
The extravasation of Evans blue dye into the tissue was used as an index of increased vascular permeability (De Matos et al., 1999). Evans blue (20 mg kg−1) was administered i.v. (1 ml kg−1) via a femoral vein 2 min prior to reperfusion of the ischaemic artery. Thirty (in the mild model) or 120 min (in the severe model) after reperfusion, fragments of the duodenum (10 cm) were cut open and allowed to dry in a petri dish for 24 h at 37°C. The dry weight of the tissue was calculated and Evans blue extracted using 3 ml of formamide (24 h at room temperature). The amount of Evans blue in the tissue was obtained by comparing the extracted absorbance with that of a standard Evans blue curve read at 620 nm in an ELISA plate reader. Results are presented as the amount of Evans blue in μg per 100 mg of tissue. The mesentery was also extracted en bloc, halved and a similar extraction procedure was performed. The right ventricle was flushed with 20 ml of phosphate buffered saline to wash the intravascular Evans blue in the lungs. The left lung was then excised and used for Evans blue extraction. The right lung was used for the determination of myeloperoxidase as described below.
Myeloperoxidase levels
The extent of neutrophil accumulation in the intestine, mesentery and right lung tissue was measured by assaying myeloperoxidase activity as previously described (Ivey et al., 1995; De Matos et al., 1999). Briefly, a fragment of duodenum, half the mesentery and the flushed right lungs of animals that had undergone I/R injury were removed and snap frozen in liquid nitrogen. Upon thawing, the tissue (1 g of tissue per 19 ml of buffer) was homogenized in pH 4.7 buffer (0.1 M NaCl, 0.02 M NaPO4, 0.015 M NaEDTA), centrifuged at 260×g for 10 min and the pellet underwent hypotonic lysis (15 ml of 0.2% NaCl solution followed 30 s later by addition of an equal volume of a solution containing NaCl 1.6% and glucose 5%). After a further centrifugation, the pellet was then resuspended in 0.05 M NaPO4 buffer (pH 5.4) containing 0.5% hexadecyltrimethylammonium bromide (HTAB) and re-homogenized. One ml aliquots of the suspension were transferred into 1.5 ml-Eppendorf tubes followed by three freeze-thaw cycles using liquid nitrogen. These were then centrifuged for 15 min at 10,000×g and the supernatants used for MPO measurements. Samples of intestine (1/30 or 1/50), mesentery (1/10) and lung (1/50 or 1/90) were diluted prior to the assay. These dilutions were determined to be optimal in preliminary experiments. Myeloperoxidase activity in the resuspended pellet was assayed by measuring the change in optical density (O.D.) at 450 nm using tetramethylbenzidine (1.6 mM) and H2O2 (0.5 mM). Results were expressed as total number of neutrophils by comparing the O.D. of tissue supernatant to the O.D. of rat peritoneal neutrophils processed in the same way. To this end, neutrophils were induced in the peritoneum of rats by injecting 3 ml of casein 5%. A standard curve of neutrophil (>95% purity) numbers versus O.D. was obtained by processing purified neutrophils as above and assaying for MPO activity.
Determination of the levels of circulating leukocytes
The total number of circulating leukocytes and neutrophils were evaluated in blood samples obtained via a cannula in the femoral artery. Samples were collected prior to ischaemia (time 0), 60 and 120 min after ischaemia and 15, 30, 60 and 120 min after reperfusion. The number of total circulating leukocytes was determined by counting leukocytes in a modified Neubauer chamber after staining with Turk's solution and differential counts by evaluating the percentage of each leukocyte on blood films stained with May-Grunwald-Giemsa.
Measurement of arterial blood pressure
Arterial blood pressure was determined via a femoral artery cannula which was connected to a pressure transducer device. The transducer was connected to an amplifier (CWE Inc.) and to a computer with a data analysis system (Windaq). Blood pressure was measured during all I/R.
Measurement of haemoglobin levels
The levels of haemoglobin in tissue were used as an index of tissue haemorrhage. After washing and perfusing the intestines to remove excess blood in the intravascular space, a sample of approximately 100 mg of duodenum was removed and homogenized in Drabkin's colour reagent according to instructions of the manufacturer (Analisa, Belo Horizonte, Brazil). The suspension was centrifuged for 15 min at 3000×g and filtered using 0.2 μm filters. The resulting solution was read using an ELISA plate reader at 520 nm and compared against a standard curve of haemoglobin.
Measurement of TNF-α levels in serum and intestine
TNF-α levels were measured in serum and intestine of animals using an ELISA technique previously described (Rees et al., 1999). Serum was obtained from coagulated blood (15 min at 37°C, then 30 min at 4°C) and stored at −20°C until further analysis. Serum samples were analysed at a 1 : 1 dilution in PBS. One hundred mg of duodenum of sham-operated and reperfused animals were homogenized in 1 ml of PBS (0.4 m NaCl and 10 mM NaPO4) containing anti-proteases (0.1 mM PMSF, 0.1 mM benzethonium chloride, 10 mM EDTA and 20 KI aprotinin A) and 0.05% Tween 20. The samples were then centrifuged for 10 min at 3000×g and the supernatant immediately used for ELISA assays at a 1 : 5 dilution in PBS. ELISA plates (Nunc MaxiSorb) were coated with a sheep anti-rat TNF-α polyclonal antibody (2 μg/ml) overnight. The plates were washed thrice and then blocked with 1% bovine serum albumin. After a further wash, plates were incubated with samples or recombinant rat TNF-α and incubated overnight. The biotinylated polyclonal antibody was used at a 1 : 2000 dilution and assay had a sensitivity of 16 pg ml−1.
