Abstract
The potential mediator role of the prostanoid PGE2 in airway smooth muscle relaxations induced by peptidic and proteolytic activators of PAR-1, PAR-2, PAR-3 and PAR-4 was investigated in carbachol-precontracted mouse isolated tracheal segments.
The tethered ligand domain sequences of murine PAR-1 (SFFLRN-NH2), PAR-2 (SLIGRL-NH2) and PAR-4 (GYPGKF-NH2), but not PAR-3 (SFNGGP-NH2), induced smooth muscle relaxation that was abolished by the non-selective cyclo-oxygenase (COX) inhibitor, indomethacin. The relative order for mean peak relaxation was SLIGRL-NH2>GYPGKF-NH2 ≈amp; SFFLRN-NH2>SFNGGP-NH2.
SFFLRN-NH2, SLIGRL-NH2 and GYPGKF-NH2, but not SFNGGP-NH2, induced significant PGE2 release that was abolished by indomethacin. Like that for relaxation, the relative order for mean PGE2 release was SLIGRL-NH2>GYPGKF-NH2>SFFLRN-NH2>SFNGGP-NH2.
In dose-response studies, SLIGRL-NH2 induced concentration-dependent increases in PGE2 release (EC50=20.4 μM) and smooth muscle relaxation (EC50=15.8 μM).
The selective COX-2 inhibitor, nimesulide, but not the COX-1 inhibitor valeryl salicylate, significantly attenuated SLIGRL-NH2-induced smooth muscle relaxation and PGE2 release.
Exogenously applied PGE2 induced potent smooth muscle relaxation (EC50=60.3 nM) that was inhibited by the mixed DP/EP1/EP2 prostanoid receptor antagonist, AH6809. SLIGRL-NH2-induced relaxation was also significantly inhibited by AH6809.
In summary, the results of this study strongly suggest that PAR-mediated relaxation in murine tracheal smooth muscle is dependent on the generation of the spasmolytic prostanoid, PGE2. PAR-stimulated PGE2 release appears to be generated preferentially by COX-2 rather than COX-1, and induces relaxation via activation of the EP2 receptor.
Keywords: Protease activated receptors, PARs, mouse trachea, cyclo-oxygenase, indomethacin, PGE2
Introduction
Protease activated receptors (PARs) are a novel group of seven transmembane-spanning, G-protein coupled receptors that can respond to extracellular proteolytic activity (Hollenberg, 1999). PAR activation is dependent on the proteolytic cleavage of the amino-terminus of the receptor, which reveals a new amino-terminus that serves to act as a ‘tethered ligand' to self-activate the receptor. The first cloned PAR (PAR-1) was activated by the coagulation enzyme thrombin, as well as a peptide sequence that mimicked the tethered ligand sequence (SFFLRN-NH2 for murine PAR-1; Connolly et al., 1996). Subsequent PARs were shown to possess a similar activation mechanism (SLIGRL-NH2, SFNGGP-NH2 and GYPGKF-NH2 for PAR-2, PAR-3 and PAR-4 respectively; Kahn et al., 1998; Nystedt et al., 1995). Thrombin (PAR-1, PAR-3 and PAR-4) and trypsin (PAR-2 and PAR-4) are the well-characterized enzymatic activators of PARs, although other potential PAR activators include mast cell tryptase (Steinhoff et al., 1999), Coagulation Factor Xa (Fox et al., 1997), Granzyme A (Suidan et al., 1994), as well as Cathepsin G (Sambrano et al., 2000). These latter proteases may play a role in PAR activation at sites where trypsin and thrombin are less abundant.
PAR activation evokes a wide array of responses in biological tissues, including platelet aggregation (Kahn et al., 1999), neuronal apoptosis (Sarker et al., 1999; Turgeon et al., 1999), smooth muscle and fibroblast mitogenesis (Bretschneider et al., 1999; McNamara et al., 1993; Akers et al., 2000), as well as regulating smooth muscle tone either directly or via the release of mediators such as cytokines, nitric oxide (NO) or prostanoids. For example, stimulation of PAR-1 and PAR-2 induces vascular smooth muscle relaxation, via the synthesis of endothelium-derived NO (Magazine & Srivastava, 1996). In contrast, in the presence of an NO synthase inhibitor or following endothelium denudation, stimulation of PAR-1 (but not PAR-2) induced vascular smooth muscle contraction (Hwa et al., 1996; Magazine & Srivastava, 1996).
In airway tissues, PAR-1, -2 and -4-stimulated relaxations also appear to be mediated via the generation of an intermediary relaxant factor(s). However, in contrast to vascular tissues, PAR-mediated relaxations were inhibited by indomethacin, suggesting the relaxant factor was a cyclo-oxygenase (COX), rather than a nitric oxide synthase, product (Cocks et al., 1999; Lan et al., 2000). These findings suggest a role of bronchorelaxant prostanoids in PAR-induced airway smooth muscle relaxation (Lan et al., 2000). To investigate the potential role of cyclo-oxygenase-derived PGE2 as a mediator of PAR-induced relaxations, simultaneous measurements of PGE2 release and smooth muscle relaxation were obtained in mouse isolated tracheal preparations in response to the selective PAR-1, -2, -3 and -4 ligands, as well as to the enzymatic activators, namely, trypsin and thrombin. PAR-2-mediated relaxations were further characterized by the use of valeryl salicylate (a selective COX-1 enzyme inhibitor, Bhattacharyya et al., 1995; Johnson et al., 1995), nimesulide (a selective COX-2 inhibitor, Pang & Knox, 1997; Range et al., 2000), indomethacin (a non-selective COX-1 and COX-2 inhibitor, Vane et al., 1998), and a mixed DP/EP1/EP2 prostanoid receptor antagonist, AH6809 (Funk et al., 1993; Keery & Lumley, 1988; Woodward et al., 1995).
