Abstract
Electrical field stimulation (EFS) of non-adrenergic non-cholinergic nerves of the mouse gastric fundus induced frequency-dependent transient relaxations which were mimicked by nitric oxide (NO), added as acidified NaNO2. The NO donors S-nitrosocysteine, S-nitrosoglutathione, SIN-1 and hydroxylamine induced sustained concentration-dependent relaxations. The NO synthase blocker L-nitro arginine (L-NOARG; 300 μM) abolished the relaxations to EFS without affecting the relaxations to NO.
The copper(I) chelator neocuproine (10 μM) enhanced the relaxations to EFS and NO but inhibited those to S-nitrosocysteine and S-nitrosoglutathione. Neocuproine potentiated the relaxations to SIN-1, which releases NO extracellularly, without affecting the relaxations to hydroxylamine, which releases NO intracellularly.
The potentiating effect of neocuproine on the relaxations to EFS was more pronounced after inhibition of catalase with 3-amino-1,2,4-triazole (1 mM) but not after inhibition of Cu/Zn superoxide dismutase (SOD) with diethyl dithiocarbamic acid (DETCA, 1 mM). The potentiating effect of neocuproine on relaxations to NO was not altered by 3-amino-1,2,4-triazole or DETCA treatment.
The relaxations to EFS were significantly inhibited by the oxidants hydrogen peroxide (70 μM) and duroquinone (10 μM) but only after inhibition of catalase with 3-amino-1,2,4-triazole or after inhibition of Cu/ZnSOD with DETCA respectively.
Our results suggest that neocuproine can act as an antioxidant in the mouse gastric fundus and that both catalase and Cu/ZnSOD protect the nitrergic neurotransmitter from oxidative breakdown. Since inhibition of catalase but not inhibition of Cu/ZnSOD potentiated the effect of neocuproine on relaxations to EFS without affecting the relaxations to NO, catalase may protect the nitrergic neurotransmitter mainly at a prejunctional site whereas Cu/ZnSOD protects at a postjunctional site.
Keywords: Antioxidant, catalase, copper, gastric fundus, neocuproine, nitrergic neurotransmission, nitric oxide, non-adrenergic non-cholinergic, S-nitrosothiols, superoxide dismutase
Introduction
We previously proposed nitric oxide (NO) as an inhibitory non-adrenergic non-cholinergic (NANC) neurotransmitter in the canine ileocolonic junction and rat gastric fundus (Boeckxstaens et al., 1990; 1991). Although nitric oxide plays an important role in the peripheral nervous system (for review see Rand & Li, 1995; Martin, 2000), the actual nature of the nitrergic neurotransmitter has remained a matter of debate. Similar to the endothelium derived relaxing factor in the vascular system, the study on the nature of the nitrergic neurotransmitter showed that some compounds failed to affect responses to nitrergic nerve stimulation while responses to exogenous NO were blocked (for reviews see: Gibson et al., 1995; Martin, 2000). This may indicate that the nitrergic factor released from NANC nerves is not free NO. Alternatively, the compound under study may not have reached the site where the nitrergic neurotransmitter is released while it may have fully reacted with exogenously added NO. In addition, the nitrergic neurotransmitter may be protected from breakdown by antioxidants (Hiraishi et al., 1994; Martin et al., 1994). A class of antioxidants that are abundantly present in cells are thiols. Thiols are also able to bind NO with concomitant formation of S-nitrosothiols and S-nitrosothiols have therefore been proposed to function as ‘reservoirs' of NO in cells. In agreement with this, cellular mechanisms of formation and decomposition of S-nitrosothiols have been reported (Stamler et al., 1992; Gaston et al., 1998a,1998b, Goçmen et al., 1998; Gordge et al., 1998; Liu et al., 1998; Jourd'heuil et al., 2000). In order to investigate further whether S-nitrosothiols are involved in nitrergic neurotransmission, suitable pharmacological compounds are needed which affect both the activity of the endogenously released nitrergic factor and that of exogenously added S-nitrosothiols. Neocuproine, which is a copper(I)-selective chelator may represent such a tool. In the rat gastric fundus, neocuproine inhibited relaxations to S-nitrosothiols while it enhanced the relaxations to nitrergic nerve stimulation (De Man et al., 1999). The differential reactivity of neocuproine towards the endogenously released nitrergic factor and exogenously added S-nitrosothiols, demonstrates that this compound may help to elucidate the nature of the nitrergic factor in the enteric nervous system and the vasculature.
The inhibitory action of neocuproine on relaxations to S-nitrosothiols most likely results from an inhibition of the copper(I)-dependent release of NO from S-nitrosothiols (Gordge et al., 1995; Dicks et al., 1996; Williams, 1996) but the mechanism behind the potentiating action of neocuproine on the relaxations to the nitrergic neurotransmitter is unknown. Emsley et al. (1999) recently demonstrated that copper ions decrease the production of endothelium-derived NO, possibly due to a copper-catalysed generation of oxidants such as superoxide and hydrogen peroxide. Although it is not known whether such a copper catalysed generation of oxidants is also present in the enteric nervous system, there is evidence that antioxidants play a major role in protecting the nitrergic factor from oxidative breakdown as mentioned above. Therefore, in the present study we have investigated in more detail the mechanism of action of neocuproine on the nitrergic neurotransmission in the mouse gastric fundus and studied whether oxidants and antioxidants are involved in this mechanism.
Methods
Tissue preparation
Male Swiss mice (30 – 40 g) were fasted for 24 h with free access to water. The animals were anaesthetized with diethyl ether and exsanguinated from the carotid artery. A laparotomy was performed and the stomach was removed and cut open. The mucosal layer was removed by sharp dissection and three longitudinal muscle strips of ≈amp;10 mm long and 3 mm wide were cut from the gastric fundus. The muscle strips were mounted in organ baths (25 ml) that were filled with Krebs-Ringer solution (mM: KH2PO4 1.2, NaCl 118.3, KCl 4.7, MgSO4 1.2, CaCl2 2.5, NaHCO3 25 and glucose 11). The solution was maintained at 37°C and aerated with a mixture of 95% O2 and 5% CO2.