Histology
Sections of duodenum were obtained from the same areas of the small intestine from representative animals in each of the treatment groups. The tissue was fixed in 10% formalin, embedded in paraffin and 4 μm-thick sections obtained. The sections were then stained with haematoxylin and eosin and examined under a light microscope. Lungs were inflated with 2 ml of 10% buffered formalin, removed from the animals and embedded and sectioned as above.
Drugs and reagents
The following drugs were obtained from Sigma (U.S.A.): urethane, Evans blue, fucoidin, hexadecyltrimethylammonium bromide. The PAF receptor antagonist UK74505 (Parry et al., 1994) was a kind gift from Dr John Parry (Pfizer, U.K.) and WEB2170 from Boehringer (Ingelheim, Germany).
Statistical analysis
Results are shown as the mean±s.e.mean. Per cent inhibition was calculated by subtracting the background levels of Evans blue extravasation or myeloperoxidase (obtained in sham-operated animals) from control and treated animals. Differences were compared by using analysis of variance (ANOVA) followed by Student-Newman-Keuls post-hoc analysis. Results with a P<0.05 were considered significant.
Results
Effects of UK74505 on the model of mild ischaemia and reperfusion injury
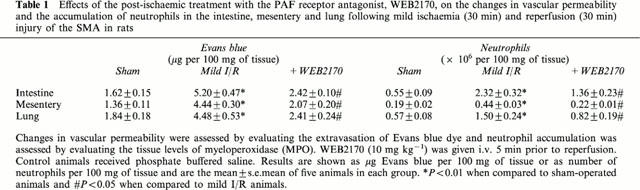
Using a previously established model of neutrophil-dependent mild I/R injury in rats (Souza et al., 2000), we evaluated the dose-dependent inhibitory effects of the PAF receptor antagonist UK74505 on the local and remote increases in vascular permeability (Figure 1) and neutrophil accumulation (Figure 2). Of note, mild I/R induced significant injury not only to the tissues irrigated by the SMA but also remote injury to the lung (Figures 1 and 2). Pretreatment with UK74505 abrogated both local and remote injury with a maximal effect (over 90% inhibition) observed when the dose of 1 mg kg−1 was used (Figures 1 and 2). This dose of UK74505 was used in all further experiments described below. For comparison, we also evaluated the effects of the PAF receptor antagonist WEB2170 (10 mg kg−1). Similarly, to the inhibitory effects of UK74505, pretreatment with WEB2170 abrogated both the local and remote reperfusion injury following ischaemia of the SMA (Table 1).
Figure 1.
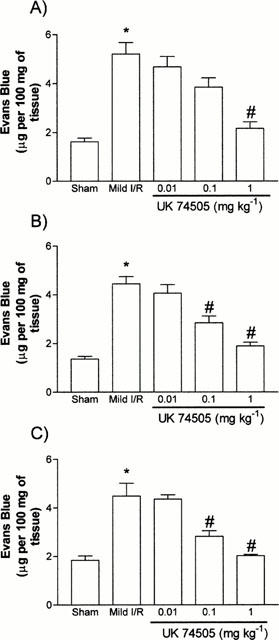
Dose-dependent effects of the post-ischaemic treatment with the PAF receptor antagonist, UK74505, on the changes in vascular permeability in the (A) intestine, (B) mesentery and (C) lung following mild ischaemia (30 min) and reperfusion (30 min) injury of the SMA in rats. Changes in vascular permeability were assessed by evaluating the extravasation of Evans blue dye. UK74505 (0.01 to 1.0 mg kg−1) was given i.v. 5 min prior to reperfusion. Control animals received phosphate buffered saline. Results are shown as μg Evans blue per 100 mg of tissue and are the mean±s.e.mean of 4–6 animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.05 when compared to mild I/R animals.
Figure 2.
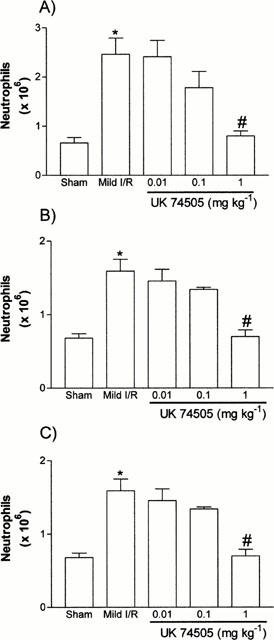
Dose-dependent effects of the post-ischaemic treatment with the PAF receptor antagonist, UK74505, on the accumulation of neutrophils in the (A) intestine, (B) mesentery and (C) lung following mild ischaemia (30 min) and reperfusion (30 min) injury of the SMA in rats. Neutrophil accumulation was assessed by evaluating the tissue levels of myeloperoxidase (MPO). UK74505 (0.01 to 1.0 mg kg−1) was given i.v. 5 min prior to reperfusion. Control animals received phosphate buffered saline. Results are shown as number of neutrophils per 100 mg of tissue and are the mean±s.e.mean of 4–6 animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.01 when compared to mild I/R animals.
Table 1.