Methods
Tissue preparation
Male CBA/CaH mice (8 – 10 weeks of age) were killed with an overdose of pentobarbitone sodium (250 mg kg−1 i.p.). The upper respiratory tract and associated alimentary tissue were rapidly excised and placed in a petri dish where the trachea was dissected free from surrounding tissue. Entire tracheal segments (4 mm long) were mounted between two stainless steel supports under a resting tension of 0.5 g in organ baths containing Krebs bicarbonate solution (composition in mM): NaCl 117, KCl 5.36, NaHCO3 25, KH2PO4 1.03, MgSO4.7H2O 0.57, CaCl2 2.5, D-glucose 11.1) that was bubbled with 5% CO2 in O2 (carbogen) and maintained at 37°C. Changes in isometric tension were detected using FTO3 Grass force displacement transducers coupled to a custom-built preamplifier and a data acquisition system. After a 45 min period of equilibration, during which time the bath fluid was changed every 15 min and the tension readjusted to 0.5 g, the tracheal segments were exposed to the cumulative additions of a submaximal (0.2 μM) and a supramaximal (10 μM) concentration of carbachol. The response to 10 μM carbachol was termed Cmax.
Effects of PAR activators on airway smooth muscle tone
Following a 20 min washout and rest period, tracheal preparations were precontracted to approximately 60% Cmax with carbachol, after which 100 μM of PAR-1, PAR-2, PAR-3 or PAR-4 scrambled peptide (FSFLRN-NH2, LSIGRL-NH2, FSNGGP-NH2 or GYPGFK-NH2 respectively) was added to the bath for 10 min. The entire bath fluid was collected and stored at −85°C prior to PGE2 analysis. The maximal relaxation response (%R, expressed as a percentage of the precontraction, i.e.: 100%R represented a total reversal of carbachol-induced contraction) was also recorded. The preparation was washed and rested for a further 15 min, and precontacted to 60% Cmax with carbachol. One hundred μM of the corresponding active peptide (SFFLRN-NH2, SLIGRL-NH2, SFNGGP-NH2 or GYPGKF-NH2 respectively) was then added for 10 min, and the bath fluid collected and relaxation response measured as described above. A similar protocol was used to measure the effects of the proteolytic activators, trypsin and thrombin. Responses evoked by PAR peptidic and enzymatic activators were compared to an appropriate time control (Figure 1). In some experiments, the role of cyclo-oxygenase (COX) and its isoforms was investigated by including 1 mM valeryl salicylate (COX-1-selective inhibitor), 1 μM nimesulide (COX-2-selective) or 3 μM indomethacin (non-selective COX-1 and COX-2 inhibitor) in the Krebs bicarbonate solution for the duration of the experiment. At the completion of all the experiments, tracheal segments were blotted dry and weighed.
Figure 1.
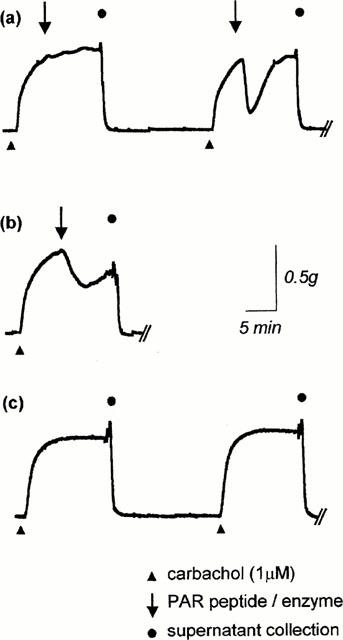
Representative tension-recording traces of PAR-induced relaxation on the mouse isolated trachea precontracted to 60% Cmax with carbachol (1 μM). (a) Relaxations induced by a scrambled PAR peptide (in this case, 100 μM LSIGRL-NH2), and then by the corresponding active PAR peptide (100 μM SLIGRL-NH2). Supernatants were collected for PGE2 quantification 10 min after addition of each peptide. (b) Relaxations induced by an enzymatic PAR activator (in this example, 100 U ml−1 trypsin). (c) Unstimulated preparations were used as appropriate time controls to determine basal PGE2 release.
Effect of AH6809 on PGE2 and SLIGRL-NH2-induced relaxation
To examine the role of relaxant prostanoid receptors in the mouse trachea, tracheal preparations were incubated with AH6809 (3 μM) or vehicle for 25 min. The preparations were then precontracted to approximately 60% Cmax with carbachol, after which a cumulative PGE2 concentration-response curve was generated (3 nM to 1 μM). A similar protocol was used to examine the maximal relaxant response of a bolus dose of PGE2 (30 nM) or SLIGRL-NH2 (30 μM).
