Abstract
CS-747 is a novel thienopyridine-type platelet ADP inhibitor which lacks in vitro activity. This study examined pharmacological profiles of R-99224, a hepatic metabolite of CS-747.
R-99224 produced a concentration-dependent inhibition of in vitro platelet aggregation in washed human platelets (0.03 – 1 μg ml−1), which was relatively specific to ADP compared to collagen and thrombin.
R-99224 (0.1 – 3 μg ml−1) also elicited a similar inhibition of ADP-induced aggregation in rat platelets. The inhibition by R-99224 (10 μg ml−1) persisted even after platelets were washed three times. Intravenous injection of R-99224 (0.1 – 3 mg kg−1) to rats resulted in a dose-dependent inhibition of ex vivo ADP-induced platelet aggregation.
R-99224 (0.1 – 100 μM) decreased binding of [3H]-2-methylthio-ADP ([3H]-2-MeS-ADP), a stable ligand for platelet ADP receptors, to washed human platelets. The inhibition by R-99224 reached a plateau at a concentration of 3 μM (1.4 μg ml−1), but complete inhibition was not achieved even at the highest concentration used (100 μM).
R-99224 (10 μM) in combination with ARL-66096 (0.3 μM), an ATP analogue-type Gi-linked P2T receptor antagonist, produced no additional inhibition of [3H]-2-MeS-ADP binding. In contrast, [3H]-2-MeS-ADP binding was completely abolished by R-99224 (10 μM) in combination with A3P5PS (300 μM), a selective P2Y1 antagonist, suggesting that R-99224 selectively binds to the Gi-linked P2T receptor.
R-99224 (0.01 – 3 μg ml−1) inhibited ADP-induced [125I]-fibrinogen binding to human platelets in a concentration-dependent manner. R-99224 (0.1 – 1 μg ml−1) also inhibited the ADP-induced decrease in cyclic AMP levels in PGE1-stimulated platelets, whereas the agent did not affect ADP (10 μM)-induced Ca2+ mobilization.
These findings suggest that R-99224 is a selective and irreversible antagonist of Gi-linked P2T receptors and that R-99224 is a responsible molecule for in vivo actions of CS-747.
Keywords: Platelet aggregation, Gi-linked P2T receptor, ADP, CS-747, R-99224, active metabolite, thienopyridine
Introduction
ADP is one of the most physiologically and pathophysiologically important platelet agonists. By inducing a number of platelet responses, including shape change from disc to sphere, aggregation, and secretion of granule contents, ADP contributes to haemostasis, pathological thrombus formation, and vascular occlusion. Transduction of the ADP-induced intracellular signalling events involves the activation of G proteins, the inhibition of adenylyl cyclase, the activation of phospholipase C, and the elevation of intracellular calcium levels (Hourani & Hall, 1994; Mills, 1996). These responses are believed to be mediated by the interaction of a series of platelet ADP receptors tentatively designated as P2T receptors (Hourani & Hall, 1994; Gachet & Cazenave, 1991). A three-receptor model has recently been proposed in which the P2T receptors are composed of three distinct receptors, i.e., the P2X1, a ligand-gated ion channel receptor and two distinct G-protein coupled ADP receptors (a Gq-linked P2Y1 receptor and a Gi-linked P2T receptor distinct from P2Y1) (for review see Kunapuli, 1998a,1998b).
Several lines of evidence indicate the importance of the Gi-linked P2T receptor for platelet aggregation, and blockers of these receptors have been the target of drug development in the field of cardiovascular diseases. Up to now, two different types of blockers against Gi-linked P2T receptors have been reported: thienopiridine analogue-and ATP analogue-type inhibitors. Ticlopidine and clopidogrel, both thienopiridine derivatives, are orally active inhibitors of ADP-induced platelet aggregation (Defreyn et al., 1991; Mills et al., 1992; Savi et al., 1994b) which are now used in clinical settings. Since ticlopidine and clopidogrel are essentially inactive in vitro, their in vivo activities may be due to putative active metabolite(s) the chemical structures of which remains to be determined (Saltiel & Ward, 1987; Savi et al., 1994a; Coukell & Markham, 1997).
We have recently reported that CS-747 (2-acetoxy-5-(α-cyclopropylcarbonyl -2-fluorobenzyl) -4,5,6,7-tetrahydrothieno [3,2-c]pyridine), a novel thienopyridine derivative, is an orally active antiplatelet agent with a fast onset and a high potency in rats (Sugidachi et al., 2000). In the same study we have demonstrated that while CS-747 is inactive in vitro, one of its hepatic metabolites R-99224 ((2Z)-[1-[2-cyclopropyl-1-( 2-fluorophenyl )- 2-oxoethyl ]- 4-mercapto-3-piperidinylidene ] (Figure 1) inhibits the in vitro aggregation of rat platelets. In the present study, we investigated the in vitro pharmacological profile of R-99224 using washed human platelets and examined the effects of injected R-99224 on platelet aggregation in rats. We now provide evidence that R-99224 is an active metabolite of CS-747, a thienopiridine-type P2T antagonist.
Figure 1.
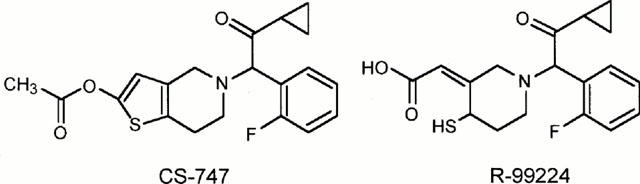
Chemical structures of CS-747 and R-99224.
Methods
Volunteers
After obtaining consent, venous blood was collected from healthy male volunteers by clean venepuncture using a 21-gauge butterfly needle and minimal stasis. All subjects avowed that they had not taken any medication in the 1 week preceding sampling.
