Abstract
This study investigates the mechanisms accounting for the adverse cholinergic effects of the antitumour drug irinotecan. The activity of irinotecan and its active metabolite, 7-ethyl-10-hydroxy-camptothecin (SN-38), was assayed in models suitable for pharmacological studies on cholinergic system.
Irinotecan moderately inhibited human or electric eel acetylcholinesterase activity, SN-38 had no effect, whereas physostigmine blocked both the enzymes with high potency and efficacy.
Irinotecan and SN-38 did not affect spontaneous or electrically-induced contractile activity of human colonic muscle. Acetylcholine and dimethylphenylpiperazinium (DMPP) caused phasic contractions or relaxations, respectively. Physostigmine enhanced the motor responses elicited by electrical stimulation.
Although irinotecan and SN-38 did not modify the basal contractile activity of guinea-pig ileum longitudinal muscle strips, irinotecan 100 μM moderately enhanced cholinergic twitch contractions. Acetylcholine or DMPP caused phasic contractions, whereas physostigmine enhanced the twitch responses. Electrically-induced [3H]-acetylcholine release was reduced by irinotecan (100 μM) or physostigmine (0.1 μM).
Intravenous irinotecan stimulated gastric acid secretion in rats, but no effects were obtained with SN-38, physostigmine or i.c.v. irinotecan. Hypersecretion induced by irinotecan was partly prevented by ondansetron, and unaffected by capsazepine. In the presence of atropine, vagotomy and systemic or vagal ablation of capsaicin-sensitive afferent fibres, irinotecan did not stimulate gastric secretion.
The present results indicate that irinotecan and SN-38 do not act as specific acetylcholinesterase blockers or acetylcholine receptor agonists. It is rather suggested that irinotecan promotes a parasympathetic discharge to peripheral organs, mediated by capsaicin-sensitive vagal afferent fibres, and that serotonin 5-HT3 receptors are implicated in the genesis of vago-vagal reflex triggered by irinotecan.
Keywords: Irinotecan, adverse effects, acetylcholinesterase, acetylcholine release, vagus nerve, capsaicin-sensitive sensory fibres, stomach, ileum, colon
Introduction
Irinotecan is a semisynthetic derivative of the plant alkaloid camptothecin and, like its parent compound, it is endowed with antitumour activity which depends on the blockade of DNA-topoisomerase I (Tanizawa et al., 1994). Irinotecan is not directly responsible for relevant cytotoxic properties, but acts as a prodrug. Upon i.v. infusion, this camptothecin analogue is subjected to the enzymatic action of esterases which, through the cleavage of the di-piperidin carbonyloxy moiety, generate the major metabolite SN-38 (7-ethyl-10-hydroxycamptothecin), which mediates most of the antitumour activity exerted by irinotecan (Rothenberg, 1997).
Irinotecan demonstrated antineoplastic activity both in preclinical and clinical studies, and it is currently undergoing phase II and phase III clinical trials for various neoplastic diseases (Rothenberg, 1997). In addition, irinotecan has been approved as second-line treatment for patients with advanced colorectal adenocarcinoma and is being investigated as first-line therapy for the same disease (Rothenberg & Blanke, 1999; Douillard et al., 2000).
Clinical studies have shown that patients treated with irinotecan frequently experience acute side effects, such as bradycardia, hypotension, hypersalivation, abdominal cramps, diarrhoea, diaphoresis, visual accommodation disturbances and other signs that are consistent with the picture of a cholinergic syndrome (Gandia et al., 1993; Rowinsky et al., 1994). In support of this view, the above symptoms can be prevented or ameliorated by administration of the anticholinergic drug atropine (Rothenberg et al., 1993).
At present, three distinct mechanisms are proposed to explain the cholinergic syndrome associated with irinotecan treatment. First, data obtained from in vitro experiments on acetylcholinesterase (AChE), isolated from human erythrocytes or electric eel, suggest that irinotecan directly inhibits the activity of this enzyme, thus preventing the breakdown of acetylcholine at level of cholinergic synapses (Kawato et al., 1993; Dodds & Rivory, 1999; Morton et al., 1999). Second, on the basis of the structural similarity between the di-piperidin carbonyloxy side chain of irinotecan and dimethylphenylpiperazinium (DMPP), a well known agonist of ganglionic nicotinic receptors (Hayashi et al., 1983), it has been speculated that the mechanism of cholinergic syndrome might be ascribed to a direct stimulation of nicotinic autonomic ganglia (Gandia et al., 1993). Third, radioligand binding experiments suggest that irinotecan may interact with cholinergic muscarinic receptors, thus causing their direct activation (Kawato et al., 1993).
Overall, the pathophysiological mechanisms underlying the cholinergic syndrome induced by irinotecan still remain obscure. In addition, it seems quite surprising that the same drug may activate the endogenous cholinergic pathways acting simultaneously at three distinct levels. Accordingly, the present study was designed in order to investigate the effects of irinotecan and SN-38 on several in vitro and in vivo preparations, all regarded as standard experimental models for pharmacological studies concerning the cholinergic system.
Methods
Acetylcholinesterase assay
The activity of AChE was determined by means of a colorimetric assay based upon the enzymatic conversion of acetylthiocholine to thiocholine, which reacts with 5,5′-dithiobis-2-nitrobenzoic acid to generate the chromogen compound 5-thio-2-nitrobenzoate (Ellman et al., 1961). Briefly, AChE obtained from human erythrocytes or electric eel was used, the assay solution was maintained at the temperature of 37°C throughout the assay and consisted of 0.1 M phosphate buffer (pH 8.0) containing 0.01 M 5,5′-dithiobis-2-nitrobenzoic acid and 3 u ml−1 AChE. The reaction was started by addition of 0.075 M acetylthiocholine iodide to the assay solution, and the formation of 5-thio-2-nitrobenzoate was followed for 5 min by means of a spectrophotometer (Uvikon 810, Kontron Instruments, Milan, Italy) as changes in absorbance at 412 nm. Irinotecan, SN-38 or physostigmine were incubated with AChE in the assay solution for 20 min, and the enzyme reaction was then initiated by addition of the substrate. The effects of test drugs were expressed as percentage of AChE activity determined in control experiments, and their potency in inhibiting AChE was expressed as EC50 (concentration of the drug that produces 50% of the maximal response for that drug). The per cent maximum inhibition of the control AChE activity (Emax) was also evaluated.
Isolated human colon
Tissue preparation
Specimens of human colon were obtained from patients undergoing surgery for benign or malignant conditions of the colon or rectum. Specimens were collected directly from the surgery room and generally consisted of a section of the whole wall of the proximal or distal colon from a macroscopically normal region. They were placed immediately into pre-oxygenated Krebs solution and transported on ice back to the laboratory. The tissue was laid flat in Krebs solution, the mesentery, mucosa and submucosa were gently removed, and muscular regions between the taenia coli were cut into strips of approximately 3 mm width and 30 mm length along the longitudinal axis (Crema et al., 1968). The experimental protocol was approved by Pisa University Hospital Ethics Committee.
Recording of contractile activity under basal conditions or electrical transmural stimulation
The motor activity of colonic muscle strips was recorded according to the procedure previously described (Crema et al., 1968). The colonic preparations were set up in organ baths of 10 ml capacity (overflow system), containing Krebs solution maintained at 37°C and bubbled continuously with 95% O2+5%CO2. The Krebs solution had the following composition (mM): NaCl 113, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, and glucose 11.5 (pH 7.4±0.1). Each colonic preparation was connected vertically to an isotonic transducer (Basile, Comerio, Italy) under a constant tension of 1 g, and was allowed to equilibrate for at least 60 min, with intervening washings at intervals of 15 min. The mechanical activity of the longitudinal muscle was recorded by a polygraph (Basile, Comerio, Italy). A pair of coaxial platinum electrodes was positioned at distance of 10 mm from the longitudinal axis of each preparation in order to deliver transmural electrical stimulation by means of a BM-ST6 stimulator (Biomedica Mangoni, Pisa, Italy). Electrical stimuli were applied as single trains lasting 30 s, consisting of square wave pulses (1 ms duration, 30 mA intensity) at the frequency of 10 Hz. At the end of the equilibration period, each colonic preparation was repeatedly challenged with electrical stimulations, and the experiments were started only when constant and reproducible contractile responses were obtained (usually after two or three electrical stimulations).
