Abstract
Although endotoxaemia induces kinin B1 receptors in several animal models, this condition is not documented in primates. This study examined the up-regulation of haemodynamic and pro-inflammatory responses to the B1 agonist des-Arg10-kallidin (dKD) in a non-human primate model.
Green monkeys (Cercopithecus aethiops St Kitts) received lipopolysaccharide (LPS; 90 μg kg−1) or saline intravenously. After 4 h, anaesthetized monkeys were canulated via the carotid artery to monitor blood pressure changes following intra-arterial injections of dKD or the B2 agonist bradykinin (BK). Oedema induced by subcutaneous kinin administration was evaluated as the increase in ventral skin folds in anaesthetized monkeys injected with captopril at 4 h to 56 days post-LPS.
LPS increased rectal temperature but did not affect blood pressure after 4 h. dKD reduced blood pressure (Emax: 27±4 mmHg; EC50: 130 pmol kg−1) and increased heart rate (Emax: 33 b.p.m.) only after LPS. In contrast, the dose-dependent fall in blood pressure with BK was comparable in all groups. The selective B1 antagonist [Leu9]dKD (75 ng kg−1 min−1, intravenously) abolished responses to dKD but not BK.
dKD injection induced oedema dose-dependently (2.4±0.1 mm at 150 nmol) only following LPS (at 4 h to 12 days but not 56 days). In contrast, BK-induced oedema was present and stable in all monkeys. Co-administration of [Leu9]dKD (150 nmol) significantly reduced oedema induced by dKD (50 nmol).
These results suggest LPS up-regulation of B1 receptor effects in green monkeys. This non-human primate model may be suitable for testing new, selective B1 antagonists with therapeutic potential as anti-inflammatory agents.
Keywords: Non-human primate, endotoxin, kinin B1 receptor, blood pressure, heart rate, oedema
Introduction
B1 receptors for kinins are considered potential therapeutic targets for the development of novel therapies aimed at controlling complications of septicemia and inflammation (Ahluwalia & Perretti, 1999; Marceau et al., 1998). Responses mediated by kinin B1 receptors are up-regulated from a null level following administration of bacterial lipopolysaccharide (LPS) or inflammatory cytokines in rabbits, rats and pigs (Marceau et al., 1998). In these models, systemic stimulation of B1 receptors reduces blood pressure. Induction followed by local stimulation of B1 receptors promotes oedema and hyperalgesia associated with chronic inflammation in the rat skin and joints (Ahluwalia & Perretti, 1999; Belichard et al., 2000). In human isolated tissues, B1 receptors appear to follow a similar pattern of up-regulation with injury or immune activation (Marceau et al., 1998). However, current evidence suggesting B1 receptor up-regulation in primates in vivo is limited. A recent study documented enhanced B1 receptor mRNA expression and B1 receptor-dependent chemotaxis in peripheral T lymphocytes isolated from patients with active multiple sclerosis as compared to non-affected individuals (Prat et al., 1999). Thus, the aim of this study was to determine whether bacterial lipopolysaccharide (LPS) administration enhances the sensitivity to a selective B1 agonist in a non-human primate model, namely green monkeys. Acute regulation of blood pressure and oedema induction by B1 receptors were chosen as relevant endpoints in this model because of the potential of B1 antagonists in the treatment of cardiovascular and inflammatory disorders.
Methods
The present studies were conducted on male green monkeys (Cercopithaecus aethiops St Kitts) at the Caribbean Primates Ltd. experimental farm (St Kitts, West Indies). Procedures were reviewed and accepted by the Animal Care Committees of the CR-CHUM (Montreal, Canada) and of Caribbean Primates Ltd. (St Kitts, West Indies). Animals weighing 6.0±0.5 kg (n=67) were anaesthetized (50 mg ketamine kg−1) and pretreated with a single intravenous injection of LPS (90 μg kg−1) or saline (1 ml) via the saphenous vein.
Blood pressure studies
At 4 h after pre-treatment, monkeys were anaesthetized again as described above, for haemodynamic measurements. Pressure data were acquired using a pressure transducer (Harvard Apparatus) connected to a Macintosh PowerBook G3 running the BioPac data acquisition software. The left common carotid artery was canulated and the changes in mean blood pressure were monitored in response to intra-arterial bolus injections of vehicle (100 μl saline), dKD (1 – 10 000 pmol kg−1) or BK (1 – 1000 pmol kg−1). All injections (saline or peptide), which were given at 1 – 3 min intervals, were immediately followed by saline (300 μl) to ensure delivery of the bolus. In a subset of LPS-treated animals, the haemodynamic response to dKD (1 nmol kg−1) and BK (0.1 nmol kg−1) were measured during and 5 min after a continuous intravenous infusion of the B1 selective antagonist [Leu9]dKD (75 nmol kg−1 min−1 via the saphenous vein). In these animals, rectal temperature was measured immediately after induction of anaesthesia, i.e. before and at 4 h after LPS or saline injection.
Inflammation studies
Kinin-induced oedema was evaluated by the ventral skin fold assay (Sciberras et al., 1987) in a separate subset of anaesthetized monkeys injected with captopril (1 mg kg−1 30 min before assay). A single subcutaneous injection of dKD, BK or the vehicle (2 mM amastatin in 100 μl Ringer's lactate) was given in the ventral area and the increase in thickness of skin folds was monitored for 30 – 45 min using a calibrated caliper. The results were expressed as the difference between the skin fold thickness before and after the subcutaneous injection. Captopril and amastatin were used to reduce degradation of kinins at the carboxyl- and amino-terminus, respectively. The time course after LPS for oedema induction by dKD (50 nmol) was determined at 4, 24 and 48 h, 12 and 56 days after LPS administration. BK (100 nmol) was used as a positive control. In following experiments, the dose-response relationship for dKD (1 – 150 nmol)-induced oedema was determined at 24 h post-LPS. A dose-response curve was obtained in each monkey studied by injecting peptides at different sites in the ventral area. To document inhibition of oedema formation by a B1 receptor antagonist, [Leu9]dKD (150 nmol) was co-injected or not with dKD (50 nmol) at 24 h post-LPS. Rectal temperature was measured in anaesthetized animals before and up to 56 days after LPS injection. Animals were used only once throughout these studies.