Isometric tension recording
One end of the muscle strip was placed between two platinum ring electrodes and connected to a strain gauge transducer (Scaime GM2, France) for continuous recording of isometric tension. The strips were brought to their optimal point of length-tension relationship (Pelckmans et al., 1989) and then allowed to equilibrate for at least 60 min before experimentation.
Experimental protocols
All experiments were performed on muscle strips contracted with 0.3 μM prostaglandin F2α and in the presence of atropine, propranolol and phentolamine (all 1 μM). After finishing each protocol, the muscle strips were washed at least three times with intervals of 5 min. Frequency-response curves were constructed by stimulating the muscle strips electrically (0.5 to 4 Hz, pulse duration of 1 ms and pulse trains of 10 s). The effect of neocuproine or cuprizone (both 10 μM) was investigated on the frequency-response curve to electrical field stimulation, on the concentration-response curves to NO (0.3 – 3 μM, applied as acidified NaNO2) and to the NO donors S-nitrosocysteine (1 nM – 0.1 μM), S-nitrosoglutathione (1 nM – 1 μM), SIN-1 (0.03 – 3 μM) and hydroxylamine (0.01 – 1 μM).
The effect of neocuproine on the frequency-response curve to electrical field stimulation and on the concentration-response curves to NO, added as acidified NaNO2, was reinvestigated on muscle strips that were pretreated with the catalase inhibitor 3-amino-1,2,4-triazole and with the Cu/Zn superoxide dismutase (Cu/Zn SOD) inhibitor DETCA. To investigate the protective mechanisms on nitrergic neurotransmission, the effect of the oxidants hydrogen peroxide and duroquinone was investigated before and after treatment of the muscle strips with 3-amino-1,2,4-triazole and DETCA. The incubation time of neocuproine and cuprizone was 10 min. The concentrations of neocuproine and cuprizone (10 μM) were chosen according to previous findings in the rat gastric fundus (De Man et al., 1999), human platelets (Gordge et al., 1995) and the rat tail artery (Al-Sa'doni et al., 1997). The incubation time of DETCA and 3-amino-1,2,4-triazole was 90 min. After treatment with DETCA and 3-amino-1,2,4-triazole, muscle strips were washed twice before starting the experiment. This does not affect the blocking activity of DETCA and 3-amino-1,2,4-triazole since their effect is irreversible (Martin et al., 1994; Mian & Martin, 1997).
All experiments were performed in parallel with muscle strips that served as time controls receiving saline instead of copper chelators. The relaxations to electrical field stimulation, NO, and the NO donors remained constant over the time course of the experiment.
Drugs
The following drugs were used: L-ascorbic acid, atropine sulphate, glyceryl trinitrate, hydrogen peroxide, sodium nitrite (Merck, Darmstadt, Germany); 3-amino-1,2,4-triazole, cuprizone, L-cysteine, diethyl dithiocarbamic acid (DETCA), D-mannitol, hydroxylamine hydrochloride, neocuproine, L-NOARG, phenylephrine, propranolol, phentolamine, reduced glutathione, 3-morpholinosydnonimine (SIN-1), superoxide dismutase (Sigma Chemical Co., St. Louis, MO, U.S.A.), prostaglandin F2α (Dinolytic® purchased from Upjohn, Puurs, Belgium as a sterile aqueous solution containing 5 mg ml−1 prostaglandin F2α and 9 mg ml−1 benzyl alcohol). All solutions were prepared freshly on the day of the experiment. S-nitrosothiols were synthesized as described previously (De Man et al., 1996) and kept sealed on ice under argon in the dark. Dilutions of the stock solutions of S-nitrosothiols were made freshly before each experiment and were used immediately after dilution. A stock solution of 0.1 mM NaNO2 was prepared freshly in distilled water on the day of the experiment. Appropriate dilutions of the stock solution were made and the solutions were adjusted to pH 3 with 5 mM HCl immediately before starting the experiments. A control solution of acidified distilled water did not affect the tension of the gastric fundus muscle strip.
Presentation of results and statistical analysis
Results are expressed as percentage decrease of the prostaglandin F2α-induced contraction of the mouse gastric fundus muscle strip. Values are shown as mean±s.e.mean for n indicating the number of mice used. Statistical significance of differences among values was determined by Student's t-test for paired values (comparison of two groups of data) or by one way ANOVA followed by post hoc Dunnett's test (comparison of three groups of data). Analysis of the effect of neocuproine before and after treatment with antioxidant inhibitors was performed by a two way ANOVA followed by post hoc testing using a paired Student's t-test to analyse the effect of neocuproine and using a one way ANOVA followed by Dunnett's test to analyse the potency of neocuproine. P values of less than 0.05 were considered as significantly different from control.
Results
Relaxations to EFS, NO and NO donors in the mouse gastric fundus
In NANC conditions, electrical field stimulation (EFS, 0.5 – 4 Hz, 1 ms duration in trains of 10 s) of the mouse gastric fundus, induced frequency-dependent relaxations (Figure 1) that were sharp and transient and which immediately returned to zero-relaxation after cessation of stimulation. Stimulation of the muscle strip at high frequency (8 Hz) during 2 min induced a sustained relaxation that also returned to zero-relaxation after cessation of stimulation (Figure 1). All relaxations to EFS, including that to prolonged stimulation at 8 Hz, were abolished by the NO synthase blocker L-NOARG (300 μM; Figure 1). After wash-out (three times) of L-NOARG and in the presence of L-arginine (1 mM), all relaxations to EFS were fully restored. This indicates that the NANC relaxations are completely nitrergic in nature. The relaxations to nitrergic nerve stimulation were mimicked by exogenous NO which was applied as acidified NaNO2 (0.3 – 3 μM) (Figures 1, 2 and 3). The NO donors S-nitrosocysteine (1 nM – 0.1 μM), S-nitrosoglutathione (1 nM – 1 μM), SIN-1 (0.03 – 3 μM) and hydroxylamine (0.01 – 1 μM) induced concentration-dependent relaxations of the mouse gastric fundus (Figure 4) but these relaxations were more sustained as compared to the relaxations to nitrergic nerve stimulation and NO.