In experiments designed to examine the effects of irinotecan, SN-38, acetylcholine or DMPP on basal motor activity, the test drugs were added to the bathing fluid according to a non-cumulative dosing schedule. Unless otherwise stated, increasing concentrations of a given drug were applied at intervals of 20 min, with at least two intervening washings, and the contact time ranged from 30 s to 5 min. The potencies of test drugs, for which full concentration-response curves could be obtained, were estimated as EC50. In agonist-antagonist interaction experiments, the antagonist was added to the bath 20 min before agonist. In experiments aiming to assay the effects of irinotecan. SN-38, physostigmine or atropine on the electrically-induced motor activity, each test drug was added to the bath 20 min before the application of electrical stimulation. In this case the drug-induced variations of contractile activity were expressed as percentage of control responses elicited by transmural electrical stimulation alone. Drugs were always given in volumes ⩽1% of total bath volume (10 ml).
Isolated guinea-pig ileum
Animal care and tissue preparation
Male albino guinea-pigs, 300 – 350 g body weight, were used throughout the study. They were fed standard laboratory chow and tap water ad libitum and were not used for at least 1 week after their delivery to the laboratory. The animals were housed, four in a cage, in temperature controlled rooms on a 12-h light cycle at 22 – 24°C and 50 – 60% humidity. Their care and handling were in accordance with the provisions of the European Community Council Directive 86 – 609, recognized and adopted by the Italian Government. At the time of the experiment, the whole ileum was excised from the small intestine with the exception of the distal 10 cm, and longitudinal muscle strips with myenteric plexus attached were prepared as previously reported (Colucci et al., 1998). The ileal preparations were then used in order to assess the effects of test drugs on basal motor activity as well as on electrically-induced twitch contractions or acetylcholine release. Experiments aiming to assay the effects of irinotecan, SN-38, acetylcholine or DMPP under basal conditions were performed as reported above. Twenty-four hours before experiments, some animals were treated with reserpine (4 μmol kg−1 s.c.) in order to promote a complete depletion of serotonin from myenteric serotonergic neurons, as previously reported by Galligan et al. (1986). Longitudinal muscle strips prepared from ileum of reserpinized animals were then used to assay the effects of irinotecan on twitch motor activity.
Recording of twitch contractions
The twitch motor activity was recorded as previously described (Colucci et al., 1998). Briefly, ileal longitudinal muscle strips, weighing 60 – 120 mg were set up in organ baths containing oxygenated Krebs solution, and connected vertically to isometric transducers (Basile, Comerio, Italy). Recurrent phasic contractions of the longitudinal muscle (twitch responses) were evoked by the application of transmural electrical stimulation consisting of square wave pulses (1 ms duration, 30 – 40 mA intensity) at the frequency of 0.1 Hz. The preparations were stimulated for 60 min and were washed four times at 15-min intervals before the beginning of experiments. Irinotecan, SN-38 or physostigmine were added cumulatively to the bathing fluid in 1−log unit increments. Unless otherwise stated, a period of 3 – 10 min was allowed to elapse between subsequent increments of concentration in order to enable the full effect of the drug to develop. The effects of test drugs were expressed as percentage of the control twitch response.
Measurement of [3H]-acetylcholine release
The measurement of [3H]-acetylcholine release was carried out as previously described (Colucci et al., 1998). Longitudinal muscle strips of guinea-pig ileum, weighing 60 – 120 mg, were incubated for 30 min in oxygenated Krebs solution at 37°C (preincubation period), and then loaded with methyl-[3H]-choline (3 μCi ml−1) for 45 min in 3 ml of Krebs solution. Electrical field stimulation of 0.1-ms duration at 1 Hz was applied to ileal preparations for 30 min during the loading period. At the end of incubation, the ileal strips were washed five times, transferred to another organ bath (5-ml capacity) and superfused at a flow rate of 1 ml min−1 with Krebs solution at 37°C, aerated with 95% O2+5% CO2. The superfusing solution contained 3 μM hemicholinium-3 in order to inhibit the reuptake of [3H]-choline liberated by the hydrolysis of released [3H]-acetylcholine. The first 60-min collection of effluent was discarded (preperfusion), after which 3-min fractions were collected for 90 min. During the superfusion period, the ileal preparations were subjected to electrical field stimulation, delivered as square wave pulses (10 V cm−1) of 1 ms duration at 1 Hz (180 pulses) in the 3rd (S1) and 20th (S2) collection periods. At the end of superfusion, the radioactivity of fractions was determined by liquid scintillation counting (Betamatic, Kontron Instruments, Milan, Italy), and the radioactive content of ileal strips was also measured. For this purpose, each preparation was incubated in 1 ml of 10% trichloroacetic acid at room temperature for 30 min. An aliquot of the supernatant (50 μl) was added to 5 ml of scintillator and the tritium content of tissue was measured by liquid scintillation spectrometry.
Irinotecan, SN-38 or physostigmine were added to the superfusing solution in the 12th collection period, namely between S1 and S2, and exposure to each drug continued until the end of experiment. Tritium efflux into the superfusate was calculated as the fraction of tritium present in the ileal strip at the onset of the respective collection period (fractional rate; min−1). The increase in tritium outflow, evoked by electrical stimulation, was calculated as percentage of the tritium content of the tissue at the onset of electrical stimulation. The effects of test drugs on the evoked tritium outflow were expressed as ratio of the percentage release during S2 over that obtained during S1 (S2/S1), namely in the presence and absence of drug respectively (Colucci et al., 1998).
In situ perfusion of rat stomach
Animal care and surgical preparation
The experiments were carried out on male Wistar rats weighing 200 – 220 g. Their care and handling were as reported above. Continuous perfusion of the stomach in situ was carried out as previously described (Blandizzi et al., 1997). Briefly, the animals were anaesthetized with urethane (13.5 mmol kg−1 i.p.) and the trachea was cannulated by a polyethylene catheter to ensure a patent airway. A polyethylene catheter was introduced into the oesophagus and advanced as far as 5 mm beyond the gastroesophageal junction. After a midline laparatomy, a second polyethylene catheter was introduced into the duodenum and pushed forward until its tip was about 5 mm beyond the pylorus.
Evaluation of gastric acid secretion
The stomach lumen was perfused continuously at a rate of 1 ml min−1 with saline solution (154 mM NaCl) at 37°C (pH=7.0±0.2), and 15-min effluent fractions were collected. The effluent samples were then used for the quantitative evaluation of acid secretion. For this purpose, after the surgical preparation of animals, basal gastric secretion was allowed to stabilize for 30 min and two consecutive 15-min effluent fractions were then collected in order to assess the basal secretory values. Acid secretion was monitored at 15-min intervals for additional 180 min. The acidity in the gastric perfusate was measured with an autotitrator pH meter (PHM 85, Radiometer, Copenhagen, Denmark) by automatic potentiometric titration to pH 7.0 with 0.01 N NaOH and was expressed as μEqH+ 15 min−1. The acid output obtained during the whole 180-min period following the collection of basal effluent samples was also calculated and expressed as μEqH+ 180 min−1.
Experimental procedures
In the first set of experiments, the effects of irinotecan, SN-38 or physostigmine on basal gastric secretion were assayed in rats with intact vagus nerves. All drugs were administered by i.v. route as a bolus immediately after the collection of basal effluent samples. In experiments investigating the involvement of muscarinic, serotonin 5-HT3 or vanilloid receptors in the gastric secretory responses elicited by irinotecan, the animals were pretreated with the respective antagonists, atropine, ondanstron or capsazepine, immediately before the collection of the second basal effluent sample.
The second set of experiments was designed in order to detect changes in gastric secretion following direct i.c.v. administration of irinotecan to rats with intact vagus nerves. For this purpose, a chronic cannula, to be used as a guide for microinjection of drugs, was implanted into the right lateral ventricle of the brain, during a short anaesthesia with pentobarbitone sodium (120 – 160 μmol kg−1 i.p.) (Blandizzi et al., 1995). Briefly, a cut was made in the scalp in order to expose the intersection of the coronal and sagittal sutures. A hole was then drilled through the skull 1.5 mm lateral and 1 mm posterior to bregma. A stainless steel cannula (24 gauge), equipped with a perspex base, was introduced through the hole in the skull to a depth of 4 mm, and fixed to the skull surface with screws and dental cement. Animals were allowed to recover from surgery for 6 days. At the time of experiment, drug solutions (2.5 μl) followed by 2.5 μl of saline solution were injected into the lateral ventricle of the brain by means of a 10-μl Hamilton microsyringe connected to the guide cannula by polyethylene tubing. The solutions were injected within 20 s. At the end of each experiment, the correct location of the cannula in the lateral ventricle was checked by injecting 10 μl of dye (0.1% toluidine). The visualization of dye on the walls of the lateral ventricle indicated the exact location of the i.c.v. cannula.