Drugs
Ketamine hydrochloride, LPS, amastatin and captopril were from Sigma (MO, U.S.A.). All peptides were from Phoenix Pharmaceuticals (CA, U.S.A.).
Statistics
Values are presented as mean±standard error of the mean (s.e.mean). In oedema studies, the pre-injection thickness of the skin folds was subtracted from the values after subcutaneous challenge. Curve fitting and EC50 calculations were obtained using the Delta Graph 4.0 software for Apple Computers. Data were compared by two-way analysis of variance followed by unpaired, one tail Student's t-test with Bonferroni correction. P<0.05 was considered statistically significant.
Results
LPS injection induced a significant increase in rectal temperature after 4 h (38.5±0.2°C; n=15) as compared to pre-LPS values (37.7±0.2°C; P<0.05) or values in saline-treated monkeys (data not shown). Rectal temperature remained elevated at 24 h (38.4±0.3°C; n=6) but not 48 h after LPS (37.7±0.2°C; n=8). At 4 h, LPS pretreatment did not affect mean blood pressure (112±10 mmHg; n=12) as compared to saline-treated animals (112±10 mmHg; n=5).
Blood pressure regulation by kinins
Administration of dKD at 4 h dose-dependently reduced blood pressure in monkeys pretreated with LPS (EC50: 130 pmol kg−1) but had a marginal effect in saline-pretreated animals (Figure 1a). In contrast, BK dose-dependently reduced blood pressure in monkeys pretreated with saline or LPS, with no difference between experimental groups (Figure 1b). There was no evidence that the maximal effect of BK was attained at the highest dose administered (1 nmol kg−1). Figure 2 shows a blood pressure tracing obtained at 4 h in an LPS-treated animal. The maximal hypotensive response was attained at 21±3 s in response to dKD (1 nmol kg−1) and 17±2 s for BK (1 nmol kg−1, not significantly different from dKD). At these time points, dKD and BK decreased blood pressure and increased heart rate similarly (control values for heart rate: 157±3 and 153±4 b.p.m., respectively; Figure 3). However, these effects were longer lasting with dKD (>1 min) than BK (⩽1 min) (Figure 3). Continuous intravenous infusion of the selective B1 antagonist [Leu9]dKD (75 nmol kg−1 min−1) did not affect blood pressure (2±3 mmHg increase at 1 min). However, [Leu9]dKD infusion abolished the response to dKD (1 nmol kg−1) but not BK (0.1 nmol kg−1) (Figure 4). In contrast, these doses of dKD and BK were equipotent in reducing blood pressure at 5 min after the end of [Leu9]dKD infusion in the same animals (Figure 4).
Figure 1.
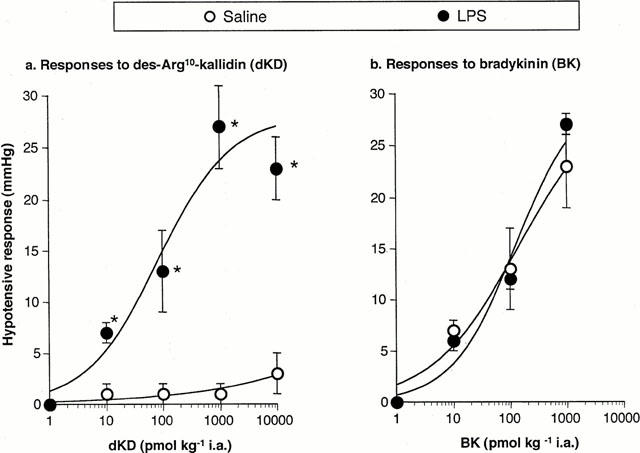
Dose-dependent decrease in mean arterial blood pressure in response to intra-arterial injections of (a) dKD or (b) BK in green monkeys anaesthetized 4 h after intravenous injection of LPS (90 ;μg kg−1) or saline vehicle. *Significantly different (P<0.05) from response in saline-pretreated monkeys (n=5–6 per group).
Figure 2.
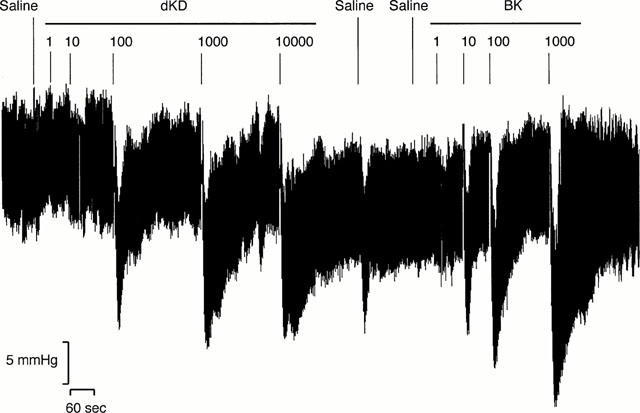
Representative tracing of blood pressure changes in response to intra-arterial bolus injection of saline vehicle (100 ;μl), des-Arg10-kallidin (dKD) or bradykinin (BK) in anaesthetized green monkeys. Animals were anaesthetized 4 h after intravenous injection of LPS (90 ;μg kg−1).
Figure 3.
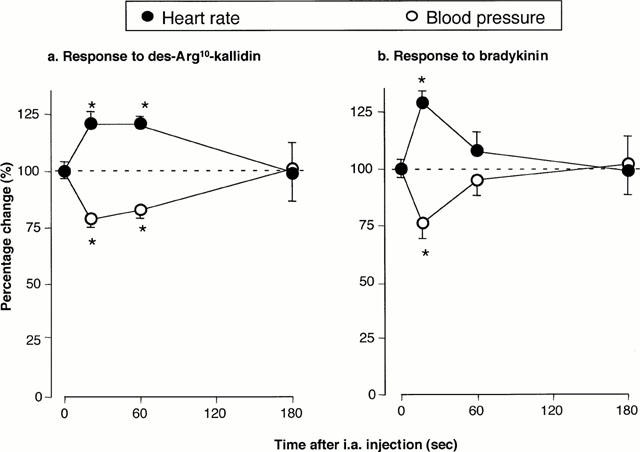
Time course for the decrease in mean arterial blood pressure and increase in heart rate in response to intra-arterial injection of (a) dKD (1 ;μmol kg−1) or (b) BK (1 ;μmol kg−1) in green monkeys. Heart rate before dKD or BK injection was 157±3 and 153±4 b.p.m., respectively. Animals were anaesthetized 4 h after intravenous injection of LPS (90 ;μg kg−1). * Significantly different (P<0.05) from pre-injection values (n=6 per group).