Figure 1.
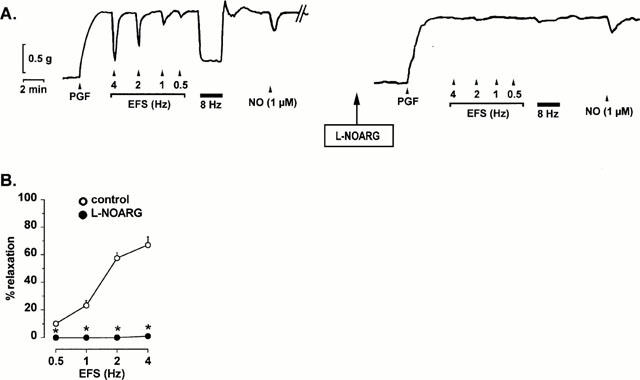
Typical tracings of the mouse gastric fundus contracted with prostaglandin F2α (PGF, 0.3 μM) showing (A) the relaxations induced by electrical field stimulation (EFS, 0.5 – 4 Hz, pulses of 1 ms during 10 s and 8 Hz, pulses of 1 ms during 2 min) and 1 μM NO, added as acidified NaNO2, and the effect of L-NOARG (300 μM) on the relaxations to EFS and NO. (B) shows the effect of L-NOARG for n=6 experiments. Results are expressed as percentage decrease of the prostaglandin F2α-induced contraction and shown as mean±s.e.mean. *P<0.05 is considered as significantly different from control, Student's t-test for paired values.
Figure 2.
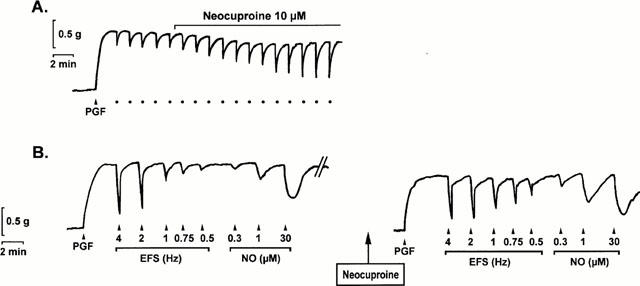
Typical tracings obtained from two different muscle strips of the mouse gastric fundus contracted with prostaglandin F2α (PGF, 0.3 μM). (A) shows the nitrergic relaxations to repetitive electrical field stimulation at 1 Hz (•) and the potentiating effect of 10 μM neocuproine on these relaxations. (B) shows the frequency- and concentration-dependent relaxations to electrical field stimulation (EFS, 0.5 – 4 Hz) and NO (0.3 – 30 μM, added as acidified NaNO2) in control conditions (left panel) and in the presence of 10 μM neocuproine (right panel).
Figure 3.
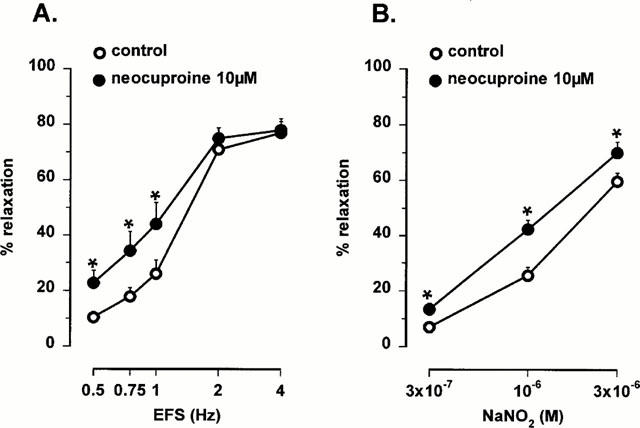
Frequency-response curves to electrical field stimulation (EFS, 0.5 – 4 Hz) and concentration-response curves to NO (0.3 – 3 μM, added as acidified NaNO2) in the mouse gastric fundus in control conditions and in the presence of 10 μM neocuproine. Results are expressed as percentage decrease of the prostaglandin F2α-induced contraction and shown as mean±s.e.mean for n=5 – 7 experiments. *P<0.05 is considered as significantly different from control, Student's t-test for paired values.
Figure 4.
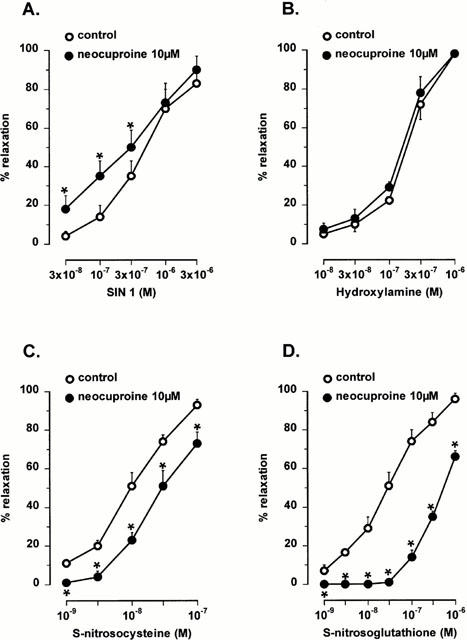
Concentration-response curves to the NO donors SIN-1 (A, 0.03 – 3 μM), hydroxylamine (B, 0.01 – 1 μM), S-nitrosocysteine (C, 1 – 100 nM) and S-nitrosoglutathione (D, 1 – 1000 nM) in the mouse gastric fundus in control conditions and in the presence of 10 μM neocuproine. Results are expressed as percentage decrease of the prostaglandin F2α-induced contraction and shown as mean±s.e.mean for n=4 – 6 experiments. *P<0.05 is considered as significantly different from control, Student's t-test for paired values.
