Abstract
Splanchnic artery occlusion shock (SAO) causes an enhanced formation of reactive oxygen species (ROS), which contribute to the pathophysiology of shock. Here we have investigated the effects of M40401, a new S,S-dimethyl substituted biscyclohexylpyridine Mn-based superoxide dismutase mimetic (SODm, kcat=1.2×10+9 M−1 s−1 at pH=7.4), in rats subjected to SAO shock.
Treatment of rats with M40401 (applied at 0.25, 2.5 or 25 μg kg−1, 15 min prior to reperfusion), attenuated the mean arterial blood and the migration of polymorphonuclear cells (PMNs) caused by SAO-shock. M40401 also attenuated the ileum injury (histology) as well as the increase in the tissue levels of myeloperoxidase (MPO) and malondialdehyde (MDA) caused by SAO shock in the ileum.
Immunohistochemical analysis for nitrotyrosine revealed a positive staining in ileum from SAO-shocked rats. The degree of staining for nitrotyrosine was markedly reduced in tissue sections obtained from SAO-shocked rats which had received M40401. Reperfused ileum tissue sections from SAO-shocked rats showed positive staining for P-selectin and for anti-intercellular adhesion molecule (ICAM-1) in the vascular endothelial cells. M40401 treatment markedly reduced the intensity and degree of P-selectin and ICAM-1 in tissue sections from SAO-shocked rats. M40401 treatment significantly improved survival.
Additionally, the very high catalytic activity of this new mimetic (comparable to the native human Cu/Zn SOD enzyme and exceeding the activity of the human Mn SOD enzyme) translates into a very low dose (∼μg kg−1) required to afford protection in this SAO model of ischemia reperfusion injury.
Taken together, our results clearly demonstrate that M40401 treatment exerts a protective effect, and part of this effect may be due to inhibition of the expression of adhesion molecules and peroxynitrite-related pathways with subsequent reduction of neutrophil-mediated cellular injury.
Keywords: Nitric oxide, peroxynitrite, poly (ADP ribose) synthetase, reperfusion, shock, superoxide dismutase mimetic, M40401
Introduction
Occlusion of the splanchnic circulation followed by reperfusion (SAO) results in a severe form of circulatory shock, characterized by severe hypotension, hemoconcentration, intestinal injury and a high mortality rate (Lefer & Lefer, 1993; Zimmermann et al., 1993). An important component of SAO shock is endothelial dysfunction (Lefer & Lefer, 1993; Altura et al., 1985; Carey et al., 1992; Zingarelli et al., 1992) originally attributed to oxygen-derived free radicals released from both the reperfused endothelium (Lefer & Lefer, 1993; Ratych et al., 1987) and from activated adherent PMNs (Granger et al., 1981; McCord, 1981; Mullane et al., 1988; Bittermann et al., 1988). Endothelial dysfunction predisposes to vasospasm, platelet deposition, and increased neutrophil adherence, which exacerbates the shock state. Ischemia-reperfusion is a stimulus for leukocyte-endothelial interaction (Granger, 1977). Leukocyte-endothelial interaction involves a complex system of adhesion molecules including the selectins, β2 integrins and the immunoglobulin superfamily (Butcher, 1993; Geng et al., 1990; von Andrian et al., 1991). Leukocyte interaction with the endothelium begins with leukocyte rolling, followed by adherence and transendothelial migration. P-selectin, a member of the selectin family of adhesion molecules, is believed to play a major role in the initial phase of leukocyte emigration, which is characterized by the rolling of leukocytes along the vascular endothelial surface. Although P-selectin is necessary for early neutrophil contact with the endothelium, P-selectin-mediated leukocyte-endothelial interaction is not sufficient to allow neutrophil emigration from the vessel. A firmer adherence of the neutrophil to the endothelial surface is required for transendothelial migration (Butcher, 1992). This firm adherence involves the interaction of β2 integrins (i.e. CD11/CD18) on the PMN surface and intercellular adhesion molecule 1 (ICAM-1) on the endothelial cell surface (Butcher, 1992; Lawrence & Springer, 1991). Experimental studies have also showed that in vivo administration of antibodies raised against ICAM-1 reduced neutrophil infiltration into the inflamed lungs in the rabbit and protects the development of SAO-induced injury. Neutrophil activation at sites of injury results in a large production of superoxide anions (.O2−) which in turn contributes to tissue damage seen post reperfusion in several ischemic organs including kidney (Morpurgo et al., 1996); stomach (Yoshikawa et al., 1990), intestine (Zimmermann et al., 1993), skin (Goossens et al., 1990) and heart (Ambrosio & Flaherty, 1992; Grill et al., 1992). Some important tissue damaging and pro-inflammatory roles attributed to .O2− include: endothelial cell damage and increased microvascular permeability (Droy-Lefaix et al., 1991; Haglind et al., 1994; Xia et al., 1995), formation of chemotactic factors such as leukotrienes B4 (Fantone & Ward 1982; Deitch et al., 1990), recruitment of neutrophils at sites of inflammation (Boughton-Smith et al., 1993; Salvemini et al., 1996a; 1999a,1999b), lipid peroxidation and oxidation and DNA single-strand damage (Dix et al., 1996). In addition, .O2− by interacting with NO destroys the biological activity of this mediator attenuating important anti-inflammatory and tissue protective properties of NO namely: maintenance of blood vessel tone and platelet reactivity, cytoprotective effect in numerous organs (including heart, intestine and kidney), and release of anti-inflammatory and cytoprotective prostacyclin (via activation of the constitutive cyclo-oxygenase enzyme (Salvemini et al., 1993; 1996b). The product formed as a result of .O2− interacting with NO is peroxynitrite (ONOO-), a well described, potent cytotoxic and pro-inflammatory molecule (Salvemini et al., 1998; 1999a,1999b; Beckman et al., 1990; Ischiropoulos et al., 1992; Beckman & Crow 1993; Crow & Beckman, 1995; Misko et al., 1998). Therefore, removal of superoxide protects NO and reduces formation of the cytotoxic ONOO-.
We have recently shown that selective removal of superoxide by M40403 exerts beneficial effects in a model of SAO (Salvemini et al., 1999a,1999b). These new low molecular weight, synthetic manganese containing superoxide dismutase mimetic (SODm) represent a breakthrough in chemical design in that they are stable in vivo, possess high activity, and are selective for superoxide with no activity toward hydrogen peroxide (H2O2), ONOO-, NO, or hypochlorite (OCl−). This novel selectivity resides in the nature of the manganese(II) center in these low molecular weight complexes. The resting oxidation state of the complex is the reduced state, Mn(II). As a consequence, the complex has no reactivity with reducing agents until it is oxidized to Mn(III) by superoxide. Since the complex is so difficult to oxidize (+0.75 v (SHE)) many oxidants will not oxidize these complexes, including nitric oxide and oxygen, and since they operate via a facile one-electron oxidation pathway other two-electron non-radical oxidants are also not able to oxidize the Mn(II) complex; e.g., OONO−, H2O2, OCl−. The unique selectivity of these complexes for superoxide in the presence of other ROS make it possible then to dissect the role of superoxide in disease models in which ROS are implicated. We have continued our computer-aided design and synthesis program so that we have recently developed M40401 (Figure 1, the S,S-dimethyl substituted derivative of the M40403 biscyclohexylpyridyl class of mimetic) which possesses a much higher catalytic activity at pH=7.4. In fact, its catalytic rate exceeds 1×10+9 M−1 s−1, comparable to the native Cu/Zn SOD enzymes and about 100 times the activity of M40403 at pH=7.4. As with M40403, M40401 has no catalase activity or reactivity with peroxynitrite.
Figure 1.
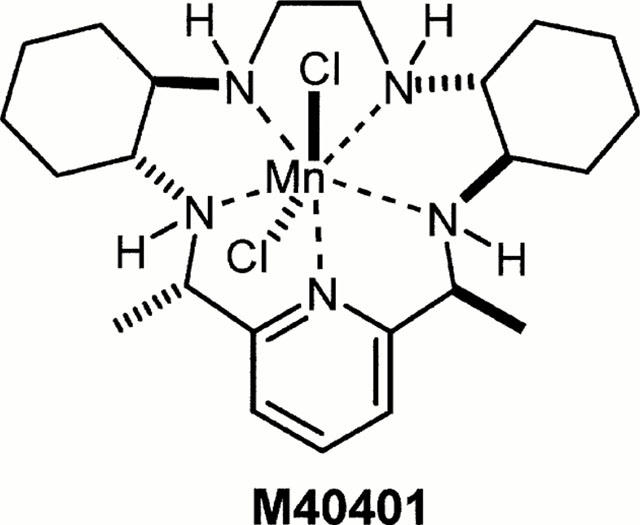
Structure of M40401.