PGE2 quantification
The collected bath fluid was thawed, and 50 μl aliquots were assayed for PGE2 content by enzyme immunoassay (Cayman Chemical Co, Cat. no. 514010), according to the manufacturer's instructions. The assay system has a high specificity for PGE2, and possesses minimal affinity (<0.01%) for other well-characterized prostanoids such as PGD2 and PGF2α. Samples taken from preparations stimulated with PAR peptides or enzymes were compared to an appropriate time control, and expressed as pg PGE2 mg−1 trachea (Table 1).
Table 1.
Effects of trypsin and thrombin on smooth muscle relaxation and PGE2 release in pre-contracted mouse trachea in the presence or absence of indomethacin
Materials
Amide-capped hexapeptides were synthesized by Dr Richard Lipscombe (Protein Facility, University of Western Australia, Perth, Australia). Thrombin was obtained from CSL Limited (Melbourne, Australia), and valeryl salicylate, nimesulide and PGE2 enzyme immunoassay kits were purchased from Cayman Chemicals (Ann Arbor, MI, U.S.A.). Indomethacin, trypsin and carbamycholine chloride (carbachol) were obtained from Sigma Chemical Company (St Louis, MO, U.S.A.), while AH6809 was purchased from BioMol Research Laboratories (Plymouth Meeting, PA, U.S.A.).
Stock solutions of PAR agonists (10 mM), thrombin (1 kU ml−1), trypsin (10 kU ml−1) and carbachol (10 mM) were made in double-distilled water and stored at −20°C. PGE2 (10 mM) was made in 100% ethanol and stored at −20°C; valeryl salicylate (100 mM) and nimesulide (10 mM) were made in 100% ethanol and stored at room temperature. AH6809 (6 mM) was made in dimethyl sulphoxide and stored at −20°C, while indomethacin was prepared in 5 mM Na2CO3 and used immediately. Dilution of drugs were prepared on the day of the experiment in double distilled water or Krebs bicarbonate solution.
Analyses
Percentage relaxation (%R) was expressed as the arithmetic mean±s.e.mean. PGE2 release and EC50 values (the concentration that induced 50% of the maximal response) were expressed as the geometric mean with associated 95% confidence limits. Differences between mean responses were assessed using one-way ANOVA and, when appropriate, paired Student's t-tests and Bonferroni's correction for multiple comparisons were used, with P<0.05 being considered statistically significant (SigmaStat, Jandel Corporation, San Rafael, CA, U.S.A.). Drug concentrations described in the text refer to the final molar concentrations in the organ bath.
Results
Relaxant effects of PAR activators
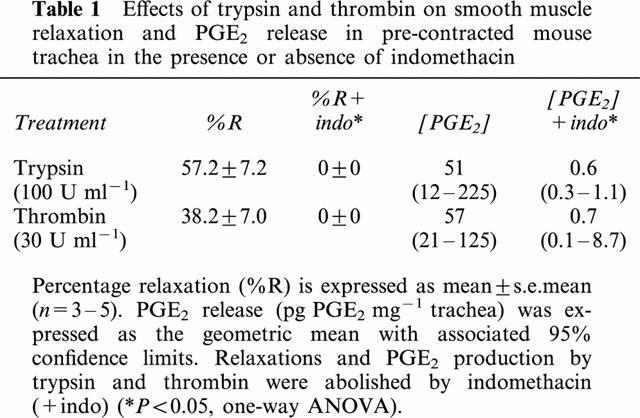
One hundred μM of PAR-1, -2 and -4 activating peptide induced significantly greater relaxations than the corresponding scrambled peptides (P<0.05, paired t-test; Figure 2). SLIGRL-NH2 induced the greatest level of relaxation (%R=78.8±2.7, n=9), followed by SFFLRN-NH2 (%R=22.9±5.6, n=5) and GYPGKF-NH2 (%R=21.3±3.7, n=4). SFNGGP-NH2 did not induce significant airway smooth muscle relaxation (%R=0.8±0.8, n=5; Figure 2). The two enzymatic PAR activators, trypsin and thrombin, induced significant relaxations (%R=57.2±7.2, n=4 and 38.2±7.0, n=5 respectively; Table 1). Relaxations induced by peptidic and enzymatic activators of PARs were abolished by the cyclo-oxygenase inhibitor, indomethacin (3 μM; Figure 2 and Table 1).
Figure 2.
Relaxant effects of 100 μM PAR-1, -2, -3 and -4 peptides on carbachol-precontracted mouse trachea in the absence or presence of 3 μM indomethacin (indo). Data are presented as mean±s.e.mean (n=4 – 5).