Effect of neocuproine
In the presence of the copper(I) chelator neocuproine (10 μM), relaxations to low frequency nitrergic nerve stimulation and to NO, added as acidified NaNO2, were significantly enhanced (Figures 2 and 3). The relaxations to SIN-1, which releases NO extracellularly, were also significantly enhanced by neocuproine (10 μM) but the relaxations to hydroxylamine, which releases NO intracellularly, were not affected (Figure 4). The relaxations to S-nitrosocysteine and S-nitrosoglutathione were inhibited by neocuproine (Figure 4). The copper(II) chelator cuprizone (10 μM) had no effect the relaxations to nitrergic nerve stimulation, NO or S-nitrosothiols (data not shown).
Involvement of oxidants and antioxidants in the effect of neocuproine
Since the biological activity of NO is dependent on the oxidative status of the tissue, we investigated the involvement of oxidants and antioxidants in the effect of neocuproine. The antioxidants superoxide dismutase (1000 u ml−1), ascorbic acid (30 μM) and D-mannitol (0.5 mM) did not mimic the effect of neocuproine as relaxations to nitrergic nerve stimulation and NO were not affected (Figure 5). 3-amino-1,2,4-triazole (1 mM) and DETCA (0.5 mM), inhibitors of the endogenous antioxidant enzymes catalase and Cu/Zn SOD respectively, had no effect by themselves on the relaxations to nitrergic nerve stimulation or NO (results not shown). However, after treatment of the muscle strips with 3-amino-1,2,4-triazole, neocuproine enhanced the relaxations to nitrergic nerve stimulation to a bigger extent as compared to neocuproine alone (Figure 6). This effect was significantly more pronounced after 3-amino-1,2,4-triazole treatment but not after DETCA treatment. On the other hand, treatment of the muscle strips with 3-amino-1,2,4-triazole or DETCA did not affect the ability of neocuproine to enhance relaxations to NO: the effect of neocuproine was similar in treated and untreated strips (Figure 6).
Figure 5.
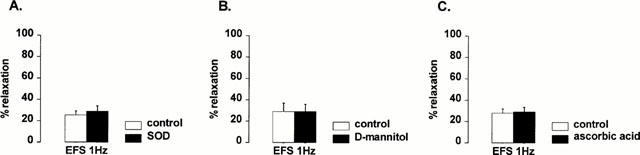
Relaxations induced by electrical field stimulation (EFS) at 1 Hz in control conditions and in the presence of (A) superoxide dismutase (SOD, 1000 u ml−1), (B) D-mannitol (0.5 mM) and (C) ascorbic acid (30 μM). Results are expressed as percentage decrease of the prostaglandin F2α -induced contraction and shown as mean±s.e.mean for n=4 – 6 experiments. Student's t-test for paired values did not reveal any significant differences.
Figure 6.
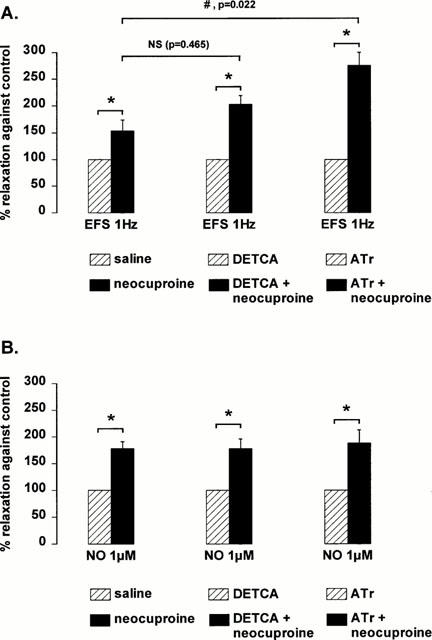
Effect of 10 μM neocuproine on the relaxations induced by (A) 1 Hz electrical field stimulation (EFS) and (B) 1 μM NO (added as acidified NaNO2) in control conditions and after treatment of the muscle strips with DETCA (0.5 mM) or 3-amino-1,2,4-triazole (Atr, 1 mM). Results are expressed as percentage enhancement induced by neocuproine of the control relaxation, which was taken as 100%, in respective conditions (saline, DETCA or 3-amino-1,2,4-triazole). Results are shown as mean±s.e.mean for n=8 – 12 experiments. *P<0.05 is considered as significantly different from respective controls (paired Student's t-test) and #P<0.05 is considered as significantly different from the effect of neocuproine in untreated (saline) muscle strips (one way ANOVA followed by post hoc Dunnett's test, NS=not significant).
These results suggest that neocuproine may have an antioxidative effect which is revealed only after inhibition of endogenous antioxidant enzymes. To investigate further whether endogenous antioxidant enzymes effectively protect nitrergic responses in the mouse gastric fundus, muscle strips were treated with the oxidants hydrogen peroxide and duroquinone. The relaxations to nitrergic nerve stimulation were not affected by hydrogen peroxide (70 μM) or duroquinone (10 μM) whereas relaxations to NO, added as acidified NaNO2, were significantly inhibited (Figure 7) After treatment of the muscle strips with 3-amino-1,2,4-triazole (1 mM) or DETCA (0.5 mM), hydrogen peroxide and duroquinone significantly inhibited the relaxations to nitrergic nerve stimulation. The inhibitory effect of duroquinone on relaxations to NO was further potentiated after treatment with DETCA (0.5 mM). However, the inhibitory effect of hydrogen peroxide on relaxations to NO was not different before and after treatment with 3-amino-1,2,4-triazole (1 mM) (Figure 7).
Figure 7.