The third series of experiments was performed on rats whose vagus nerves were carefully separated from the carotid arteries and cut at the cervical level 30 min before the collection of basal effluent samples began. The effects of irinotecan on gastric secretion were reassessed in animals that underwent this vagotomy procedure.
The fourth set of experiments was designed in order to assess whether irinotecan was able to affect gastric acid secretion in the presence of systemic ablation of capsaicin-sensitive sensory nerve fibres (Holzer, 1991). For this purpose, animals were given a total dose of 400 μmol kg−1 capsaicin s.c., as previously reported (Blandizzi et al., 1997). Ten days after this treatment the animals were used for measurement of acid secretion. One day before the experiment, the efficacy of capsaicin treatment was checked by instilling a drop of capsaicin solution (0.3 μmol ml−1 in saline solution) into one eye of each rat. Capsaicin-treated rats were expected not to react by wiping their eyes but, whenever an animal responded with wiping, the afflicted eye was immediately and extensively rinsed with water.
The fifth series of experiments was performed to investigate the effects of irinotecan on acid secretion in rats subjected to perivagal application of capsaicin, which produces a selective functional impairment of vagal capsaicin-sensitive afferent fibres (Holzer, 1991). In accordance with the procedure reported by Raybould & Taché (1989), rats were anaesthetized with pentobarbitone sodium (120 – 160 μmol kg−1 i.p.). Under full aseptic conditions, the cervical vagi were exposed by making a midline neck incision and carefully freeing the vagal trunk from the carotid artery for a distance of 2 – 3 mm. Parafilm was placed around the vagus nerve and a pledget of cotton wool soaked in capsaicin was placed around the nerve trunk for 30 min. Further drops of capsaicin were applied every 5 min, the maximum amount of capsaicin applied being 0.1 ml (3 μmol per rat). The area was then thoroughly rinsed with olive oil followed by saline and dried with sterile swabs, and the neck incision was closed. Animals in which the vagi were treated with 10% Tween 80 in olive oil served as vehicle controls. Ten days after this treatment the animals were used for measurement of acid secretion.
In an additional group of experiments, performed on rats subjected to either systemic or vagal selective capsaicinization, the vagus nerves were cut at cervical level, as reported above. The distal end of the left vagus nerve was then placed on a bipolar platinum electrode and immersed in paraffin oil. After the collection of basal samples, gastric acid secretion was evoked by continuous electrical stimulation of the left vagus nerve (180 min). The stimulus parameters were square-wave pulses 0.5 ms in duration, delivered at 5 Hz with supramaximal intensity (10 V) by means of a Grass S5 stimulator (Grass Instruments, Quincy, MA, U.S.A.).
Drugs
The following drugs and reagents were used: acetylcholine chloride, acetylthiocholine iodide, human erythrocyte acetylcholinesterase, electric eel acetylcholinesterase, 5,5′-dithiobis-2-nitrobenzoic acid, 1,1-dimethyl-4-phenylpiperazinium iodide, urethane ethyl carbamate, physostigmine sulphate, tetrodotoxin, hemicholinium-3 bromide, capsaicin, aminophylline, terbutaline, capsazepine, reserpine (Sigma Chemicals Co., St. Louis, MO, U.S.A.); hexamethonium 2-chloride (RBI, Natick, MA, U.S.A.); methyl-[3H]-choline chloride (80 Ci mM) (Amersham Laboratories, Des Plaines, IL, U.S.A.); atropine sulphate (BDH Chemicals, Poole, U.K.); pentobarbitone sodium (Clin-Midy, Paris, France); ondansetron (Glaxo Wellcome, Verona, Italy); irinotecan, SN-38 [7-ethyl-10-hydroxycamptothecin] (kindly provided by Rhone-Poulenc Rorer, Vitry-Alfortville, France). Other reagents were of analytical grade. Unless otherwise stated, the drugs were dissolved in distilled water or saline solution. SN-38 was dissolved in dimethylsulphoxide and then diluted in water. 5,5′-Dithiobis-2-nitrobenzoic acid was dissolved in phosphate buffer 0.1 M (pH 7.0). Human erythrocyte acetylcholinesterase was dissolved in water by 5% Triton X-100. Electric eel acetylcholinesterase was provided as aqueous solution containing ammonium sulphate. All drugs administered by i.v., i.p. or s.c. route were injected in a volume of 0.25 ml per rat. Capsaicin to be administered by s.c. route was dissolved (37.5 μmol ml−1) in a vehicle composed by 10% ethanol, 10% Tween 80 and 80% saline solution (v/v/v). The total dose of capsaicin (400 μmol kg−1) was administered under ether anaesthesia in four injections over two consecutive days (first day: 80 μmol kg−1 in the morning and 80 μmol kg−1 in the late afternoon; second day: 80 μmol kg−1 in the morning and 160 μmol kg−1 in the late afternoon). Capsaicin (30 μmoles) to be used for perivagal application was sonicated for 10 min in 0.1 ml Tween 80, then 0.9 ml of olive oil were added and the mixture sonicated for further 10 min. In order to counteract the respiratory impairment induced by capsaicin, the rats received atropine (0.6 μmol kg−1 i.p.), terbutaline (0.5 μmol kg−1 i.p.) and aminophylline (72 μmol kg−1 i.p.) 10 min before the first and third s.c. injection as well as the perivagal application of capsaicin.
Statistical analysis
The results are given as means of 6 – 8 experiments±s.e.mean. The significance of differences was evaluated by Student's t-test or one-way analysis of variance (ANOVA) followed by post hoc analysis by Student-Newman-Keuls test, and P values lower than 0.05 were considered significant: ‘n' indicates the number of experiments. EC50 values were interpolated from concentration-response curves. All statistical procedures, curve fitting and calculations of 95% confidence limits (95% CL) for EC50 values were performed by means of personal computer programs (GraphPad Prism™, software package version 2.01 for Windows 95, and InStat™, software package version 2.01 for MacIntosh; both from GraphPad Software Inc., San Diego, CA, U.S.A.).
Results
Acetylcholinesterase activity
Irinotecan, at concentrations ranging from 0.01 to 100 μM, slightly reduced the activity of human erythrocyte AChE, with an inhibitory effect of −21.5% observed at the highest concentration tested (Figure 1A). Under the same conditions, no significant changes in the enzymatic activity were detected when SN-38 (0.01 – 100 μM) was incubated with human erythrocyte AChE in the assay solution for 20 min. By contrast, physostigmine (0.0001 – 30 μM), used as reference drug, caused a marked and concentration-dependent inhibition of AChE function, with an EC50 value of 26.6 nM (95%CL: 11.5 – 61.2) and the maximal effect (−93.4%) at 3 μM (Figure 1A). Similar results were obtained on the activity of electric eel AChE: SN-38 was without effect, irinotecan caused an inhibition of −15.7% at 100 μM, and physostigmine blocked the enzymatic activity with an EC50 of 16.3 nM (95%CL: 13.6 – 19.7) and a maximal inhibition at 1 μM (−96.1%) (Figure 1B).
Figure 1.
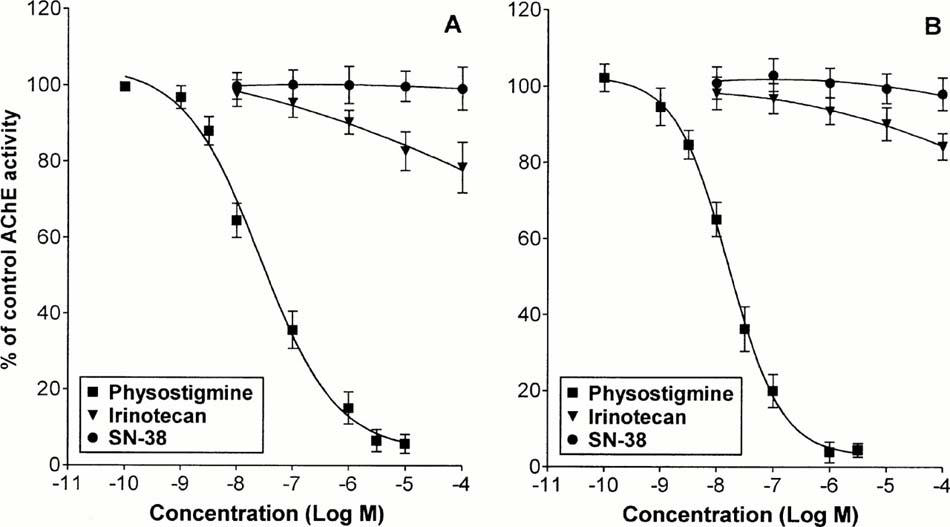
Effects of irinotecan (0.01 – 100 μM), SN-38 (0.01 – 100 μM) or physostigmine (0.0001 – 30 μM) on the enzymatic activity of human erythrocyte (A) or electric eel (B) AChE. Each point represents the mean of 6 – 8 experiments±s.e.mean (vertical lines).