Figure 4.
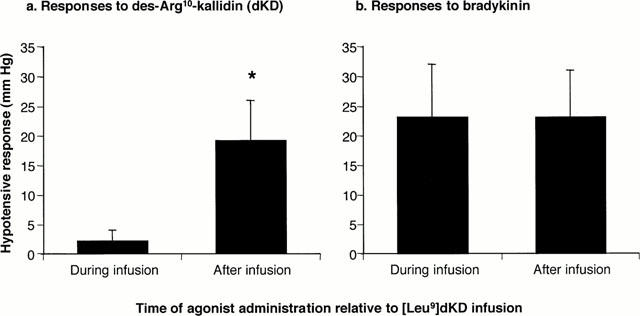
Decrease in mean arterial blood pressure in response to intra-arterial injection of (a) dKD (1 nmol kg−1) or (b) BK (100 pmol kg−1) during intravenous infusion of [Leu9]dKD (75 nmol kg−1 min−1) or 5 min after the end of [Leu9]dKD infusion in green monkeys. Animals were anaesthetized 4 h after intravenous injection of LPS (90 μg kg−1). * Significantly different (P<0.05) from response obtained during [Leu9]dKD infusion (n=6 per group).
Pro-inflammatory effects of kinins
The mean thickness of ventral skin folds in naïve monkeys was 2.3±0.2 mm (n=50). This parameter did not change significantly after LPS (data not shown). Administration of vehicle slightly increased skin fold thickness (by 0.5 – 1.0 mm), with no difference between experimental groups (Figure 5). In LPS- but not in saline-pretreated monkeys, dKD (50 nmol) induced a time-related increase in skin fold thickness with a maximum at 10 – 20 min (Figure 5). Responses to dKD were weaker at 4 h as compared to 24 h. Similar dKD responses were observed at 24 and 48 h and 12 days post-LPS (data at 48 h not shown), suggesting that maximal induction of B1 sensitivity was reached within 24 h post-LPS. In contrast, BK (100 nmol) induced comparable oedema in all experimental groups including saline-pretreated animals. At 56 days post-LPS, oedema formation was observed in response to BK (2.1±0.6 mm within 20 min post-challenge) but not dKD (0.9±0.5 mm) as compared to vehicle (0.8±0.6 mm; n=8). At 24 h post-LPS, maximal oedema in response to dKD (within 20 min post-challenge) was dose-dependent (Figure 6) and the effect induced by dKD (50 nmol) was significantly reduced by co-injection of [Leu9]dKD (150 nmol) (Figure 7).
Figure 5.
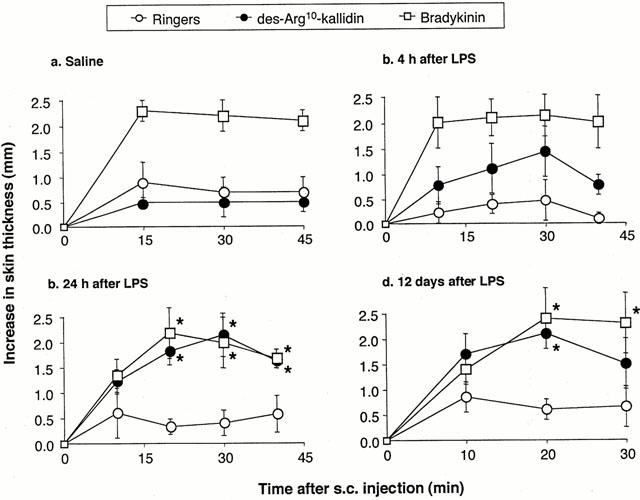
Oedema development in response to sub-cutaneous injections of Ringer's lactate vehicle (100 μl), dKD (75 nmol) or BK (100 nmol) in ventral skin of anaesthetized green monkeys at (a) 24 h following pre-treatment with saline (1 ml; n=7), or at (b) 4 h (n=3) (c) 24 h (n=6) and (d) 12 days (n=8) following pre-treatment with LPS (90 μg kg−1). * Significantly different (P<0.05) from response induced by vehicle.
Figure 6.
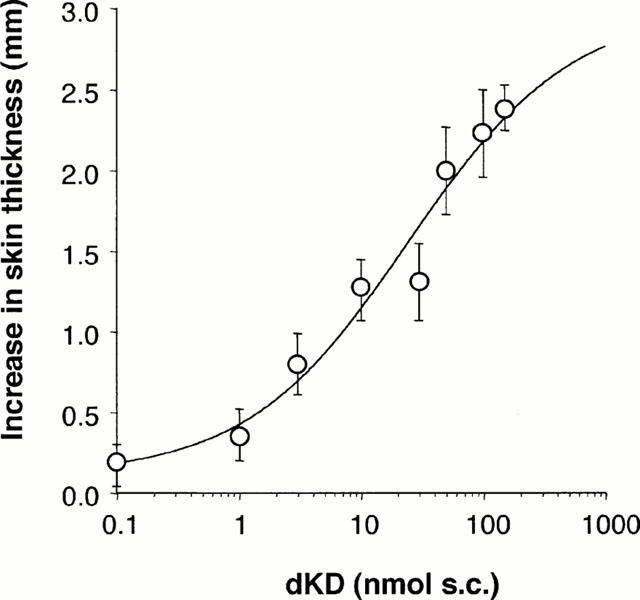
Dose-response relationship for maximal oedema development in response to subcutaneous injection of dKD (1–150 >nmol) in ventral skin of green monkeys anaesthetized 4 h after pre-treatment with LPS (90 μg kg−1; n=7).
Figure 7.
