Abstract
The differential responsiveness of various sections and regions in the vascular system to the vasodilator activity of organic nitrates is important for the beneficial antiischaemic effects of these drugs. In this study we examined the vasodilator activity of organic nitrates in cerebral arteries, where vasodilation causes substantial nitrate induced headache.
Isolated porcine basilar and coronary arteries were subjected to increasing concentrations of glyceryl trinitrate (GTN), isosorbide-5-nitrate (ISMN) and pentaerythritol tetranitrate (PETN). S-nitroso-N-acetyl-D,L-penicillamine (SNAP) and endothelium-dependent vasodilation was investigated for comparison purpose.
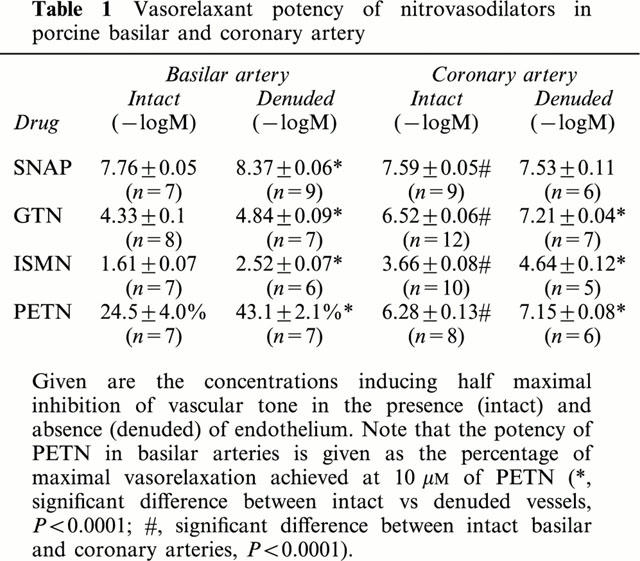
The vasodilator potency (halfmaximal effective concentration in −logM) of GTN (4.33±0.1, n=8), ISMN (1.61±0.07, n=7) and PETN (>10 μM, n=7) in basilar arteries was more than 100 fold lower than that of GTN (6.52±0.06, n=12), ISMN (3.66±0.08, n=10) and PETN (6.3±0.13, n=8) observed in coronary arteries.
In striking contrast, the vasodilator potency of SNAP (halfmaximal effective concentration in −logM) was almost similar in basilar (7.76±0.05, n=7) and coronary arteries (7.59±0.05, n=9). Likewise, no difference in endothelium dependent relaxation was observed.
Denudation of the endothelium resulted in a small increase of the vasodilator potency (halfmaximal effective concentration in −logM) of GTN (4.84±0.09, n=7, P<0.03) in basilar arteries and similar results were obtained in the presence of the NO-synthase inhibitor Nω-nitro-L-arginine (4.59±0.05, n=9, P<0.03).
These results suggest that cerebral conductance blood vessels such as porcine basilar arteries seems to have a reduced expression and/or activity of certain cellular enzymatic electron transport systems such as cytochrome P450 enzymes, which are necessary to bioconvert organic nitrates to NO.
Keywords: Coronary arteries, cerebral arteries, glyceryl trinitrate, isosorbide-5-nitrate, pentaerythritol tetranitrate, metabolism, bioactivation, hemodynamics
Introduction
Organic nitrates are still important drugs for the treatment of cardiovascular disorders (Parker & Parker, 1998). In addition, these drugs are also increasingly used to treat gastrointestinal disorders such as esophagal spasm, biliary colic, functional dyspepsia and liver cirrhosis or complications in pregnancy such as preterm labour and severe foetal distress (Kojda, 2000). Organic nitrates are smooth muscle relaxants acting after enzymatic bioconversion to nitric oxide (NO) in the target cells (Ahlner et al., 1991). In the cardiovascular system the most prominent effect is vasodilation. This leads to a unique pattern of haemodynamic changes consisting in preferential venodilation, vessel size selective coronary vasodilation and, at higher doses, also reduction of peripheral resistance (Bassenge & Stuart, 1986). The most common side effect of organic nitrates, namely headache, is caused by cerebral vasodilation (Olesen et al., 1994).
The specificity of the haemodynamic changes induced by organic nitrates is most likely caused by striking differences in the responsiveness of blood vessels from different body sections and regions (Bassenge & Stuart, 1986). Many years ago, Winbury et al. (1971) have shown that organic nitrates selectively increase blood flow to endocardial regions of the ischaemic heart. This effect is most likely caused by a poor vasodilator response of coronary resistance vessels as compared to coronary conductance arteries (Sellke et al., 1990). Furthermore, substantial venodilation has been shown to occur at doses of organic nitrates that have no effect on peripheral resistance (Bassenge & Stuart, 1986). Preferential venodilation is most likely related to genuine production of NO by the vascular endothelium, which suppresses the activity of soluble guanylate cyclase and the enzymatic bioconversion of organic nitrates to NO (Moncada et al., 1991; Kojda et al., 1998). Both actions are less pronounced in venous vessels, since blood flow induced shear stress, which is the most important stimulator of endothelial NO production, is apparently lower in venes. In accordance, aortic segments of mice lacking the endothelial NO-synthase gene show a substantial hyperresponsiveness to glyceryl trinitrate (GTN) (Kojda et al., 1999).