Isolated human colon
Spontaneous contractile activity
During the 60-min equilibration period in carbogenated Krebs solution, most human colonic muscle strips developed varying degrees of spontaneous activity, which was variable among different tissues and often among different preparations from the same colon specimen. Most of the strips displayed a rapid spontaneous activity, with an amplitude less than 10% of the contractile response obtained by electrical stimulation, and generally stable throughout the experiment or with a trend to reduce. When the spontaneous activity was greater than 30% of contractions evoked by electrical stimulations, the strips were discarded. Some colonic preparations which displayed slow phasic contractions with shorter and more frequent contractions superimposed were also discarded.
The reference drugs used in the present study changed the spontaneous contractile activity of human colonic muscle in different ways: (1) exogenously applied acetylcholine (0.01 – 30 μM) evoked concentration-dependent phasic contractions (Figure 2A) which were antagonized by atropine 1 μM (not shown). Maximal responses occurred at the concentration of 10 μM, with an estimated EC50 value of 0.47 μM (95% CL: 0.41 – 0.54; n=8); (2) DMPP (0.01 – 100 μM) elicited phasic relaxations, at concentrations of 10 – 100 μM (Figure 2B), which were prevented by hexamethonium 10 μM (not shown). By contrast, 0.1 to 100 μM irinotecan or SN-38 failed to modify the spontaneous motor activity of colonic preparations (Figure 2C,D).
Figure 2.
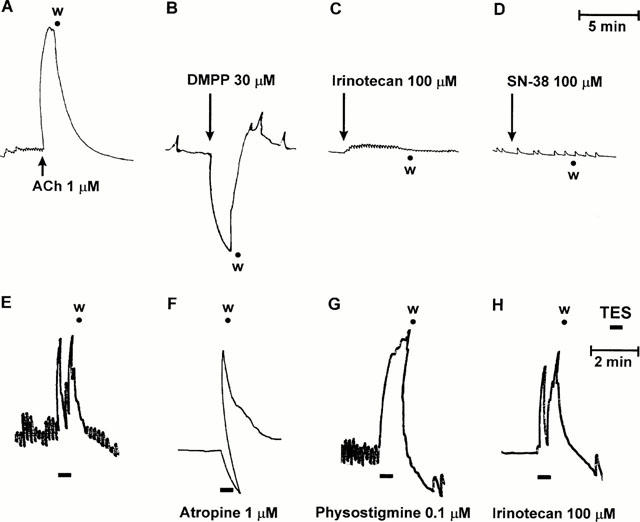
Isolated human colon. Upper panels: Representative trace recordings showing the effects of acetylcholine (ACh, 1 μM) (A), dimethylphenylpiperazinium (DMPP, 30 μM) (B), irinotecan (100 μM) (C) or SN-38 (100 μM) (D) on the spontaneous contractile activity of longitudinal muscle preparations. Lower panels: Representative trace recordings showing the contractile responses of longitudinal muscle strips to the application of transmural electrical stimulation (TES: 1 ms, 10 Hz, 30 s), either alone (E) or in the presence of atropine (1 μM) (F), physostigmine (0.1 μM) (G) or irinotecan (100 μM) (H), w=washing.
Contractile activity induced by transmural electrical stimulation
Trains lasting 30 s evoked motor responses which consisted of two components: a fast phasic contraction observed during the delivery of electrical stimulation, followed by a further contraction at the end of stimulation (aftercontraction) (Figure 2E). In the presence of atropine (1 μM), the initial phasic contraction was abolished, or reversed into a relaxation, and only the aftercontraction was evident (Figure 2F). In this respect, it has been previously shown that, besides acetylcholine, the application of electrical stimulation to smooth muscle preparations of human colon promotes also the release of non-adrenergic non-cholinergic (NANC) neural mediators which account for the occurrence of relaxations and aftercontractions (Crema et al., 1968; Maggi et al., 1997).
Under these conditions, physostigmine (0.01 – 0.1 μM) greatly enhanced the stimulated motor responses (+46.3±5.1% at 0.1 μM; P<0.05), without affecting the resting tone (Figure 2G). Concentrations higher than 0.1 μM could not be tested since they were associated with increments of basal contractile tone. Irinotecan or SN-38, both applied at 0.01 – 100 μM for 20 min, did not significantly modify phasic contractile responses elicited by electrical stimulation of human colonic preparations (Figure 2H).
Isolated guinea-pig ileum
Spontaneous contractile activity
During the equilibration period, longitudinal muscle strips of guinea-pig ileum developed a spontaneous contractile activity, which remained stable throughout the experiment without interfering with the motor responses elicited by test drugs. Exogenous acetylcholine (0.01 – 30 μM) as well as DMPP (0.1 – 100 μM) caused fast and concentration-dependent phasic contractions (Figure 3A,B), which were antagonized by atropine 1 μM and hexamethonium 10 μM, respectively (not shown). Acetylcholine 10 μM caused maximal responses with an EC50 value of 0.35 μM (95% CL: 0.21 – 0.59). DMPP induced maximal contractions at 30 μM, with an EC50 value of 1.94 μM (95% CL: 1.38 – 2.74). Under the same conditions, basal motor activity of longitudinal muscle strips did not change in the presence of irinotecan or SN-38 (0.01 – 300 μM for each drug) (Figure 3C,D).
Figure 3.
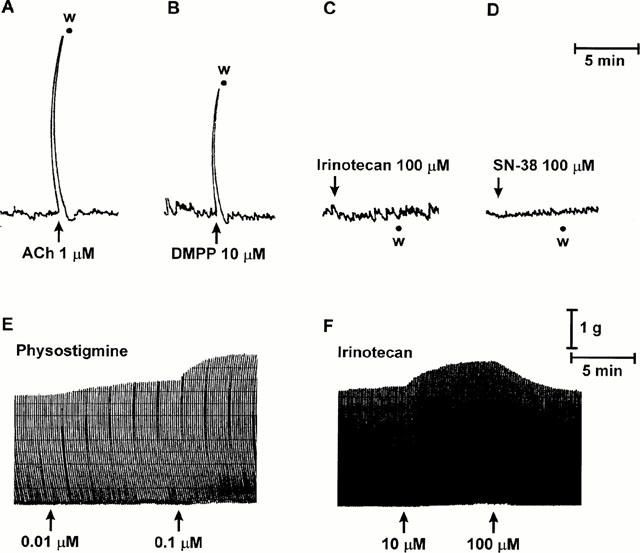
Isolated guinea-pig ileum. Upper panels: Representative trace recordings showing the effects of acetylcholine (ACh, 1 μM) (A), dimethylphenylpiperazinium (DMPP, 10 μM) (B), irinotecan (100 μM) (C) or SN-38 (100 μM) (D) on the spontaneous contractile activity of longitudinal muscle preparations. Lower panels: Representative trace recordings showing the effects of physostigmine (0.01 – 0.1 μM) (E) or irinotecan (10 – 100 μM) (F) on the cholinergic twitch contractions evoked by transmural electrical stimulation of longitudinal muscle preparations (1 ms, 0.1 Hz), w=washing.
Twitch contractions
Electrical stimulation of ileal preparations at low frequency induced recurrent phasic contractions of longitudinal muscle, which were abolished by tetrodotoxin (1 μM), or atropine (0.1 μM), but unaffected by hexamethonium (10 μM), indicating, the involvement of postganglionic cholinergic nerves (not shown). Physostigmine 0.01 – 0.1 μM increased the amplitude of twitch responses (+39.6±5.4%; P<0.05) (Figure 3E). Higher concentrations of physostigmine could not be tested, since they caused marked and persistent increments of the basal tone associated with a parallel decrease in the amplitude of the superimposed twitch activity. Under these conditions, irinotecan or SN-38, both tested at 0.01 – 100 μM, did not modify the cholinergic twitch contractions (not shown). However, in a minority of cases (24%) the amplitude of twitch responses increased moderately following the application of irinotecan 10 μM (+18.4±2.3%), and returned toward the basal level when the drug concentration was raised to 100 μM (Figure 3F). Similar results were obtained when irinotecan was assayed on twitch contractions evoked by electrical stimulation of ileal preparations obtained from animals pretreated with reserpine (not shown).