The aim of the study reported here was to ascertain if such a highly superoxide specific catalyst with enhanced activity would be useful as a human therapeutic agent for reperfusion injury by assessing its activity in pharmacologically relevant animal models of reperfusion injury and to compare its in vivo activity to that of an SOD mimetic with similar stability and lipophilicity, but with the 100 fold less catalytic activity; i.e. to the precursor mimetic M40403. Such a study should enable us to determine the relative importance and role of superoxide-mediated injury in reperfusion in these models.
Methods
Animals
Male Sprague-Dawley rats (250 – 300 g; Charles River; Milan; Italy) were housed in a controlled environment and provided with standard rodent chow and water. Animal care was in compliance with Italian regulations on protection of animals used for experimental and other scientific purposes (D.M. 116192), as well as with the EEC regulations (O.J. of E.C. L358/1 12/18/1986).
Surgical procedures
Male Sprague-Dawley rats weighing 250 – 300 g were allowed access to food and water ad libitum. The rats were anaesthetized with sodium pentobarbital (45 mg kg−1 i.p.). Following anaesthesia, catheters were placed in the carotid artery and jugular vein as described previously (Caputi et al., 1980). Blood pressure was monitored continuously by a Maclab A/D converter (Ugo Basile, Varese, Italy), and stored and displayed on a Macintosh personal computer. After midline laparotomy, the celiac and superior mesenteric arteries were isolated near their aortic origins. During this procedure, the intestinal tract was maintained at 37°C by placing it between gauze pads soaked with warmed 0.9% NaCl solution. Rats were observed for a 30 min stabilization period before either splanchnic ischemia or sham ischemia. SAO shock was induced by clamping both the superior mesenteric artery and the celiac trunk, resulting in a total occlusion of these arteries for 45 min. After this period of occlusion, the clamps were removed. In one study, the various groups of rats were sacrificed at 60 min for histological examination of the bowel and for biochemical studies, as described below.
Experimental groups
In the treated groups of animals (n=10), SOD mimetic (M40401) or corresponding volume of vehicle (26 mM sodium bicarbonate buffer, pH 8.1 – 8.3) was given as an intravenous bolus 0.25, 2.5 or 25 μg kg−1, 15 min prior to reperfusion (SAO+M40401 groups). In separate groups of rats, surgery was performed identically to the SAO group, except that the blood vessels were not occluded (time-controlled sham group; Sham).
Measurement of nitrite/nitrate (NOx) in plasma
Nitrate/nitrate (NOx) levels, an indicator of NO synthesis, was measured in plasma samples from sham or SAO-shocked rats at 60 min after reperfusion as previously described (Cuzzocrea et al., 1997). First, nitrate in the plasma was reduced to nitrite by incubation with nitrate reductase (670 mu ml−1 and NADPH (160 μM) at room temperature for 3 h. After 3 h, nitrite concentration in the samples was measured by the Griess reaction, by adding 100 μl of Griess reagent (0.1% naphthalethylenediamine dihydrochloride in H2O and 1% sulphanilamide in 5% concentrated H3PO4; vol. 1 : 1) to 100 μl samples. The optical density at 550 nm (OD550) was measured using a Spectramax 250 microplate reader (Molecular Devices Sunnyvale, CA, U.S.A). Nitrate concentrations were calculated by comparison with OD550 of standard solutions of sodium nitrate prepared in saline solution.
Measurement of peroxynitrite production
The formation of peroxynitrite was estimated by the peroxynitrite-dependent oxidation of dihydrorhodamine 123 to rhodamine, using a previously described method (Cuzzocrea et al., 1997). In separate groups, animals were injected with dihydrorhodamine 123 (2 μmol kg−1 in 0.3 ml saline i.v.) 40 min after reperfusion. Twenty minutes later, rats were sacrificed and plasma samples taken for rhodamine fluorescence evaluation using a Perkin-Elmer fluorimeter (Model LS50B; Perkin-Elmer, Norwalk, CT, U.S.A.) at an excitation wavelength of 500 nm, emission wavelength of 536 nm (slit widths 2.5 and 3.0 nm, respectively). The rate of rhodamine formation, an index of peroxynitrite production, was calculated using a standard curve obtained with authentic rhodamine (1 – 30 nM) prepared in plasma obtained from untreated rats. Background plasma fluorescence was subtracted from all samples.
Immunofluorescence localization for nitrotyrosine, P-selectin and ICAM-I
Indirect immunofluorescence staining was performed on 7 μm thick sections of unfixed rat ileum. Sections were cut in with a Slee and London cryostat at −30°C, transferred into clean glass slides and dried overnight at RT. Sections were permeabilized with acetone at −20°C for 10 min and rehydrated in PBS (phosphate buffered saline, 150 mM NaCI, 20 mM sodium phosphate pH 7.2) at RT for 45 min. Sections were incubated overnight: (1) with anti-nitrotyrosine rabbit polyclonal antibody (1 : 500 in PBS, v v−1); or (2) with rabbit anti-human polyclonal antibody directed at P-selectin (CD62P) which react with rat and with mouse anti-rat antibody directed at ICAM-1 (CD54) (1 : 500 in PBS, v v−1) (DBA, Milan, Italy). Sections were washed with PBS, and incubated with secondary antibody (TRITC-conjugated anti-rabbit and with FITC-conjugated anti-mouse (Jackson, West Grove, PA, U.S.A.) or with TRITC-conjugated anti-goat antibody (1 : 80 in PBS, v v−1) for 2 h at RT. Sections were washed as before, mounted with 90% glycerol in PBS, and observed with a Nikon RCM8000 confocal microscope equipped with a 40× oil objective.
Myeloperoxidase activity
Myeloperoxidase activity, an index of PMN accumulation, was determined as previously described (Mullane et al., 1988). Intestinal and lung tissues, collected 60 min after reperfusion, were homogenized in a solution containing 0.5% hexa-decyl-trimethyl-ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) and centrifuged for 30 min at 20,000×g at 4°C. An aliquot of the supernatant was then allowed to react with a solution of tetra-methyl-benzidine (1.6 mM) and 0.1 mM H2O2. The rate of exchange in absorbance was measured by a spectrophotometer at 650 nm. Myeloperoxidase activity was defined as the quantity of enzyme degrading 1 μmol of peroxide min−1 at 37°C and was expressed in μ-units per gram weight of wet tissue.
Leukocyte count
Tail vein blood samples for leukocyte count were taken at 60 min after reperfusion. The number of leukocytes (WBC×103 mm3) is shown as mean±s.d.
Malondialdehyde (MDA) measurement
Levels of malondialdehyde (MDA) in the plasma and in the intestinal tissues was determined as an index of lipid peroxidation, as described by Okhawa et al. (1979). Intestinal tissues, collected 60 min after reperfusion, were homogenized in 1.15% KCl solution. An aliquot (100 μl) of the homogenate was added to a reaction mixture containing 200 μl of 8.1% SDS, 1500 μl of 20% acetic acid (pH 3.5), 1500 μl of 0.8% thiobarbituric acid and 700 μl distilled water. Samples were then boiled for 1 h at 95°C and centrifuged at 3000×g for 10 min. The absorbance of the supernatant was measured by spectrophotometry at 650 nm.
Measurement of cytokines
TNFα and IL-1β levels were evaluated in plasma samples at 60 min after reperfusion. The assay was carried out by using a colorimetric commercial kit (Calbiochem-Novabiochem Corporation, U.S.A.).
Light microscopy
For histopathological examination, biopsies of small intestine were taken 60 min after reperfusion. The tissue were fixed in Dietric solution (14.25% ethanol, 1.85% formaldehyde, 1% acetic acid) for 1 week at room-temperature, dehydrated by graded ethanol and embedded in Paraplast (Sherwood Medical, Mahwah, N.J., U.S.A.). From each biopsy, 7 μm thick slices were obtained and stained with trichromic Van Gieson and studied using light microscopy (Dialux 22 Leitz).