The effects of organic nitrates on isolated cerebral blood vessels have rarely been studied, although cerebral vasodilation is believed to be the major reason causing nitrate induced headache (Olesen et al., 1994). The aim of this study was to comparatively investigate the responsiveness of isolated cerebral and coronary arteries to organic nitrates, spontaneous NO-donors and endothelium dependent vasodilation. To study the influence of endothelial NO on the effect of organic nitrates we used a perfusion system that mimicks physiologic blood flow and pressure. Our results suggest that cerebral arteries have a low capacity to bioconvert organic nitrates to NO.
Methods
Tissue preparation
We investigated isolated pig right coronary and basilar arteries. The arteries were obtained from the local slaughterhouse and taken from the hearts and brains of freshly slaughtered female pigs (5 – 7 months). Intraluminal blood and thrombi were rapidly removed by rinsing the vessels in Krebs-Henseleit buffer (see below). More time was needed to prepare coronary arteries (10 min) as compared to basilar arteries (5 min), while the transport of the vessel segments in precooled Krebs-Henseleit buffer to the laboratory and the time to finally prepare the segments studied was nearly identical.
Porcine coronary arteries
Right coronary arteries were prepared free from the aorta to the ramus interventricularis posterior and perfused with cold preoxygenated Krebs-Henseleit solution (KHP-buffer, pH 7.4) of the following composition (in mM): Na+ 143.07, K+ 5.87, Ca2+ 1.6, Mg2+ 1.18, Cl− 125.96, HCO3− 25.00, H2PO4− 1.18, SO42− 1.18 and glucose 5.05. Then the arteries were cut from their muscle foundation, immediately stored in cooled KHP-buffer and transferred into the laboratory, where they were carefully dissected free from all surrounding tissue. The proximal ends were rejected and the rest of the arteries were cut in rings (length 5 mm). Great care was taken to preserve the intimal endothelium. Porcine coronary arteries were cut into ring segments (4 mm) and fixed between stainless-steel hooks in a water jacked organ bath (37°C) as described previously (Kojda et al., 1991). Resting tension was 2 g. After equilibration (1 h), contractile function was tested by addition of KCl (60 mM) and prostaglandin F2α (PGF2α 0.1 – 100 μM) to reach a maximal tension of approximately 5 g. The presence of intact endothelium was verified by complete, transient relaxation of PGF2α-precontracted (10 μM) segments after application of 3 nM substance P (Cocks & Angus, 1983). Vasorelaxing activities of GTN (1 nM – 100 μM), ISMN (1 nM – 100 μM), PETN (0.1 nM – 10 μM) and SNAP (1 nM – 100 μM) were evaluated by cumulative application after precontraction with PGF2α (50 μM).
Porcine basilar arteries
After carefully removing all loosely adherent tissue of the basilar arteries, measurement of vessel diameter changes was performed using a perfusion method. Artery segments (2 cm length) were mounted between stainless steel tubes connected to the perfusion system and perfused oxygenated Krebs-Henseleit buffer (pH 7.4, 37°C) at a constant flow rate of 10 ml min−1 (pulse free flow). This produced an initial intraluminal pressure of 60 mmHg during equilibration of the vessel segments. Two heatable buffer containers were used to allow drug application without changing flow. As for the measurement of vessel diameter changes, two ultrasound recipient crystals mounted on a thin wire (0.5 mm diameter) were attached with a low pressure (<20 mg cm−2) on opposite sites of the adventitia. Ultrasound transmitted from transmitter crystals to the recipient crystals was used to measure the vascular diameter. The ultrasound transmitting time was measured for each site of the vessel separately and directly transferred into a mean value which was the key measurement signal. Vasodilatation corresponded to a shorter transmitting time, while vasoconstrictions corresponded to a longer transmitting time. The ultrasound measuring unit was calibrated with a special device allowing the exact calculation of vessel diameter variations in μm. Changes of the vessel diameter were recorded using a standard plotter (SE 120 BBC Goerz Metrawatt, Austria). Equilibration experiments revealed a high resolution allowing reproducible detection of changes in vessel diameter of 2.5 μm.
During equilibration the basilar entry segments developed a spontaneous tone (myogenic tone) which was reproducibly and completely inhibited by 10 μM noradrenaline (Figure 1). Control experiments showed that this myogenic tone was stable for more than 4 h and, thus, longer than the duration of the experiments. In selected cases it was necessary to remove the endothelium. This was achieved by intraluminal perfusion with carbogen gas for 2 min. The absence of intact endothelium was verified by the lack of vasorelaxation upon application of substance P (3 nM) to the basilar artery segments preconstricted with 10 μM PGF2α. Preconstricted endothelium intact basilar artery segments showed a vasorelaxation of 45±6.4% (n=89). In a subset of experiments the effects of glyceryl trinitrate (1 nM – 100 μM) were investigated in ring preparations of endothelium intact porcine basilar arteries at similar conditions than coronary arteries (see above) described in detail elsewhere (Kojda et al., 1992).
Figure 1.