[3H]-Acetylcholine release
In control experiments, after a 60-min initial preperfusion period, the spontaneous tritium overflow approached a rate of 0.0021±0.00014 min−1 and remained unchanged throughout the experiment. When the superfused ileum strips were stimulated electrically, the tritium efflux increased from 0.0020±0.00012 to 0.0061±0.00028 min−1 (P<0.001). The increase in tritium outflow evoked by electrical stimulation was observed in four consecutive 3-min fractions: the release reached a peak during this time, and then declined exponentially to the prestimulation value. Under control conditions, the evoked tritium outflow was 2.86±0.34% for S1 and 2.69±0.37% for S2, not significantly different from each other, and the calculated ratio S2/S1 was 0.94±0.05 (Figure 4A). It has been previously shown that, after incubation of ileal preparations with radiolabelled choline, the application of electrical stimulation at 1 ms and 1 Hz evokes a significant release of radioactivity, which is tetrodotoxin-sensitive and consists mostly of radiolabelled acetylcholine (Vizi et al., 1984).
Figure 4.
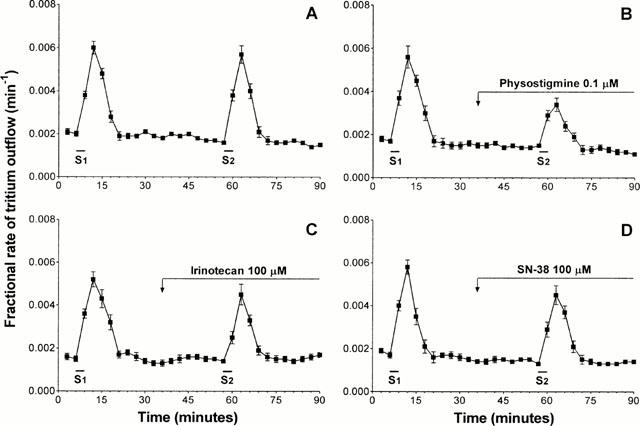
Isolated guinea-pig ileum. Tritium efflux from longitudinal muscle strips preincubated with methyl-[3H]-choline. Abscissa: time of superfusate collection. Ordinate: efflux of tritium at the onset of the respective collection period. Electrical field stimulation during S1 and S2 consisted of 180 pulses (1 ms, 1 Hz). (A) Control experiments. (B, C, D) Effects of physostigmine (0.1 μM), irinotecan (100 μM) and SN-38 (100 μM), respectively. The drugs were present in the superfusion medium during the time indicated by the horizontal bar. Each point represents the mean of 6 – 8 experiments±s.e.mean (vertical lines).
Neither physostigmine (0.1 μM) nor irinotecan (0.1 – 100 μM) or SN-38 (0.1 – 100 μM) significantly modified the resting overflow of tritium (not shown). When tested on tritium outflow evoked by electrical stimulation, physostigmine 0.1 μM significantly inhibited the release of [3H]-acetylcholine (−47.7±5.6%) (Figure 4B). Under the same conditions, irinotecan (10 – 100 μM) reduced the electrically-induced tritium outflow (−31.9±4.2% at 100 μM) (Figure 4C), and SN-38 (1 – 100 μM) was without effect (S2/S1=0.90±0.07 at 100 μM) (Figure 4D).
Acid secretion from in situ perfused rat stomach
In control animals with intact vagus nerves, basal acid secretion, assessed after 30-min stabilization, accounted for 3.92±0.47 μEqH+ 15 min−1, and this value remained nearly constant until the end of the experiments (180 min). In addition, when control animals underwent bilateral cervical vagotomy, basal secretion was 3.4±0.73 μEqH+ 15 min−1. This value was similar to that obtained in control rats with intact vagus nerves, and remained at a steady level throughout the experiment.
In animals with intact vagus nerves, irinotecan (5, 10 and 20 μmol kg−1 i.v.) caused a dose-dependent increase in acid secretion, with the maximal effect at the dose of 10 μmol kg−1 (Figure 5). No significant changes in the gastric secretory rate were observed when irinotecan was injected by i.c.v. route at 0.01 or 0.1 μmol kg−1 or when animals were treated with SN-38 (20 μmol kg−1 i.v.) or physostigmine (3 μmol kg−1 i.v.) (Figure 6A). In addition, the excitatory effect of irinotecan no longer occurred when injected to animals subjected to bilateral vagotomy or pretreated with atropine (3 μmol kg−1 i.v.), whereas it was partly prevented by ondansetron (15 μmol kg−1 i.v.) and unaffected by capsazepine (50 μmol kg−1 i.v.) (Figure 6B).
Figure 5.
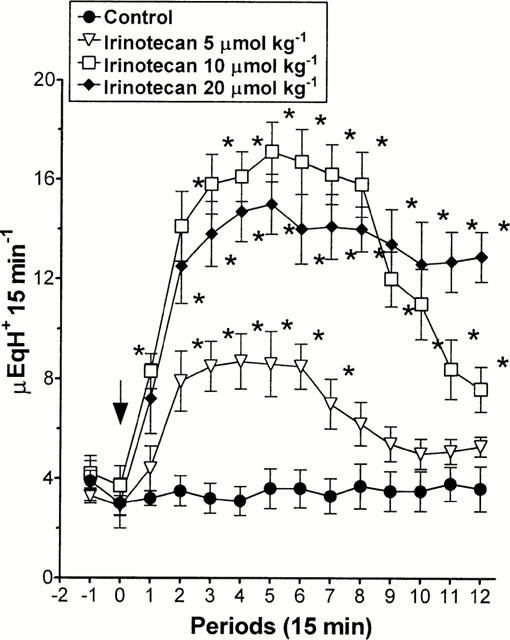
Anaesthetized rats with in situ perfusion of gastric lumen. Effects of irinotecan (5, 10 and 20 μmol kg−1 i.v.) on gastric acid secretion. Each point represents the mean value obtained from 6 – 8 animals±s.e.mean (vertical lines). The arrow indicates the time of irinotecan administration. *P<0.05: significant difference from control values.
Figure 6.
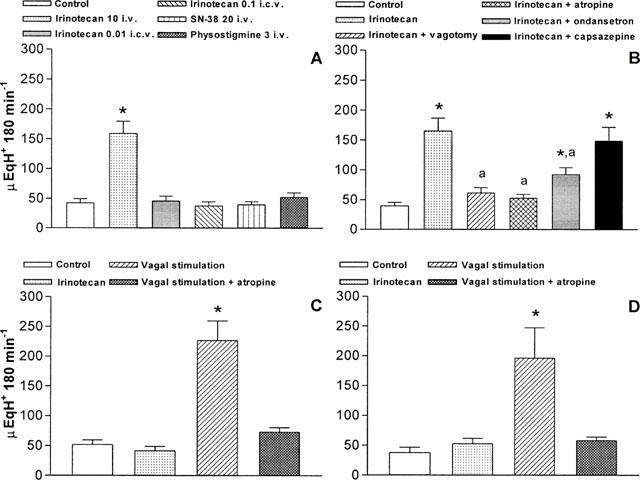
Anaesthetized rats with in situ perfusion of gastric lumen. (A) Effects of irinotecan (10 μmol kg−1 i.v. or 0.01 – 0.1 μmol kg−1 i.c.v.), SN-38 (20 μmol kg−1 i.v.) or physostigmine (3 μmol kg−1 i.v.) on gastric acid secretion. (B) Effects of irinotecan (10 μmol kg−1 i.v.) either alone or in the presence of bilateral cervical vagotomy, atropine (3 μmol kg−1 i.v.), ondansetron (15 μmol kg−1 i.v.) or capsazepine (50 μmol kg−1 i.v.) on gastric acid secretion. (C) Effects of irinotecan (10 μmol kg−1 i.v.) and electrical vagal stimulation, either alone or in the presence of atropine (3 μmol kg−1 i.v.), on gastric acid secretion in rats subjected to systemic ablation of capsaicin-sensitive sensory nerve fibres. (D) Effects of irinotecan (10 μmol kg−1 i.v.) and electrical vagal stimulation, either alone or in the presence of atropine (3 μmol kg−1 i.v.), on gastric acid secretion in rats subjected to perivagal application of capsaicin. Each column represents the mean value obtained from 6 – 8 animals±s.e.mean (vertical lines). *P<0.05: significant difference from control values: aP<0.05: significant difference from irinotecan alone.