PGE2 release by PAR activators
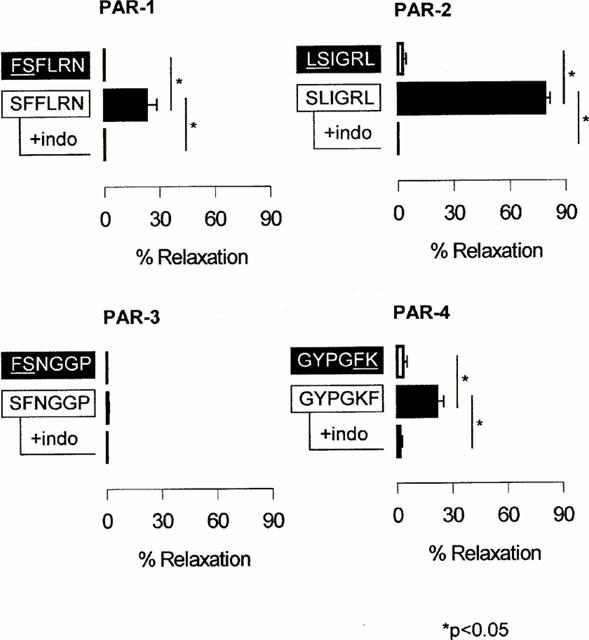
Over a 15 min collection period, the basal release of PGE2 from carbachol-contracted mouse isolated trachea was 3.0 pg mg−1 tissue (95% c.l., 2.3 – 4.0 pg mg−1 trachea, n=3). One hundred μM of PAR-1, -2 and -4 activating peptides, but not scrambled PAR peptides, stimulated PGE2 release above basal levels (P<0.05; Figure 3). SLIGRL-NH2 produced the greatest increase in PGE2 release (18.4 fold above basal, 95% c.l., 7.9 – 47.8 fold increase, n=9), followed by GYPGKF-NH2 (5.7 fold above basal, 95% c.l., 2.7 – 16.7 fold increase, n=4) and SFFLRN-NH2 (2.3 fold above basal, 95% c.l., 1.1 – 9.8 fold increase, n=5). SFNGGP-NH2 did not significantly modulate PGE2 release relative to basal secretions (1.2 fold above basal, 95% c.l., 0.9 – 5.8 fold increase, n=5; Figure 3). The two enzymatic activators of PAR, trypsin and thrombin, produced similar increases in PGE2 release from the mouse trachea (15.9 fold above basal, 95% c.l., 3.9 – 74.1 fold increase, n=4, and 17.9 fold above basal, 95% c.l., 6.9 – 51.6 fold increase, n=5, respectively; Table 1). Indomethacin (3 μM) inhibited PGE2 release induced by peptidic and enzymatic activators of PARs (Figure 3 and Table 1).
Figure 3.
Effects of 100 μM PAR-1, -2, -3 and -4 peptides on PGE2 release from carbachol-precontracted mouse trachea in the absence or presence of 3 μM indomethacin (indo). Data are presented as mean±s.e.mean (n=4 – 9).
Relationship between SLIGRL-NH2, COX-isoforms, PGE2 release and smooth muscle relaxation
Over the concentration range of 3 to 100 μM, SLIGRL-NH2 concentration-dependently induced smooth muscle relaxation (EC50=15.8 μM, 95% c.l., 9.2 – 27.2 μM, n=6) and PGE2 release (EC50=20.3 μM, 95% c.l., 4.4 – 93.5 μM, n=4; Figure 4a). The mean EC50 values of SLIGRL-NH2-induced smooth muscle relaxation and SLIGRL-NH2-induced PGE2 release were not statistically different (one-way ANOVA of log EC50). One hundred μM SLIGRL-NH2-induced PGE2 release and smooth muscle relaxation were markedly attenuated by the selective COX-2 inhibitor nimesulide (1 μM), and by the non-selective COX inhibitor indomethacin (3 μM). In contrast, the selective COX-1 inhibitor valeryl salicylate (1 mM) had no significant effect on SLIGRL-NH2-induced relaxations or PGE2 release (P<0.05; Figure 4b,c).
Figure 4.
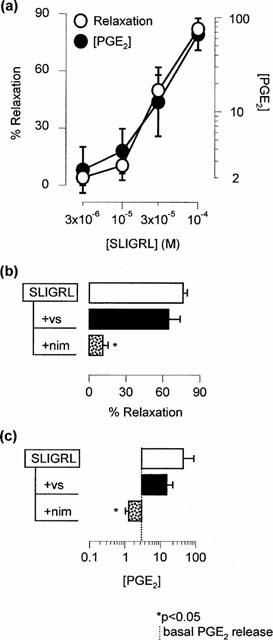
(a) Concentration-response curve of SLIGRL-NH2-induced smooth muscle relaxation and PGE2 release from precontracted mouse isolated trachea (n=4 – 6). (b,c) Effects of 1 mM valeryl salicylate (vs), 1 μM nimesulide (nim), or 3 μM indomethacin (indo) on SLIGRL-NH2-induced smooth muscle relaxation and PGE2 release (n=5 – 7).
Effect of AH6809 on PGE2 and SLIGRL-NH2-induced relaxations
Concentration-dependent relaxations induced by the cumulative addition of exogenous PGE2 (EC50=60.3 nM, 95% c.l., 34.9 – 104.0 nM, n=10) were inhibited by the DP/EP1/EP2 antagonist, AH6809 (3 μM; Figure 5a). AH6809 also significantly attenuated the relaxant responses to single-concentration additions of PGE2 (30 nM) or SLIGRL-NH2 (30 μM; Figure 5b,c).