Animals
Male Sprague-Dawley rats purchased from Japan SLC (Shizuoka, Japan) were used. The animals were allowed free access to standard rat chow and water. The experimental procedures employed in this study were in accordance with the guidelines of the Institutional Animal Care and Use Committee at Sankyo Research Laboratories (Tokyo, Japan).
Preparation of platelets
Blood was drawn from the healthy volunteers and rats anaesthetized with sodium pentobarbital (40 mg kg−1, i.p.) using 3.8% (w v−1) sodium citrate (nine parts blood, one part citrate) as an anticoagulant. Washed platelets were used in experiments with human platelets, and either platelet-rich plasma (PRP) or washed platelets were used in experiments with rat platelets. PRP was prepared by centrifugation at 180×g (human) or 230×g (rat) for 15 min at room temperature. Platelet-poor plasma (PPP) was obtained by centrifugation of the remaining blood at 2000×g for 10 min. Platelet counts in rat PRP were adjusted to 5×108 platelets ml−1 by adding PPP.
Washed platelets were prepared as described previously (Sugidachi et al., 1998) with slight modifications. The PRP was centrifuged at 1200×g for 6 min, and the resulting platelet pellet was resuspended in washing buffer containing (in mM): NaCl 140, KCl 2.7, NaH2PO4· 2H2O 0.4 NaHCO3 12, MgCl2· 6H2O 1 glucose 5 HEPES 10 and 3.5 mg ml−1 fatty acid-free bovine serum albumin (BSA), pH 6.7. Next, this platelet suspension was washed two more times and resuspended in the suspension buffer (same composition as the washing buffer, pH 7.4). PGE1 (100 – 200 nM) and/or apyrase (0.05 – 0.5 u ml−1) were used to prevent platelet activation while the platelets were being washed.
Measurement of platelet aggregation
All aggregation studies were performed in Mebanix aggregometers (model PAM-6C and PAM-8C, Tokyo, Japan). In studies on washed platelet aggregation, the washed platelet suspension was supplemented with human fibrinogen (1 mg ml−1 for human and 0.068 mg ml−1 for rat) and 1 mM Ca2+. The washed platelet suspension (3×108 platelets ml−1) or PRP (5×108 platelets ml−1) was incubated at 37°C for 1.5 min in the aggregometer with continuous stirring at 1000 r.p.m. and then stimulated with ADP, collagen, or thrombin. Changes in light transmission were recorded for at least 5 min and the maximum aggregation was estimated. The extent of aggregation was expressed as a percentage of the maximum light transmittance, obtained with the suspension buffer (washed platelet aggregation) or PPP (PRP aggregation). In the experiment to determine the duration of action in vitro, rat platelet aggregation was measured before and after each of up to three platelet washings.
[3H]-2-MeS-ADP binding
The washed human platelet suspension (3×108 platelets ml−1) was incubated with 10 nM [3H]-2-MeS-ADP at room temperature. After 60 min, the reaction mixture was layered onto a suspension buffer containing 20% sucrose and the bound ligand was separated by centrifugation at 10,000×g for 3 min at room temperature. After careful aspiration of the supernatant, the platelet pellet was dissolved in NCS-II (Amersham, Buckinghamshire, U.K.) and its radioactivity was measured by scintillation counting. Specific binding was defined as the difference between the total binding and nonspecific binding determined by the addition of unlabelled 2-MeS-ADP at 100 μM.
[125I]-Fibrinogen binding
The ability of washed platelets to bind fibrinogen in response to ADP was assessed with [125I]-fibrinogen according to the method previously described (Sugidachi et al., 1998) with slight modifications. [125I]-Fibrinogen (70 μg ml−1) was added to the washed human platelets in the presence of 1 mM Ca2+. The mixtures containing R-99224 or vehicle (total volume of 240 μl) were incubated for 1.5 min, and fibrinogen binding was initiated by adding 10 μM ADP. Following a 10-min incubation, a 200 μl aliquot from each reaction mixture was layered on 400 μl of the suspension buffer containing 20% sucrose, and centrifuged at 10,000×g for 3 min. The supernatants were aspirated, and the radioactivity was measured using a gamma counter (Riastar, Packard). Specific fibrinogen binding was calculated by subtracting the nonspecific binding measured in the resting platelets from the total binding. The data were expressed as the per cent (%) of binding in ADP (10 μM)-stimulated control platelets.
Measurement of cyclic AMP concentration
Cyclic AMP levels were determined according to the method of Defreyn et al. (1991) with slight modifications. A mixture of 1 ml buffer (mM): Tris 15, NaCl 120, KCl 4, MgSO4 1.6, NaH2PO4·2H2O 2, glucose 10 (0.2% BSA), IBMX 1.5, pH 7.4, and 2 ml PRP (3×108 platelets ml−1) was incubated for 1.5 min, and then PGE1 (10 μM), a stimulator of adenylyl cyclase, was added. Three min after the PGE1 stimulation, ADP (10 μM) or saline was added to the reaction mixture. Aliquots in a volume of 0.5 ml were taken from the reaction mixture before and 3 and 6 min after the PGE1 stimulation. These samples were supplemented with 50 μl of 6N HCl and 50 μM EDTA solution and boiled for 5 min. After rapid cooling on ice, the samples were centrifuged at 10,000×g for 5 min at 4°C. The supernatants (300 μl) were incubated after adding CaCO3 (60 mg) at room temperature for 15 min and then centrifuged again at 10,000×g for 5 min at 4°C. The final supernatants were assayed for cyclic AMP concentrations using an EIA kit (Amersham, Buckinghamshire, U.K.).