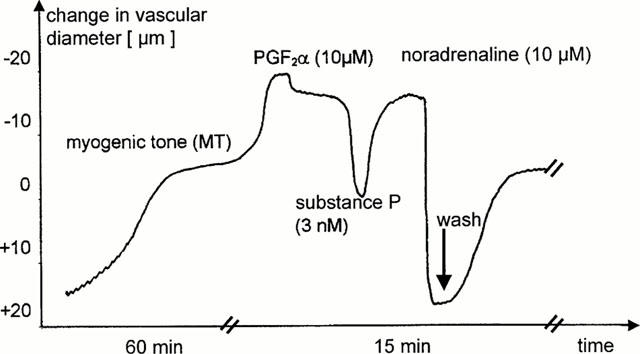
Original registration showing vascular responses of isolated perfused porcine basilar artery segments. Spontaneous vasoconstriction (myogenic tone) developed within 60 min of perfusion. Further vasoconstriction could be achieved by 10 μM prostaglandin F2α (PGF2α), while addition of 10 μM noradrenaline induced maximal vasodilation. This vasodilation was completely reversible, since the myogenic tone fully recovered after washout of noradrenaline (see washout arrow). Substance P (3 nM) induced an endothelium-dependent relaxation, which reversed the vascular constriction induced by exogenous vasoconstrictors. Please note that the interruptions of the ordinate indicate different time scales.
Substances and solutions
ISMN and PETN were generously provided by ISIS Pharma GmbH, Zwickau, Germany. SNAP was synthesized according to Field et al. (1978) as described previously (Kojda et al., 1998). GTN (4.404 mM in 154 mM NaCl, directly used as stock solution) was generously provided by Schwarz Pharma AG (Monheim, Germany). All other chemicals (analytical grade) were purchased from either Sigma Chemicals (Deisenhofen, Germany) or Merck (Darmstadt, Germany). All concentrations indicated in the text, figure and tables are expressed as final organ bath concentrations.
Statistics
Vasorelaxation is expressed as remaining percentage of the contractile response achieved with PGF2α (porcine coronary artery) or of the spontaneous contractile tone (myogenic tone, basilar artery) at the beginning of the cumulative application. Maximal vasorelaxation in basilar arteries refers to the vascular tone achieved after addition of 10 μM noradrenaline (Figure 1). The concentrations for halfmaximal inhibition of precontraction (IC50) were calculated from the individual concentration-effect curves and are given in −logM. All data were analysed by either one-way or two-way analysis of variance (ANOVA, GraphPad Prism® 3.0) and are expressed as mean values and standard error of the mean (s.e.mean). Significant differences were evaluated using either paired two-tailed Student's t-test or ANOVA and a P value below 0.05 was considered significant.
Results
Myogenic tone
All segments of porcine basilar artery showed a strong myogenic tone that fully developed during 1 h (Figure 1) and remained stable for at least 4 h. Application of PGF2α elicited a further vasoconstriction, which induced on average a similar reduction of the vessel diameter. The maximal reduction of diameter of endothelium intact basilar artery segments induced by both the myogenic tone and 10 μM PGF2α was 221±83.9 μm. The contribution of the myogenic tone to this maximal vasoconstriction was 46.9±11.8% (n=72). Similar results were obtained in a second set of experiments performed with endothelium denuded basilar artery segments. The maximal reduction of vessel diameter (myogenic tone plus PGF2α) was 228.4±64.8 μm and the contribution of the myogenic tone was 46.3±13.1% (n=32). Thus, the spontaneous myogenic tone accounts for half of the maximal vasoconstriction of isolated perfused basilar arteries. Its development is independent of the presence of intact endothelium.
Endothelium-dependent vasodilation
Endothelium-dependent vasodilation was achieved by application of 3 nM substance P (Figure 1). Coronary artery segments preconstricted with PGF2α showed a full vasorelaxant response, while in basilar arteries the vasodilation to 3 nM substance P contrasted 45.2±17.1% (n=89) of the maximal vasoconstriction (Figure 1). The vasodilator response to substance P was completely abolished after removal of the endothelium or preincubation with the NO-synthease inhibitors Nω-monomethyl-L-arginine (L-NMMA, 100 μM) or Nω-nitro-L-arginine (L-NA, 100 μM). Furthermore, addition of NO-synthase inhibitors (100 μM) induced a small vasoconstriction in basilar arteries that did not reach statistical significance. The reduction of vessel diameter induced by L-NMMA and L-NA were 33.1±17.3% (n=10, P>0.05) and 26.1±10.6% (n=9, P>0.05), respectively.
Vasorelaxant activity of nitrovasodilators inbasilar arteries
Cumulative addition of GTN, PETN, ISMN and SNAP resulted in a concentration-dependent vasorelaxation of endothelium intact basilar artery segments (Figure 2). While SNAP induced a complete vasorelaxation with a halfmaximal effective concentration in the nanomolar range, the organic nitrates were surprisingly much less effective. The concentrations needed for halfmaximal effective vasodilation were more than 100 fold greater (Table 1).
Figure 2.