Figure 5.
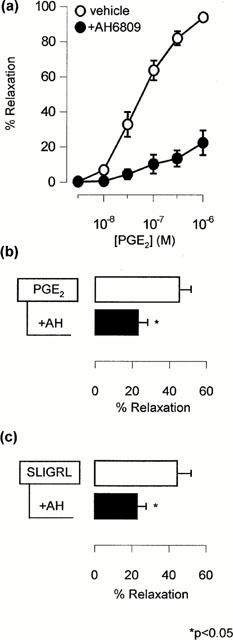
(a) Effect of 3 μM AH6809 on the cumulative concentration-response relationship generated by exogenous PGE2 in the precontracted mouse isolated trachea (n=5 – 10). (b,c) Effect of 3 μM AH6809 (AH) on the single concentration addition of PGE2 (30 nM) or SLIGRL-NH2 (30 μM) in the precontracted mouse isolated trachea (n=6 – 13).
Discussion
In this study, synthetic peptidic activators of PAR-1, -2 and -4, as well as trypsin and thrombin, induced indomethacin-sensitive release of PGE2 and airway smooth muscle relaxation of murine isolated trachea. The PAR-2 activator, SLIGRL-NH2, induced concentration-dependent PGE2 release and smooth muscle relaxation, and exogenously applied PGE2 induced marked relaxation responses in this isolated airway preparation. Furthermore, SLIGRL-NH2-induced relaxations were inhibited by the COX-2 selective inhibitor nimesulide and a mixed DP/EP1/EP2 receptor antagonist, AH6809. Taken together, these findings provide strong supportive evidence for COX-2-derived PGE2 playing an important mediator role in PAR-induced relaxations in mouse isolated trachea.
Several recent studies have demonstrated that stimulation of airway PARs modulates airway smooth muscle tone (Cocks et al., 1999; Lan et al., 2000; Ricciardolo et al., 2000). For example, PAR-2 activators have been reported to induce relaxation in isolated smooth muscle preparations from mouse bronchi and trachea (Cocks et al., 1999; Lan et al., 2000), guinea-pig trachea and main bronchi (Ricciardolo et al., 2000) and rat trachea, main bronchi and interpulmonary bronchi (Chow et al., 2000). In contrast, PAR-2 activation induced contractile responses in guinea-pig intrapulmonary bronchi and epithelium-denuded main bronchi (Ricciardolo et al., 2000). In the current study, PAR-2 activation was associated solely with relaxation responses, although PAR-1- and PAR-4-mediated relaxations were typically preceded by a transient contractile response (Lan et al., 2000). PAR-1-mediated contractile responses have also been reported in epithelium-denuded mouse bronchi (Cocks et al., 1999) and rat trachea, and main and intrapulmonary bronchi (Chow et al., 2000). Furthermore, alpha-thrombin, which is an enzymatic activator of PAR-1 (-3 and -4), induced contraction of human isolated bronchial rings in a receptor-specific and dose-dependent manner (Hauck et al., 1999). Thus, stimulation of PARs in the airways may result in profound changes in bronchomotor tone, but the magnitude and nature of the response depends on which particular PAR is stimulated, and is subject to significant regional and species differences, many of which have yet to be fully elucidated in human airways.
Indomethacin, a non-selective inhibitor of cyclo-oxygenase, completely prevented PAR-1, -2 and -4-mediated relaxation responses in mouse trachea (current study; Lan et al., 2000). PAR-2-mediated relaxations were also at least partially inhibited by indomethacin in isolated airways preparations of mouse and rat bronchi (Cocks et al., 1999; Chow et al., 2000), and guinea-pig trachea (Ricciardolo et al., 2000), suggesting that these PAR-induced responses were mediated via the actions of cyclo-oxygenase product(s). Moreover, results from the current study provide strong evidence that the cyclo-oxygenase product responsible for PAR-1, -2 and -4-mediated relaxations in mouse isolated tracheal preparations was PGE2. Firstly, each of the PAR stimulants that induced a significant relaxant response (SFFLRN-NH2, SLIGRL-NH2, GYPGKF-NH2, trypsin and thrombin) also induced a significant increase in PGE2 release. In contrast, the synthetic peptidic activator of PAR-3, SFNGGP-NH2, induced neither relaxation nor PGE2 release. Secondly, there was a strong positive relationship between the amount of PGE2 released by each of the synthetic peptidic PAR stimulants and the magnitude of the relaxant response. The relative order of effect for both relaxation and PGE2 release was SLIGRL-NH2 > GYPGKF-NH2 ≈amp; SFFLRN-NH2 > SFNGGP - NH2. Thirdly, the PAR-2 stimulant SLIGRL-NH2 induced concentration-dependent increases in both PGE2 release and relaxation. Moreover, the concentration-effect relationships were superimposable, indicating that SLIGRL-NH2-induced increases in PGE2 release and smooth muscle relaxation occurred over the same concentration range. Finally, the exogenous application of PGE2 induced potent, concentration-dependent relaxations in carbachol-contracted mouse isolated tracheal preparations, confirming previous studies that PGE2 is an effective spasmolytic agent in this preparation (Li et al., 1998). Thus, these findings collectively suggest that the prostanoid PGE2 mediates PAR-induced relaxations in the mouse airway.