Intracellular Ca2+ concentration ([Ca2+]i)
[Ca2+]i was determined after loading human platelets with a fluorescent dye, fura 2-AM. PRP was incubated with 2 μM fura 2-AM at 37°C for 40 min, and this was then washed twice by centrifugation and resuspended at 3×108 platelets ml−1 in a suspension buffer containing 0.05 u ml−1 apyrase. Measurement of fura 2 fluorescence was performed in a Hitachi F-2000 fluorescence spectrophotometer. ADP (10 μM) was added to the platelet suspension in the presence of 1 mM Ca2+. Fluorescence from fura 2 in platelets was excited with two excitation wavelengths of 340 and 380 nm, and the relative intensities of fluorescence were measured at 510 nm. Maximum fluorescence was achieved by lysing the platelets with 0.1% Triton X-100, and the minimum fluorescence was obtained in the presence of 3 mM EGTA. [Ca2+]i was calculated by the equation of Grynkiewicz et al. (1985).
Agents and administration
R-99224 ((2Z)-[1-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4-mercapto-3-piperidinylidene], acetic acid, trifluoroacetate) and CS-747 (2-acetoxy-5-(α-cyclopropylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno [3,2-c]pyridine) were synthesized by Ube Industries (Yamaguchi, Japan). ARL-66096 was synthesized by Chemtech Labo., Inc. (Tokyo, Japan). Adenosine 3′-phosphate 5′-phosphosulfate (A3P5PS), ADP (sodium salt), human fibrinogen, fatty-acid-free BSA, apyrase and gum arabic were purchased from Sigma (St. Louis, MO, U.S.A.). PGE1 was from Funakoshi (Tokyo, Japan). [125I]-Fibrinogen (297 μCi mg−1) and 2-[methyl-3H]methylthio-adenosine-5′-diphosphate ([3H]-2-MeS-ADP, ammonium salt, specific activity 85 Ci mmol−1) were obtained from Amersham (Buckinghamshire, U.K.), and 2-MeS-ADP (trisodium salt) was obtained from Research Biochemicals International (Natick, MA, U.S.A.). Fura 2-AM was obtained from Dojin (Kumamoto, Japan).
In ex vivo studies, R-99224 (0.1 – 3 mg ml−1) dissolved in saline was injected intravenously to rats anaesthetized with pentobarbital sodium (40 mg kg−1, i.p.) in a volume of 1 ml kg−1. Blood was drawn from the rats 1 h post-dose, and the ADP (0.3 – 30 μM)-induced platelet aggregation was measured in PRP prepared by the method described above. In the experiment to examine the duration of action, CS-747 (3 mg ml−1) suspended in 5% gum arabic solution was orally administered to non-fasted, conscious rats in a volume of 1 ml kg−1. Blood was drawn from the rats 4 h post-dose, and ADP (10 μM)-induced platelet aggregation was measured in PRP prepared by the method described above.
Statistics
Results are expressed as the mean±s.e.mean unless otherwise stated. Differences between two experimental groups were assessed by the unpaired t-test, and differences between multiple groups were assessed by Dunnett's multiple comparison test or Turkey multiple comparison test (SAS statistical computer package, SAS Institute Inc., Cary, NC, U.S.A.). A P value of less than 0.05 was considered statistically significant.
Results
In vitro platelet aggregation in human washed platelets
The addition of R-99224 to washed human platelets at concentrations up to 100 μg ml−1 did not elicit any aggregating responses, indicating that this agent is devoid of agonist activity (data not shown). To determine the inhibitory potency and specificity of R-99224, we examined the effects of R-99224 on platelet aggregation in washed human platelets. As shown in Figure 2, pretreatment of platelets with R-99224 (0.03 – 1 μg ml−1) inhibited ADP-induced platelet aggregation. This inhibition was concentration-dependent, and the IC50 value against 3 μM ADP was 0.11 μg ml−1. R-99224 did not affect the ADP-induced shape change even at the highest concentration used (1 μg ml−1) (data not shown).
Figure 2.
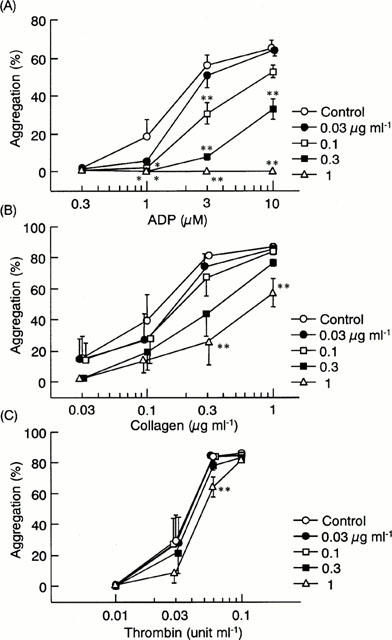
In vitro effects of R-99224 (0.03 – 1 μg ml−1) on platelet aggregation induced by ADP (A), collagen (B) and thrombin (C) in washed human platelets. Results are expressed as the mean±s.e.mean (n=5 – 6). *P<0.05, **P<0.01 vs each control.
Platelet aggregation induced by low concentrations of collagen and thrombin (0.06 u ml−1) was slightly inhibited at the highest concentration of R-99224 (1 μg ml−1), but platelet aggregation induced by higher concentrations of thrombin was negligible. Similar results of specificity to ADP were observed for apyrase (data not shown).
In vitro and ex vivo platelet aggregation of rat platelets
The in vitro effect of R-99224 on platelet aggregation was also examined in washed rat platelets. R-99224 (0.1 – 3 μg ml−1) inhibited ADP-induced platelet aggregation in a concentration-dependent manner with an IC50 value of 0.42 μg ml−1 against 3 μM ADP (Figure 3A). R-99224 also inhibited collagen-induced platelet aggregation in a similar concentration range (Figure 3B). In contrast, the effects of R-99224 on thrombin-induced aggregation were moderate (Figure 3C). Similar specificities to ADP were also observed in the results for apyrase (data not shown).