Evaluation of survival
The various groups of rats were monitored for 4 h after SAO and reperfusion, and survival rates and survival times were evaluated.
Reagents
Biotin blocking kit, biotin-conjugated goat anti-rabbit IgG and avidin-biotin peroxidase complex were obtained from Vector Laboratories (Burlingame, CA, U.S.A.). Primary anti-nitrotyrosine antibody was purchased from Upstate Biotech (Saranac Lake, NY, U.S.A.). Primary anti-PARP, anti-ICAM-I and anti-P-selectin were purchased from Santa Cruz (DBA, Milan, Italy). Secondary antibody (FITC-conjugated or Tric-conjugated) were obtained from Jackson (West Grove, PA, U.S.A.). Dihydrorhodamine 123 and rhodamine 123 were purchased from Molecular Probes (Eugene, OR, U.S.A.). All the other reagents were obtained from Sigma (Milan, Italy).
The complex M40401 is synthesized in a manner analogous to that previously reported for the SOD mimetic complexes M40403 and M40404 (Salvemini et al., 1998). Unlike M40404, which is inactive as an SOD mimetic and is the R,R-dimethyl substituted derivative of M40403, this new highly active mimetic is the S,S-dimethyl derivative, and its preparation in a stereocontrolled manner is accomplished via reduction using ammonium formate with Pd on charcoal catalyst in place of NaBH4, which was used to generate the R,R-dimethyl derivative, M40404. Complete details of this synthetic procedure and the X-ray crystallographic characterization of this complex is the subject of a separate account to be submitted to Inorganic Chemistry.
Selectivity of M40401
In a previous series of papers (Riley et al., 1997), we have shown that the pentaaza macrocyclic ligand complexes of Mn(II) can not only be highly active catalysts for the dismutation of .O2−, but that they are also highly selective. The complex biscyclohexyl complex, SC-55858, for example, has been shown to catalytically dismute .O2− at a rate exceeding 10+8 molecules of O2−. per molecule of complex per second at pH=7.4 and 21°C, at a rate comparable to the native Mn SOD enzyme. Remarkably, this complex and others of this pentaaza macrocyclic ligand class, such as M40403 or M40401, do not react with hydrogen peroxide under the same conditions (Riley et al., 1997; Salvemini et al., 1999a,1999b), nor do they react with other biologically relevant oxidants such as ONOO- or nitric oxide. Thus, in our assays to assess catalytic catalase activity using oxygen electrodes (Marshall & Worsfold, 1978; Pasternack & Pysnik, 1983) in which total oxygen concentration evolved from the reaction of hydrogen peroxide with catalase (or any putative catalase mimic) is quantitatively monitored, no catalytic activity is observed between SC-55858, M40403, or M40401 and H2O2, and further, no stoichiometric reaction is observed to occur between these complexes and H2O2 as monitored via spectrophotometric or electrochemical (cyclic voltammetric) techniques. The stopped flow assay developed for monitoring peroxynitrite catalytic activity (Stern et al., 1996) was also utilized to assess the PN activity of these agents. No catalytic or stoichiometric reactivity of PN with these complexes is observed. These substrate specificities allow us to probe directly the biological roles that the free radical, .O2−, plays by studying the effects that such selective catalysts exhibit in vivo.
Data analysis
All values in the figures and text are expressed as mean±standard error of the mean of n observations, where n represents the number of animals studied. In the experiments involving histology or immunohistochemistry, the figures shown are representative of at least three experiments performed on different experimental days. Data sets were examined by one- and two-way analysis of variance and individual group means were then compared with Student's unpaired t-test. Non-parametric data were analysed with the Fisher's exact test. A P-value less than 0.05 was considered significant.
Results
Protective effects of superoxide dismutase mimetic in sphlanchnic artery occlusion shock
Occlusion of the splanchnic arteries produced an increase in MAP which then decreased until death (Figure 2). The mean survival time was found to be 69±3.7% min (n=27) whereas control sham animals survived for the entire period of observation (4 h, n=24; Table 1). Having established the survival time, in another series of experiments, animals were sacrificed either after the period of ischemia or 60 min post reperfusion in order to collect blood and tissues for biochemical analysis. Reperfusion of the ischemic splanchnic circulation led to the following events: a substantial increase in plasma and intestinal lipid peroxidation products as determined by increased levels of MDA (Figure 3), TNFα and IL1β (Figure 4) and a profound infiltration of neutrophils into the intestine and lung (Figure 5). These inflammatory events were triggered by the reperfusion phase since no changes were observed when blood or tissues were removed after the period of ischemia alone (data not shown).
Figure 2.
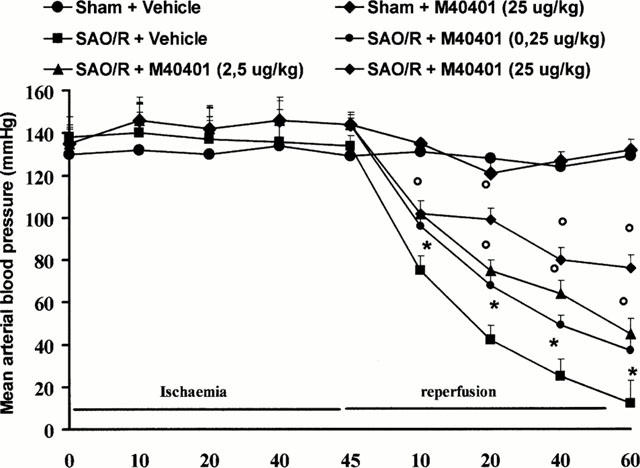
The fall in mean arterial blood pressure (MAP) in SAO rats (n=6) is blocked in a dose dependent manner by M40401. *P<0.05 versus sham, °P<0.05 versus IR.
Table 1.
Effect of vehicle or M40401 on survival rate, percentage survival, and survival time in sham shocked rats or splanchnic artery occlusion (SAO) shocked rats
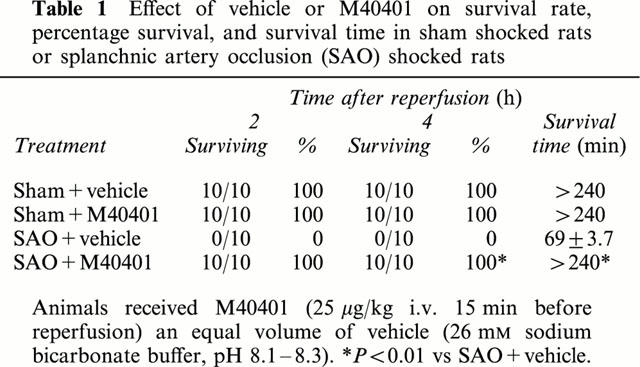
Figure 3.
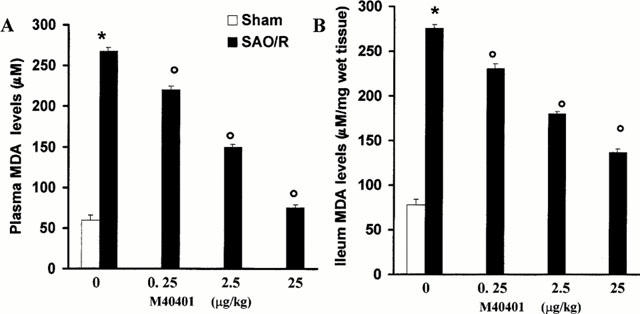
Reperfusion of the ischemic splanchnic circulation leads to profound increase in plasma (A) and ileum (B) levels of MDA and this is inhibited in a dose-dependent manner by M40401. Each point is the mean±s.e.mean for n=10 experiments. *P<0.05 versus sham, °P<0.05 versus IR.
Figure 4.