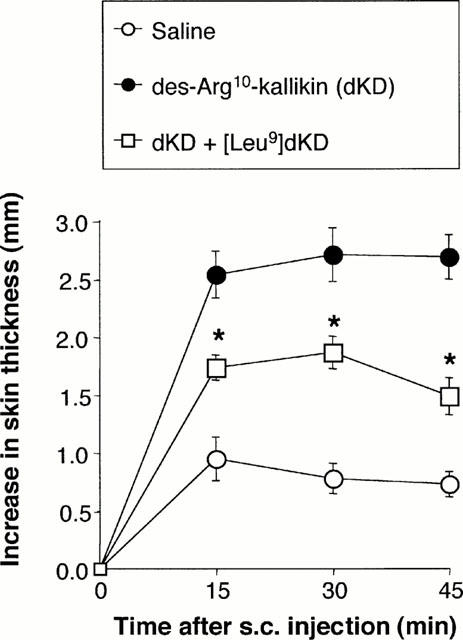
Inhibition by [Leu9]dKD (150 nmol) of oedema induced by subcutaneous injection of dKD (50 nmol) in green monkeys. [Leu9]dKD was co-injected with dKD. * Significantly different (P−0.05) from response without [Leu9]dKD (n=7).
Discussion
A growing body of evidence suggests a role for kinin B1 receptors in immunopathology, particularly in promoting chronic inflammatory disorders (Marceau et al., 1998). In contrast with kinin B2 receptors, current evidence suggests that B1 receptor-mediated cardiovascular or inflammatory responses are normally low or absent in humans, rabbits and rats (Ahluwalia & Perretti, 1999; Marceau et al., 1998; Prat et al., 1999). To date, constitutive B1 receptor expression has been documented in vascular tissues of dogs (Belichard et al., 1996; Staszewska-Woolley & Woodman, 1991), the central nervous system of spontaneously hypertensive rats (Emanueli et al., 1999), the hindlimb and pulmonary vasculature in cats (Santiago et al., 1995; Seyedi et al., 1997) and human ocular tissues (Ma et al., 1996) and T lymphocytes (Prat et al., 1999). Factors implicated in B1 receptor induction include bacterial endotoxin, pro-inflammatory cytokines (e.g., interleukin-1) as well as intracellular pathways regulating the cell response to environmental stress (e.g., the p38 MAP kinase and NF-κB pathways) (Bastian et al., 1998; Larrivee et al., 1998; Marceau et al., 1998; Phagoo et al., 1999; Prat et al., 1999; Sardi et al., 1998; Schanstra et al., 1998; Tsukagoshi et al., 1999). In turn, B1 receptor stimulation may activate further these same pathways (Campos et al., 1999; Naraba et al., 1998; Schanstra et al., 1998; Tsukagoshi et al., 1999). B1 receptors may also interact with cytokines to promote further B1 receptor up-regulation (Phagoo et al., 1999). The role of B1 receptors during haemodynamic disorders associated with septicaemia is still unclear (Siebeck et al., 1996). However, studies in rodent models of inflammation have provided fairly convincing evidence implicating B1 receptors as mediators of chronic oedema and hyperalgesia in the skin and joints (Ahluwalia & Perretti, 1999). By virtue of its selective induction at sites of inflammation, the B1 receptor is considered a potential therapeutic target for the control of tissue response to immune activation or mechanical injury, with minimal secondary effects in non-affected tissues. To date, however, there is no primate model of in vivo B1 receptor sensitization. Moreover, it remains unclear whether B1 agonists are pro-inflammatory in these species, although the enhanced B1 receptor-mediated migration shown by T lymphocytes from patients with active multiple sclerosis (Prat et al., 1999) support this hypothesis.
Functional studies of human B1 receptor sensitization in the cardiovascular system come in large part from in vitro studies with cultured cells or incubated smooth muscle preparations (Marceau et al., 1998). To our knowledge, the present study in green monkeys provides the first evidence that endotoxin up-regulates B1 receptor-mediated responses in primates in vivo, a condition associated with increased haemodynamic and pro-inflammatory responses to a selective B1 agonist. This interpretation is based on three lines of observations. (1) dKD induced significant, dose-dependent haemodynamic or inflammatory responses only in LPS-pretreated monkeys. (2) The increased sensitivity to kinins was specific for dKD (responses to BK were significant in control monkeys and unaffected after LPS). (3) The selective B1 antagonist dLKD inhibited the responses to dKD. Although supportive molecular evidence has not been obtained in monkeys, the present functional evidence is compatible with the notion that LPS increased tissue expression of the B1 receptor gene in sensitized tissues, as shown in rats and rabbits (Marceau et al., 1999; Marin-Castano et al., 1998; McLean et al., 1999). Beside LPS, several other conditions known to up-regulate sensitivity to B1 agonists are also associated with increased B1 receptor mRNA levels (Bastian et al., 1998; Belichard et al., 1999; Phagoo et al., 1999; Prat et al., 1999; Tsukagoshi et al., 1999). Notably, increased B1 gene expression and biological activity were recently observed in a model of inflammatory hyperalgesia induced by zymosan injection in the plantar tissue of the rat (Belichard et al., 2000). Based on current data (Davis et al., 1996; Marceau et al., 1998; McLean et al., 2000), candidate cell types expressing functional B1 receptors in LPS-treated monkeys include endothelial cells, vascular smooth muscle cells, fibroblasts, mast cells and peripheral sensory neurons.