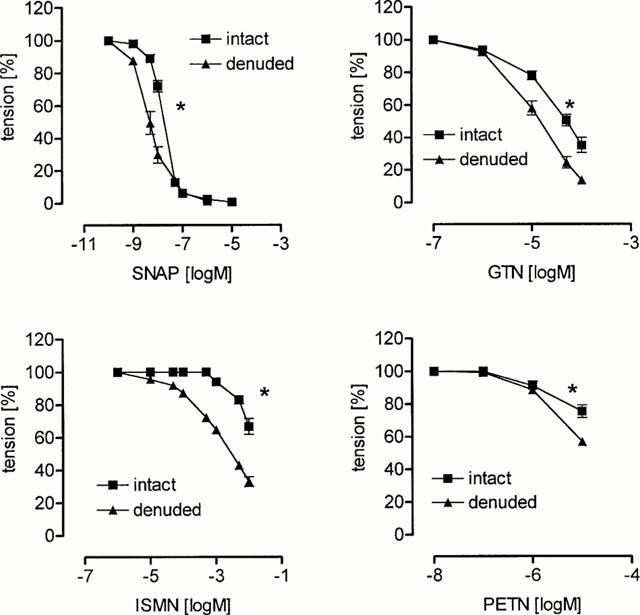
Nitrovasodilator induced relaxation of isolated porcine basilar artery segments. The vasodilator activity of S-nitroso-N-acetyl-D,L-penicillamine (SNAP), glyceryl trinitrate (GTN), isosorbide-5-nitrate (ISMN) and pentaerythritol tetranitrate (PETN) was assessed in the presence (intact) and absence (denuded) of intact endothelium. Each concentration-response curve is plotted by taking the respective mean values of 6 – 9 individual experiments (s.e.mean indicated by bars, *P<0.05 compared to intact endothelium, halfmaximal effective concentrations, see Table 1).
Table 1.
Vasorelaxant potency of nitrovasodilators in porcine basilar and coronary artery
Removal of the endothelium significantly increased the activity of the nitrovasodilators as indicated by the decrease of the halfmaximal effective concentration (Figure 2, Table 1). A similar increase of the halfmaximal effective concentration of GTN (in −logM) was observed after preincubation of the isolated basilar artery segments with 100 μM L-NA (4.59±0.01, n=9). These data indicate that isolated perfused basilar artery segments show a normal response to either endothelial NO or NO extracellularly released by SNAP, while being relatively insensitive to organic nitrates which have to undergo enzymatic cleavage to release NO.
A low potency of organic nitrates was also observed in basilar artery ring studies, which were done to investigate any influence of the vessel preparation on the vasodilator potency of organic nitrates. The halfmaximal vasodilator concentration in −logM of glyceryl trinitrate in porcine basilar ring segments was 4.868±0.104 (n=6). Thus, the potency of glyceryl trinitrate in basilar ring segments was slightly greater than that in perfused basilar segments (see Table 1, P<0.05, ANOVA). However, at the maximal concentration of glyceryl trinitrate (100 μM) basilar rings segments relaxed on average 65.0±2.34% and this value was not significantly different from that obtained in perfused basilar artery segments (64.8±4.71%, P=0.9217, t-test).
Vasorelaxant activity of nitrovasodilators incoronary arteries
Cumulative addition of GTN, PETN, ISMN and SNAP resulted in a concentration-dependent vasorelaxation of endothelium intact coronary artery segments that was increased in the absence of endothelium (Figure 3). In striking contrast to the results obtained in basilar arteries, coronary arteries responded very sensitive to organic nitrates. The order of potency was PETN>SNAP>GTN>ISMN. Thus, the response of coronary arteries to organic nitrates was much greater than that of basilar arteries, while the response to the NO-donor SNAP was similar (Figure 4).
Figure 3.
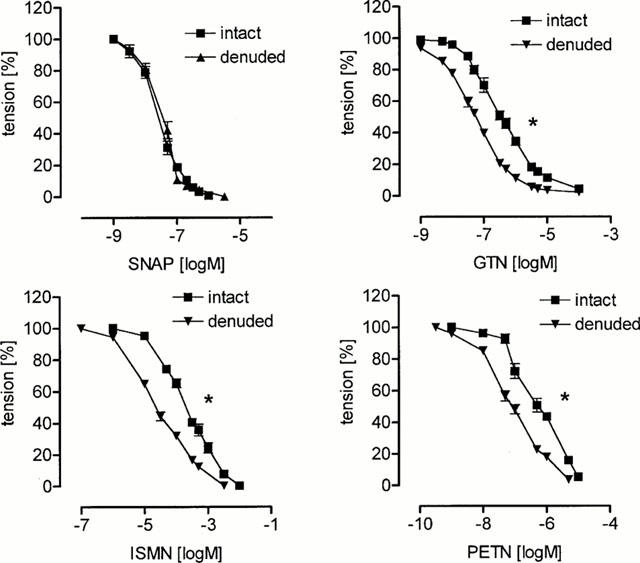
Nitrovasodilator induced relaxation of isolated porcine coronary artery segments. The vasodilator activity of S-nitroso-N-acetyl-D,L-penicillamine (SNAP), glyceryl trinitrate (GTN), isosorbide-5-nitrate (ISMN) and pentaerythritol tetranitrate (PETN) was assessed in the presence (intact) and absence (denuded) of intact endothelium. Each concentration-response curve is plotted by taking the respective mean values of 6 – 12 individual experiments (s.e.mean indicated by bars, *P<0.05 compared to intact endothelium, halfmaximal effective concentration, see Table 1).