Figure 3.
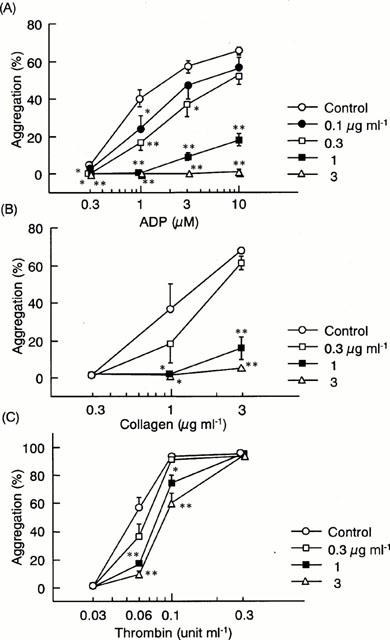
In vitro effects of R-99224 (0.1 – 3 μg ml−1) on platelet aggregation induced by ADP (A), collagen (B) and thrombin (C) in washed rat platelets. Results are expressed as the mean±s.e.mean (n=5 – 6). *P<0.05, **P<0.01 vs each control.
To investigate the in vivo potency of R-99224, ex vivo platelet aggregation was determined using platelets collected from rats that received intravenous bolus injection of R-99224 (0.1 – 3 mg kg−1, i.v.). Ex vivo platelet aggregation induced by ADP (0.3 – 30 μM) was inhibited by R-99224 (0.1 – 3 mg kg−1, i.v.) in a dose-dependent manner (Figure 4). The ED50 value against 3 μM ADP was approximately 0.48 mg kg−1 (i.v.).
Figure 4.

Ex vivo effect of single intravenous administration of R-99224 (0.1 – 3 mg kg−1) on ADP (0.3 – 30 μM)-induced platelet aggregation in rats. R-99224 was intravenously administered to rats 1 h before the blood collection. Results are presented as the mean±s.e.mean (n=6).
Duration of action
The in vitro duration of action of R-99224 was investigated in comparison with PGE1, a potent but reversible inhibitor of platelet aggregation. ADP-induced aggregation of rat platelets was completely inhibited in the presence of PGE1 (1 μM), but this inhibition was not evident after platelets were washed to eliminate PGE1 in the plasma (Figure 5A). In contrast, the inhibition of platelet aggregation by R-99224 (10 μg ml−1) persisted even after the platelets were washed three times (Figure 5A). Likewise, inhibition of ex vivo platelet aggregation of rats treated with CS-747 (3 mg kg−1, p.o.) was not affected by washing the platelets up to three times (Figure 5B).
Figure 5.
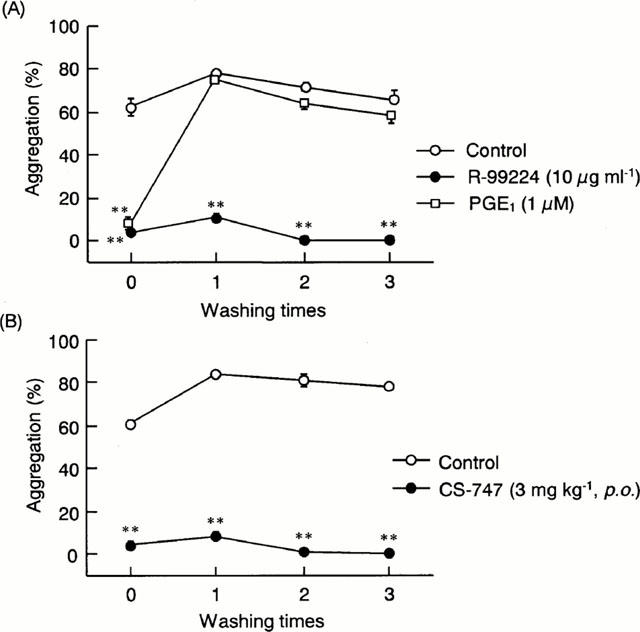
In vitro duration of antiaggregatory effects: (A) Rat platelet-rich plasma was preincubated with R-99224 (10 μg ml−1) or PGE1 (1 μM) for 1 min, and then ADP (10 μM)-induced platelet aggregation was measured before and after washing the platelets; (B) ADP (10 μM)-induced ex vivo aggregation of platelets from vehicle- or CS-747 (3 mg kg−1, p.o.)-treated rats was measured before and after washing the platelets. Results are presented as the mean±s.e.mean (n=6). **P<0.01 vs each control.
[3H]-2-MeS-ADP binding
To determine the effects of R-99224 on platelet ADP receptors, we examined the effects of R-99224 on the binding of [3H]-2-MeS-ADP, a stable ADP analogue, to human platelets. Our preliminary study showed that [3H]-2-MeS-ADP binding to human platelets was time-related and saturable. The [3H]-2-MeS-ADP (10 nM) binding to vehicle-treated (control) platelets was 96.5±9.5 fmol 108 platelets−1 (n=6). Treatment with R-99224 (0.1 – 100 μM) produced a concentration-related inhibition of the [3H]-2-MeS-ADP binding and the inhibition reached a plateau at 3 μM (1.4 μg ml−1) of R-99224 (Figure 6). However, this inhibition by R-99224 was partial (84% of control) even at the highest concentration used (100 μM=46 μg ml−1). Our preliminary experiment showed that CS-747 has no in vitro inhibitory activity at concentrations of up to 100 μM (data not shown).
Figure 6.