Effects of the post-ischaemic treatment with the PAF receptor antagonist, WEB2170, on the changes in vascular permeability and the accumulation of neutrophils in the intestine, mesentery and lung following mild ischaemia (30 min) and reperfusion (30 min) injury of the SMA in rats
Model of severe ischaemia and reperfusion injury
In order to induce a more severe reperfusion injury following ischaemia of the SMA, animals were made ischaemic for a period of 120 min and allowed to reperfuse for the same time. These time points were chosen based on preliminary experiments demonstrating these to be optimal for substantial injury to the small intestine mucosa (data not shown). There were significant local (intestine and mesentery) and remote (lung) increases in vascular permeability (Figure 3) and neutrophil accumulation (Figure 4). Overall, these increases, specially the increases in MPO levels (with the exception of the mesentery, tissue homogenates from severe I/R were diluted for the MPO assay in general 1.5 times more than those of mild I/R animals), were greater in magnitude than those observed after mild I/R injury. Histological examination of the small intestine revealed marked submucosal oedema and hyperaemia, neutrophil influx, globet cell depletion, extensive areas of villous epithelial shedding, and areas of tissue necrosis (Figure 5A). There was also significant tissue haemorrhage, as assessed histologically (Figure 5A) and by measuring the levels of haemoglobin in tissue (Figure 5C).
Figure 3.
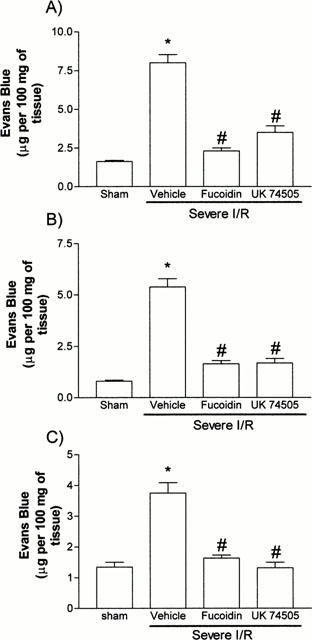
Effects of the post-ischaemic treatment with the selectin blocker, fucoidin, or the PAF receptor antagonist, UK74505, on the changes in vascular permeability in the (A) intestine, (B) mesentery and (C) lung following prolonged ischaemia (120 min) and reperfusion (120 min) of the SMA in rats. Changes in vascular permeability were assessed by evaluating the extravasation of Evans blue dye. Fucoidin (10 mg kg−1) or UK74505 (1 mg kg−1) were given i.v. 5 min prior to reperfusion. Control animals received phosphate buffered saline. Results are shown as μg Evans blue per 100 mg of tissue and are the mean±s.e.mean of 6–7 animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.01 when compared to severe I/R animals.
Figure 4.
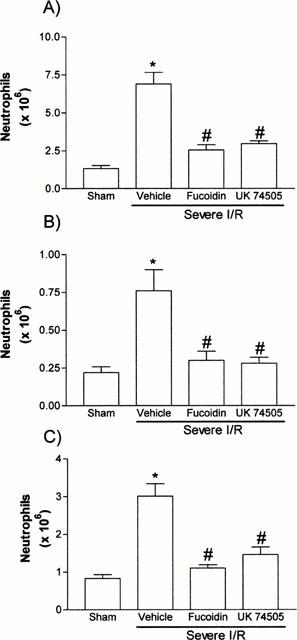
Effects of the post-ischaemic treatment with the selectin blocker, fucoidin, or the PAF receptor antagonist, UK74505, on the accumulation of neutrophils in the (A) intestine, (B) mesentery and (B) lung following prolonged ischaemia (120 min) and reperfusion (120 min) of the SMA in rats. Neutrophil accumulation was assessed by evaluating the tissue levels of myeloperoxidase. Fucoidin (10 mg kg−1) or UK74505 (1 mg kg−1) were given i.v. 5 min prior to reperfusion. Control animals received phosphate buffered saline. Results are shown as number of neutrophils per 100 mg of tissue and are the mean±s.e.mean of 6–7 animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.01 when compared to severe I/R animals.
Figure 5.
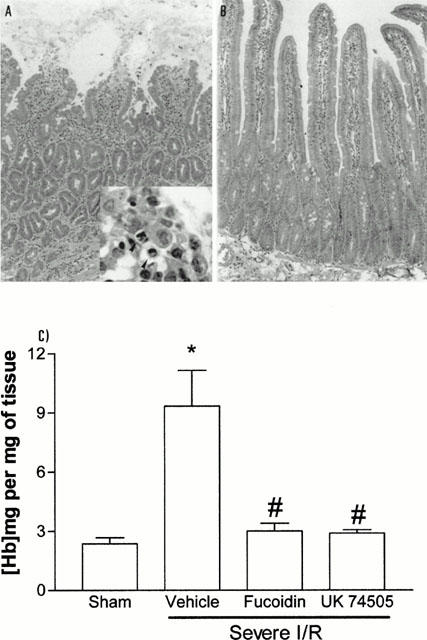
Effects of the post-ischaemic treatment with the PAF receptor antagonist, UK74505, on the histopathological changes and tissue haemorrhage following prolonged ischaemia (120 min) and reperfusion (120 min) of the SMA in rats. Light photomicrographs of transverse sections of duodenum of vehicle-treated animals (A). In (A), note the increased number of infiltrating neutrophils, epithelial shedding, tissue oedema and haemorrhage (Haematoxylin and eosin stain, ×33). In the insert, note the presence of neutrophils (arrow heads) and loose epithelial cells (×200). Following treatment with UK74505 (B), note only the very mild oedema and scarce number of cells scattered throughout the tissue (×33). Tissue haemorrhage (C) was assessed by evaluating the tissue levels of haemoglobin. Fucoidin (10 mg kg−1) or UK74505 (1 mg kg−1) were given i.v. 5 min prior to reperfusion. Control animals received phosphate buffered saline. Results are shown as mg haemoglobin per mg of tissue and are the mean±s.e.mean of 6–7 animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.01 when compared to severe I/R animals.