An initial step in the synthesis of PGE2 is the metabolism of arachidonic acid by the two cyclo-oxygenase isoforms, COX-1 and COX-2. Despite earlier indications that COX-2 only plays a role in pathological conditions, there is accumulating evidence of both COX-1 and COX-2 being required for normal cellular function. Constitutive COX-2 expression has been detected in non-inflamed bronchial tissue including the airway epithelium (Watkins et al., 1999), as well as smooth muscle cells of the pulmonary vasculature (Ermert et al., 1998). In the present study, we showed that SLIGRL-NH2-induced PGE2 release and smooth muscle relaxation in tracheal preparations obtained from non-diseased mice were largely attenuated by the selective COX-2 inhibitor nimesulide (Pang & Knox, 1997; Range et al., 2000), but not by selective COX-1 inhibitor valeryl salicylate (Bhattacharyya et al., 1995; Johnson et al., 1995). These findings suggest that PAR-2-mediated PGE2 generation and subsequent smooth muscle relaxation was dependent on COX-2 rather than COX-1. However, the COX isoform(s) that regulate PAR-1 and PAR-4-induced PGE2 release and smooth muscle relaxation remain unknown and is currently being investigated.
PGE2 is the predominant prostaglandin in the upper respiratory tract (Karim et al., 1967; Knight et al., 1995a) and is the most potent relaxant prostaglandin in airway preparations (Coleman & Kennedy, 1980, Knight et al., 1995b). In the current study, PGE2 induced potent concentration-dependent relaxation in murine isolated tracheal preparations. These findings are consistent with a recent report by Li et al. (1998) using murine trachea, and with a raft of other studies using animal (Kennedy et al., 1982; Gardiner, 1986; Lydford & McKechnie, 1994) and human (Norel et al., 1999) isolated airway smooth muscle preparations.
Somewhat less clear is the subtype(s) of prostanoid receptor that mediate these relaxant effects, since PGE2 can activate four different subtypes of EP receptor (EP1, EP2, EP3 and EP4) and the distribution of these receptors in the murine airway is unknown. Nevertheless, EP1 and EP3 receptor subtypes are unlikely to be involved in PGE2-induced airway smooth muscle relaxation since they are typically linked to transduction mechanisms that induce smooth muscle contraction via the increase of phosphoinositide tunover, elevation of intracellular free Ca2+ and inhibition of adenylate cyclase (Coleman et al., 1994).
In contrast, stimulation of EP2 and EP4 receptors is associated with increases in adenylate cyclase activity and intracellular cyclic AMP levels, a well-established pathway for mediating airway smooth muscle relaxation. Although the EP4 receptor has been implicated in PGE2-induced relaxations in rat isolated trachea (Lydford & McKechnie, 1994), it is the EP2 receptor that has been proposed to be the predominant relaxant EP receptor in human bronchus (Norel et al., 1999) and several animal airway preparations (Kennedy et al., 1982; Gardiner, 1986). Our findings that AH6809, an antagonist at EP2 but not EP4 receptors, markedly inhibited PGE2-induced relaxations suggests that EP2 rather than EP4 receptors were also involved in relaxation of murine trachea. Although further studies are required to clearly classify the relaxant EP receptor in murine trachea, it was interesting to note that AH6809 inhibited relaxations induced by PGE2 and SLIGRL-NH2 to a similar extent. These latter findings provide further support for the postulate that the relaxant actions of the PAR-2 activating peptide are mediated by PGE2.
In addition to bronchodilatory effects, PGE2 also possesses anti-inflammatory properties. For example, PGE2 inhibits leukotriene production (Christman et al., 1993) and IgE synthesis (Pene et al., 1988), and blocks both the early and late responses to allergen challenge in asthmatics (Pavord et al., 1993). Furthermore, the recruitment, activation and survival of various inflammatory cells into the lung is inhibited by PGE2 and PGE2-mimetics (Smith et al., 1996; Chouaib et al., 1987; Alam et al., 1993). PGE2 may also exert favourable effects on airway remodelling since PGE2 (Florio et al., 1994) and COX-2 induction (Belvisi et al., 1998) both exert powerful anti-proliferative effects on airway smooth muscle cells and fibroblasts (McAnulty et al., 1997). In light of the evidence that endogenous PGE2 may possess bronchoprotective and anti-inflammatory roles in the airway (for review, see Pavord & Tattersfield, 1995), release of PGE2 by PAR activation may possess potential therapeutic benefit in respiratory diseases such as allergic rhinitis and asthma.