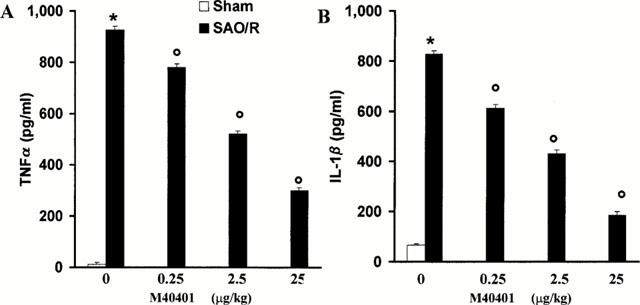
Reperfusion of the ischemic splanchnic circulation leads to profound increase in plasma TNFα (A) and IL1β (B) and this is inhibited in a dose-dependent manner by M40401. Each point is the mean±s.e.mean for n=10 experiments. *P<0.05 versus sham, °P<0.05 versus IR.
Figure 5.
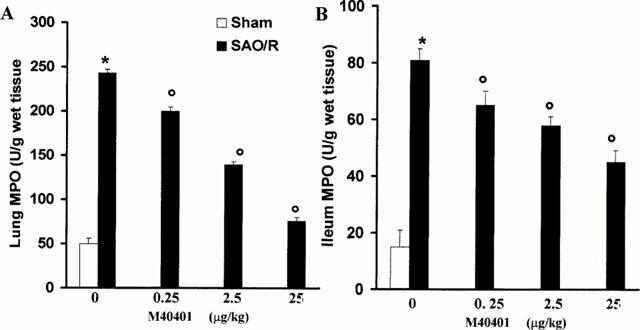
Reperfusion of the ischemic splanchnic circulation leads to the infiltration of neutrophils in the lung (A) and ileum (B). M40401 significantly inhibit in a dose-dependent manner the neutrophils infiltration. Each point is the mean±s.e.mean for n=10 experiments. *P<0.05 versus sham, °P<0.05 versus IR.
The SOD mimetic, M40401, (0.25, 2.5 or 25 μg kg−1, n=10) when given i.v. 15 min prior to reperfusion, inhibited in a dose dependent manner the increased plasma and ileum levels of MDA (Figure 3A,B) as well as TNFα and IL1β (Figure 4). M40401 significantly reduced the neutrophils infiltration into the ileum and in the lung (Figure 5). Also, M40401 (n=10) in a dose dependent manner prevented the fall in blood pressure (Figure 2) seen after reperfusion and increased the survival rate (100% survival at 4 h in M40401-treated rats vs 0% survival at 4 h in non-treated rats; Table 1).
NO and peroxynitrite production in splanchnic artery occlusion shock
There was no change in the plasma levels of nitrate/nitrite at 60 min of the reperfusion period (Figure 6A), in agreement with previous observations where we have demonstrated that the current protocol of ischemia and reperfusion does not trigger the expression of the inducible isoform of NOS (iNOS) (Cuzzocrea et al., 1997). The treatment with M40401 did not affect baseline nitrite/nitrate levels (Figure 6A). In agreement with previous observations (Cuzzocrea et al., 1997), SAO shock caused a significant increase in the rhodamine fluorescence of plasma, indicative of peroxynitrite-induced oxidation of dihydrorhodamine during the reperfusion phase (Figure 6B). In vivo treatment with M40401 reduced in a dose dependent manner the oxidation of dihydrorhodamine 123 during reperfusion (Figure 6B).
Figure 6.
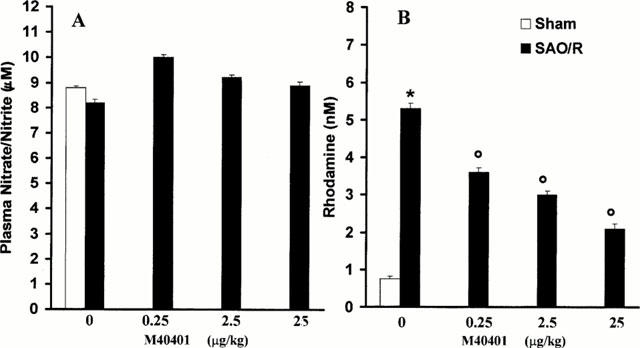
Plasma NOx levels (A); Plasma peroxynitrite production assessed by oxidation of dihydrorhodamine 123 to rhodamine (B). There was no change in the plasma levels of nitrate/nitrite during occlusion or 60 min of reperfusion period. Peroxynitrite production in the SAO-shocked rats were significantly increased versus sham group. M40401 significantly inhibit in a dose-dependent manner the elevation of the plasma peroxynitrite production. Each point is the mean±s.e.mean for n=10 experiments. *P<0.05 versus sham, °P<0.05 versus IR.
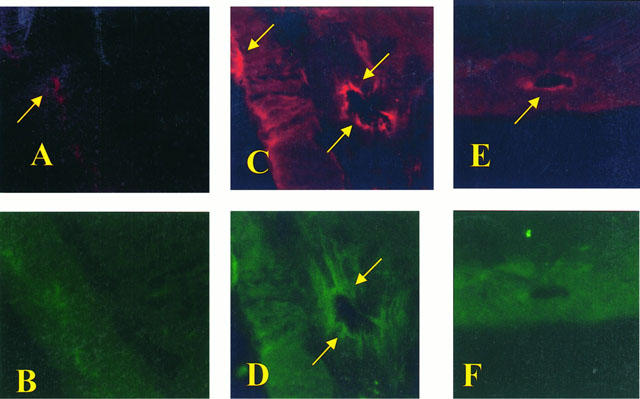
At 60 min after reperfusion, ileum sections were taken from sham or shocked rats in order to determine the immunohistological staining for nitrotyrosine. While there was negligible staining in the intestinal sections of control animals (Figure 7A), immunohistochemical analysis, using a specific anti-nitrotyrosine antibody, revealed a positive staining in the sub-mucosa vessels (Figure 7B, arrows). M40401 (25 μg kg−1) treatment reduced the degree of immunostaining for nitrotyrosine (Figure 7C) in the reperfused intestine. In order to confirm that the immunoreaction for the nitrotyrosine was specific some sections were also incubated with the primary antibody (anti-nitrotyrosine) in the presence of excess nitrotyrosine (10 mM) to verify the binding specificity. In this situation, no positive staining was found in the sections indicating that the immunoreaction was positive in all the experiments carried out.
Figure 7.
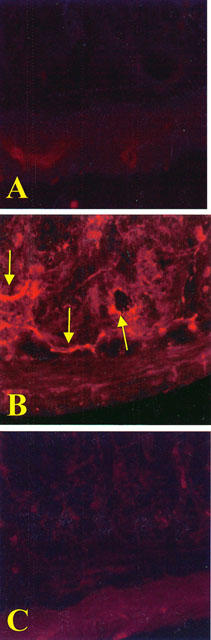
No positive staining for nitrotyrosine (A) was found in the ileum section from sham-administered rats. Sixty minutes after reperfusion immunohistochemical for nitrotyrosine (B) show positive staining localised in the vascular wall and in the inflammatory cells (arrows) in the injured area from a SAO-shocked rats. The intensity of the positive staining for nitrotyrosine (C) was significantly reduced in the ileum from M40401-treated rats. Original magnification: ×145. Figure is representative of at least three experiments performed on different experimental days.
Leukocyte count
The administration of M40401 did not modify the leukocyte count in sham-shocked rats. In contrast, SAO shock produced a marked leukopenia. Our data show that the leukocyte count was markedly decreased at 60 min after reperfusion. The administration of M40401 (25 μg kg−1) significantly ameliorated leukopenia (Table 2).
Table 2.
Effect of vehicle or M40401 on white blood cell count (WBC) of rats subjected to splanchnic artery occlusion (SAO) shock
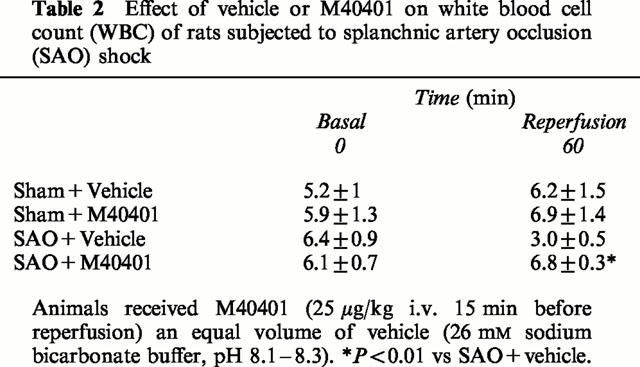
Immunohistochemical localization of ICAM-1 and P-selectin in the reperfused intestine
Staining of ileum tissue sections obtained from sham-operated rats with anti-ICAM-1 antibody showed a specific staining along vessels (arrows), demonstrating that ICAM-1 is constitutively expressed (Figure 8A). After 1 h of reperfusion, the staining intensity substantially increased in the vessels (Figure 8C; see arrows). Sections from M40401-treated rats did not reveal any up-regulation of the constitutive ICAM-1, which was normally expressed in the endothelium along the vascular wall (Figure 8E; see arrows). Ileum tissue sections obtained from SAO-shocked rats undergoing 45 min of ischemia followed by 1 h reperfusion showed positive staining for P-selectin localized in the vessels (Figure 8C; see arrows). No staining was observed in sham-operated rats (Figure 8B). In tissue obtained at 1 h after reperfusion from M40401-treated rats, no positive staining for P-selectin was found (Figure 8F). To verify the binding specificity for ICAM-1 or P-selectin, some sections were also incubated with only the primary antibody (no secondary) or with only the secondary antibody (no primary). In these situations no positive staining was found in the sections, indicating that the immunoreaction was positive in all the experiments carried out.