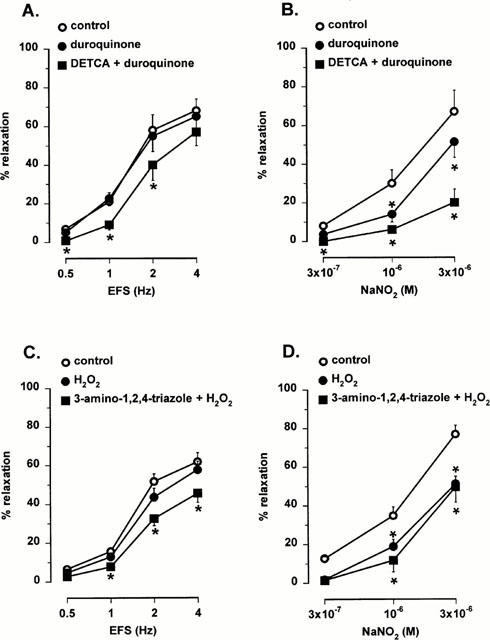
Effect of duroquinone (10 μM, A,B) and hydrogen peroxide (H2O2, 70 μM, C,D) on the relaxations to electrical field stimulation (EFS, 0.5 – 4 Hz) and NO (0.3 – 3 μM, added as acidified NaNO2) before and after treatment of the muscle strips with DETCA (A,B) or 3-amino-1,2,4 triazole (C,D). Results are expressed as percentage decrease of the prostaglandin F2α -induced contraction and shown as mean±s.e.mean for n=4 – 5 experiments. *P<0.05 is considered as significantly different from control, one way ANOVA followed by post hoc Dunnett's test.
Discussion
In this study we investigated the effect and mechanism of action of the copper(I)-selective chelator neocuproine on the nitrergic neurotransmitter in the mouse gastric fundus. Our results indicate that neocuproine protects the biological activity of the nitrergic neurotransmitter by acting as an antioxidant both at a prejunctional and postjunctional level.
In the mouse gastric fundus, short and prolonged periods of electrical field stimulation induced transient and sustained relaxations respectively. L-NOARG, a specific inhibitor of nitric oxide synthase, completely blocked these relaxations, an effect that was readily reversed by L-arginine. These results indicate that in this tissue, the NANC relaxations to both short and prolonged periods of electrical field stimulation are completely nitrergic in nature. This is in contrast to the gastric fundus of a number of other animals in which NANC relaxations are not solely mediated by NO but also by VIP, ATP and possibly other mediators (De Beurme & Lefebvre, 1987; Li & Rand, 1990; Boeckxstaens et al., 1992; Dick et al., 2000; Jenkinson & Reid, 2000). Since such additionally released NANC mediators may complicate the study of nitrergic neuronal events, we propose the mouse gastric fundus as a suitable model to study the mechanisms of nitrergic neurotransmission.
Neocuproine enhanced the nitrergic relaxations to NANC nerve stimulation of the mouse gastric fundus and enhanced the relaxations to NO, added as acidified NaNO2. We investigated whether this enhancement resulted from an antioxidative action of neocuproine. Such an action may be related to the divalent nature of the copper ion: free radicals that are generated by a copper(I)/copper(II) based redox cycle may destroy NO. In the vasculature, Emsley et al. (1999) recently demonstrated that copper ions catalyse the generation of oxidants resulting in a decreased activity of the endothelium derived relaxing factor. Neocuproine chelates Cu(I) ions and may therefore protect the nitrergic neurotransmitter from oxidative breakdown. To study an antioxidant effect of neocuproine, we first compared the effect of neocuproine with that of the antioxidants superoxide dismutase (SOD), ascorbic acid and D-mannitol. However, these antioxidants did not mimic the effect of neocuproine. Possibly, the endogenous concentration of tissue antioxidants is sufficient for an optimal protection of the nitrergic neurotransmitter. Antioxidants such as SOD may protect the tissue against oxidative attack but such a protective mechanism was revealed only after blockade of endogenous SOD with DETCA (Hiraishi et al., 1994; Martin et al., 1994). We therefore investigated whether inhibition of tissue antioxidant enzymes would affect the ability of neocuproine to enhance relaxations to endogenous and exogenous NO. After inhibition of catalase with 3-amino-1,2,4-triazole, neocuproine induced a more pronounced potentiation of the relaxations to nitrergic nerve stimulation whereas the potentiation of the relaxations to NO, added as acidified NaNO2, was similar to untreated control strips. After inhibition of Cu/Zn SOD with DETCA, the neocuproine-induced enhancement of relaxations to nitrergic nerve stimulation and NO was not significantly different from untreated control strips. The additional potentiating effect of neocuproine on endogenous but not exogenous NO, that was observed only after inhibition of catalase, indicates that neocuproine may have a dual action. The first action is sensitive to inhibition of catalase and results in an enhancement of relaxations to the nitrergic neurotransmitter but not to an enhancement of the direct smooth muscle response to exogenous NO. This suggests that this effect of neocuproine is prejunctional at the neuron and not at the smooth muscle. The second action of neocuproine is insensitive to inhibition of antioxidant enzymes and results in an enhancement of relaxations to both the nitrergic neurotransmitter and exogenous NO suggesting that this second effect of neocuproine is postjunctional at the smooth muscle.