Not only was there remote injury to the lung, but also, following 120 min of ischaemia, there was a significant and rapid drop of arterial blood pressure as soon as the ischaemia arterial bed was reperfused (Figure 6). Blood pressure dropped by approximately 50 mmHg at 5 min following reperfusion and remained low throughout the first 30 min (Figure 6). There were little further changes in blood pressure thereafter (data not shown).
Figure 6.
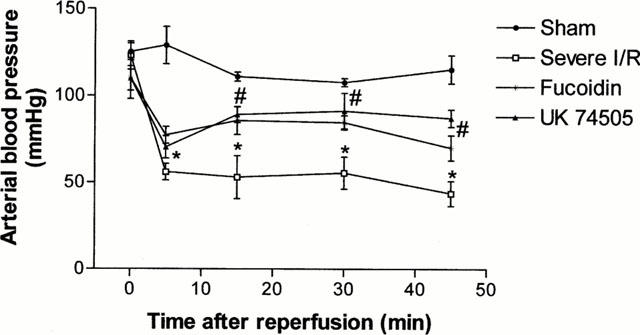
Effects of the post-ischaemic treatment with the selectin blocker, fucoidin, or the PAF receptor antagonist, UK74505, on the rapid fall of blood pressure following prolonged ischaemia (120 min) and reperfusion (120 min) of the SMA in rats. Time 0 represents the time when reperfusion occurred. Fucoidin (10 mg kg−1) or UK74505 (1 mg kg−1) were given i.v. 5 min prior to reperfusion. Control animals received phosphate buffered saline. Results are shown as mmHg and are the mean±s.e.mean of five animals in each group. *P<0.05 when compared to sham-operated animals and #P<0.05 when compared to severe I/R animals.
Examination of total blood leukocytes demonstrated a slow and steady increase in leukocyte numbers following the onset of ischaemia and a rapid and maintained fall immediately after reperfusion (data not shown). These alterations in blood leukocyte levels were explained almost entirely by changes in the circulating levels of neutrophils (Figure 7) with little change in other cell types (data not shown).
Figure 7.
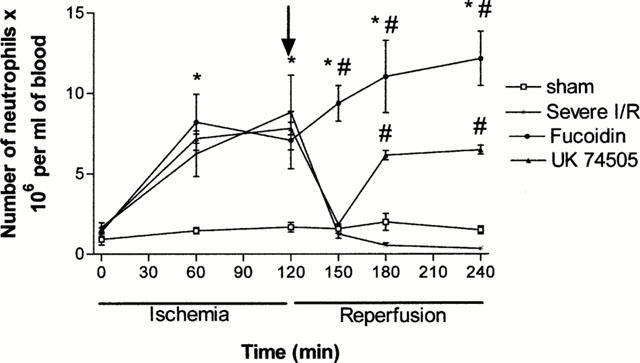
Effects of the post-ischaemic treatment with the selectin blocker, fucoidin, or the PAF receptor antagonist, UK74505, on the levels of circulating neutrophils following prolonged ischaemia (120 min) and reperfusion (120 min) of the SMA in rats. The arrow represents the time when reperfusion started. Fucoidin (10 mg kg−1) or UK74505 (1 mg kg−1) were given i.v. 5 min prior to reperfusion. Control animals received phosphate buffered saline. Results are shown as the number of neutrophils×106 per ml of blood and are the mean±s.e.mean of 5–6 animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.05 when compared to severe I/R animals.
TNF-α levels were evaluated in serum and duodenal homogenates at the end of the reperfusion period in sham operated and severe I/R animals. As seen in Figure 8, TNF-α levels were significantly elevated in the serum and intestine after reperfusion.
Figure 8.
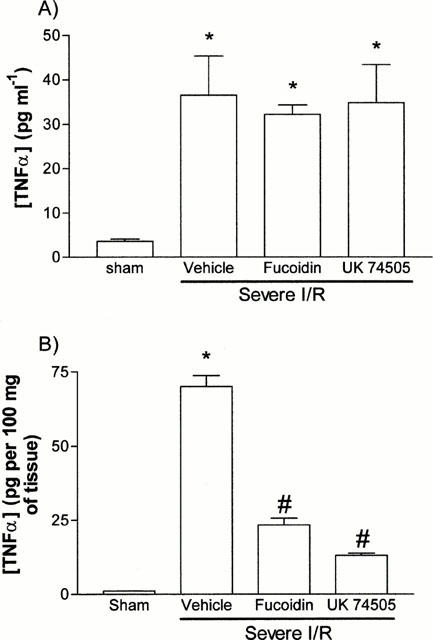
Effects of the post-ischaemic treatment with the selectin blocker, fucoidin, or the PAF receptor antagonist, UK74505, on the (A) systemic and (B) intestinal levels of TNF-α following prolonged ischaemia (120 min) of the SMA in rats. Fucoidin (10 mg kg−1) or UK74505 (1 mg kg−1) were given i.v. 5 min prior to reperfusion. Control animals received phosphate buffered saline. Results are shown as pg TNF-α per ml of plasma or as pg TNF-α per 100 mg of tissue and are the mean±s.e.mean of 5–6 animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.05 when compared to severe I/R animals.