In conclusion, we have shown that peptidic and enzymatic activators of PAR-1, -2 and -4 induced concomitant release of PGE2 and smooth muscle relaxation in murine trachea. Strong, positive relationships were observed between the amount of PGE2 released and the extent of smooth muscle relaxation produced. Moreover, inhibition of PAR-2-induced PGE2 release by the COX-2 inhibitors indomethacin and nimesulide was associated with marked attenuation of smooth muscle relaxation. Finally, exogenous PGE2 induced marked concentration-dependent relaxations in mouse isolated tracheal smooth muscle, and both PGE2- and SLIGRL-NH2-mediated relaxations were inhibited by AH6809. These findings strongly suggest that COX-2-derived PGE2 is an important mediator of PAR-2-induced relaxations in mouse isolated trachea.
Acknowledgments
This study was supported by grants from the Australian Research Council, Asthma Foundation and the National Health and Medical Research Council of Australia.
Abbreviations
- ANOVA
analysis of variance
- c.l.
confidence limits
- Cmax
contractile response to 10 μM carbachol
- COX
cyclo-oxygenase
- DP
prostaglandin D2 receptor
- EP
prostaglandin E2 receptor
- PAR
protease activated receptor
- PGE2
prostaglandin E2
- %R
percentage relaxation, expressed as a percentage of carbachol-induced pre-contraction
References
- AKERS I.A., PARSONS M., HILL M.R., HOLLENBERG M.D., SANJAR S., LAURENT G.J., MCANULTY R.J. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am. J. Physiol. 2000;278:L193–L201. doi: 10.1152/ajplung.2000.278.1.L193. [DOI] [PubMed] [Google Scholar]
- ALAM R., DEJARNATT A., STAFFORD S., FORSYTHE P.A., KUMAR D., GRANT J.A. Selective inhibition of the cutaneous late but not immediate allergic response to antigens by misoprostol, a PGE analog. Results of a double-blind, placebo-controlled randomized study. Am. Rev. Respir. Dis. 1993;148:1066–1070. doi: 10.1164/ajrccm/148.4_Pt_1.1066. [DOI] [PubMed] [Google Scholar]
- BELVISI M.G., SAUNDERS M., YACOUB M., MITCHELL J.A. Expression of cyclo-oxygenase-2 in human airway smooth muscle is associated with profound reductions in cell growth. Br. J. Pharmacol. 1998;125:1102–1108. doi: 10.1038/sj.bjp.0702104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATTACHARYYA D.K., LECOMTE M., DUNN J., MORGANS D.J., SMITH W.L. Selective inhibition of prostaglandin endoperoxide synthase-1 (cyclooxygenase-1) by valerylsalicyclic acid. Arch. Biochem. Biophys. 1995;317:19–24. doi: 10.1006/abbi.1995.1130. [DOI] [PubMed] [Google Scholar]
- BRETSCHNEIDER E., KAUFMANN R., BRAUN M., WITTPOTH M., GLUSA E., NOWAK G., SCHROR K. Evidence for proteinase-activated receptor-2 (PAR-2)-mediated mitogenesis in coronary artery smooth muscle cells. Br. J. Pharmacol. 1999;126:1735–1740. doi: 10.1038/sj.bjp.0702509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOUAIB S., ROBB R.J., WELTE K., DUPONT B. Analysis of prostaglandin E2 effect on T lymphocyte activation. Abrogation of prostaglandin E2 inhibitory effect by the tumor promotor 12.0 tetradecanoyl phorbol-13 acetate. J. Clin. Invest. 1987;80:333–340. doi: 10.1172/JCI113077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOW J.M., MOFFAT J.D., COCKS T.D. Effect of PAR-2-activating peptide SLIGRL-NH2 on airway smooth muscle in vitro and in vivo. Am. J. Respir. Crit. Care Med. 2000;161:A438. [Google Scholar]
- CHRISTMAN B.W., CHRISTMAN J.W., DWORSKI R., BLAIR I.A., PRAKASH C. Prostaglandin E2 limits arachidonic acid availability and inhibits leukotriene B4 synthesis in rat alveolar macrophages by a nonphospholipase A2 mechanism. J. Immunol. 1993;151:2096–2104. [PubMed] [Google Scholar]
- COCKS T.M., FONG B., CHOW J.M., ANDERSON G.P., FRAUMAN A.G., GOLDIE R.G., HENRY P.J., CARR M.J., HAMILTON J.R., MOFFATT J.D. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., KENNEDY I. Contractile and relaxant actions of prostaglandins on guinea-pig isolated trachea. Br. J. Pharmacol. 1980;68:533–539. doi: 10.1111/j.1476-5381.1980.tb14569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- CONNOLLY A.J., ISHIHARA H., KAHN M.L., FARESE R.V., JR, COUGHLIN S.R. Role of the thrombin receptor in development and evidence for a second receptor. Nature. 1996;381:516–519. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- ERMERT L., ERMERT M., GOPPELT-STRUEBE M., WALMRATH D., GRIMMINGER F., STEUDEL W., GHOFRANI H.A., HOMBERGER C., DUNCKER H., SEEGER W. Cyclooxygenase isoenzyme localization and mRNA expression in rat lungs. Am. J. Respir. Cell. Mol. Biol. 1998;18:479–488. doi: 10.1165/ajrcmb.18.4.2939. [DOI] [PubMed] [Google Scholar]