In animals with intact vagus nerve, the functional ablation of capsaicin-sensitive sensory neurons by systemic pretreatment with capsaicin did not modify basal acid secretion, but fully prevented gastric hypersecretory effect of irinotecan (10 μmol kg−1 i.v.) (Figure 6C). Analogously, in rats subjected to selective ablation of vagal capsaicin-sensitive afferent fibres, irinotecan (10 μmol kg−1 i.v.) failed to modify basal secretory output (Figure 6D). In both types of preparations, the application of electrical stimuli to the distal trunk of the left vagus nerve, in animals subjected to bilateral vagotomy, was still able to promote significant and atropine-sensitive increments of acid secretion (Figure 6C,D).
Discussion
In a previous investigation, Kawato et al. (1993) suggested that irinotecan might directly activate muscarinic receptors, based on the ability of this drug to compete with the binding of radiolabelled quinuclidinyl benzylate to cell membranes isolated from rat brain. In the present study, exogenously applied acetylcholine caused atropine-sensitive phasic contractions of both ileal and colonic muscle strips, with EC50 values in the nanomolar range. However, these stimulant actions could not be reproduced in the presence of irinotecan or SN-38, even when they were applied at high concentrations, suggesting that these drugs are devoid of direct muscarinic agonistic properties.
Similar conclusions to those drawn for muscarinic receptors can be applied to the present experiments in which the effects of irinotecan and SN-38 were compared with motor responses elicited by DMPP. The opposite effects exerted by DMPP on guinea-pig ileum or human colon preparations in the present series are in agreement with other studies showing that nicotinic receptor agonists may induce hexamethomium-sensitive contractile or relaxant responses, depending on the intestinal region and species investigated (Bucknell & Whitney, 1964; Hayashi et al., 1983). Since, in the present study, irinotecan and SN-38 did not cause relaxations of human colonic preparations, neither had they mimicked the fast contractions of longitudinal muscle evoked by application of DMPP to guinea-pig ileal strips, a possible implication of neuronal nicotinic receptors in the genesis of cholinergic symptoms associated with irinotecan treatment appears to be unlikely. The present results do not support the hypothesis of Gandia et al. (1993), who speculated that irinotecan might act as agonist on ganglion nicotinic receptors, due to the structural similarity of its di-piperidin carbonyloxy group with DMPP.
An attractive mechanism, proposed to explain the cholinergic syndrome induced by irinotecan, is based on the assumption that this camptothecin analogue may block the activity of AChE, thus preventing the enzymatic breakdown of endogenous acetylcholine and promoting the tissue accumulation of this neuromediator over physiological concentrations (Kawato et al., 1993; Rivory et al., 1996; Morton et al., 1999). Previous investigations supported this view showing that, when tested in vitro, irinotecan inhibited the activity of AChE with potency values ranging from 0.19 to 0.41 μM (Kawato et al., 1993; Rivory et al., 1996; Morton et al., 1999). In addition, it has been recently proposed that irinotecan interacts directly with both human erythrocyte and electric eel AChE, leading to a non-competitive inhibition of these enzymes (Dodds & Rivory, 1999).
In the present study, in vitro assays performed on AChE isolated from human erythocytes or electric eel revealed that irinotecan acted as a very weak inhibitor of AChE activity, since the activities of these enzymes decreased by about 15 – 20% only when irinotecan was used at 100 μM. The remarkable discrepancies between our results and those obtained in previous studies can be hardly explained. However, in the present experiments, physostigmine, a well known AChE blocker (Taylor, 1996), inhibited the activity of both human erythrocyte and electric eel AChE with EC50 values similar to those reported by other authors (Yu et al., 1988; Rampa et al., 1998), indicating that our assays were carried out under standard and suitable experimental conditions. In addition, the following points must be taken into account: (1) no significant inhibition of AChE activity was observed either in isolated erythrocytes or in whole blood samples obtained from cancer patients exposed to i.v. infusion of irinotecan (Gandia et al., 1993; Blandizzi et al., 2000; (2) AChE is widely expressed in mammalian tissues, and symptoms associated with its pharmacological blockade usually result not only from the indirect activation of peripheral muscarinic receptors, but also from an involvement of cholinergic receptors at autonomic ganglia, skeletal muscle and central nervous system (Taylor, 1996); nevertheless, the acute cholinergic syndrome evoked by irinotecan is consistent with a picture of systemic parasympathetic activation (Gandia et al., 1993; Rowinsky et al., 1994), a feature which can be hardly reconciled with a drug-induced widespread inhibition of AChE activity.
In the present study, additional evidence to support the view that irinotecan exerts a negligible inhibitory activity on AChE came from the experiments performed on longitudinal muscle preparations of guinea-pig ileum or human colon. The electrically-induced contractile activity of ileal strips was weakly stimulated by irinotecan in a minority of experiments when this drug was applied at high concentrations, whereas SN-38 was always ineffective. By contrast, physostigmine, used at concentrations which do not cause a direct muscarinic receptor activation (Yamada et al., 1982), significantly enhanced the cholinergic motor responses of both ileal and colonic muscles to electrical stimulation. These findings are in full agreement with previous studies, where the potentiating actions of physostigmine on human or guinea-pig intestinal preparations were related to the accumulation of endogenous acetylcholine following AChE blockade (Cox & Stobart, 1972; Frigo et al., 1973; Yamada et al., 1982). Moreover, since the electrical stimulation of human colon is also associated with the activation of intramural NANC pathways (Crema et al., 1968; Maggi et al., 1997), and in the present study irinotecan did not modify the pattern of electrically-induced motor responses from colonic preparations, it can be suggested that irinotecan does not interfere with functions mediated by myenteric NANC transmitters.
A useful model for testing the effects of drugs on cholinergic neurotransmission consists of the measurement of acetylcholine release from longitudinal muscle strips preincubated with radiolabelled choline and subjected to electrical stimulation (Vizi et al., 1984). Previous studies have clearly established that AChE blockers reduce the electrically-induced [3H]-acetylcholine output from ileal preparations, since the reduced enzymatic breakdown of endogenous acetylcholine enhances its feedback inhibitory control on its own release through the activation of muscarinic autoreceptors (Somogyi & Vizi, 1988). Accordingly, the results of our experiments, showing that physostigmine significantly decreased the evoked [3H]-acetylcholine release, whereas irinotecan exerted only moderate inhibitory effects when tested at concentrations of 100 μM, further strengthen the view that irinotecan does not act as a specific inhibitor of AChE. Moreover, it is unlikely that such weak anti-cholinesterase activity may be relevant in clinical settings, since i.v. infusion of irinotecan to cancer patients usually results in drug peak plasma concentrations of 1.8 – 4.5 μM (Rowinsky et al., 1994; Falcone et al., 1999).
Since mechanisms related to cholinergic receptor activation or AChE blockade do not support the cholinergic syndrome induced by irinotecan, we hypothesized that this antitumour drug might stimulate a cholinergic parasympathetic dicharge either by acting at central nervous sites or triggering reflex autonomic responses. Therefore, an in vivo model was used in order to test this hypothesis, as the anaesthetized rat represents a useful preparation to assay the influence of centrally or peripherally applied cholinergic stimuli on gastric secretory functions (Maggi & Meli, 1986; Blandizzi et al., 1997). Data obtained from these experiments provided consistent evidence that irinotecan, but not SN-38, acts at peripheral sites to promote a reflex activation of vagal cholinergic efferent outflow and that capsaicin-sensitive fibres are involved in the afferent branch of this reflex response. In particular, the following findings support this conclusion: (1) when administered parenterally, at doses comparable with those used in clinical practice (Rowinsky et al., 1994; O'Leary & Muggia, 1998), irinotecan caused a gastric hypersecretory effect which could not be reproduced by its direct injection into the central nervous system; (2) the stimulant action of irinotecan was no longer observed in rats pretreated with atropine or subjected to bilateral vagotomy; (3) in animals where the functional ablation of capsaicin-sensitive afferent fibres was induced by systemic pretreatment with capsaicin, irinotecan did not modify the acid output, whereas, under the same conditions, electrical stimulation of the vagus nerve elicited significant atropine-sensitive secretory responses, indicating that capsaicin did not affect the functional integrity of vagal efferent fibres.