We investigated further the interaction of neocuproine with exogenously added NO and studied the effect of neocuproine on relaxations to the NO donors SIN-1 and hydroxylamine. SIN-1 releases NO extracellularly (Feelisch & Noack, 1987) whereas hydroxylamine, which is a precursor of NO in biological systems, releases NO intracellularly (DeMaster et al., 1989). Neocuproine enhanced the relaxations to SIN-1 but did not affect the relaxations to hydroxylamine. This suggests that the interaction of neocuproine with exogenously added NO is at an extracellular site. Since physiological solutions can contain trace amounts of copper, neocuproine may prevent an interaction of exogenous NO with copper in solution. However, copper in solution is present as copper(II) and in our study we found that the copper(II) chelator cuprizone did not mimic the effect of neocuproine. In addition, we previously observed that EDTA, which scavenges copper(II) ions, did not affect the relaxations to NO in the rat gastric fundus (De Man et al., 1998). Although copper may also react directly with NO to form nitrosyls, such reactions are reported to be very slow and not significant for cellular communication (Wink et al., 2000). These results therefore indicate that the tissue itself – and not the Krebs solution – is the primary source of copper(I). Possibly, neocuproine may prevent an indirect reaction of exogenous NO with copper: tissue antioxidants may reduce copper(II) to copper(I) and the radicals generated by this redox cycle may inhibit the activity of NO.
To investigate further the effect of antioxidants and radicals on endogenous and exogenous NO, tissues were treated with the radical generators hydrogen peroxide and duroquinone. These compounds did not affect the relaxations to nitrergic nerve stimulation whereas relaxations to NO, added as acidified NaNO2, were significantly inhibited. However, after treatment of the muscle strips with the catalase inhibitor 3-amino-1,2,4-triazole, hydrogen peroxide also inhibited the relaxations to nitrergic nerve stimulation. Similarly, after treatment of the muscle strips with the Cu/ZnSOD inhibitor DETCA, duroquinone inhibited the nitrergic relaxations to electrical stimulation. The inhibitory effect of hydrogen peroxide on relaxations to NO which acts directly on the smooth muscle, was not altered after inhibition of catalase. On the other hand, the inhibitory effect of duroquinone on relaxations to NO was potentiated after inhibition of Cu/Zn SOD. These results further indicate that catalase may act mainly at a prejunctional neuronal site whereas Cu/Zn SOD may act mainly at a postjunctional smooth muscle site. These results also indicate that tissue antioxidants protect the nitrergic neurotransmitter in the mouse gastric fundus from oxidative breakdown, confirming previous findings in smooth muscle from the anoccocygeus (Martin et al., 1994; Lilley & Gibson, 1996; Liu et al., 1997; La & Rand, 1999), retractor penis (Paisley & Martin, 1996), gastric region (Hiraishi et al., 1994; De Man et al., 1996; Lefebvre, 1996), small intestine (Correia et al., 1999) and urether (Garcia-Pascual et al., 2000).
Apart from catalase and SOD, additional antioxidants such as ascorbate (Lilley & Gibson, 1997) and bilirubin (Colpaert & Lefebvre, 2000) may protect the nitrergic neurotransmitter but it remains unclear whether thiols are also involved in this protective mechanism. Thiols are the most abundantly present antioxidants in cells and they have the capacity to bind NO and to form S-nitrosothiols. S-nitrosothiols may function as NO carriers that protect NO against oxidative attack. In our study we found that neocuproine inhibited the relaxations to S-nitrosothiols. Since Cu(I) ions catalyse the release of NO from S-nitrosothiols (Gordge et al., 1995; Dicks et al., 1996; Williams, 1996), chelation of Cu(I) ions by neocuproine inhibits the release of NO from S-nitrosothiols resulting in a diminished relaxation to S-nitrosothiols. If S-nitrosothiols do mediate NANC relaxations in the mouse gastric fundus, neocuproine should affect NANC relaxations similar as relaxations to S-nitrosothiols. However, as discussed above, neocuproine enhanced, but did not inhibit, the nitrergic relaxations to NANC nerve stimulation. This differential effect of neocuproine suggests that S-nitrosothiols do not function as NO carries in the nitrergic neurotransmission of the mouse gastric fundus.
In conclusion, the copper(I)-specific chelator neocuproine potentiates relaxations to the nitrergic neurotransmitter and to exogenous NO by acting at two sites. Neocuproine acts on the nitrergic neurotransmitter by a mechanism that is sensitive to inhibition of catalase. The site of action is most likely prejunctional. In addition to this prejunctional action, neocuproine acts at a postjunctional level on responses to exogenous NO at a site that is insensitive to inhibition of catalase or Cu/ZnSOD. Our results substantiate the hypothesis that antioxidants play an important role in protecting the biological activity of the nitrergic neurotransmitter in the enteric nervous system. On the other hand, our results argue against S-nitrosothiols as intermediates in such protective mechanisms. Finally, our results demonstrate that neoucuproine is a useful tool to elucidate the nature of the nitrergic neurotransmitter in the enteric nervous system.
Acknowledgments
This work was supported by the Fund for Scientific Research Flanders (Grant no. G. 0220.96) and by the Interuniversity Pole of Attraction Programme (Grant no. P4/16, Services of the Prime Minister - Federal Services for scientific, technical and cultural Affairs).