The haemodynamics effects of B1 receptor agonists are complex and may involve the modulation of cardiac contractility and vasodilatation following the release of prostaglandins or nitric oxide depending on the species and vascular bed. In conscious instrumented dogs under ganglionic blockade, infusion of des-Arg9-BK or BK reduces mean arterial pressure and coronary vascular resistance without changing cardiac contractility (Belichard et al., 1996). In contrast, both cardiac contractility and arterial pressure are reduced (even under ganglionic blockade) in response to B1 receptor stimulation in rabbits anaesthetized following LPS pretreatment (Audet et al., 1997). In anaesthetized monkeys however, kinin-induced tachycardia was evident, an effect that was significantly more sustained with the B1 agonist. These differences may depend on the species or experimental conditions such as the type of anaesthesia used. Possible mechanisms for kinin-induced tachycardia in monkeys include reflex sympathetic activation in response to the fall in blood pressure, and pre-synaptic stimulation of catecholamine release as shown in rats, guinea-pigs and cats following stimulation with a B2 agonist (Kurz et al., 1997; Loro et al., 1998; Seyedi et al., 1997) or a B1 agonist (DeWitt et al., 1994). Vascular B1 receptors coupled to nitric oxide release mediate relaxation in bovine (Drummond & Cocks, 1995), porcine (Pruneau et al., 1996) and human (Su et al., 2000) coronary arteries, rabbit carotid arteries (Pruneau & Belichard, 1993) and cat pulmonary arteries (DeWitt et al., 1994). Alternatively, B1 receptors coupled to prostaglandin release mediate vascular relaxation in rabbit mesenteric arteries (deBlois & Marceau, 1987), rabbit coeliac arteries (Ritter et al., 1989), dog mesenteric veins (Toda et al., 1987) and rat coronary arteries (McLean et al., 1999). Proposed mechanisms for kinin-induced vasorelaxation also include the release of endothelial hyperpolarizing factor(s) (Mombouli et al., 1992). In dogs and LPS-treated rabbits, inhibition of prostaglandin or nitric oxide production does not elicit a marked reduction in maximal hypotensive response to B1 receptor stimulation (Audet et al., 1997; Belichard et al., 1996). The duration of the hypotension is reduced, however, by cyclo-oxygenase inhibition in rabbits (Audet et al., 1994; Drapeau et al., 1991), suggesting a significant haemodynamic role for prostaglandins. Based on these data, it is difficult to speculate on the possible role of the cyclo-oxygenase and nitric oxide pathways in B1 receptor-mediated hypotensive responses in monkeys, although a role for reduced cardiac output can be ruled out.
The present results are consistent with observations made in rat models of oedema development (Campos et al., 1996; 1997; de Campos et al., 1998). At 24 h following systemic administration of LPS in rats, intra-plantar administration of des-Arg9-BK promotes paw oedema (EC50: 42 nmol), an effect blocked by co-injection of des-Arg9[Leu8]-BK (IC50: 134 nmol) (Campos et al., 1996). Injection of rats with Mycobacterium bovis bacillus Calmette-Guerin results in long-term sensitization to B1 agonists in the paw oedema assay (for up to 75 days) (Campos et al., 1997). Although no live bacteria were used in monkeys, B1 sensitization in the skin was sustained for up to 12 days (but less than 56 days) following LPS. In contrast, the pyrogenic response to LPS lasted less than 2 days. Together, these data suggest a slow rate of receptor turnover in vivo, a feature consistent with known characteristics of B1 receptors which include lack of ligand-induced receptor desensitization and sequestration (Faussner et al., 1999). A role for prostaglandins in the present model is likely. Pro-inflammatory prostaglandins were implicated in mediating B1 receptor-induced oedema in rats (Campos & Calixto, 1995; Campos et al., 1996). Moreover, several studies showed B1 receptor activation of the cyclo-oxygenase pathway in vascular cells or fibroblasts (Lerner & Modeer, 1991; Marceau et al., 1998). New, potent, peptide and non-peptide antagonists of B1 receptors are being developed, with inflammation-associated hyperalgesia as a primary target condition (Altamura et al., 1999; Galoppini et al., 1999; Gobeil et al., 1999; Horlick et al., 1999). The observation that dKD mediates oedema in green monkeys supports the assumption that B1 receptor antagonists may be effective anti-inflammatory tools in humans. In non-human primates, B1 receptor-induced oedema may offer a simple and ethical surrogate assay for B1-mediated hyperalgesia.
Several studies provided evidence that the structure-relationship of B1 antagonists is species-dependent (MacNeil et al., 1997; Nsa Allogho et al., 1998; Wohlfart et al., 1997). Notably, peptide B1 antagonists such as [Leu8]-des-Arg9-BK act as partial agonists on the murine B1 receptor (MacNeil et al., 1997; Nsa Allogho et al., 1998). In hexamethonium-treated dogs, administration of [Leu8]des-Arg9-BK (25 μg kg−1) elicits a significant fall in blood pressure (Belichard et al., 1996). In LPS-treated monkeys, however, we observed no blood pressure change with infusion of a larger dose of the extended peptide antagonist [Leu9]dKD, as previously reported in rabbits (Marceau et al., 1998). In the skin fold assay, dKD-induced oedema was partially inhibited by [Leu9]dKD (150 nmol), i.e. at a dose comparable to the IC50 of des-Arg9[Leu8]-BK (134 nmol) in suppressing des-Arg9-BK-induced oedema in LPS-treated rats (Campos et al., 1996). Collectively, these data suggest that the partial inhibition of oedema observed with [Leu9]dKD in monkeys was due to incomplete blockade of B1 receptors rather than partial agonist activity of [Leu9]dKD.
In summary, LPS administration to green monkeys increased from a null level their sensitivity to a B1 receptor agonist in a blood pressure regulation assay and an oedema formation assay. Comparatively, responses to the B2 receptor agonist BK were not affected. A selective B1 receptor antagonist inhibited the effects of dKD. Taken together with the previous results showing an association between increased B1 receptor mRNAs and biological activity, the present data suggest that LPS up-regulates B1 receptor expression in green monkeys. This non-human primate model may be suitable for testing new, selective B1 antagonists with therapeutic potential as anti-inflammatory agents in a species with a high degree of genetic homology with humans.
Acknowledgments
This work was supported by a grant from Pharmacopeia Inc. D deBlois is a scholar from the Fonds de la Recherche en Santé du Québec. Fruitful discussions with Dr Maria L. Webb are gratefully acknowledged.