Figure 8.
Staining of ileum tissue sections obtained from sham-operated rats with anti-ICAM-1 antibody showed a specific staining along vessels (arrow), demonstrating that ICAM-1 is constitutively expressed (A), no P-selectin staining was seen in sham animals (B). Section obtained from SAO shocked-rats showed intense positive staining (see arrows) for ICAM-1 (C) and for P-selectin (D) on vascular wall. The degree of endothelial staining for ICAM-1 (E) and for P-selectin (F) was markedly reduced in tissue section obtained from M40401-treated rats. Original magnification: ×145. Figure is representative of at least three experiments performed on different experimental days.
Histological change
Histological examinations of the small intestine at 60 min of reperfusion (see representative sections in Figure 9) revealed pathologic changes. Ileum section showed inflammatory infiltration by inflammatory extending through the wall and concentrated below the epithelial layer (Figure 9B). M40401-treated rats show a significant reduction in organ injury (Figure 8C).
Figure 9.
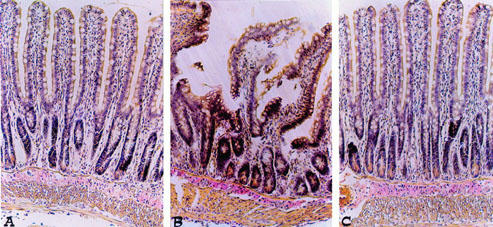
Distal ileum section from a sham rat demonstrating the normal architecture of the intestinal epithelium and wall (A). Distal ileum section from a SAO shocked-rats mice demonstrating oedema of the distal portion of the villi (B). Distal ileum from M40401-treated rats shows reduced SAO-induced organ injury (C) Original magnification: ×125. Figure is representative of at least three experiments performed on different experimental days.
Discussion
The major goal of our work was to compare the efficacy of M40401, a recently developed SODm possessing a structure similar to, but having a much higher catalytic SOD activity than M40403 (Salvemini et al., 1999a,1999b) in splanchnic artery occlusion shock. Splanchnic artery occlusion shock is a well-described model of circulatory shock that arises after reperfusion of a prolonged ischemia of the splanchnic circulation (Lefer & Lefer 1993; Zimmermann et al., 1993; Cuzzocrea et al., 1997). The end result is a high and rapid mortality rate, with most animals dying within the first 2 h of reperfusion (Cuzzocrea et al., 1997). Some of the local alterations that occur in this model include upregulation of adhesion molecules (ICAM-1 and P-selectin (Butcher, 1992; Lawrence & Springer, 1991; Geng et al., 1990; Dreyer et al., 1991), neutrophil infiltration in the intestine, nitrotyrosine staining and PARP activation (Cuzzocrea et al., 1997) and a profound peroxidation of membranes leading to elevated plasma levels of lipid peroxidation products such as MDA (Salvemini et al., 1999a,1999b). Systemic alterations, on the other hand, include elevated plasma levels of the cytokines TNFα and IL1β, infiltration of neutrophils in lung and severe hypotension. The role of NO from the inducible form of iNOS is not involved in the pathogenesis of SAO shock (Cuzzocrea et al., 1998) whereas it seems that loss of NO from the constitutive enzyme (ecNOS) accounts at least in part for the damage seen in this model of reperfusion injury (Kanwar et al., 1994). This is not surprising in light of the beneficial effects ascribed to date to NO released from ecNOS (Gauthier et al., 1994) and from the protective effects seen in reperfusion injury with nitric oxide donors (Gauthier et al., 1994; Naseem et al., 1995). The role of superoxide as a key driver of the damage associated with reperfusion of the ischemic sphlanchnic circulation has been demonstrated by the use of M40403, a stable superoxide dismutase mimetic which selectively removes .O2− without directly interacting with other reactive oxygen species that have been implicated in SAO such as ONOO-. Thus, M40403 at doses ranging from 0.1 3 μg kg−1 inhibited the infiltration of PMNs in lung and ileum, cytokines (TNFα and IL1β) release, lipid peroxidation, hypotension and improved survival at the highest dose tested (Salvemini et al., 1999a,1999b). Here we have clearly shown that M40401 had similar protective effects except at doses at least 100 fold lower. The magnitude of the potency difference directly correlates with the catalytic rate constant for the dismutation of superoxide which these two molecules possess. Thus, at pH=7.4 the ratio of the two catalytic rate constants is approximately 80 fold. It is possible that the beneficial and protective effects seen with SODm are due at least in part to inhibition of TNFα and IL1β as well as attenuation of lipid peroxidation, since pharmacological inhibition of either TNFα/IL1β release or lipid peroxidation is protective in this model of SAO (Cuzzocrea et al., 1999; Squadrito et al., 1999; Sun et al., 1999; Campo et al., 1998). In this study we have also extended our previous finding by showing that M40401 inhibits peroxynitrite formation, nitration of tyrosine residues in the intestine and the upregulation of adhesion molecules.
One mechanism by which M40401 will attenuate the damage seen after reperfusion of the ischemic intestine is by reducing ONOO- formation by simply removing .O2− before it reacts with NO. This is important since the pro-inflammatory and cytotoxic effects of ONOO- are numerous (Beckman et al., 1990; Ischiropoulos et al., 1992; Beckman & Crow, 1993; Crow & Beckman 1995; Salvemini et al., 1998; Misko et al., 1998). The production of ONOO- has been demonstrated in the perfused heart (Matheis et al., 1992; Schulz & Wambolt, 1995), liver (Ma et al., 1995), kidney (Morpurgo et al., 1996), intestine (Cuzzocrea et al., 1997; 1998; Squadrito et al., 1999), brain (Casevieille et al., 1993) and lung (Kooy et al., 1995). Furthermore, removal of ONOO- by agents such as FeTMPS, a porphyrin-containing molecule which increases the rate of isomerization of ONOO- to nitrate (Misko et al., 1998; Salvemini et al., 1998), significantly attenuates damage associated with SAO (Cuzzocrea et al., 2000). Peroxynitrite (and the products of the reactions of both nitrite with hypochlorous acid and the reaction of myeloperoxidase with hydrogen peroxide, Halliwell, 1997) will nitrate tyrosine residues in proteins. The nitrotyrosine formation, as detected by immunofluorescence, is considered to be an indicator of ‘increased nitrosative stress' (Eiserich et al., 1998). Consistent with previous results (Cuzzocrea et al., 1997; 1998; Squadrito et al., 1991) we have found that increased immunohistochemical expression of nitrotyrosine is mostly localized on vessels and in the areas of infiltrated inflammatory cells, suggesting that ONOO- or other nitrogen derivatives and oxidants are formed in vivo and may contribute to tissue injury. Indeed, we have found a marked increase in the plasma levels of peroxynitrite after reperfusion of the ischemic splanchnic circulation. This was inhibited in a dose-dependent manner by M40401, which also attenuated nitrotyrosine staining. Overall these findings indicate that part of the beneficial effect of M40401 is through inhibition of ONOO- formation, attenuation of nitrosative stress, and lack of an effect on NO released from ecNOS.