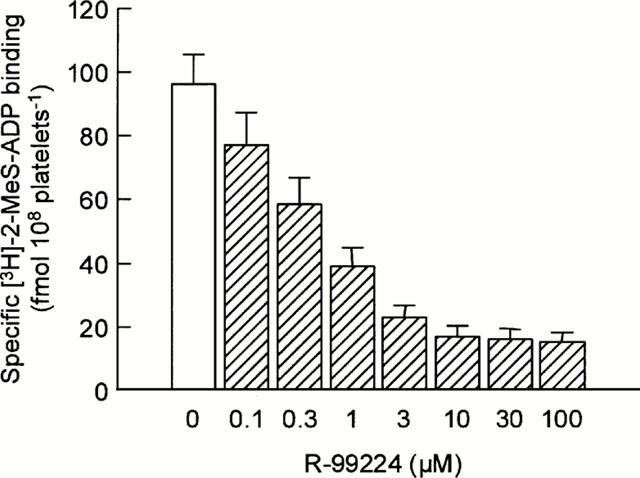
In vitro effect of R-99224 (0.1 – 100 μM) on [3H]-2-MeS-ADP binding to washed human platelets. Results are expressed as the mean±s.e.mean (n=6).
To further characterize the binding properties of R-99224, we used ARL-66096, an ATP-type antagonist for a Gi-linked P2T (Kunapuli & Daniel, 1998; Daniel et al., 1998), and A3P5PS, a selective P2Y1 antagonist (Boyer et al., 1996; Kunapuli & Daniel, 1998). ARL-66096 and A3P5PS inhibited [3H]-2-MeS-ADP binding in a concentration-dependent manner, with maximal inhibition observed at 0.3 μM (ARL-66096) and 300 μM (A3P5PS), respectively (data not shown). As shown in Figure 7A, R-99224 (10 μM=4.6 μg ml−1) in combination with ARL-66096 (0.3 μM) caused no additional inhibition of [3H]-2-MeS-ADP binding. In contrast, R-99224 (10 μM) in combination with A3P5PS (300 μM) completely abolished [3H]-2-MeS-ADP binding (Figure 7B). Likewise, ARL-66096 (0.3 μM) combined with A3P5PS abolished [3H]-2-MeS-ADP binding.
Figure 7.

(A) In vitro effect of R-99224 (10 μM) combined with ARL-66096 (0.3 μM) on [3H]-2-MeS-ADP binding to washed human platelets; (B) In vitro effects of A3P5PS (300 μM) combined with R-99224 (10 μM) or ARL-66096 (0.3 μM) on [3H]-2-MeS-ADP binding to washed human platelets. Results are expressed as the mean±s.e.mean (n=6). **P<0.01, NS, not significant.
[125I]-Fibrinogen binding
We examined the effects of R-99224 on fibrinogen binding to platelets, the final common step of platelet activation. The ADP-induced [125I]-fibrinogen binding to control platelets was 1.097±0.144 pmol 108 platelets−1 (n=6). R-99224 (0.01 – 3 μg ml−1) produced a concentration-related inhibition of the [125I]-fibrinogen binding (Figure 8). In vitro treatment with CS-747 (30 and 100 μg ml−1) had no effect on [125I]-fibrinogen binding to the washed human platelets (data not shown).
Figure 8.

In vitro effect of R-99224 (0.01 – 3 μg ml−1) on [125I]-fibrinogen binding to washed human platelets. Results are expressed as the mean±s.e.mean (n=6). **P<0.01 vs control.
Cyclic AMP levels in platelets
Since ADP inhibits adenylyl cyclase via activation of Gi protein (Defreyn et al., 1991), we examined if R-99224 attenuates ADP-mediated suppression of PGE1-induced cyclic AMP elevation. An addition of PGE1 (10 μM) produced a progressive increase of intraplatelet cyclic AMP levels, indicating the activation of adenylyl cyclase. The elevated cyclic AMP levels were suppressed by ADP (10 μM) added 3 min after PGE1 stimulation. As shown in Figure 9, the inhibitory effect of ADP (10 μM) on elevated cyclic AMP levels was inhibited substantially in R-99224 (0.1 – 1 μg ml−1)-treated platelets in a concentration-related manner. There was no difference in the basal cyclic AMP levels between R-99224-treated and vehicle-treated (control) platelets.
Figure 9.
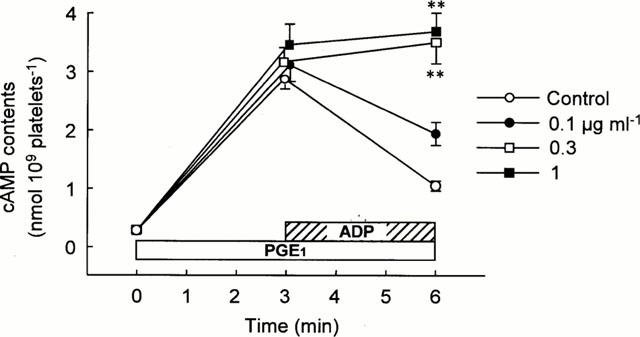
In vitro effect of R-99224 (0.1 – 1 μg ml−1) on ADP (10 μM)-induced cyclic AMP decrease in PGE1 (10 μM)-stimulated human platelets. ADP was added to the reaction mixture 3 min after PGE1 stimulation. Results are expressed as the mean±s.e.mean (n=6). **P<0.01 vs control.
Ca2+ mobilization
Since ADP causes elevation of intracellular Ca2+ ([Ca2+]i), we examined the effects of R-99224 on [Ca2+]i using fura 2-loaded platelets. There were no differences in basal [Ca2+]i between control and R-99224 (up to 1 μg ml−1)-treated platelets (data not shown). In control platelets, ADP (10 μM) induced a marked increase in [Ca2+]i that peaked 5 s after the addition of ADP (264.3±17.5 nM, n=6). R-99224 (0.03 – 1 μg ml−1) had minimal effects on the ADP-induced increase in [Ca2+]i at all time points (5, 10, 15, 30 and 60 s) (Figure 10).