There is evidence in the literature to support the view that abdominal viscera, including the stomach, are supplied with capsaicin-sensitive afferent nerve-fibres (Sengupta & Gebhart, 1994; Szallasi & Blumberg, 1999). These sensory neurons carry out several regulatory functions by means of bioactive peptides, whose release can be promoted by activation of vanilloid receptors or application of various chemicophysical stimuli (Holzer, 1991; Szallasi & Blumberg, 1999). Moreover, capsaicin-sensitive nerves constitute the afferent branch of autonomic reflexes and express specific receptors on their peripheral endings, through which various mediators, including cholecystokinin and serotonin, can modulate the activity of central nervous pathways implicated in the control of peripheral organs (Bozkurt et al., 1999; Szallasi & Blumberg, 1999). In line with these findings, three major mechanisms can be postulated to account for irinotecan-induced activation of abdominal afferents: (1) direct activation of afferent nerves; (2) sensitization of the afferents to endogenous mediators; (3) release of endogenous mediators which then stimulate peripheral afferent fibres. Although a direct activation or sensitization of capsaicin-sensitive afferent nerves by irinotecan cannot be ruled out on the basis of the present results, these mechanisms are not supported by previous studies dealing with other cytotoxic drugs. In particular, cisplatin failed to directly stimulate vagal afferent fibres or to sensitize them to the depolarizing action exerted by serotonin (Woods & Andrews, 1995).
In the present study, a series of experimental data indicates that the gastric stimulant action of irinotecan was abolished in animals subjected to perivagal capsaicin application, it was partly prevented by blockade of 5-HT3 serotonin receptors with ondansetron and was not modified by capsazepine, a selective antagonist of vanilloid receptors (Szallasi & Blumberg, 1999). Therefore, on the basis of these results, it can be suggested that the capsaicin-sensitive afferent fibres, which mediate the cholinergic responses triggered by irinotecan, belong to the vagus nerves, and that endogenous serotonergic pathways could be involved, at least in part, in the activation of this reflex.
At the level of gastrointestinal tract, serotonin can be released from enterochromaffin cells or intramural serotonergic neurons (Gershon et al., 1994). In the present study, the results of in vitro experiments, showing that the weak enhancing action exerted by irinotecan on twitch contractions could be reproduced also in ileal preparations obtained from reserpinized animals, argue against the possibility that irinotecan promotes the release of serotonin from myenteric serotonergic neurons. On the other hand, previous studies have shown a close relationship between the cytotoxic action of various antineoplastic drugs and the peripheral serotonergic system (Andrews et al., 1990, Cubeddu, 1996). In particular, it has been proposed that enterochromaffin cells, located in the mucosa of the upper gut, may act as chemoreceptors which are able to release serotonin once challenged by cytotoxic drugs. The released serotonin then activates 5-HT3 receptors on vagal afferent fibres, which terminate in close proximity of enterochromaffin cells, and hence triggers the emetic reflex (Andrews et al., 1990; Cubeddu, 1996).
Clinical studies have shown that treatment with irinotecan may induce nausea and vomiting, which respond favourably to serotonin 5-HT3 receptor antagonists (Rowinsky et al., 1994), indicating that this camptothecin analogue has the potential for activating serotonin-mediated emetogenic reflexes. Accordingly, taking into account the results obtained with ondansetron in the present in vivo experiments, it is conceivable that irinotecan acts on peripheral chemoreceptors, including enterochromaffin cells, and promotes the activation of a vago-vagal reflex which causes a centrally driven cholinergic outflow. However, since other cytotoxic drugs known to induce nausea and vomiting, such as cisplatin, cyclophosphamide, epirubicin and 5-fluorouracil, did not affect, or rather inhibited, gastric acid secretion in previous studies (Fabrin et al., 1991, Kakinuma & Ohwada, 1997), it is also likely that, besides serotonin, other endogenous mediators and pathways can be involved in the vagal activation promoted by irinotecan.
In conclusion, the present results indicate that the acute cholinergic syndrome observed in cancer patients during treatment with irinotecan is not likely to depend on the blockade of AChE activity by irinotecan or SN-38, and that both these drugs are devoid of direct cholinomimetic properties. According to our findings, it is rather proposed that irinotecan is able to promote a sustained vagal efferent discharge to peripheral effector organs, mediated by the activation of capsaicin-sensitive sensory afferent fibres. It is also suggested that serotonin 5-HT3 receptors are implicated, at least in part, in the genesis of the vago-vagal reflex triggered by irinotecan.
Abbreviations
- AChE
acetylcholinesterase
- DMPP
dimethylphenylpiperazinium
- SN-38
7-ethyl-10-hydroxycamptothecin
References
- ANDREWS P.L., DAVIS C.J., BINGHAM S., DAVIDSON H.I., HAWTHORN J., MASKELL L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can. J. Physiol. Pharmacol. 1990;68:325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- BLANDIZZI C., COLUCCI R., CARIGNANI D., LAZZERI G., DEL TACCA M. Positive modulation of pepsinogen secretion by gastric acidity after vagal cholinergic stimulation. J. Pharmacol. Exp. Ther. 1997;283:1043–1050. [PubMed] [Google Scholar]
- BLANDIZZI C., COLUCCI R., CARIGNANI D., NATALE G., LAZZERI G., CREMA F., DEL TACCA M. Role of peripheral GABAB receptors in the regulation of pepsinogen secretion in anaesthetized rats. Eur. J. Pharmacol. 1995;294:191–200. doi: 10.1016/0014-2999(95)00538-2. [DOI] [PubMed] [Google Scholar]
- BLANDIZZI C., DE PAOLIS B., DANESI R., DI PAOLO A., FALCONE A., DEL TACCA M. Assay of acetylcholinesterase activity in cancer patients treated with irinotecan. Br. J. Clin. Pharmacol. 2000;50 Suppl.:245. [Google Scholar]
- BOZKURT A., OKTAR B.K., KURTEL H., ALICAN I., COSKUN T., YEGEN B.C. Capsaicin-sensitive vagal fibres and 5-HT3-, gastrin releasing peptide- and cholecystokinin A-receptors are involved in distension-induced inhibition of gastric emptying in the rat. Regul. Pept. 1999;83:81–86. doi: 10.1016/s0167-0115(99)00050-6. [DOI] [PubMed] [Google Scholar]
- BUCKNELL A., WHITNEY B. A preliminary investigation of the pharmacology of the human isolated taenia coli preparation. Br. J. Pharmacol. 1964;23:164–175. doi: 10.1111/j.1476-5381.1964.tb01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLUCCI R., BLANDIZZI C., CARIGNANI D., PLACANICA G., LAZZERI G., DEL TACCA M. Effects of imidazoline derivatives on cholinergic motility in guinea-pig ileum: involvement of presynaptic α2-adrenoceptors or imidazoline receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;357:682–691. doi: 10.1007/pl00005225. [DOI] [PubMed] [Google Scholar]
- COX B., STOBART G. Effects of four cholinesterase inhibitors on the response of the guinea-pig isolated ileum to transmural electrical stimulation. J. Pharm. Pharmacol. 1972;24:828–829. doi: 10.1111/j.2042-7158.1972.tb08894.x. [DOI] [PubMed] [Google Scholar]
- CREMA A., DEL TACCA M., FRIGO G.M., LECCHINI S. Presence of a non-adrenergic inhibitory system in the human colon. Gut. 1968;9:633–637. doi: 10.1136/gut.9.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUBEDDU L.X. Serotonin mechanisms in chemotherapy-induced emesis in cancer patients. Oncology. 1996;53 Suppl. 1:18–25. doi: 10.1159/000227636. [DOI] [PubMed] [Google Scholar]