Figure 4.
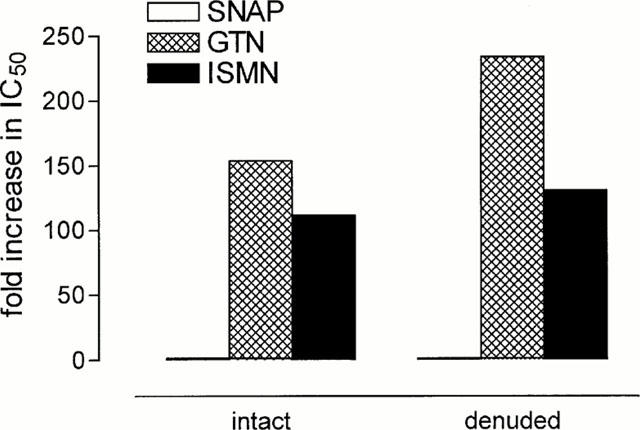
Low potency of organic nitrates in basilar versus coronary artery segments as indicated by the fold increase in the IC50-values. Given are the ratios of the halfmaximal effective concentration of the spontaneous NO-donor S-nitroso-N-acetyl-D,L-penicillamine (SNAP) and the organic nitrates glyceryl trinitrate (GTN) and isosorbide-5-nitrate (ISMN) in cardinal numbers (halfmaximal effective concentration, see Table 1).
Discussion
In this study the vasorelaxant potency of organic nitrates was investigated in isolated cerebral arteries. The new finding is that these vessels show a poor vasorelaxant response to this particular group of nitrovasodilators, while having a normal sensitivity to endothelium-dependent vasodilation or relaxation induced by spontaneous NO-donors such as SNAP. In striking contrast, studies in isolated coronary conductance vessels showed a similar relaxant response to endogenous NO, SNAP and organic nitrates. These results suggest that enzymatic bioactivation of organic nitrates to NO is largely impaired in cerebral vessels.
The major finding of this study is an approximately 100 fold lower vasodilator potency of organic nitrates such as GTN in basilar compared to coronary arteries, while there was no such difference for the nitrosothiol SNAP. Although there is some debate as to the rate and mechanism of NO-release from various nitrosothiols such as SNAP, there is general agreement that these compounds release NO in buffer-solutions (Ignarro et al., 1981; Noack & Feelisch, 1991; Kowaluk & Fung, 1990; Kojda et al., 1998). In preliminary experiments we were able to confirm previous studies showing that SNAP indeed spontaneously releases NO in our buffer solutions. Thus, a comparable vasorelaxant response of coronary and basilar artery segments to SNAP suggests a similar sensitivity of the NO signal transduction pathway in these vessels. We also examined the vasorelaxant effect of organic nitrates in both vessel types and found a strongly reduced activity in basilar arteries. Again, there is debate regarding the mechanisms responsible for the release of NO from these drugs, but it is generally agreed that they undergo enzymatic biotransformation to NO in the vascular smooth muscle cells (Feelisch & Kelm, 1991). Thus, the results of our study suggest that the apparent reduction of the potency of organic nitrates in cerebral vessels is caused by a low ability of cerebral endothelial and/or vascular smooth muscle cells to bioconvert these drugs to nitric oxide.
Previous investigations on the efficiency of organic nitrates in isolated cerebral arteries are rare and – for the most part – restricted to GTN. Subjection of cultured smooth muscle cells derived from porcine basilar and middle cerebral artery to GTN results in a concentration-dependent increase of cGMP-levels with a halfmaximal concentration of approximately 10 μM (Tao et al., 1997). Furthermore, the maximal increase in cGMP following GTN treatment was 6 fold lower than that observed after treatment with the NO-donor sodium nitroprusside. These data show a poor response of cerebral vascular smooth muscle cells to the organic nitrate GTN and are consistent with our results. In accordance, another investigation with guinea-pig basilar arteries obtained similar results. Vasorelaxation experiments demonstrated a poor activity of GTN, while endothelium-dependent vasodilation was normal (You et al., 1995). The maximal vasodilator response to GTN (100 μM) was approximately 50% of maximal vasodilation. Thus, not only porcine but also guinea-pig isolated cerebral vessels are less responsive to organic nitrates.
Generation of NO from organic nitrates requires the reduction of a nitrate nitrogen from +5 to +2 and thus a flow of three electrons. In tissues this reaction is most likely catalyzed by intracellular membrane-bound enzymes (Bennett et al., 1992; Chung & Fung, 1990; Salvemini et al., 1992; Feelisch & Kelm, 1991). So far, the identity of these enzymes are unknown. Preliminary evidence indicates an involvement of cytochrome P450 enzymes (Servent et al., 1989; McDonald & Bennett, 1990; Schröder & Schrör, 1990) and of glutathion-S-transferase (Hill et al., 1992; Kenkare et al., 1994; Lau & Benet, 1990; Nigam et al., 1993), but these findings are not consistent (Sakanashi et al., 1991; Liu et al., 1993; Kurz et al., 1993). It is therefore most likely that a variety of enzymes or isozymes are capable to produce NO by reducing a nitrate moiety of organic nitrates. Recent results indicate a particular role for the cytochrome P450 isozyme CYP3A4 (Minamiyama et al., 1999). Lymphoblast microsomes transfected with CYP3A4, which is also abundantly present in the vascular wall of the human coronary circulation, showed the most effective generation of NO from isosorbide dinitrate. According to these results, it is suggested that cerebral blood vessels may have an expression and/or activity pattern of either cytochrome P450 enzymes or glutathion-S-transferase which is different from that of conductance blood vessels e.g. in the coronary circulation. A similar situation might hold true for coronary resistance vessels compared to conductance blood vessels (Selke et al., 1990). In view of these possible mechanistic explanations it might appear as a drawback of this study that we didn't measure the vascular bioconversion of GTN to NO and the metabolites 1,2-glyceryl dinitrate and 1,3-glyceryl dinitrate directly.