Abbreviations
- DETCA
diethyl dithiocarbamic acid
- EFS
electrical field stimulation
- L-NOARG
L-nitro arginine
- NANC
non-adrenergic non-cholinergic
- NO
nitric oxide
- SIN-1
3-morpholinosydnonimine
- SOD
superoxide dismutase
References
- AL-SA'DONI H.H., MEGSON I.L., BISLAND S., BUTLER A.R., FLITNEY F.W. Neocuproine, a selective Cu(I) chelator, and the relaxation of rat vascular smooth muscle by S-nitrosothiols. Br. J. Pharmacol. 1997;121:1047–1050. doi: 10.1038/sj.bjp.0701218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., PELCKMANS P.A., BOGERS J.J., BULT H., DE MAN J.G., OOSTERBOSCH L., HERMAN A.G., VAN MAERCKE Y.M. Release of nitric oxide upon stimulation of nonadrenergic noncholinergic nerves in the rat gastric fundus. J. Pharmacol. Exp. Ther. 1991;256:441–447. [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., PELCKMANS P.A., BULT H., DE MAN J.G., HERMAN A.G., VAN MAERCKE Y.M. Non-adrenergic non-cholinergic relaxation mediated by nitric oxide in the canine ileocolonic junction. Eur. J. Pharmacol. 1990;190:239–246. doi: 10.1016/0014-2999(90)94132-h. [DOI] [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., PELCKMANS P.A., DE MAN J.G., BULT H., HERMAN A.G., VAN MAERCKE Y.M. Evidence for a differential release of nitric oxide and vasoactive intestinal polypeptide by nonadrenergic noncholinergic nerves in the rat gastric fundus. Arch. Int. Pharmacodyn. Ther. 1992;318:107–115. [PubMed] [Google Scholar]
- COLPAERT E.E., LEFEBVRE R.A. Influence of bilirubin and other antioxidants on nitrergic relaxation in the pig gastric fundus. Br. J. Pharmacol. 2000;129:1201–1211. doi: 10.1038/sj.bjp.0703176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORREIA N.A., CAVALCANTI P.M., OLIVEIRA R.B., BALLEJO G. Effect of hydroquinone, hydroxocobalamin and carboxy-PTIO on non-adrenergic non-cholinergic nerve mediated relaxations of the rat duodenum. J. Auton. Pharmacol. 1999;19:233–240. doi: 10.1046/j.1365-2680.1999.00139.x. [DOI] [PubMed] [Google Scholar]
- DE BEURME F.A., LEFEBVRE R.A. Influence of alpha-chymotrypsin and trypsin on the non-adrenergic non-cholinergic relaxation in the rat gastric fundus. Br. J. Pharmacol. 1987;91:171–177. doi: 10.1111/j.1476-5381.1987.tb08996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MAN J.G., DE WINTER B.Y., BOECKXSTAENS G.E., HERMAN A.G., PELCKMANS P.A. Effect of thiol modulators and Cu/Zn superoxide dismutase inhibition on nitrergic relaxations in the rat gastric fundus. Br. J. Pharmacol. 1996;119:1022–1028. doi: 10.1111/j.1476-5381.1996.tb15773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MAN J.G., DE WINTER B.Y., MOREELS T.G., HERMAN A.G., PELCKMANS P.A. S-nitrosothiols and the nitrergic neurotransmitter in the rat gastric fundus: effect of antioxidants and metal chelation. Br. J. Pharmacol. 1998;123:1039–1046. doi: 10.1038/sj.bjp.0701692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MAN J.G., MOREELS T.G., DE WINTER B.Y., HERMAN A.G., PELCKMANS P.A. Neocuproine potentiates the activity of the nitrergic neurotransmitter but inhibits that of S-nitrosothiols. Eur. J. Pharmacol. 1999;381:151–159. doi: 10.1016/s0014-2999(99)00564-6. [DOI] [PubMed] [Google Scholar]
- DEMASTER E.G., RAIJ L., ARCHER S.L., WEIR E.K. Hydroxylamine is a vasorelaxant and a possible intermediate in the oxidative conversion of L-arginine to nitric oxide. Biochem. Biophys. Res. Commun. 1989;163:527–533. doi: 10.1016/0006-291x(89)92169-4. [DOI] [PubMed] [Google Scholar]
- DICK J.M.C., VAN GELDRE L.A., TIMMERMANS J.P., LEFEBVRE R.A. Investigation of the interaction between nitric oxide and vasoactive intestinal polypeptide in the guinea-pig gastric fundus. Br. J. Pharmacol. 2000;129:751–763. doi: 10.1038/sj.bjp.0703089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKS A.P., SWIFT H.R., WILLIAMS D.L.H., BUTLER A.R., AL-SA'DONI H.H., COX B.G. Identification of Cu+ as the effective reagent in nitric oxide formation from S-nitrosothiols (RSNO) J. Chem. Soc., Perkin Trans. 1996;2:481–487. [Google Scholar]
- EMSLEY A.M., JEREMY J.Y., GOMES G.N., ANGELINI G.D., PLANE F. Investigation of the inhibitory effects of homocysteine and copper on nitric oxide-mediated relaxation of rat isolated aorta. Br. J. Pharmacol. 1999;126:1034–1040. doi: 10.1038/sj.bjp.0702374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEELISCH M., NOACK E. Nitric oxide (NO) formation from nitrovasodilators occurs independently of hemoglobin or non-heme iron. Eur. J. Pharmacol. 1987;142:465–469. doi: 10.1016/0014-2999(87)90090-2. [DOI] [PubMed] [Google Scholar]
- GARCIA-PASCUAL A., LABADIA A., COSTA G., TRIGUERO D. Effects of superoxide anion generators and thiol modulators on nitrergic transmission and relaxation to exogenous nitric oxide in the sheep urethra. Br. J. Pharmacol. 2000;129:53–62. doi: 10.1038/sj.bjp.0703000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASTON B., FRY E., SEARS S., HEROMAN W.M., IGNARRO L., STAMLER J.S. Umbilical arterial S-nitrosothiols in stressed newborns: role in perinatal circulatory transition. Biochem. Biophys. Res. Commun. 1998a;253:899–901. doi: 10.1006/bbrc.1998.9865. [DOI] [PubMed] [Google Scholar]
- GASTON B., SEARS S., WOODS J., HUNT J., PONAMAN M., MCMAHON T., STAMLER J.S. Bronchodilator S-nitrosothiol deficiency in asthmatic respiratory failure. Lancet. 1998b;351:1317–1319. doi: 10.1016/S0140-6736(97)07485-0. [DOI] [PubMed] [Google Scholar]