- DODDS H.M., RIVORY L.P. The mechanism for the inhibition of acetylcholinesterases by irinotecan (CPT-11) Mol. Pharmacol. 1999;56:1346–1353. doi: 10.1124/mol.56.6.1346. [DOI] [PubMed] [Google Scholar]
- DOUILLARD J.Y., CUNNINGHAM D., ROTH A.D., NAVARRO M., JAMES R.D., KARASEK P., JANDIK P., IVESON T., CARMICHAEL J., ALAKL M., GRUIA G., AWAD L., ROUGIER P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- ELLMAN G.L., COURTNEY K.D., ANDRES V., FEATHERSTONE R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- FABRIN B., HOJGAARD L., JOHANSEN A., OLESEN H.P., MADSEN J., MAURIDSEN H.T. Electrical potential difference across the stomach wall and gastric morphology in anaesthetized pigs after intravenous administration of cytotoxic drugs. Acta Oncol. 1991;30:803–806. doi: 10.3109/02841869109091824. [DOI] [PubMed] [Google Scholar]
- FALCONE A., DANESI R., ALLEGRINI G., MASI G., DI PAOLO A., LENCIONI M., PFANNER E., COMIS S. Escalating dose irinotecan (CPT-11) immediately prior or after 5-fluorouracil (5-FU) 48 hours infusion+leucovorin (LV): pharmacokinetic and pharmacodynamic interactions in chemotherapy naïve metastatic colorectal cancer patients. J. Clin. Oncol. 1999;18:241a. [Google Scholar]
- FRIGO G.M., DEL TACCA M., LECCHINI S., CREMA A. Some observations on the intrinsic nervous mechanism in Hirschprung's disease. Gut. 1973;14:35–40. doi: 10.1136/gut.14.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLIGAN J.J., FURNESS J.B., COSTA M. Effects of cholinergic blockade and sympathetic denervation on gastrointestinal myoelectric activity in guinea pig. J. Pharmacol. Exp. Ther. 1986;238:1114–1125. [PubMed] [Google Scholar]
- GANDIA D., ABIGERGES D., ARMAND J.-P., CHABOT G., DA COSTA L., DE FORNI M., MATHIEU-BOUE A., HERAIT P. CPT-11 induced cholinergic effects in cancer patients (letter) J. Clin. Oncol. 1993;11:196–197. doi: 10.1200/JCO.1993.11.1.196. [DOI] [PubMed] [Google Scholar]
- GERSHON M.D., KIRCHGESSNER A.L., WADE P.R.Functional anatomy of the enteric nervous system Physiology of the Gastrointestinal Tract 1994Raven Press: New York; 381–422.ed. Johnson, L.R. [Google Scholar]
- HAYASHI E., SHINOZUKA K., MAESA T., TAKEDA M. Effect of ascorbate on the contractile response induced by DMPP in guinea-pig ileal longitudinal muscle strip. Eur. J. Pharmacol. 1983;89:229–234. doi: 10.1016/0014-2999(83)90498-3. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- KAKINUMA S., OHWADA S. Gastric mucosal blood flow and gastric secretion following intravenous administration of 5-fluorouracil in anesthetized rats. Cancer Chemother. Pharmacol. 1997;39:357–360. doi: 10.1007/s002800050583. [DOI] [PubMed] [Google Scholar]
- KAWATO Y., SEKIGUCHI M., AKAHANE K., TSUTOMI Y., HIROTA Y., KUGA H., SUZUKI W., HAKUSUI H., SATO K. Inhibitory activity of camptothecin derivatives against acetylcholinesterase in dogs and their binding activity to acetylcholine receptors in rats. J. Pharm. Pharmacol. 1993;45:444–448. doi: 10.1111/j.2042-7158.1993.tb05573.x. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., MELI A. Suitability of urethane anaesthesia for physiopharmacological investigation in various systems. Part. 3: Other systems and conclusions. Experientia. 1986;42:531–537. doi: 10.1007/BF01946692. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., CATALIOTO R.M., CRISCUOLI M., CUCCHI P., GIULIANI S., LECCI A., LIPPI A., MEINI S., PATACCHINI R., RENZETTI A.R., SANTICIOLI P., TRAMONTANA M., ZAGORODNYUK V., GIACHETTI A. Tachykinin receptors and intestinal motility. Can. J. Physiol. Pharmacol. 1997;75:696–703. [PubMed] [Google Scholar]
- MORTON C.L., WADKINS R.M., DANKS M.K., POTTER P.M. The anticancer prodrug CPT-11 is a potent inhibitor of acetylcholinesterase but is rapidly catalyzed to SN-38 by butyrylcholinesterase. Cancer Res. 1999;59:1458–1463. [PubMed] [Google Scholar]
- O'LEARY J., MUGGIA F.M. Camptothecins: a review of their development and schedules of administration. Eur. J. Cancer. 1998;34:1500–1508. doi: 10.1016/s0959-8049(98)00229-9. [DOI] [PubMed] [Google Scholar]
- RAMPA A., BISI A., VALENTI P., RECANATINI M., CAVALLI A., ANDRISANO V., CAVRINI V., FIN L., BURIANI A., GIUSTI P. Acetylcholinesterase inhibitors: synthesis and structure-activity relationships of ω-[N-methyl-N-(3-alkylcarbamoyloxyphenyl)-methyl]-aminoalkoxy-heteroaryl derivatives. J. Med. Chem. 1998;41:3976–3986. doi: 10.1021/jm9810046. [DOI] [PubMed] [Google Scholar]
- RAYBOULD H.E., TACHÉ Y. Capsaicin-sensitive vagal afferent fibers and stimulation of gastric acid secretion in anesthetized rats. Eur. J. Pharmacol. 1989;167:237–243. doi: 10.1016/0014-2999(89)90584-0. [DOI] [PubMed] [Google Scholar]
- RIVORY L.P., RIOU J.-F., HAAZ M.-C., SABLE S., VUILHORGNE M., COMMERÇON A., POND S.M., ROBERT J. Identification and properties of a major plasma metabolite of irinotecan (CPT-11) isolated from the plasma of patients. Cancer Res. 1996;56:3689–3694. [PubMed] [Google Scholar]
- ROTHENBERG M.L. Topoisomerase I inhibitors: Review and update. Ann. Oncol. 1997;8:837–855. doi: 10.1023/a:1008270717294. [DOI] [PubMed] [Google Scholar]
- ROTHENBERG M.L., BLANKE C.D. Topoisomerase I inhibitors in the treatment of colorectal cancer. Semin. Oncol. 1999;26:632–639. [PubMed] [Google Scholar]
- ROTHENBERG M.L., KUHN J.G., BURRIS H., NELSON J., ECKARDT J.R., TRISTAN-MORALES M., HILSENBECK S.G., WEISS G.R., SMITH L.S., RODRIGUEZ G.I. Phase I and pharmacokinetic trial of weekly CPT-11. J. Clin. Oncol. 1993;11:2194–2204. doi: 10.1200/JCO.1993.11.11.2194. [DOI] [PubMed] [Google Scholar]
- ROWINSKY E.K., GROCHOW L.B., ETTINGER D.S., SARTORIUS S.E., LUBEJKO B.G., CHEN T.-L., ROCK M.K., DONEHOWER R.C. Phase I and pharmacological study of the novel topoisomerase I inhibitor 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothecin (CPT-11) administered as a ninety-minute infusion every 3 weeks. Cancer Res. 1994;54:427–436. [PubMed] [Google Scholar]
- SENGUPTA J.N., GEBHART G.F. Physiology of the Gastrointestinal Tract. Johnson, L.R. Raven Press: New York; 1994. Gastrointestinal afferent fibers and sensation; pp. 483–519. [Google Scholar]
- SOMOGYI G.T., VIZI E.S. Evidence that cholinergic axon terminals are equipped with both muscarinic and adenosine receptors. Brain Res. Bull. 1988;21:575–579. doi: 10.1016/0361-9230(88)90195-5. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- TANIZAWA A., FUJIMORI A., FUJIMORI Y., POMMIER Y. Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. J. Natl. Cancer Inst. 1994;86:836–842. doi: 10.1093/jnci/86.11.836. [DOI] [PubMed] [Google Scholar]
- TAYLOR P.Anticholinesterase agents The Pharmacological Basis of Therapeutics 1996McGraw-Hill: New York; 161–176.ed. Hardman, J.G., Limbird, L.E., Molinoff, P.B., Ruddon, R.W. & Goodman Gilman, A [Google Scholar]
- VIZI E.S., ONO K., ADAM-VIZI V., DUNCALF D., FOLDES F.F. Presynaptic inhibitory effect of Met-enkephalin on [14C]acetylcholine release from the myenteric plexus and its interaction with muscarinic negative feedback inhibition. J. Pharmacol. Exp. Ther. 1984;230:493–499. [PubMed] [Google Scholar]
- WOODS A.J., ANDREWS P.L.R. Cisplatin acutely reduces 5-hydroxytryptamine-induced vagal depolarization in the rat: protective action of dexamethasone. Eur. J. Pharmacol. 1995;278:275–278. doi: 10.1016/0014-2999(95)00174-j. [DOI] [PubMed] [Google Scholar]
- YAMADA S., KATSUOKA M., OKUDAIRA H., HAYASHI E. A pharmacological comparison of organophosphorus and carbamate anti-cholinesterase agents on guinea pig ileum. Toxicol. Appl. Pharmacol. 1982;64:79–87. doi: 10.1016/0041-008x(82)90324-6. [DOI] [PubMed] [Google Scholar]
- YU Q.-S., ATACK J.R., RAPOPORT S.I., BROSSI A. Carbamate analogues of (−)-physostigmine: in vitro inhibition of acetyl- and butyrylcholinesterase. FEBS Letters. 1988;234:127–130. doi: 10.1016/0014-5793(88)81317-6. [DOI] [PubMed] [Google Scholar]