Abbreviations
- BK
bradykinin
- dKD
des-Arg10-kallidin
- [Leu9]dKD
[Leu9]des-Arg10-kallidin
- LPS
lipopolysaccharide
- s.e.mean
standard error of the mean
References
- AHLUWALIA A., PERRETTI M. B1 receptors as a new inflammatory target. Could this B the 1. Trends Pharmacol. Sci. 1999;20:100–104. doi: 10.1016/s0165-6147(99)01321-8. [DOI] [PubMed] [Google Scholar]
- ALTAMURA M., MEINI S., QUARTARA L., MAGGI C.A. Nonpeptide antagonists for kinin receptors. Regul. Pept. 1999;80:13–26. doi: 10.1016/s0167-0115(99)00003-8. [DOI] [PubMed] [Google Scholar]
- AUDET R., PETITCLERC E., DRAPEAU G., RIOUX F., MARCEAU F. Further analysis of the upregulation of bradykinin B1 receptors in isolated rabbit aorta by using metabolic inhibitors. Europ. J. Pharmacol. 1994;271:551–555. doi: 10.1016/0014-2999(94)90819-2. [DOI] [PubMed] [Google Scholar]
- AUDET R., RIOUX F., DRAPEAU G., MARCEAU F. Cardiovascular effects of Sar-[D-Phe8]des-Arg9-bradykinin, a metabolically protected agonist of B1 receptor for kinins, in the anesthetized rabbit pretreated with a sublethal dose of bacterial lipopolysaccharide. J. Pharmacol. Exp. Ther. 1997;280:6–15. [PubMed] [Google Scholar]
- BASTIAN S., PAQUET J.L., ROBERT C., CREMERS B., LOILLIER B., LARRIVEE J.F., BACHVAROV D.R., MARCEAU F., PRUNEAU D. Interleukin 8 (IL-8) induces the expression of kinin B1 receptor in human lung fibroblasts. Biochem. Biophys. Res. Commun. 1998;253:750–755. doi: 10.1006/bbrc.1998.9848. [DOI] [PubMed] [Google Scholar]
- BELICHARD P., LANDRY M., FAYE P., BACHVAROV D.R., BOUTHILLIER J., PRUNEAU D., MARCEAU F. Inflammatory hyperalgesia induced by zymosan in the plantar tissue of the rat: effect of kinin receptor antagonists. Immunopharmacol. 2000;46:139–147. doi: 10.1016/s0162-3109(99)00165-4. [DOI] [PubMed] [Google Scholar]
- BELICHARD P., LOILLIER B., PAQUET J.L., LUCCARINI J.M., PRUNEAU D. Haemodynamic and cardiac effects of kinin B1 and B2 receptor stimulation in conscious instrumented dogs. Br. J. Pharmacol. 1996;117:1565–1571. doi: 10.1111/j.1476-5381.1996.tb15322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELICHARD P., LUCCARINI J.M., DEFRENE E., FAYE P., FRANCK R.M., DUCLOS H., PAQUET J.L., PRUNEAU D. Pharmacological and molecular evidence for kinin B1 receptor expression in urinary bladder of cyclophosphamide-treated rats. Br. J. Pharmacol. 1999;128:213–219. doi: 10.1038/sj.bjp.0702769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., CALIXTO J.B. Involvement of B1 and B2 receptors in bradykinin-induced rat paw oedema. Br. J. Pharmacol. 1995;114:1005–1013. doi: 10.1111/j.1476-5381.1995.tb13305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., HENRIQUES M.G., CALIXTO J.B. The role of B1 and B2 kinin receptors in oedema formation after long-term treatment with Mycobacterium bovis bacillus Calmette-Guerin (BCG) Br. J. Pharmacol. 1997;120:502–508. doi: 10.1038/sj.bjp.0700914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E., CALIXTO J.B. In vivo B1 kinin-receptor upregulation. Evidence for involvement of protein kinases and nuclear factor kappaB pathways. Br. J. Pharmacol. 1999;127:1851–1859. doi: 10.1038/sj.bjp.0702715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E., CALIXTO J.B. Upregulation of B1 receptor mediating des-Arg9-BK-induced rat paw oedema by systemic treatment with bacterial endotoxin. Br. J. Pharmacol. 1996;117:793–798. doi: 10.1111/j.1476-5381.1996.tb15262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS C.L., NAEEM S., PHAGOO S.B., CAMPBELL E.A., URBAN L., BURGESS G.M. B1 bradykinin receptors and sensory neurones. Br. J. Pharmacol. 1996;118:1469–1476. doi: 10.1111/j.1476-5381.1996.tb15562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE CAMPOS R.O., HENRIQUES M.G., CALIXTO J.B. Systemic treatment with Mycobacterium bovis bacillus Calmette-Guerin (BCG) potentiates kinin B1 receptor agonist-induced nociception and oedema formation in the formalin test in mice. Neuropeptides. 1998;32:393–403. doi: 10.1016/s0143-4179(98)90062-2. [DOI] [PubMed] [Google Scholar]
- DEBLOIS D., MARCEAU F. The ability of des-Arg9-bradykinin to relax rabbit isolated mesenteric arteries is acquired during in vitro incubation. Eur. J. Pharmacol. 1987;142:141–144. doi: 10.1016/0014-2999(87)90664-9. [DOI] [PubMed] [Google Scholar]
- DEWITT B.J., CHENG D.Y., KADOWITZ P.J. des-Arg9-bradykinin produces tone-dependent kinin B1 receptor-mediated responses in the pulmonary vascular bed. Circ. Res. 1994;75:1064–1072. doi: 10.1161/01.res.75.6.1064. [DOI] [PubMed] [Google Scholar]
- DRAPEAU G., DEBLOIS D., MARCEAU F. Hypotensive effects of Lys-des-Arg9-bradykinin and metabolically protected agonists of B1 receptors for kinins. J. Pharmacol. Exp. Ther. 1991;259:997–1003. [PubMed] [Google Scholar]