Figure 10.
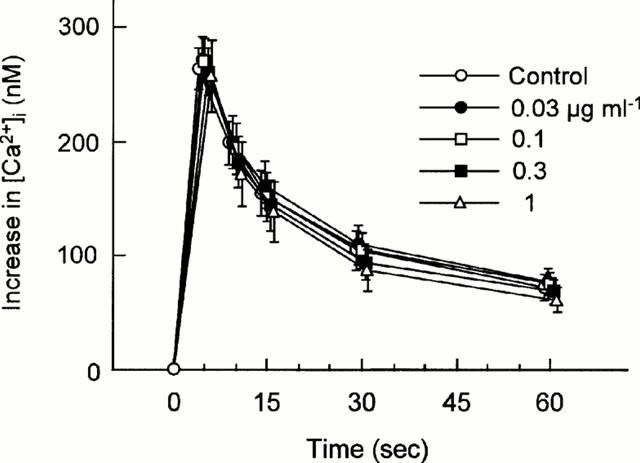
In vitro effect of R-99224 (0.03 – 1 μg ml−1) on ADP (10 μM)-induced increase in intracelular Ca2+ concentration in washed human platelets. Results are expressed as the mean±s.e.mean (n=6). There are no statistically significant differences at any points.
Discussion
CS-747, a novel thienopyridine derivative, is an orally active antiplatelet and antithrombotic agent with a potency higher than that of clopidogrel and ticlopidine (Sugidachi et al., 2000). Like clopidogrel and ticlopidine, CS-747 is inactive in vitro, but R-99224, a metabolite of CS-747, is active in vitro in inhibiting ADP-induced aggregation of rat platelets. The purpose of the present investigation was to identify the subtype of ADP receptors to which R-99224 binds in human platelets. The results of this study have demonstrated that R-99224 binds to Gi-linked P2T receptors selectively and irreversibly, and that R-99224 is a responsible metabolite for in vivo actions of CS-747.
Several lines of evidence suggest that the in vivo antiplatelet effects of the thienopyridine derivatives, clopidogrel and ticlopidine, are due to their active metabolite(s), but no such active metabolites have been identified (Saltiel & Ward, 1987; Savi et al., 1994a; Coukell & Markham, 1997). Recently, one group (Weber et al., 1999) claimed that the antiaggregatory effects of clopidogrel could be due to its direct interaction with human platelets in vitro. However, this might be artifactual due to the long period of incubation of the washed platelets with clopidogrel (Herbert & Savi, 1999). R-99224 was originally isolated from the incubation medium of CS-747 and rat hepatocytes. R-99224 has been detected in the plasma of various animals treated with CS-747 (unpublished data). In the present study, intravenous administration of R-99224 to rats potently inhibited ADP-induced platelet aggregation. To our knowledge, therefore, R-99224 is the first thienopyridine-derived metabolite that is active in vitro and in vivo.
The present study has shown that R-99224 is a potent inhibitor of ADP-induced platelet aggregation both in human and rat platelets. Collagen- and thrombin-induced aggregation was moderately inhibited by R-99224 at high concentrations. This is consistent with ex vivo results of CS-747 orally administered to rats (Sugidachi et al., 2000), and also with the ex vivo results of ticlopidine on human platelet aggregation (Cattaneo et al., 1991). In addition, apyrase, an ADP scavenger, produced a similar inhibitory profile on human and rat platelet aggregation. Thus, the antiaggregatory effects of R-99224 against collagen- and thrombin-induced aggregation are most likely attributable to its inhibitory effects on ADP released from the dense granules of activated platelets.
Previous study has shown that CS-747 exerts long-lasting ex vivo antiaggregatory effects (Sugidachi et al., 2000). The duration of action of CS-747 is comparable to the life span of circulating platelets in the rat (Cattaneo et al., 1985; Jackson et al., 1992) suggesting that CS-747 interacts with platelets in an irreversible manner. Indeed, the present study showed that the inhibition of platelet aggregation in PRP prepared from rats given an oral dose of CS-747 were maintained even after the platelets were washed three times. Likewise, the in vitro treatment of platelets with R-99224 was maintained after platelets were washed up to three times to remove R-99224 from the medium. In contrast, the inhibition by PGE1, a reversible antiaggregating agent, was eliminated after the first washing. These findings strongly suggest that R-99224 interacts with platelets in an irreversible manner. Our structure-activity relation study showed that the thiol group is essential for achieving antiaggregatory effects (data not shown). Although further studies will be necessary to elucidate the molecular mechanism of the antiaggregatory action of R-99224, its irreversible mode of action may derive from the formation of a disulfur bridge between the reactive thiol group and that of a cysteine residue of the platelet ADP receptor.
Recent studies have demonstrated that the platelet ADP receptor is not homogeneous. In addition to the ligand-gated ion channel P2X1 receptor (MacKenzie et al., 1996), two subclasses of G-protein coupled ADP receptors have been proposed to exist on human platelet membranes: the Gq-linked P2Y1 receptor and the Gi-linked P2T receptor (Fagura et al., 1998; Daniel et al., 1998; Jantzen et al., 1999). 2-MeS-ADP is a stable agonist for P2Y1 and Gi-linked P2T receptor (Kunapuli, 1998a,1998b), and radiolabelled 2-MeS-ADP has been widely used for the study of ADP receptors (Léon et al., 1999; Mills et al., 1992; Savi et al., 1994b). In the present study, R-99224 produced a significant inhibition of [3H]-2-MeS-ADP binding to human platelets in a concentration-dependent manner. This finding is consistent with the ex vivo effects of CS-747 in rats (Sugidachi et al., 2000) and suggests that R-99224 is a specific platelet ADP receptor antagonist.