Effects of fucoidin on the model of severe ischaemia and reperfusion injury
The effects of fucoidin, an algal derivative with selectin blocking activity (see Teixeira & Hellewell, 1997 and references therein), were evaluated in order to demonstrate a role for neutrophil migration in the injuries observed. In animals treated with fucoidin just prior to reperfusion, there was a virtually complete inhibition of neutrophil accumulation in the intestine, mesentery and lung, as assessed histologically (data not shown) and by measuring the levels of MPO in tissues (Figure 4). Of interest, the number of circulating neutrophils did not drop after the reperfusion in fucoidin-treated animals (Figure 7), again arguing for the ability of this pharmacological strategy to inhibit the accumulation of neutrophils in sites of inflammation. In these animals, the local and remote increase in vascular permeability returned to baseline levels (Figure 3). Similarly, the local increase in haemoglobin, an index of haemorrhage, was normalized by fucoidin treatment (Figure 5C). Furthermore, fucoidin treatment significantly, but only partially, reversed the rapid fall in blood pressure which occurred after reperfusion (Figure 6). Overall these data argue for an important role of migrating neutrophils for the local and remote injuries and the systemic manifestations of reperfusion.
The effects of fucoidin on the intestinal and systemic levels of TNF-α are depicted in Figure 8. Fucoidin treatment had no effect on the systemic levels of TNF-α, but significantly reversed the increase of TNF-α levels in the intestine following local I/R.
Effects of UK74505 on the model of severe ischaemia and reperfusion injury
Next, we examined the effects of the PAF receptor antagonist UK74505 on the local and remote injuries following severe I/R injury. Similarly to the effects of fucoidin described above, post-ischaemic treatment with UK74505 abrogated the local (intestine and mesentery) and remote (lung) increase in vascular permeability following I/R of the SMA (Figure 3). These beneficial effects of UK74505 appeared to be secondary to its ability to inhibit neutrophil influx (Figure 4) and were also reflected in amelioration of tissue histopathology (Figure 5B) and the inhibition of tissue haemorrhage (Figure 5C). In addition, blockade of PAF receptors was accompanied by a significant, but partial, reversal in the hypotension (Figure 6) and leukopenia (Figure 7) which followed the reperfusion of the SMA. The effects of UK74505 on the latter parameter were interesting as the initial fall in circulating neutrophil numbers equalled that of vehicle-treated animals and was followed by a partial return to levels seen in the ischaemic period (Figure 7).
Finally and similarly to the effects of fucoidin, pre-treatment with UK74505 had no significant effect on the levels of TNF-α in plasma (Figure 8A) but reversed the increase in local TNF-α levels (Figure 8B).
Discussion
In addition to promoting local tissue injury, reperfusion of an ischaemic vascular bed is frequently accompanied by significant systemic manifestations and injury to remote organs. These injuries prevent part of the beneficial effects of reperfusion in saving ischaemic tissue (Lefer & Lefer, 1996). Thus, there is much interest in the development of pharmacological strategies which limit the damage of I/R syndrome. Here, we evaluate the effects of the long-acting and specific PAF receptor antagonist, UK74505 (Alabaster et al., 1991; Parry et al., 1994), both in a mild and in a severe model of I/R injury.
Post-ischaemic treatment with UK74505 effectively and dose-dependently inhibited the local and remote increase in vascular permeability in the mild model of ischaemia. Maximal inhibition occurred at 1 mg kg−1 and this is in agreement with previous studies demonstrating anti-inflammatory effects of similar doses of UK74505 in vivo (Alabaster et al., 1991; Miotla et al., 1998). Similarly to UK74505, the PAF receptor antagonist WEB2170 also inhibited the increase in vascular permeability following I/R injury. These results are in agreement with previous studies demonstrating the effectiveness of PAF receptor antagonists at inhibiting both local and remote tissue injury in various models of I/R (e.g. Canale et al., 1994; Carter et al., 1996; Riera et al., 1997).
We have previously shown that neutrophils appear to play an essential role in mediating local and remote reperfusion injury in our mild model (Souza et al., 2000). Following pre-treatment with UK74505 or WEB2170 we observed a virtually complete inhibition of neutrophil accumulation in the intestine, mesentery and lung following I/R injury. These results suggest that inhibition of neutrophil influx played a major role in the effects of PAF receptor antagonists on I/R injury. Interestingly, PAF appears to induce neutrophil influx by mediating their firm adherence, but not rolling, to the endothelium (Kubes et al., 1990) and neutrophil locomotion in extravascular tissue (Werr et al., 1998). However, in addition to mediating neutrophil influx, PAF may also play a role in activating neutrophils which have migrated to the site of injury (Nagase et al., 1999; Prescott et al., 1999; Au et al., 1994).
Next, we designed a series of experiments to assess whether UK74505 would also be inhibitory in a more severe model of I/R injury. To this end, we developed a model of reperfusion following ischaemia of the SMA in which in addition to the more severe local and remote increases in vascular permeability and neutrophil accumulation, we could observe a more significant tissue injury, as demonstrated by local haemorrhage, and systemic manifestations, such as hypotension and neutropenia. These remote and systemic injuries in addition to the local injuries mimic the clinical situation in severe cases and are important to evaluate the true benefit of pharmacological treatment with PAF receptor antagonists. Furthermore, in this model we could also assess local and systemic TNF-α levels as this cytokine appears to play an important role in mediating tissue injury in various models (e.g. Gilmont et al., 1996; Yao et al., 1995).