Superoxide and peroxynitrite can also cause DNA single-strand damage that is the obligatory trigger for PARP activation (Inoue & Kawanishi, 1995; Salgo et al., 1995), which ultimately leads to cell injury (Szabó & Dawson, 1998). Furthermore, substantial evidence exists to support the fact that PARP activation is important in damage associated with reperfusion injury of the ischemic gut. Thus, Szabo and coworkers provided evidence that formation of ONOO- following reperfusion of the ischemic splanchnic circulation, damages DNA which in turn leads to PARP activation and endothelial cell injury (Cuzzocrea et al., 1997; Szabó et al., 1997). PARP inhibitors such as nicotinamide and 3-aminobenzamide attenuate damage (Cuzzocrea et al., 1997; Chaterjee et al., 2000). Furthermore, recent studies have demonstrated that PARP−/− mice are significantly protected from SAO shock when compared with PARP+/+ mice (Liaudet et al., 2000). Thus, we may propose that the observed beneficial effects of M40403 and M40401 are (at least in part) due to the prevention of the activation of PARP.
Inhibition of superoxide prevents the infiltration of neutrophils at inflamed sites as shown by the use of the native SOD enzyme, experiments performed in transgenic mice that overexpress the human CuZnSOD enzyme (Li et al., 1995; Lebovitz et al., 1996; Melov et al., 1999), and by SODm such as SC-55858 (Salvemini et al., 1999a,1999b; Lowe et al., 1996, M40403 and M40401 (Salvemini et al., 1999a,1999b and this study). Endothelial cells appear to be major regulators of neutrophil trafficking, regulating the process of neutrophil chemotaxis, adhesion and emigration from the vasculature to the tissue. During the early phase of reperfusion, P-selectin is rapidly released to the cell surface from preformed storage pools after exposure to certain stimuli, such as hydrogen peroxide, thrombin, histamine, or complement, and allows the leukocytes to roll along the endothelium (Geng et al., 1990). ICAM-1 constitutively expressed on the surface of endothelial cells, is then involved in neutrophil adhesion (Bittermann et al., 1988; Dreyer et al., 1991). Hypoxic endothelial cells synthesize proinflammatory cytokines, which can up-regulate endothelial expression of the constitutive adhesion molecule ICAM-1 in autocrine fashion (Farhood et al., 1995). The expression of P-selectin and ICAM-1 corresponds with the induction of neutrophil recruitment, which is maximal within the first hour of reperfusion, and persists at a lower rate in the late phase of reperfusion (Shreeniwas et al., 1992). In accordance with these findings, we observed that a 45-min occlusion of the splanchnic artery followed by 1-h reperfusion induced the appearance of P-selectin on the endothelial vascular wall and upregulated the surface expression of ICAM-1 on endothelial cells. Treatment with M40401 reduced proinflammatory cytokines production (Figure 4) and abolished the expression of P-selectin and the upregulation of ICAM-1 (Figure 8E,F), while not affecting the constitutive levels of ICAM-1 on endothelial cells (data not shown). Our data suggest that superoxide production contributes to the regulation of neutrophil infiltration through the upregulation of these adhesion molecules. Cytokines are most likely involved in this process, since it is known that TNFα induces the upregulation of ICAM-1 and P-selectin (Farhood et al., 1995; Shreeniwas et al., 1992).
Finally, histological examination of the ileum revealed significant preservation of the architecture by M40401.
In conclusion, we have observed that M40401 exerts its protective effects at doses considerably lower than that of M40403. Such increased efficacy may be due to increased catalytic rate or differential tissue distribution. This lends additional credence to the conclusion that superoxide anion plays a major contributory role in the pathogenesis of ischemia-reperfusion injury and thus points to the potential clinical use of SOD mimetics, such as M40401, in ischemia-reperfusion injury.
Abbreviations
- ICAM-1
intercellular adhesion molecules
- MDA
malonaldehyde
- MPO
myeloperoxidase
- NO
nitric oxide
- O2− superoxide anions; PBS
phosphate-buffered saline
- PMN
polymorphonuclear leukocyte
- SAO
splanchnic artery occlusion
- SOD
superoxide dismutase
References
- ALTURA B.M., GEBREWOLDA A., BURTON R.W. Reactive hyperaemic responses of single arterioles are attenuated markedly after intestinal ischemia, endotoxemia and traumatic shock: possible role of endothelial cells. Microcirc. Endothelium Lymphatics. 1985;2:3–14. [PubMed] [Google Scholar]
- AMBROSIO G., FLAHERTY J.T. Effects of the superoxide radical scavenger superoxide dismutase, and of the hydroxyl radical scavenger mannitol, on reperfusion injury in isolated rabbit hearts. Cardiovasc. Drugs. Ther. 1992;6:623–632. doi: 10.1007/BF00052564. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN J., MARSHALLAND P.A., FREEMAN B.A. Apparent hydroxyl radical production by peroxynitrite: implication for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMAN J.S., CROW L.P. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem. Soc. Trans. 1993;21:330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- BITTERMANN H., AOKI N., LEFER A.M. Anti-shock effects of human superoxide dismutase in splanchnic occlusion shock. Proc. Soc. Exp. Biol. Med. 1988;188:256–271. doi: 10.3181/00379727-188-42734. [DOI] [PubMed] [Google Scholar]
- BOUGHTON-SMITH N.K., EVANS S.M., LASZLO F., WHITTLE B.J., MONCADA S. The induction of nitric oxide synthase and intestinal vascular permeability by endotoxin in the rat. Br. J. Pharmacol. 1993;110:1189–1195. doi: 10.1111/j.1476-5381.1993.tb13940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTCHER E.C. Leukocyte-endothelial cell adhesion as an active, multi-step process: a combinatorial mechanism for specificity and diversity in leukocyte targeting. Adv. Exp. Med. Biol. 1992;323:181–194. doi: 10.1007/978-1-4615-3396-2_23. [DOI] [PubMed] [Google Scholar]
- BUTCHER E.C. Specificity of leukocyte-endothelial interactions and diapedesis: physiologic and therapeutic implications of an active decision process. Res. Immunol. 1993;144:695–698. doi: 10.1016/s0923-2494(93)80053-2. [DOI] [PubMed] [Google Scholar]
- CAMPO G.M., SQUADRITO F., CAMPO S., ALTAVILLA D., LONGONI B., SAITTA A., SQUADRITO G., CAPUTI A.P. Protective effects of the new lazaroid ‘U-83836E' in splanchnic artery occlusion (SAO) shock. Free Radic. Res. 1998;28:477–484. doi: 10.3109/10715769809066885. [DOI] [PubMed] [Google Scholar]
- CAPUTI A.P., ROSSI F., CARNEY K., BREZENOFF H.E. Modulatory effect of brain acetylcholine on reflex-induced bradycardia and tachycardia in conscious rats. J. Pharmacol. Exp. The. 1980;215:309–316. [PubMed] [Google Scholar]
- CAREY C., SIEGFRIED M.R., MA X.L., WEYRICH A.S., LEFER A.M. Antishock and endothelial protective actions of a NO donor in mesenteric and reperfusion. Circ. Shock. 1992;38:209–216. [PubMed] [Google Scholar]
- CAZEVIEILLE C., MULLER, MEYNIER F., BONNE C. Superoxide and nitric oxide cooperation in hypoxia/reoxigenation-induced neuron injury. Free Radical. Biol. Med. 1993;14:389–395. doi: 10.1016/0891-5849(93)90088-c. [DOI] [PubMed] [Google Scholar]
- CHATTERJEE P.K., ZACHAROWSKI K., CUZZOCREA S., OTTO M., THIEMERMANN C. Inhibitors of poly (ADP-ribose) polymerase reduce renal ischemia-reperfusion injury in the anesthetized rat in vivo. FASEB J. 2000;14:641–651. doi: 10.1096/fasebj.14.5.641. [DOI] [PubMed] [Google Scholar]