However, the inhibition of [3H]-2-MeS-ADP binding by R-99224 was partial: 16% of [3H]-2-MeS-ADP binding still remained even at the highest concentration used (100 μM=46 μg ml−1). This suggests that there are two binding sites, one of which is resistant to R-99224. Thus, we further characterized binding properties of R-99224 using ARL-66096, a selective Gi-linked P2T receptor antagonist (Kunapuli & Daniel, 1998; Daniel et al., 1998) and A3P5PS, a selective P2Y1 receptor antagonist (Boyer et al., 1996; Kunapuli & Daniel, 1998). If the two antagonists bound to two separate receptors, an additive inhibition could be anticipated. As expected, R-99224 abolished [3H]-2-MeS-ADP binding, when it was combined with A3P5PS, but not with ARL-66096. These results are consistent with the contention that R-99224 binds selectively to Gi-linked P2T receptors.
To further determine if R-99224 inhibits Gi-linked P2T receptor functions, we investigated the effects of R-99224 on ADP-induced intracellular signal events. P2Y1 receptors are linked to heterodimeric G proteins that stimulate PLC, which leads to mobilization of Ca2+ ions. Activation of P2X1 receptors linked to Ca2+ channels also leads to elevation of [Ca2+]i. The present study showed that R-99224 had no effects on ADP-induced Ca2+ mobilization. In contrast, R-99224 neutralized ADP-induced inhibition of cyclic AMP elevation by PGE1. Since Gi-linked P2T receptors are linked G proteins that stimulate adenylyl cyclase, these results are consistent with R-99224 being a selective inhibitor of Gi-linked P2T receptors. This contention is further supported by the fact that R-99224 had no effects on ADP-induced shape change (Sugidachi et al., 2000), a process mediated via the P2Y1 receptor (Kunapuli, 1998a,1998b). Taken together, in the present study, inhibitions of [3H]-2-MeS-ADP binding, ADP-induced fibrinogen binding, and cyclic AMP reduction were achieved by treatment with R-99224 at a similar concentration range. These data suggest that R-99224 irreversibly binds to Gi-linked P2T receptors, thereby inhibiting signaling events and fibrinogen binding, and ultimately resulting in a long-lasting inhibition of ADP-induced platelet aggregation. Our results may partially resolve ongoing controversy regarding the importance of the Gi-linked P2T receptor (Léon et al., 1999; Jarvis et al., 2000) and P2Y1 receptor (Fabre et al., 1999; Jarvis et al., 2000) in ADP-induced platelet aggregation, by providing additional evidence that the former is in fact essential in this process (Jin & Kunapuli, 1998).
In summary, the present study demonstrated that R-99224 is an irreversible and selective Gi-linked P2T receptor antagonist. The potent antiaggregtory and antithrombotic effects of CS-747 seem to be mediated by the interaction of its hepatic metabolite, R-99224, with circulating platelets in vivo. Whether R-99224 is the sole active metabolite of CS-747 remains to be elucidated.
Acknowledgments
We thank Ms Naoko Suzuki, Ms Junko Fukuoka, Ms Yumiko Kawamura and Ms Takako Nagasawa for their expert technical assistance.
Abbreviations
- ADP
adenosine 5′-diphosphate
- A3P5PS
adenosine 3′-phosphate 5′-phosphosulfate
- BSA
bovine serum albumin
- EGTA
ethylene glycol-bis (β-aminoethyl ether) N,N,N′,N′-tetraacetic acid
- HEPES
N-2-hydroxylethylpiperazine-N′-2-ethanesulfonic acid
- IBMX
3-isobutyl-1-methylxanthine
- 2-MeS-ADP
2-methylthio-adenosine 5′-diphosphate
- PGE1
prostaglandin E1
- PPP
platelet-poor plasma
- PRP
platelet-rich plasma
References
- BOYER J.L., ROMERO-AVILA T., SCHACHTER J.B., HARDEN T.K. Identification of competitive antagonists of the P2Y1 receptor. Mol. Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- CATTANEO M., AKKAWAT B., LECCHI A., CIMMINIELLO C., CAPITANIO A.M., MANNUCCI P.M. Ticlopidine selectively inhibits human platelet responses to adenosine diphosphate. Thromb. Haemost. 1991;66:694–699. [PubMed] [Google Scholar]
- CATTANEO M., WINOCOUR P.D., SOMERS D.A., GROVES H.M., KINLOUGH-RATHBONE R.L., PACKHAM M.A., MUSTARD J.F. Effect of ticlopidine on platelet aggregation, adherence to damaged vessels, thrombus formation and platelet survival. Thromb. Res. 1985;37:29–43. doi: 10.1016/0049-3848(85)90030-1. [DOI] [PubMed] [Google Scholar]
- COUKELL A.J., MARKHAM A. Clopidogrel. Drugs. 1997;54:745–750. doi: 10.2165/00003495-199754050-00006. [DOI] [PubMed] [Google Scholar]
- DANIEL J.L., DANGELMAIER C., JIN J., ASHBY B., SMITH J.B., KUNAPULI S.P. Molecular basis for ADP-induced platelet activation. I. Evidence for three distinct ADP receptors on human platelets. J. Biol. Chem. 1998;273:2024–2029. doi: 10.1074/jbc.273.4.2024. [DOI] [PubMed] [Google Scholar]
- DEFREYN G., GACHET C., SAVI P., DRIOT F., CAZENAVE J.P., MAFFRAND J.P. Ticlopidine and clopidogrel (SR 25990C) selectively neutralize ADP inhibition of PGE1-activated platelet adenylyl cyclase in rats and rabbits. Thromb. Haemost. 1991;65:186–190. [PubMed] [Google Scholar]