Another possible reason for the apparent lack of activity of GTN in cerebral vessel might be a direct interaction with NO produced by endothelial NO-synthase. It has been reported that permanent endogenous NO-production maintained by blood flow induced shearing of endothelial cells leads to a desensitization of vascular soluble guanylate cyclase (Moncada et al., 1991) and to an impairment of GTN-bioactivation to nitric oxide (Kojda et al., 1998). These suggestions are consistent with the results of our study, since the effects of both organic nitrates and the NO-donor SNAP were enhanced after endothelial denudation (Figures 2 and 3). Possibly, these mechanisms might have contributed to the low activity of GTN in porcine basilar artery segments. We have used a perfusion method to mimic physiologic blood flow and pressure. As expected, endothelial denudation resulted in significant increase in potency of GTN and SNAP. However, the effect of endothelial denudation was too small to explain the substantial lack of activity of GTN in porcine basilar arteries.
Despite the finding of a low vasodilator potency of organic nitrates in cerebral blood vessels it is doubtless that cerebral vasodilation underlies the typical nitrate headache (Olesen et al., 1994). According to our results, cerebral vasodilation in vivo cannot be explained by a direct vasodilator effect of organic nitrates. It is more likely that other indirect mechanisms are responsible for the reduction of cerebral vascular tone. Interestingly, Wei et al. (1992) showed that organic nitrates can activate sensoric nerve fibres to release calcitonin gene related peptide, which then induces cerebral vasodilation via the NO signal transduction pathway. Furthermore, calcitonin gene related peptide is also involved in the inhibition by GTN of whole blood aggregation (Booth et al., 1997) and relaxation of rat aorta (Fung HL, personal communication). However, the in vivo implications of our findings need to be addressed in further studies.
Our results might have been influenced by the different vessel preparations we used for basilar and coronary arteries. To investigate this possible influence, we have made control experiments in ring segments of basilar arteries, and studied them in the same manner as the coronary artery rings. As shown in Results, the efficiency of glyceryl trinitrate in basilar ring segments was only slightly different to that observed in perfused basilar artery preparations suggesting that the type of vessel preparation was of minor influence on our results. In accordance, the vasodilator SNAP, which does not undergo a reductive metabolism to release NO showed no difference between basilar and coronary arteries suggesting that the NO-signal transduction system is equally effective in both artery types. This is consistent with our finding of a full relaxant response after activation of endothelial NO-production by substance P. Finally, it appears to be unlikely that alterations in the preparation procedure of vascular tissues such as time needed for dissection, pH and temperature of the transport buffer, the preparation of ring segments and the time needed to mount the tissues into the measuring unit are the cause for the observed differences (see Methods). According to these remarks, it is unlikely that differences in the method of vessel preparation and/or the technique to measure vasomotion are the major cause of the observed difference in GTN efficiency in basilar and coronary arteries.
In summary, our results indicate that cerebral conductance blood vessels are highly unresponsive to organic nitrates as compared to coronary conductance blood vessels. In striking contrast, the responsiveness to spontaneous NO-donors or endothelium derived NO does not differ between these blood vessel types. These results suggest that cerebral conductance blood vessels such as porcine basilar arteries seems to have a reduced expression and/or activity of certain cellular enzymatic electron transport systems such as cytochrome P450 enzymes, which are necessary to bioconvert organic nitrates to NO.