- GIBSON A., BRAVE S.R., MCFADZEAN I., TUCKER J.F., WAYMAN C. The nitrergic transmitter of the anococcygeus: NO or not. Arch. Int. Pharmacodyn. Ther. 1995;329:39–51. [PubMed] [Google Scholar]
- GOCMEN C., SECILMIS A., UCAR P., KARATAS Y., ONDER S., DIKMEN A., BAYSAL F. A possible role of S-nitrosothiols at the nitrergic relaxations in the mouse corpus cavernosum. Eur. J. Pharmacol. 1998;361:85–92. doi: 10.1016/s0014-2999(98)00703-1. [DOI] [PubMed] [Google Scholar]
- GORDGE M.P., ADDIS P., NORONHA-DUTRA A.A., HOTHERSALL J.S. Cell-mediated biotransformation of S-nitrosoglutathione. Biochem. Pharmacol. 1998;55:657–665. doi: 10.1016/s0006-2952(97)00498-x. [DOI] [PubMed] [Google Scholar]
- GORDGE M.P., MEYER D.J., HOTHERSALL J., NEILD G.H., PAYNE N.N., NORONHA-DUTRA A. Copper chelation-induced reduction of the biological activity of S-nitrosothiols. Br. J. Pharmacol. 1995;114:1083–1089. doi: 10.1111/j.1476-5381.1995.tb13317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAISHI H., TERANO A., SUGIMOTO T., HARADA T., RAZANDI M., IVEY K.J. Protective role of intracellular superoxide dismutase against extracellular oxidants in cultured rat gastric cells. J. Clin. Invest. 1994;93:331–338. doi: 10.1172/JCI116964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON K.M., REID J.J. Evidence that adenosine 5′-triphosphate is the third inhibitory non-adrenergic non-cholinergic neurotransmitter in the rat gastric fundus. Br. J. Pharmacol. 2000;130:1627–1631. doi: 10.1038/sj.bjp.0703481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOURD'HEUIL D., HALLEN K., FEELISCH M., GRISHAM M.B. Dynamic state of S-nitrosothiols in human plasma and whole blood. Free Radic. Biol. Med. 2000;28:409–417. doi: 10.1016/s0891-5849(99)00257-9. [DOI] [PubMed] [Google Scholar]
- LA M., RAND M.J. Effects of pyrogallol, hydroquinone and duroquinone on responses to nitrergic nerve stimulation and NO in the rat anococcygeus muscle. Br. J. Pharmacol. 1999;126:342–348. doi: 10.1038/sj.bjp.0702277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEBVRE R.A. Influence of superoxide dismutase inhibition on the discrimination between NO and the nitrergic neurotransmitter in the rat gastric fundus. Br. J. Pharmacol. 1996;118:2171–2177. doi: 10.1111/j.1476-5381.1996.tb15659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur. J. Pharmacol. 1990;191:303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Antioxidant protection of NO-induced relaxations of the mouse anococcygeus against inhibition by superoxide anions, hydroquinone and carboxy-PTIO. Br. J. Pharmacol. 1996;119:432–438. doi: 10.1111/j.1476-5381.1996.tb16004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Release of the antioxidants ascorbate and urate from a nitrergically-innervated smooth muscle. Br. J. Pharmacol. 1997;122:1746–1752. doi: 10.1038/sj.bjp.0701571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Z.G., RUDD M.A., FREEDMAN J.E., LOSCALZO J. S-transnitrosation reactions are involved in the metabolic fate and biological actions of nitric oxide. J. Pharmacol. Exp. Ther. 1998;284:526–534. [PubMed] [Google Scholar]
- LIU X., MILLER S.M., SZURSZEWSKI J.H. Protection of nitrergic neurotransmission by and colocalization of neural nitric oxide synthase with copper zinc superoxide dismutase. J. Auton. Nerv. Syst. 1997;62:126–133. doi: 10.1016/s0165-1838(96)00113-0. [DOI] [PubMed] [Google Scholar]
- MARTIN W.The role of nitric oxide in the peripheral nervous system Nitric Oxide 2000Berlin-Heidelberg: Springer-Verlag; 277–313.ed. Mayer B. pp [Google Scholar]
- MARTIN W., MCALLISTER K.H.M., PAISLEY K. NANC neurotransmission in the bovine retractor penis muscle is blocked by superoxide anion following inhibition of superoxide dismutase with diethyldithiocarbamate. Neuropharmacology. 1994;33:1293–1301. doi: 10.1016/0028-3908(94)90029-9. [DOI] [PubMed] [Google Scholar]
- MIAN K.B., MARTIN W. Hydrogen peroxide-induced impairment of reactivity in rat isolated aorta: potentiation by 3-amino-1,2,4-treatment. Br. J. Pharmacol. 1997;121:813–819. doi: 10.1038/sj.bjp.0701187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAISLEY K., MARTIN W. Blockade of nitrergic transmission by hydroquinone, hydroxocobalamin and carboxy-PTIO in bovine retractor penis: role of superoxide anion. Br. J. Pharmacol. 1996;117:1633–1638. doi: 10.1111/j.1476-5381.1996.tb15333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELCKMANS P.A., BOECKXSTAENS G.E., VAN MAERCKE Y.M., HERMAN A.G., VERBEUREN T.J. Acetylcholine is an indirect inhibitory transmitter in the canine ileocolonic junction. Eur. J. Pharmacol. 1989;170:235–242. doi: 10.1016/0014-2999(89)90544-x. [DOI] [PubMed] [Google Scholar]
- RAND M.J., LI C.G. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Annu. Rev. Physiol. 1995;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- STAMLER J.S., JARAKI O., OSBORNE J., SIMON D.I., KEANEY J., VITA J., SINGEL D., VALERI C.R., LOSCALZO J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS D.L.S. Nitrosothiols and role of metal ions in decomposition to nitric oxide. Methods Enzymol. 1996;268:299–308. doi: 10.1016/s0076-6879(96)68032-x. [DOI] [PubMed] [Google Scholar]
- WINK D.A., MIRANDA K.M., ESPEY M.G., MITCHELL J.B., GRISHAM M.B., FUKUTO J., FEELISCH M.The chemical biology of nitric oxide. Balancing nitric oxide with oxidative and nitrosative stress Nitric Oxide 2000Berlin-Heidelberg: Springer-Verlag; 7–29.ed. Mayer B. pp [Google Scholar]