Initial experiments were designed to confirm a role for neutrophils in mediating the I/R injuries. To this end, animals were pretreated with the selectin blocker, fucoidin, at doses known to interfere with neutrophil rolling and accumulation in vivo (Ley et al., 1993). The effectiveness of this strategy was confirmed by the inability of neutrophils to accumulate in tissues after I/R and by the capacity of the drug to prevent the reperfusion-induced neutropenia. In fact, the number of circulating neutrophils rose steadily and plateaued even after the SMA was reperfused, suggesting that leukocyte rolling was an essential step for leukopenia to occur. In these animals, there was an almost complete reversal of the increase in vascular permeability, tissue haemorrhage and hypotension following I/R injury. These results are in agreement with other studies showing the effectiveness of fucoidin in preventing I/R injury in other models (Omata et al., 1997; Ritter et al., 1998; Cornejo et al., 1997; Souza et al., 2000) and demonstrate an essential role of the accumulation of neutrophils for the local and systemic injuries in the severe model of I/R. Moreover, our studies are in agreement with the ability of selectins to mediate neutrophil sequestration in the lung in vivo (Kubo et al., 1998).
In PAF receptor antagonist-treated animals, local and systemic inhibition of injuries were also observed. As in the mild model, UK74505 effectively inhibited local neutrophil accumulation and oedema formation following I/R injury. UK74505 also reversed the local increase in haemoglobin content, an index of tissue haemorrhage, and ameliorated the histopathological changes observed after severe I/R injuries. Overall, our studies are in agreement with other work using PAF receptor antagonists (e.g. Canale et al., 1994; Carter et al., 1996; Riera et al., 1997) and argue for a central role of PAF in mediating the local damages following mild or severe I/R injury. In addition, pulmonary damage and other systemic manifestations (i.e. blood neutrophilia and hypotension) following severe I/R injury were significantly blocked by the PAF receptor antagonist. Whereas lung injury was completely reversed by UK74505, the drug only partially inhibited the fall in blood pressure and leukopenia that accompanied the onset of reperfusion. These results suggest that other mediators (eg. LTB4, C5a) are released and may mediate part of these systemic manifestations. We are currently investigating a role for LTB4 in this model.
The ability of PAF receptor antagonists to inhibit the development of lung injury in the present study and in other work (Miotla et al., 1998; Nagase et al., 1999; Tavares-de-Lima et al., 1998) suggests an important role for PAF in the events leading to lung injury. Not only PAF appears to mediate the accumulation of neutrophils in the lung (see Figures 1 and 3), but the locally-produced PAF also has the potential to activate neutrophils to degranulate or release reactive oxygen species, which in turn may ensue lung damage. Interestingly, at least one study has shown that PAF may be produced by locally infiltrating neutrophils (Riera et al., 1997), thus having the potential to initiate tissue cycles of further neutrophil accumulation/activation.
Previous studies have suggested a role for PAF as an intermediate molecule for the production of TNF-α and other cytokines by both neutrophils and macrophages in vitro (Au et al., 1994; Yamada et al., 1999). As TNF-α appears to play a role in the development of I/R injury, it was of interest to examine whether UK74505 would interfere with the production of this cytokine in vivo. Interestingly, pretreatment with UK74505 abrogated the increase in TNF-α levels in the intestine, but not in serum. Moreover, virtually identical results were obtained with the selectin blocker fucoidin. Overall, these results suggest that the local (in the intestine) infiltration of neutrophils is essential for the local production of TNF-α. These latter results are in agreement with a recent hypothesis proposed by Sedgwick et al. (2000) who suggest that haematopoietically, but not resident cell, derived TNF-α that appears to regulate further leukocyte movement during inflammatory processes. In our experiments, it appears that blood derived haematopoietic cells are more relevant than tissue cells. Finally, inasmuch as systemic levels of TNF-α were unaltered by the PAF receptor antagonist, our results suggest that PAF is not a necessary intermediate molecule for the production of TNF-α in vivo as it has been demonstrated in vitro (Yamada et al., 1999).
In conclusion, we demonstrate that the long acting and specific PAF receptor antagonist, UK74505, is very effective at inhibiting the local and systemic injury following I/R of the superior mesenteric artery in rats. The effects of UK74505 were clinically relevant (Loucks et al., 2000) as demonstrated by the ability of the drug to block injuries when given just prior to the reperfusion. The ability of PAF receptor antagonists to block the migration of neutrophils to the site of reperfusion injury may account for the inhibition of injuries following I/R of SMA, in addition to any possible effect on the blockade of the effects of PAF on neutrophil activation. Finally, our results demonstrate an important role of migrating cells for the production of TNF-α by the reperfused intestine, but do not support a role for PAF as an intermediate molecule in the production of TNF-α. The beneficial effects of PAF receptor antagonists in various models of I/R injury in animals and the safety and long duration of action of UK74505 use in man (Kuitert et al., 1995; Jezequel et al., 1996) warrant further investigations of the use of this drug as preventive measure for I/R injury in humans.
Acknowledgments
We are grateful to FAPEMIG and CNPq for financial support and thank Dr Fernando Cunha for his critical appraisal of the manuscript.