Abbreviations
- GTN
glyceryl trinitrate
- ISMN
isosorbide-5-nitrate
- L-NA
Nω-nitro-L-arginine
- L-NMMA
Nω-monomethyl-L-arginine
- NO
nitric oxide, nitrogen monoxide
- PETN
pentaerythritol tetranitrate
- PGF2α
prostaglandin F2α
- SNAP
S-nitroso-N-acetyl-D,L-penicillamine
References
- AHLNER J., ANDERSON R.G.G., TORFGÅRD K., AXELSSON K.L. Organic nitrate esters: Clinical use and mechanisms of actions. Pharmacol. Rev. 1991;43:351–423. [PubMed] [Google Scholar]
- BASSENGE E., STUART D.J. Effects of nitrates in various vascular sections and regions. Z. Kardiol. 1986;75 Suppl 3:1–7. [PubMed] [Google Scholar]
- BENNETT B.M., MCDONALD B.J., NIGAM R., LONG P.G., SIMON W.C. Inhibition of nitrovasodilator- and acetylcholine-induced relaxation and cyclic GMP accumulation by the cytochrome P-450 substrate, 7-ethoxyresorufin. Can. J. Physiol. Pharmacol. 1992;70:1297–1303. doi: 10.1139/y92-181. [DOI] [PubMed] [Google Scholar]
- BOOTH B.P., NOLAN T.D., FUNG H.L. Nitroglycerin-inhibited whole blood aggregation is partially mediated by calcitonin gene-related peptide – a neurogenic mechanism. Br. J. Pharmacol. 1997;122:577–583. doi: 10.1038/sj.bjp.0701408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG S.-J., FUNG H.-L. Identification of the subcellular site for nitroglycerin metabolism to nitric oxide in bovine coronary smooth muscle cells. J. Pharmacol. Exp. Ther. 1990;253:614–619. [PubMed] [Google Scholar]
- COCKS T.M., ANGUS J.A. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983;305:627–630. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., KELM M. Biotransformation of organic nitrates to nitric oxide by vascular smooth muscle and endothelial cells. Biochem. Biophys. Res. Commun. 1991;180:286–293. doi: 10.1016/s0006-291x(05)81290-2. [DOI] [PubMed] [Google Scholar]
- FIELD L., DILTS R.V., RAVICHANDRAN R., LENHERT G., CARNAHAN G.E. An unusual stable thionitrite from N-acetyl-D,L-penicillamine; x-ray crystal and molecular structure of 2-(acetylamino)-2-carboxy-1,1-dimethyl thionitrite. JCS Chem. Commun. 1978;1157:249–250. [Google Scholar]
- HILL K.E., HUNT R.W., JR, JONES R., HOOVER R.L., BURK R.F. Metabolism of nitroglycerin by smooth muscle cells. Involvement of glutathione and glutathione S-transferase. Biochem. Pharmacol. 1992;43:561–566. doi: 10.1016/0006-2952(92)90579-8. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., LIPTON H., EDWARDS J.C., BARRICOS W.H., HYMAN A.L., KADOWITZ P.J., GRUETTER C.A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J. Pharmacol. Exp. Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- KENKARE S.R., HAN C., BENET L.Z. Correlation of the response to nitroglycerin in rabbit aorta with the activity of the mu class glutathione S-transferase. Biochem. Pharmacol. 1994;48:2231–2235. doi: 10.1016/0006-2952(94)00415-3. [DOI] [PubMed] [Google Scholar]
- KOJDA G. Therapeutic importance of nitrovasodilators. Handbook of Pharmacology Nitric Oxide 2000143Berlin, New York, Tokyo: Springer Verlag; 365–384.ed. Mayer, B. pp [Google Scholar]
- KOJDA G., KLAUS W., WERNER G., FRICKE U. The influence of 3-ester side chain variation on the cardiovascular profile of nitrendipine in porcine isolated trabeculae and coronary arteries. Naunyn Schmiedeberg's Arch. Pharmacol. 1991;344:488–494. doi: 10.1007/BF00172590. [DOI] [PubMed] [Google Scholar]
- KOJDA G., KLAUS W., WERNER G., FRICKE U. Intervascular and stimulus selectivity of nitrendipine and related derivatives in KCl and prostaglandin F2a precontracted porcine arteries. Br. J. Pharmacol. 1992;106:85–90. doi: 10.1111/j.1476-5381.1992.tb14297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOJDA G., LAURSEN J.B., RAMASAMY S., KENT J.D., KURZ S., BURCHFIELD J., SHESELY E.G., HARRISON D.G. Protein expression, vascular reactivity and soluble guanylate cyclase activity in mice lacking the endothelial nitric oxide synthase: contributions of NOS isoforms to blood pressure and heart rate control. Cardiovasc. Res. 1999;42:206–213. doi: 10.1016/s0008-6363(98)00315-0. [DOI] [PubMed] [Google Scholar]
- KOJDA G., PATZNER M., HACKER A., NOACK E. Nitric oxide inhibits vascular bioactivation of glyceryl trinitrate. A novel mechanism to explain preferential venodilation of organic nitrates. Mol. Pharmacol. 1998;53:547–554. doi: 10.1124/mol.53.3.547. [DOI] [PubMed] [Google Scholar]
- KOWALUK E.A., FUNG H.-L. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J. Pharmacol. Exp. Ther. 1990;225:1256–1264. [PubMed] [Google Scholar]