- FABRE J.E., NGUYEN M., LATOUR A., KEIFER J.A., AUDOLY L.P., COFFMAN T.M., KOLLER B.H. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat. Med. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- FAGURA M.S., DAINTY I.A., MCKAY G.D., KIRK I.P., HUMPHRIES R.G., ROBERTSON M.J., DOUGALL I.G., LEFF P. P2Y1-receptors in human platelets which are pharmacologically distinct from P2Y (ADP)-receptors. Br. J. Pharmacol. 1998;124:157–164. doi: 10.1038/sj.bjp.0701827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GACHET C., CAZENAVE J.P. ADP induced blood platelet activation: a review. Nouv. Rev. Fr. Hematol. 1991;33:347–358. [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3550. [PubMed] [Google Scholar]
- HERBERT J.M., SAVI P. Non-specific inhibition of ADP-induced platelet antiaggregation by clopidogrel in vitro. Thromb. Haemost. 1999;82:156–157. [PubMed] [Google Scholar]
- HOURANI S.M., HALL D.A. Receptors for ADP on human blood platelets. Trends Pharmacol. Sci. 1994;15:103–108. doi: 10.1016/0165-6147(94)90045-0. [DOI] [PubMed] [Google Scholar]
- JACKSON C.W., STEWARD S.A., ASHMUN R.A., MCDONALD T.P. Megakaryocytopoiesis and platelet production are stimulated during late pregnancy and early postpartum in the rat. Blood. 1992;79:1672–1678. [PubMed] [Google Scholar]
- JANTZEN H.M., GOUSSET L., BHASKAR V., VINCENT D., TAI A., REYNOLDS E.E., CONLEY P.B. Evidence for two distinct G-protein-coupled ADP receptors mediating platelet activation. Thromb. Haemost. 1999;81:111–117. [PubMed] [Google Scholar]
- JARVIS G.E., HUMPHRIES R.G., ROBERTSON M.J., LEFF P. ADP can induce aggregation of human platelets via both P2Y1 and P2T receptors. Br. J. Pharmacol. 2000;129:275–282. doi: 10.1038/sj.bjp.0703046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN J., KUNAPULI S.P. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNAPULI S.P. Multiple P2 receptor subtypes on platelets: a new interpretation of their function. Trends Pharmacol. Sci. 1998a;19:391–394. doi: 10.1016/s0165-6147(98)01248-6. [DOI] [PubMed] [Google Scholar]
- KUNAPULI S.P. Functional characterization of platelet ADP receptors. Platelets. 1998b;9:343–351. doi: 10.1080/09537109876401. [DOI] [PubMed] [Google Scholar]
- KUNAPULI S.P., DANIEL J.L. P2 receptor subtypes in the cardiovascular system. Biochem. J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LÉON C., VIAL C., GACHET C., OHLMANN P., HECHLER B., CAZENAVE J.P., LECCHI A., CATTANEO M. The P2Y1 receptor is normal in a patient presenting a severe deficiency of ADP-induced platelet aggregation. Thromb. Haemost. 1999;81:775–781. [PubMed] [Google Scholar]
- MACKENZIE A.B., MAHAUT-SMITH M.P., SAGE S.O. Activation of receptor-operated cation channels via P2X1 not P2T purinoceptors in human platelets. J. Biol. Chem. 1996;271:2879–2881. doi: 10.1074/jbc.271.6.2879. [DOI] [PubMed] [Google Scholar]
- MILLS D.C. ADP receptors on platelets. Thromb. Haemost. 1996;76:835–856. [PubMed] [Google Scholar]
- MILLS D.C., PURI R., HU C.J., MINNITI C., GRANA G., FREEDMAN M.D., COLMAN R.F., COLMAN R.W. Clopidogrel inhibits the binding of ADP analogues to the receptor mediating inhibition of platelet adenylate cyclase. Arterioscler. Thromb. 1992;12:430–436. doi: 10.1161/01.atv.12.4.430. [DOI] [PubMed] [Google Scholar]
- SALTIEL E., WARD A. Ticlopidine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in platelet-dependent disease states. Drugs. 1987;34:222–262. doi: 10.2165/00003495-198734020-00003. [DOI] [PubMed] [Google Scholar]
- SAVI P., COMBALBERT J., GAICH C., ROUCHON M.C., MAFFRAND J.P., BERGER Y., HERBERT J.M. The antiaggregating activity of clopidogrel is due to a metabolic activation by the hepatic cytochrome P450-1A. Thromb. Haemost. 1994a;72:313–317. [PubMed] [Google Scholar]
- SAVI P., LAPLACE M.C., MAFFRAND J.P., HERBERT J.M. Binding of [3H]-2-methylthio ADP to rat platelets - effect of clopidogrel and ticlopidine. J. Pharmacol. Exp. Ther. 1994b;269:772–777. [PubMed] [Google Scholar]
- SUGIDACHI A., ASAI F., OGAWA T., INOUE T., KOIKE H. The in vivo pharmacological profile of CS-747, a novel antiplatelet agent with platelet ADP receptor antagonist properties. Br. J. Pharmacol. 2000;129:1439–1446. doi: 10.1038/sj.bjp.0703237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGIDACHI A., OGAWA T., ASAI F., SAITO S., OZAKI H., FUSETANI N., KARAKI H., KOIKE H. Inhibition of rat platelet aggregation by mycalolide-B, a novel inhibitor of actin polymerization with a different mechanism of action from cytochalasin-D. Thromb. Haemost. 1998;79:614–619. [PubMed] [Google Scholar]
- WEBER A.A., REIMANN S., SCHRÖR K. Specific inhibition of ADP-induced platelet aggregation by clopidogrel in vivo. Br. J. Pharmacol. 1999;126:415–420. doi: 10.1038/sj.bjp.0702276. [DOI] [PMC free article] [PubMed] [Google Scholar]