Abbreviations
- I/R
ischaemia and reperfusion
- MPO
myeloperoxidase
- SMA
superior mesenteric artery
References
- ALABASTER V.A., KEIR R.F., PARRY M.J., DE SOUZA R.N. UK-74505, a novel and selective PAF antagonist, exhibits potent and long lasting activity in vivo. Agents Actions (Suppl.) 1991;34:221–227. [PubMed] [Google Scholar]
- AU B.T., WILLIAMS T.J., COLLINS P.D. Zymosan-induced IL-8 release from human neutrophils involves activation via the CD11b/CD18 receptor and endogenous platelet-activating factor as an autocrine modulator. J. Immunol. 1994;152:5411–5419. [PubMed] [Google Scholar]
- CANALE P., SQUADRITO F., ALTAVILLA D., IOCULANO M., ZINGARELLI B., CAMPO G.M., URNA G., SARDELLA A., SQUADRITO G., CAPUTI A.P. TCV-309, a novel platelet activating factor antagonist, inhibits leukocyte accumulation and protects against splanchnic artery occlusion shock. Agents Actions. 1994;42:128–134. doi: 10.1007/BF01983478. [DOI] [PubMed] [Google Scholar]
- CARTER M.B., WILSON M.A., WEAD W.B., GARRISON R.N. Platelet-activating factor mediates pulmonary macromolecular leak following intestinal ischaemia-reperfusion. J. Surg. Res. 1996;60:403–408. doi: 10.1006/jsre.1996.0066. [DOI] [PubMed] [Google Scholar]
- CORNEJO C.J., WINN R.K., MARLAN J.M. Anti-adhesion therapy. Adv. Pharmacol. 1997;39:99–142. doi: 10.1016/s1054-3589(08)60070-8. [DOI] [PubMed] [Google Scholar]
- DE MATOS I.M., SOUZA D.G., SEABRA D.G., FREIRE-MAIA L., TEIXEIRA M.M. Effects of tachykinin NK1- or PAF-receptor blockade on the Lung Injury Induced by Scorpion Venom. Eur. J. Pharmacol. 1999;376:293–300. doi: 10.1016/s0014-2999(99)00382-9. [DOI] [PubMed] [Google Scholar]
- GILMONT R.R., DARDANO A., ENGLE J.S., ADAMSON B.S., WELSH M.J., LI T., REMICK D.G., SMITH D.J., REES R.S. TNFα potentiates oxidant and reperfusion-induced endothelial cell injury. J. Surg. Res. 1996;61:175–182. doi: 10.1006/jsre.1996.0101. [DOI] [PubMed] [Google Scholar]
- IVEY C.L., WILLIAMS F.M., COLLINS P.D., JOSE P.J., WILLIAMS T.J. Neutrophil chemoattractants generated in two phases during reperfusion of ischaemia myocardium in the rabbit: evidence for a role for C5a and interleukin-8. J. Clin. Invest. 1995;95:2720–2728. doi: 10.1172/JCI117974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEZEQUEL S.G., UDEN S., WALSTALL P. Modipafant, a new PAF antagonist: pharmacokinetics and disposition in rat, dog and man. Xenobiotica. 1996;26:963–975. doi: 10.3109/00498259609052498. [DOI] [PubMed] [Google Scholar]
- KIM F.J., MOORE E.E., MOORE F.A., BIFFL W.L., FONTES B., BANERJEE A. Reperfused gut elaborates PAF that chemoattracts and primers neutrophils. J. Surg. Res. 1995;58:636–640. doi: 10.1006/jsre.1995.1100. [DOI] [PubMed] [Google Scholar]
- KUBES P., IBBOTSON G., RUSSELL J., WALLACE J.L., GRANGER D.N. Role of platelet-activating factor in ischaemia/reperfusion-induced leukocyte adherence. Am. J. Physiol. 1990;259:G300–G305. doi: 10.1152/ajpgi.1990.259.2.G300. [DOI] [PubMed] [Google Scholar]
- KUBES P., JUTILA M., PAYNE D. Therapeutic potential of inhibiting leukocyte rolling in ischaemia/reperfusion. J. Clin. Invest. 1995;95:2510–2519. doi: 10.1172/JCI117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUBO H., GRAHAM L., DOYLE N.A., QUINLAN W.M., HOGG J.C., DOERSCHUK C.M. Complement fragment-induced release of neutrophils from bone marrow and sequestration within pulmonary capillaries in rabbits. Blood. 1998;92:283–290. [PubMed] [Google Scholar]
- KUITERT L.M., ANGUS R.M., BARNES N.C., BARNES P.J., BONE M.F., CHUNG K.F., FAIRFAX A.J., HIGENBOTHAM T.W., O'CONNOR B.J., PIOTROWSKA B. Effect of a novel potent platelet-activating factor antagonist, modipafant, in clinical asthma. Am. J. Respir. Crit. Care Med. 1995;151:1331–1335. doi: 10.1164/ajrccm.151.5.7735582. [DOI] [PubMed] [Google Scholar]
- LEFER A.M., LEFER D.J. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischaemia-reperfusion. Cardiovasc. Res. 1996;32:743–751. [PubMed] [Google Scholar]
- LEY K., LINNEMANN G., MEINEN M., STOOLMAN L.M., GAEHTGENS P. Fucoidin, but not yeast polyphosphomannan PPME, inhibits leukocyte rolling in venules of the rat mesentery. Blood. 1993;81:177–185. [PubMed] [Google Scholar]
- LOUCKS E.B., SYMERSKY P., QAYUMI A.K. Platelet-activating factor antagonism: a new concept in the management of regional myocardial ischaemia-reperfusion injury. J. Invest. Surg. 1997;10:321–338. doi: 10.3109/08941939709099596. [DOI] [PubMed] [Google Scholar]