- KURZ M.A., BOYER T.D., WHALEN R., PETERSON T.E., HARRISON D.G. Nitroglycerin metabolism in vascular tissue: Role of glutathione S-transferases and relationship between NO• and NO2− formation. Biochem. J. 1993;292:545–550. doi: 10.1042/bj2920545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAU D.T.W., BENET L.Z. Nitroglycerin metabolism in subcellular fractions of rabbit liver. Dose dependency of glyceryl dinitrate formation and possible involvement of multiple isoenzymes of glutathione S-transferases. Drug Metab. Dispos. 1990;18:292–297. [PubMed] [Google Scholar]
- LIU Z., BRIEN J.F., MARKS G.S., MCLAUGHLIN B.E., NAKATSU K. Lack of evidence for the involvement of cytochrome P-450 or other hemoproteins in metabolic activation of glyceryl trinitrate in rabbit aorta. J. Pharmacol. Exp. Ther. 1993;264:1432–1439. [PubMed] [Google Scholar]
- MCDONALD B.J., BENNETT B.M. Cytochrome P-450 mediated biotransformation of organic nitrates. Can. J. Physiol. Pharmacol. 1990;68:1552–1557. doi: 10.1139/y90-236. [DOI] [PubMed] [Google Scholar]
- MINAMIYAMA Y., TAKEMURA S., AKIYAMA T., IMAOKA S., INOUE M., FUNAE Y., OKADA S. Isoforms of cytochrome P450 on organic nitrate-derived nitric oxide release in human heart vessels. FEBS Lett. 1999;452:165–169. doi: 10.1016/s0014-5793(99)00612-2. [DOI] [PubMed] [Google Scholar]
- MONCADA S., REES D.D., SCHULZ R., PALMER R.M.J. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc. Natl. Acad. Sci. USA. 1991;88:2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIGAM R., WHITING T., BENNETT B.M. Effect of inhibitors of glutathione S-transferase on glyceryl trinitrate activity in isolated rat aorta. Can. J. Physiol. Pharmacol. 1993;71:179–184. doi: 10.1139/y93-025. [DOI] [PubMed] [Google Scholar]
- NOACK E., FEELISCH M.Molecular mechanism of nitrovasodilator bioactivation Endothelial mechanism of vasomotor control 1991Darmstadt: Steinkopff Verlag; 37–50.ed. Drexler, H., Zeiher, A.M., Bassenge, E. & Just, H. pp [DOI] [PubMed] [Google Scholar]
- OLESEN J., THOMSEN L.L., IVERSEN H. Nitric oxide is a key molecule in migraine and other vascular headaches. Trends Pharmacol. Sci. 1994;15:149–153. doi: 10.1016/0165-6147(94)90075-2. [DOI] [PubMed] [Google Scholar]
- PARKER J.D., PARKER J.O. Nitrate therapy for stable angina pectoris. N. Engl. J. Med. 1998;338:520–531. doi: 10.1056/NEJM199802193380807. [DOI] [PubMed] [Google Scholar]
- SAKANASHI M., MATSUZAKI T., ANIYA Y. Nitroglycerin relaxes coronary artery of the pig with no change in glutathione content or glutathione S-transferase activity. Br. J. Pharmacol. 1991;103:1905–1908. doi: 10.1111/j.1476-5381.1991.tb12350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., MOLLACE V., PISTELLI A., ANGGARD E., VANE J. Metabolism of glyceryl trinitrate to nitric oxide by endothelial cells and smooth muscle cells and its induction by Escherichia coli lipopolysaccharide. Proc. Natl. Acad. Sci. USA. 1992;89:982–986. doi: 10.1073/pnas.89.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRÖDER H., SCHRÖR K. Inhibitors of cytochrome P-450 reduce cGMP stimulation by glyceryl trinitrate in LLC-PK1 kidney epithelial cells. Naunyn Schmiedeberg's Arch. Pharmacol. 1990;342:616–618. doi: 10.1007/BF00169054. [DOI] [PubMed] [Google Scholar]
- SELLKE F.W., MYERS P.R., BATES J.N., HARRISON D.G. Influence of vessel size on the sensitivity of porcine microvessels to nitroglycerin. Am. J. Physiol. 1990;258:H515–H520. doi: 10.1152/ajpheart.1990.258.2.H515. [DOI] [PubMed] [Google Scholar]
- SERVENT D., DELAFORGE M., DUCROCQ C., MANSUY D., LENFANT M. Nitric oxide formation during microsomal hepatic denitration of glyceryl trinitrate: involvement of cytochrome P-450. Biochem. Biophys. Res. Commun. 1989;163:1210–1216. doi: 10.1016/0006-291x(89)91106-6. [DOI] [PubMed] [Google Scholar]
- TAO H., ZHANG L.M., CASTRESANA M.R., NEWMAN W.H., SHILLCUTT S.D. Response of cultured cerebral artery smooth muscle cells to the nitric oxide vasodilators, nitroglycerin and sodium nitroprusside. J. Neurosurg. Anesthesiol. 1997;9:58–64. doi: 10.1097/00008506-199701000-00013. [DOI] [PubMed] [Google Scholar]
- WEI E.P., MOSKOWITZ M.A., BOCCALINI P., KONTOS H.A. Calcitonin gene-related peptide mediates nitroglycerin and sodium nitroprusside-induced vasodilation in feline cerebral arterioles. Circ. Res. 1992;70:1313–1319. doi: 10.1161/01.res.70.6.1313. [DOI] [PubMed] [Google Scholar]
- WINBURY M.M., HOWE B.B., WEISS H.R. Effect of nitroglycerin and dipyridamole on epicardial and endocardial oxygen tension-further evidence for redistribution of myocardial blood flow. J. Pharmacol. Exp. Ther. 1971;176:184–199. [PubMed] [Google Scholar]
- YOU J., ZHANG W., JANSEN-OLESEN I., EDVINSSON L. Relation between cyclic GMP generation and cerebrovascular reactivity: modulation by NPY and alpha-trinositol. Pharmacol. Toxicol. 1995;77:48–56. doi: 10.1111/j.1600-0773.1995.tb01913.x. [DOI] [PubMed] [Google Scholar]