- DRUMMOND G.R., COCKS T.M. Endothelium-dependent relaxation to the B1 kinin receptor agonist des- Arg9-bradykinin in human coronary arteries. Br. J. Pharmacol. 1995;116:3083–3085. doi: 10.1111/j.1476-5381.1995.tb15108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMANUELI C., CHAO J., REGOLI D., CHAO L., NI A., MADEDDU P. The bradykinin B1 receptor and the central regulation of blood pressure in spontaneously hypertensive rats. Br. J. Pharmacol. 1999;126:1769–1776. doi: 10.1038/sj.bjp.0702527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAUSSNER A., BATHON J.M., PROUD D. Comparison of the responses of B1 and B2 kinin receptors to agonist stimulation. Immunopharmacol. 1999;45:13–20. doi: 10.1016/s0162-3109(99)00052-1. [DOI] [PubMed] [Google Scholar]
- GALOPPINI C., MEINI S., TANCREDI M., DI FENZA A., TRIOLO A., QUARTARA L., MAGGI C.A., FORMAGGIO F., TONIOLO C., MAZZUCCO S., PAPINI A., ROVERO P. A new class of pseudopeptide antagonists of the kinin B1 receptor containing alkyl spacers. J. Med. Chem. 1999;42:409–414. doi: 10.1021/jm980495r. [DOI] [PubMed] [Google Scholar]
- GOBEIL F., JR, CHARLAND S., FILTEAU C., PERRON S.I., NEUGEBAUER W., REGOLI D. Kinin B1 receptor antagonists containing alpha-methyl-L-phenylalanine: in vitro and in vivo antagonistic activities. Hypertension. 1999;33:823–829. doi: 10.1161/01.hyp.33.3.823. [DOI] [PubMed] [Google Scholar]
- HORLICK R.A., OHLMEYER M.H., STROKE I.L., STROHL B., PAN G., SCHILLING A.E., PARADKAR V., QUINTERO J.G., YOU M., RIVIELLO C., THORN M.B., DAMAJ B., FITZPATRICK V.D., DOLLE R.E., WEBB M.L., BALDWIN J.J., SIGAL N.H. Small molecule antagonists of the bradykinin B1 receptor. Immunopharmacol. 1999;43:169–177. doi: 10.1016/s0162-3109(99)00130-7. [DOI] [PubMed] [Google Scholar]
- KURZ T., TOLG R., RICHARDT G. Bradykinin B2-receptor-mediated stimulation of exocytotic noradrenaline release from cardiac sympathetic neurons. J. Mol. Cell. Cardiol. 1997;29:2561–2569. doi: 10.1006/jmcc.1997.0492. [DOI] [PubMed] [Google Scholar]
- LARRIVEE J.F., BACHVAROV D.R., HOULE F., LANDRY J., HUOT J., MARCEAU F. Role of the mitogen-activated protein kinases in the expression of the kinin B1 receptors induced by tissue injury. J. Immunol. 1998;160:1419–1426. [PubMed] [Google Scholar]
- LERNER U.H., MODEER T. Bradykinin B1 and B2 receptor agonists synergistically potentiate interleukin-1-induced prostaglandin biosynthesis in human gingival fibroblasts. Inflammation. 1991;15:427–436. doi: 10.1007/BF00923340. [DOI] [PubMed] [Google Scholar]
- LORO J.F., ZHANG J., PFAFFENDORF M., VAN ZWIETEN P.A. Positive chronotropic activity of bradykinin in the pithed normotensive rat. Fundam. Clin. Pharmacol. 1998;12:77–81. doi: 10.1111/j.1472-8206.1998.tb00927.x. [DOI] [PubMed] [Google Scholar]
- MA J.X., SONG Q., HATCHER H.C., CROUCH R.K., CHAO L., CHAO J. Expression and cellular localization of the kallikrein-kinin system in human ocular tissues. Exp. Eye Res. 1996;63:19–26. doi: 10.1006/exer.1996.0087. [DOI] [PubMed] [Google Scholar]
- MACNEIL T., FEIGHNER S., HRENIUK D.L., HESS J.F., VAN DER PLOEG L.H. Partial agonists and full antagonists at the human and murine bradykinin B1 receptors. Can. J. Physiol. Pharmacol. 1997;75:735–740. [PubMed] [Google Scholar]
- MARCEAU F., HESS J.F., BACHVAROV D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- MARCEAU F., LARRIVEE J.F., BOUTHILLIER J., BACHVAROVA M., HOULE S., BACHVAROV D.R. Effect of endogenous kinins, prostanoids, and NO on kinin B1 and B2 receptor expression in the rabbit. Am. é J. Physiol. 1999;277:R1568–R1578. doi: 10.1152/ajpregu.1999.277.6.R1568. [DOI] [PubMed] [Google Scholar]
- MARIN-CASTANO M.E., SCHANSTRA J.P., PRADDAUDE F., PESQUERO J.B., ADER J.L., GIROLAMI J.P., BASCANDS J.L. Differential induction of functional B1-bradykinin receptors along the rat nephron in endotoxin induced inflammation. Kidney Int. 1998;54:1888–1898. doi: 10.1046/j.1523-1755.1998.00201.x. [DOI] [PubMed] [Google Scholar]
- MCLEAN P.G., AHLUWALIA A., PERRETTI M. Association between kinin B(1) receptor expression and leukocyte trafficking across mouse mesenteric postcapillary venules. J. Exp. Med. 2000;192:367–380. doi: 10.1084/jem.192.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEAN P.G., PERRETTI M., AHLUWALIA A. Inducible expression of the kinin B1 receptor in the endotoxemic heart: mechanisms of des-Arg9bradykinin-induced coronary vasodilation. Br. J. Pharmacol. 1999;128:275–282. doi: 10.1038/sj.bjp.0702743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOMBOULI J.V., ILLIANO S., NAGAO T., SCOTT-BURDEN T., VANHOUTTE P.M. Potentiation of endothelium-dependent relaxations to bradykinin by angiotensin I converting enzyme inhibitors in canine coronary artery involves both endothelium-derived relaxing and hyperpolarizing factors. Circ. Res. 1992;71:137–144. doi: 10.1161/01.res.71.1.137. [DOI] [PubMed] [Google Scholar]
- NARABA H., UENO A., KOSUGI Y., YOSHIMURA M., MURAKAMI M., KUDO I., OH-ISHI S. Agonist stimulation of B1 and B2 kinin receptors causes activation of the MAP kinase signaling pathway, resulting in the translocation of AP-1 in HEK 293 cells. FEBS Lett. 1998;435:96–100. doi: 10.1016/s0014-5793(98)01045-x. [DOI] [PubMed] [Google Scholar]