- LOUCKS E.B., QAYUMI A.K., GODIN D.V., ENGLISH J.C., LIM S.P., AL MAHMEED T., GUL S. Therapeutic potential of platelet-activating factor antagonism in the management of myocardial infarction. Can. J. Cardiol. 2000;16:497–504. [PubMed] [Google Scholar]
- MIOTLA J.M., JEFFERY P.K., HELLEWELL P.G. Platelet-activating factor plays a pivotal role in the induction of experimental lung injury. Am. J. Respir. Cell Mol. Biol. 1998;18:197–204. doi: 10.1165/ajrcmb.18.2.2846. [DOI] [PubMed] [Google Scholar]
- NAGASE T., ISHII S., KUME K., UOZUMI N., IZUMI T., OUCHI Y., SHIMIZU T. Platelet-activating factor mediates acid-induced lung injury in genetically engineered mice. J. Clin. Invest. 1999;104:1071–1076. doi: 10.1172/JCI7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMATA M., MATSUI N., INOMATA N., OHNO T. Protective effects of polysaccharide fucoidin on myocardial ischaemia-reperfusion injury in rats. J. Cardiovasc. Pharmacol. 1997;30:717–724. doi: 10.1097/00005344-199712000-00003. [DOI] [PubMed] [Google Scholar]
- PARRY M.J., ALABASTER V.A., CHEESEMAN H.E., COOPER K., DE SOUZA R.N., KEIR R.F. Pharmacological profile of UK-74,505, a novel and selective PAF antagonist with potent and prolonged oral activity. J. Lipid Mediat. Cell Signal. 1994;10:251–268. [PubMed] [Google Scholar]
- PRESCOTT S.M., MCINTYRE T.M., ZIMMERMAN G. Two of the usual suspects, platelet-activating factor and its receptor, implicated in acute lung injury. J. Clin. Invest. 1999;104:1019–1020. doi: 10.1172/JCI8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES G.S., GEE C.K., WARD H.L., BALL C., TARRANT G.M., POOLE S., BRISTOW A.F. Rat tumour necrosis factor-alpha: expression in recombinant Pichia pastoris, purification, characterization and development of a novel ELISA. Eur. Cytokine Netw. 1999;10:383–392. [PubMed] [Google Scholar]
- RIERA M., TORRAS J., HERRERO I., VALLES J., PAUBERT-BRAQUET M., CRUZADO J.M., ALSINA J., GRINYO J.M. Neutrophils accentuate renal cold ischaemia-reperfusion injury. Dose-dependent protective effect of a platelet-activating factor receptor antagonist. J. Pharmacol. Exp. Ther. 1997;280:786–794. [PubMed] [Google Scholar]
- RITTER L.S., COPELAND J.S., MCDONAGH P.F. Fucoidin reduces coronary microvascular leukocyte accumulation early in reperfusion. Ann. Thorac. Surg. 1998;66:2063–2071. doi: 10.1016/s0003-4975(98)00823-6. [DOI] [PubMed] [Google Scholar]
- SEDGWICK J.D., RIMINTON D.S., CYSTER J.G., KORNER H. Tumor necrosis factor: a master-regulator of leukocyte movement. Immunol. Today. 2000;21:110–113. doi: 10.1016/s0167-5699(99)01573-x. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., COUTINHO S.F., SILVEIRA M.R., CARA D.C., TEIXEIRA M.M. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischaemia of the superior mesenteric artery in rats. Eur. J. Pharmacol. 2000;403:121–128. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- TAVARES DE LIMA W., STEIL A.A., RUSSO M., STAROBINAS N., TEIXEIRA C.F., JANCAR S. Lipid mediators, tumor necrosis factor and nitric oxide and their interactions in immune-complex-induced lung injury. Eur. J. Pharmacol. 1998;358:69–75. doi: 10.1016/s0014-2999(98)00594-9. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA M.M., HELLEWELL P.G. The selectin binding polysaccharide fucoidin inhibits eosinophil recruitment in vivo. Br. J.Pharmacol. 1997;120:1059–1066. doi: 10.1038/sj.bjp.0701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERR J., XIE X., HEDQVIST P., RUOSLAHTI E., LINDBOM L. β1 integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J. Exp. Med. 1998;187:2091–2096. doi: 10.1084/jem.187.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLERSON J.T. Pharmacologic approaches to reperfusion injury. Adv. Pharmacol. 1997;39:291–312. doi: 10.1016/s1054-3589(08)60074-5. [DOI] [PubMed] [Google Scholar]
- YAMADA M., TANIMOTO A., ICHINOWATARI G., YAGINUMA H., OHUCHI K. Possible participation of intracellular platelet-activating factor in tumor necrosis factor-α production by rat peritoneal macrophages. Eur. J. Pharmacol. 1999;374:341–350. doi: 10.1016/s0014-2999(99)00337-4. [DOI] [PubMed] [Google Scholar]
- YAO Y.M., SHENG Z.Y., YU Y., TIAN H.M., WANG Y.P., LU L.R., XU S.H. The potential etiologic role of tumor necrosis factor in mediating multiple organ dysfunction in rats following intestinal ischaemia-reperfusion injury. Resuscitation. 1995;29:157–168. doi: 10.1016/0300-9572(95)00831-d. [DOI] [PubMed] [Google Scholar]