- CROW J.P., BECKMAN J.S. The role of peroxynitrite in nitric oxide-mediated toxicity. Current Top. Microbiol. Immunol. 1995;196:57–73. doi: 10.1007/978-3-642-79130-7_7. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., DE SARRO G., COSTANTINO G., CILIBERTO G., MAZZON E., DE SARRO A., CAPUTI A.P. IL-6 knock-out mice exhibit resistance to splanchnic artery occlusion shock. J. Leukoc. Biol. 1999;66:471–480. doi: 10.1002/jlb.66.3.471. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., MISKO T.P., COSTANTINO G., MAZZON E., MICALI A., CAPUTI A.P., MACARTHUR H., SALVEMINI D. Beneficial effects of peroxynitrite decomposition catalyst in a rat model of splanchnic artery occlusion and reperfusion. FASEB J. 2000;14:1061–1072. doi: 10.1096/fasebj.14.9.1061. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., CAPUTI A.P. Role of constitutive nitric oxide synthase activation and peroxynitrite production in a rat model of splanchnic artery occlusion shock. Life Sci. 1998;63:789–800. doi: 10.1016/s0024-3205(98)00334-8. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., COSTANTINO G., SZABÓ A., SALZMAN A.L., CAPUTI A.P., SZABÓ C. Beneficial effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) polymerase in a rat model of splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 1997;121:1065–1074. doi: 10.1038/sj.bjp.0701234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEITCH E.A., BRIDGES W., BERG R., SPECIAN R.D., GRANGER N. Hemorrhagic Shock-induced Bacterial Translocation: The role of neutrophils and hydroxyl radicals. J. Trauma. 1990;30:942–951. doi: 10.1097/00005373-199008000-00002. [DOI] [PubMed] [Google Scholar]
- DIX T.A., HESS K.M., MEDINA M.A., SULLIVAN R.W., TILLY S.L., WEBB T.L.L. Mechanism of site-selective DNA nicking by the hydrodioxyl (perhydroxyl) radical. Biochemistry. 1996;35:4578–4583. doi: 10.1021/bi952010w. [DOI] [PubMed] [Google Scholar]
- DREYER W.J., MICHAEL L.H., WEST M.S., SMITH C.W., ROTHLEIN R., ROSSEN R.D., ANDERSON D.C., ENTMAN M.L. Neutrophil accumulation in ischemic canine myocardium. Insights into time course, distribution, and mechanism of localisation during early reperfusion. Circulation. 1991;84:400–411. doi: 10.1161/01.cir.84.1.400. [DOI] [PubMed] [Google Scholar]
- DROY-LEFAIX M.T., DROUET Y., GERAUD G., HOSFOD D., BRAQUET P. Superoxide dismutase (SOD) and the PAF-antagonist (BN 52021) reduce small intestinal damage induced by ischemia-reperfusion. Free Rad. Res. Commun. 1991;12–13:725–735. doi: 10.3109/10715769109145852. [DOI] [PubMed] [Google Scholar]
- EISSERICH J.P., HRISTOVA M., CROSS C.E., JONES A.D., FREEMAN B.A., HALLIWELL B., VAN DER VLIET A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- FANTONE J.C., WARD P.A. A review: role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am. J. Pathol. 1982;107:395–418. [PMC free article] [PubMed] [Google Scholar]
- FARHOOD A., MCGUIRE G.M., MANNING A.M., MIYASAKA M., SMITH C.W., JAESCHKE H. Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J. Leukoc. Biol. 1995;57:368–374. [PubMed] [Google Scholar]
- GAUTHIER T.W., DAVENPECK K.L., LEFER A.M. Nitric oxide attenuates leukocyte-endothelial interaction via P-selectin in splanchnic ischemia-reperfusion. Am. J. Physiol. 1994;267:562–568. doi: 10.1152/ajpgi.1994.267.4.G562. [DOI] [PubMed] [Google Scholar]
- GENG J.G., BEVILACQUA M.P., MOORE K.L., MCINTYRE T.M., PRESCOTT S.M., KIM J.M., BLISS G.A., ZIMMERMAN G.A., MCEVER R.P. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990;343:757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- GOOSSENS D.P., RAO V.K., HARMS B.A., STARLING J.R. Superoxide dismutase and catalase in skin flaps during venous occlusion and reperfusion. Ann. Plast. Surg. 1990;1:21–25. doi: 10.1097/00000637-199007000-00005. [DOI] [PubMed] [Google Scholar]
- GRANGER D.N. Cell adhesion and migration. II. Leukocyte-endothelial cell adhesion in the digestive system. Am. J. Physiol. 1977;273:G982–G986. doi: 10.1152/ajpgi.1997.273.5.G982. [DOI] [PubMed] [Google Scholar]
- GRANGER D.N., RUTILI G., MCCORD J.M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–23. [PubMed] [Google Scholar]
- GRILL H.P., ZWEIER J.L., KUPPUSAMY P., WEISFELDT M.L., FLAHERTY J.T. Direct measurement of myocardial free radical generation in an in vivo model: effects of postischemic reperfusion and treatment with human recombinant superoxide dismutase. J. Am. Coll. Cardiol. 1992;20:1604–1611. doi: 10.1016/0735-1097(92)90457-x. [DOI] [PubMed] [Google Scholar]
- HAGLIND E., XIA G., RYLANDER R. Effects of antioxidants and PAF receptor antagonist in intestinal shock in the rat. Circ. Shock. 1994;42:83–91. [PubMed] [Google Scholar]
- HALLIWELL B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo. FEBS Lett. 1997;411:157–160. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- INOUE S., KAWANISHI S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 1995;371:86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., ZHU L., BECKMAN I.S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- KANWAR S., TEPPERMAN B.L., PAYNE D., SUTHERLAND L.R., KUBES P. Time course of nitric oxide production and epithelial dysfunction during ischemia/reperfusion of the feline small intestine. Circ. Shock. 1994;42:135–140. [PubMed] [Google Scholar]
- KOOY N.W., ROYALL J.A., YE Y.Z., KELLY D.R., BECKMAN J.S. Evidence for in vivo peroxynitrite production in human acute lung injury. Am. J. Respir. Crit. Care Med. 1995;151:1250–1254. doi: 10.1164/ajrccm/151.4.1250. [DOI] [PubMed] [Google Scholar]
- LAWRENCE M.B., SPRINGER T.A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- LIAUDET L., SZABO A., SORIANO F.G., ZINGARELLI B., SZABO C., SALZMAN A.L. Poly (ADP-ribose synthetase mediates intestinal mucosal barrier dysfunction after mesenteric ischemia. Shock. 2000;14:134–141. doi: 10.1097/00024382-200014020-00010. [DOI] [PubMed] [Google Scholar]
- LEBOVITZ R.M., ZHANG H., VOGEL H., CARTWRIGHT J., JR, DIONNE L., LU N., HUANG S., MATZUK M.M. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFER A.M., LEFER D.J. Pharmacology of the endothelium in ischemia-reperfusion and circulatory shock. Ann. Rev. Pharmacol. Toxicol. 1993;33:71–90. doi: 10.1146/annurev.pa.33.040193.000443. [DOI] [PubMed] [Google Scholar]
- LI Y., HUANG T.T., CARLSON E.J., MELOV S., URSELL P.C., OLSON J.L., NOBLE L.J., YOSHIMURA M.P., BERGER C., CHAN P.H. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- LOWE D., PAGEL P.S., MCGOUGH M.F., HETRRICK D.A., WARLTIER D.C. Comparison of the cardiovascular effects of two novel superoxide dismutase mimetics, SC-55858 and SC-54417, in conscious dogs. Eur. J. Pharmacol. 1996;304:81–86. doi: 10.1016/0014-2999(96)00120-3. [DOI] [PubMed] [Google Scholar]
- MA T.T., ISCHIROPOULOS H., BRASS C.A. Endotoxin-stimulated nitric oxide production increases injury and reduces rat liver chemiluminescence during reperfusion. Gastroenterology. 1995;108:463–469. doi: 10.1016/0016-5085(95)90075-6. [DOI] [PubMed] [Google Scholar]
- MARSHALL M.J., WORSFOLD W. Superoxide dismutase: a direct, continuous linear assay using the oxygen electrode. Anal. Biochem. 1978;86:561–567. doi: 10.1016/0003-2697(78)90783-2. [DOI] [PubMed] [Google Scholar]