- NSA ALLOGHO S., GOBEIL F., PERRON S.I., HESS J.F., REGOLI D. Effects of kinins on isolated stomachs of control and transgenic knockout B2 receptor mice. Naun. Schmied. Arch. Pharmacol. 1998;357:191–196. doi: 10.1007/pl00005157. [DOI] [PubMed] [Google Scholar]
- PHAGOO S.B., POOLE S., LEEB-LUNDBERG L.M. Autoregulation of bradykinin receptors: agonists in the presence of interleukin-1beta shift the repertoire of receptor subtypes from B2 to B1 in human lung fibroblasts. Mol. Pharmacol. 1999;56:325–333. doi: 10.1124/mol.56.2.325. [DOI] [PubMed] [Google Scholar]
- PRAT A., WEINRIB L., BECHER B., POIRIER J., DUQUETTE P., COUTURE R., ANTEL J.P. Bradykinin B1 receptor expression and function on T lymphocytes in active multiple sclerosis. Neurology. 1999;53:2087–2092. doi: 10.1212/wnl.53.9.2087. [DOI] [PubMed] [Google Scholar]
- PRUNEAU D., BELICHARD P. Induction of bradykinin B1 receptor-mediated relaxation in the isolated rabbit carotid artery. Eur. J. Pharmacol. 1993;239:63–67. doi: 10.1016/0014-2999(93)90976-o. [DOI] [PubMed] [Google Scholar]
- PRUNEAU D., LUCCARINI J.M., DEFRENE E., PAQUET J.L., BELICHARD P. Characterisation of bradykinin receptors from juvenile pig coronary artery. Eur. J. Pharmacol. 1996;297:53–60. doi: 10.1016/0014-2999(95)00720-2. [DOI] [PubMed] [Google Scholar]
- RITTER J.M., DOKTOR H.S., CRAGOE E.J., , JR Actions of bradykinin and related peptides on rabbit coeliac artery rings. Br. J. Pharmacol. 1989;96:23–28. doi: 10.1111/j.1476-5381.1989.tb11779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTIAGO J.A., GARRISON E.A., CHAMPION H.C., SMITH R.E., DEL RIO O., KADOWITZ P.J. Analysis of responses to kallidin, DABK, and DAK in feline hindlimb vascular bed. Am. J. Physiol. 1995;269:H2057–H2064. doi: 10.1152/ajpheart.1995.269.6.H2057. [DOI] [PubMed] [Google Scholar]
- SARDI S.P., ARES V.R., ERRASTI A.E., ROTHLIN R.P. Bradykinin B1 receptors in human umbilical vein: pharmacological evidence of up-regulation, and induction by interleukin-1 beta. Eur. J. Pharmacol. 1998;358:221–227. doi: 10.1016/s0014-2999(98)00609-8. [DOI] [PubMed] [Google Scholar]
- SCHANSTRA J.P., BATAILLE E., MARIN CASTANO M.E., BARASCUD Y., HIRTZ C., PESQUERO J.B., PECHER C., GAUTHIER F., GIROLAMI J.P., BASCANDS J.L. The B1-agonist [des-Arg10]-kallidin activates transcription factor NF-kappaB and induces homologous upregulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J. Clin. Invest. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCIBERRAS D.G., GOLDENBERG M.M., BOLOGNESE J.A., JAMES I., BABER N.S. Inflammatory responses to intradermal injection of platelet activating factor, histamine and prostaglandin E2 in healthy volunteers: a double blind investigation. Br. J. Clin. Pharmacol. 1987;24:753–761. doi: 10.1111/j.1365-2125.1987.tb03242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEYEDI N., WIN T., LANDER H.M., LEVI R. Bradykinin B2-receptor activation augments norepinephrine exocytosis from cardiac sympathetic nerve endings. Mediation by autocrine/paracrine mechanisms. Circ. Res. 1997;81:774–784. doi: 10.1161/01.res.81.5.774. [DOI] [PubMed] [Google Scholar]
- SIEBECK M., SPANNAGL E., SCHORR M., STUMPF B., FRITZ H., WHALLEY E.T., CHERONIS J.C. Effect of combined B1 and B2 kinin receptor blockade in porcine endotoxin shock. Immunopharmacol. 1996;33:81–84. doi: 10.1016/0162-3109(96)00060-4. [DOI] [PubMed] [Google Scholar]
- STASZEWSKA-WOOLLEY J., WOODMAN O.L. Kinin receptors mediating the effects of bradykinin on the coronary circulation in anaesthetized greyhounds. Eur. J. Pharmacol. 1991;196:9–14. doi: 10.1016/0014-2999(91)90402-c. [DOI] [PubMed] [Google Scholar]
- SU J.B., HOUEL R., HELOIRE F., BARBE F., BEVERELLI F., SAMBIN L., CASTAIGNE A., BERDEAUX A., CROZATIER B., HITTINGER L. Stimulation of bradykinin B(1) receptors induces vasodilation in conductance and resistance coronary vessels in conscious dogs: comparison with B(2) receptor stimulation. Circulation. 2000;101:1848–1853. doi: 10.1161/01.cir.101.15.1848. [DOI] [PubMed] [Google Scholar]
- TODA N., BIAN K., AKIBA T., OKAMURA T. Heterogeneity in mechanisms of bradykinin action in canine isolated blood vessels. Eur. J. Pharmacol. 1987;135:321–329. doi: 10.1016/0014-2999(87)90681-9. [DOI] [PubMed] [Google Scholar]
- TSUKAGOSHI H., SHIMIZU Y., HORIE T., FUKABORI Y., IWAMAE S., HISADA T., ISHIZUKA T., IIZUKA K., DOBASHI K., MORI M. Regulation by interleukin-1beta of gene expression of bradykinin B1 receptor in MH-S murine alveolar macrophage cell line. Biochem. Biophys. Res. Commun. 1999;259:476–482. doi: 10.1006/bbrc.1999.0798. [DOI] [PubMed] [Google Scholar]
- WOHLFART P., DEDIO J., WIRTH K., SCHOLKENS B.A., WIEMER G. Different B1 kinin receptor expression and pharmacology in endothelial cells of different origins and species. J. Pharmacol. Exp. Ther. 1997;280:1109–1116. [PubMed] [Google Scholar]