- MATHEIS G., SHERMAN M.P., BUCKBERG G.D., HAYBRON D.M., YOUNG H.N., IGNARRO L.J. Role of L-arginine-nitric oxide pathway in myocardial reoxygeneration injury. Am. J. Physiol. 1992;262:H616–H620. doi: 10.1152/ajpheart.1992.262.2.H616. [DOI] [PubMed] [Google Scholar]
- MCCORD J.M. Oxygen-derived free radicals in post ischemic tissue injury. N. Engl. J. Med. 1981;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- MELOV S., COSKUN P., PATEL M., TUINSTRA R., COTTRELL B., JUN A.S., ZASTAWNY T.H., DIZDAROGLU M., GOODMAN S.I., HUANG T.T., MIZIORKO H., EPSTEIN C.J., WALLACE D.C. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:846–851. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISKO T.P., HIGHKIN M.K., VEENHUIZEN A.W., MANNING P.T., STERN M.K., CURRIE M.G., SALVEMINI D. Characterization of the cytoprotective action of peroxynitrite decomposition catalysts. J. Biol. Chem. 1998;273:15646–15653. doi: 10.1074/jbc.273.25.15646. [DOI] [PubMed] [Google Scholar]
- MORPURGO E., CADROBBI R., MORPURGO M., RIGOTTI P., SCHIAVON F., SCHIAVON O., CALICETI P., ANCONA E., VERONESE F.M. Protective efect of superoxide dismutase and polyethylene glycol-linked superoxide dismutase against renal warm ischemia/reperfusion injury. Transplantation. 1996;62:1221–1223. doi: 10.1097/00007890-199611150-00006. [DOI] [PubMed] [Google Scholar]
- MULLANE K.M., WESTLIN W., KRAEMER R. Activated neutrophils release mediators that may contribute to myocardial injury and dysfunction associated with ischemia and reperfusion. Ann. NY Acad. Sci. 1988;524:103–121. doi: 10.1111/j.1749-6632.1988.tb38534.x. [DOI] [PubMed] [Google Scholar]
- NASEEM S.A., KONTOS M.C., RAO P.S., JESSE R.L., HESS M.L., KUKREJA R.C. Sustained inhibition of nitric oxide by NG-nitro-arginine improves myocardial function following ischemia/reperfusion in isolated perfused rats heart. J. Mol. Cell. Cardiol. 1995;27:419–424. doi: 10.1016/s0022-2828(08)80038-7. [DOI] [PubMed] [Google Scholar]
- OHKAWA H., OHISHI N., YAGI K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- PASTERNACK R.F., PYSNIK D.Catalase activity of metalloporphyrin Oxy Radicals and Their Scavenger Systems 1983Elsevier: The Netherlands; p. 151(eds) Cohen, G. & Greenwald, R [Google Scholar]
- RATYCH R.E., CHUKNYSKA R.S., BURKLEY G.B. The primary localisation of free radical generation after anoxia/reoxygenation in isolated endothelial cells. Surgery. 1987;102:122–131. [PubMed] [Google Scholar]
- RILEY D.P., LENNON P.J., NEUMANN W.L., WEISS R.H. Toward the rational design of superoxide dismutase mimics: Mechanistic studies for the elucidation of substituent effects on the catalytic activity of macrocyclic manganese(II) complexes. J. Am. Chem. Soc. 1997;119:6522–6533. [Google Scholar]
- SALGO M.G., BERMUDEZ E., SQUADRITO G., PRYOR W. DNA damage and oxidation of thiols peroxynitrite causes in rat thymocytes. Arch. Biochem. Biophys. 1995;322:500–505. doi: 10.1006/abbi.1995.1493. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., CURRIE M.G., MOLLACE V. Nitric oxide-mediated cyclooxygenase activation. A key event in the antiplatelet effects of nitrovasodilators. J. Clin. Invest. 1996b;97:2562–2568. doi: 10.1172/JCI118704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., MISKO T.P., MASFERRER J., SEIBERT K., CURRIE M.G., NEEDLEMAN P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., RILEY D.P., LENNON P.J., WANG Z.Q., CURRIE M.G., MACARTHUR H., MISKO T.P. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br. J. Pharmacol. 1999a;127:685–692. doi: 10.1038/sj.bjp.0702604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., STERN M.K., CURRIE M.G., MISKO T.P. Peroxynitrite decomposition catalysts: novel therapeutics for peroxynitrite-mediated pathology. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2659–2663. doi: 10.1073/pnas.95.5.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., WYATT P., BOURDON D.M., MARINO M.H., MANNING P.T., CURRIE M.G. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996a;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., ZWEIER J.L., SAMOUILOV A., MACARTHUR H., MISKO T.P., CURRIE M.G., CUZZOCREA S., SIKORSKI J.A., RILEY D.P. A nonpeptidyl mimetic of superoxide dismutase with therapeutic activity in rats. Science. 1999b;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- SCHULZ R., WAMBOLT T. Inhibition of nitric oxide synthesis protects the isolated working rabbit heart from ischemia-reperfusion injury. Cardiovas. Res. 1995;30:432–439. [PubMed] [Google Scholar]
- SHREENIWAS R., KOGA S., KARAKURUM M., PINSKY D., KAISER E., BRETT J., WOLITZKY B.A., NORTON C., PLOCINSKI J., BENJAMIN W., KURNS D.K., GOLDESTEIN A., STERN D. Hypoxia-mediated induction of endothelial cell interleukin-1 alpha. An autocrine mechanism promoting expression of leukocyte adhesion molecules on the vessel surface. J. Clin. Invest. 1992;90:2333–2339. doi: 10.1172/JCI116122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SQUADRITO F., ALTAVILLA D., SQUADRITO G., FERLITO M., CAMPO G.M., ARLOTTA M., GRIMALDI S., QUARTARONE C., SAITTA A., CAPUTI A.P. Tacrolimus suppresses tumour necrosis factor-alpha and protects against splanchnic artery occlusion shock. Br. J. Pharmacol. 1999;127:498–504. doi: 10.1038/sj.bjp.0702528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN M.K., JENSEN M.P., KRAMER K. Peroxynitrite decomposition catalysts. J. Am. Chem. Soc. 1996;118:8735–8736. [Google Scholar]
- SUN Z., WANG X., LASSON A., BORJESSON A., LEVEAU P., HARALDSEN P., ANDERSSON R. Roles of platelet-activating factor, interleukin-1beta and interleukin-6 in intestinal barrier dysfunction induced by mesenteric arterial ischemia and reperfusion. J. Surg. Res. 1999;87:90–100. doi: 10.1006/jsre.1999.5746. [DOI] [PubMed] [Google Scholar]
- SZABO C., CUZZOCREA S., ZINGARELLI B., O'CONNOR M., SALZMAN A.L. Endothelial dysfunction in a rat model of endotoxic shock. Importance of the activation of poly (ADP-ribose) polymerase by peroxynitrite. J. Clin. Invest. 1997;100:723–735. doi: 10.1172/JCI119585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABÓ C., DAWSON V.L. Role of poly(ADP-ribose) polymerase in inflammation and ischaemia-reperfusion. Trends Pharmacol. Sci. 1998;19:287–298. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- VON ANDRIAN U.H., CHAMBERS J.D., MCEVOY L.M., BARGATZE R.F., ARFORS K.E., BUTCHER E.C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIA Z.F., HOLLYOAK M., BARROW R.E., HE F., MULLER M.J., HERNDON D.N. Superoxide dismutase and leupeptin prevent delayed reperfusion injury in the rat small intestine during burn shock. J. Burn Care and Rehab. 1995;16:111–117. doi: 10.1097/00004630-199503000-00004. [DOI] [PubMed] [Google Scholar]
- YOSHIKAWA T., NAITO Y., UEDA S., TAKAHASHI S., OYAMADA H., YONETA T., SUGINO S., KONDO M. Ischemia-reperfusion injury and free radical involvement in gastric mucosal disorders. Adv. Exp. Med. Biol. 1990;264:401–410. doi: 10.1007/978-1-4684-5730-8_64. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN B.J., ARNDT H., KUBES P., KURTEL H., GRANGER D.N. Pathophysiology of Shock, Sepsis and Organ Failure 1993Berlin: Springer-Verlag; 322–335.Reperfusion injury in the small intestine. In:eds Schlag, G., and Redl, H. pp [Google Scholar]
- ZINGARELLI B., SQUADRITO F., IOCULANO M.P., ALTAVILLA D., BUSSOLINO F., CAMPO G.M., CAPUTI A.P. Platelet activating factor in splanchnic artery occlusion shock. Eur. J. Pharmacol. 1992;222:13–19. doi: 10.1016/0014-2999(92)90456-e. [DOI] [PubMed] [Google Scholar]