- FLORIO C., MARTIN J.G., STYHLER A., HEISLER S. Antiproliferative effect of prostaglandin E2 in cultured guinea pig tracheal smooth muscle cells. Am. J. Physiol. 1994;266:L131–L137. doi: 10.1152/ajplung.1994.266.2.L131. [DOI] [PubMed] [Google Scholar]
- FOX M.T., HARRIOTT P., WALKER B., STONE S.R. Identification of potential activators of proteinase-activated receptor-2. FEBS Lett. 1997;417:267–269. doi: 10.1016/s0014-5793(97)01298-2. [DOI] [PubMed] [Google Scholar]
- FUNK C.D., FURCI L., FITZGERALD G.A., GRYGORCZYK R., ROCHETTE C., BAYNE M.A., ABRAMOVITZ M., ADAM M., METTERS K.M. Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. J. Biol. Chem. 1993;268:26767–26772. [PubMed] [Google Scholar]
- GARDINER P.J. Characterization of prostanoid relaxant/inhibitory receptors (psi) using a highly selective agonist, TR4979. Br. J. Pharmacol. 1986;87:45–56. doi: 10.1111/j.1476-5381.1986.tb10155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUCK R.W., SCHULZ C., SCHOMIG A., HOFFMAN R.K., PANETTIERI R.A. Alpha-Thrombin stimulates contraction of human bronchial rings by activation of protease-activated receptors. Am. J. Physiol. 1999;277:L22–L29. doi: 10.1152/ajplung.1999.277.1.L22. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG M.D. Protease-activated receptors: PAR4 and counting: how long is the course. Trends Pharmacol. Sci. 1999;20:271–273. doi: 10.1016/s0165-6147(99)01333-4. [DOI] [PubMed] [Google Scholar]
- HWA J.J., GHIBAUDI L., WILLIAMS P., CHINTALA M., ZHANG R., CHATTERJEE M., SYBERTZ E. Evidence for the presence of a proteinase-activated receptor distinct from the thrombin receptor in vascular endothelial cells. Circ. Res. 1996;78:581–588. doi: 10.1161/01.res.78.4.581. [DOI] [PubMed] [Google Scholar]
- JOHNSON J.L., WIMSATT J., BUCKEL S.D., DYER R.D., MADDIPATI K.R. Purification and characterization of prostaglandin H synthase-2 from sheep placental cotyledons. Arch. Biochem. Biophys. 1995;324:26–34. doi: 10.1006/abbi.1995.9934. [DOI] [PubMed] [Google Scholar]
- KAHN M.L., NAKANISHI-MATSUI M., SHAPIRO M.J., ISHIHARA H., COUGHLIN S.R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAHN M.L., ZHENG Y.W., HUANG W., BIGORNIA V., ZENG D., MOFF S., FARESE R.V., JR, TAM C., COUGHLIN S.R. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- KARIM S.M.M., SANDLER M., WILLIAMS E.D. Distribution of postaglandins in human tissues. Br. J. Pharmacol. Chemother. 1967;31:340–344. doi: 10.1111/j.1476-5381.1967.tb02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEERY R.J., LUMLEY P. AH6809, a prostaglandin DP-receptor blocking drug on human platelets. Br. J. Pharmacol. 1988;94:745–754. doi: 10.1111/j.1476-5381.1988.tb11584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY I., COLEMAN R.A., HUMPHREY P.P., LEVY G.P., LUMLEY P. Studies on the characterisation of prostanoid receptors: a proposed classification. Prostaglandins. 1982;24:667–689. doi: 10.1016/0090-6980(82)90036-3. [DOI] [PubMed] [Google Scholar]
- KNIGHT D.A., STEWART G.A., LAI M.L., THOMPSON P.J. Epithelium-derived inhibitory prostaglandins modulate human bronchial smooth muscle responses to histamine. Eur. J. Pharmacol. 1995a;272:1–11. doi: 10.1016/0014-2999(94)00601-3. [DOI] [PubMed] [Google Scholar]
- KNIGHT D.A., STEWART G.A., THOMPSON P.J. Prostaglandin E2, but not prostacyclin inhibits histamine-induced contraction of human bronchial smooth muscle. Eur. J. Pharmacol. 1995b;272:13–19. doi: 10.1016/0014-2999(94)00602-4. [DOI] [PubMed] [Google Scholar]
- LAN R.S., STEWART G.A., HENRY P.J. Modulation of airway smooth muscle tone by protease activated receptor-1,-2,-3 and -4 in trachea isolated from influenza A virus-infected mice. Br. J. Pharmacol. 2000;129:63–70. doi: 10.1038/sj.bjp.0703007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI L., VAALI K., PAAKKARI I., VAPAATALO H. Involvement of bradykinin B1 and B2 receptors in relaxation of mouse isolated trachea. Br. J. Pharmacol. 1998;123:1337–1342. doi: 10.1038/sj.bjp.0701741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYDFORD S.J., MCKECHNIE K. Characterization of the prostaglandin E2 sensitive (EP)-receptor in the rat isolated trachea. Br. J. Pharmacol. 1994;112:133–136. doi: 10.1111/j.1476-5381.1994.tb13042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGAZINE H.I., SRIVASTAVA K.D. Thrombin-induced vascular reactivity is modulated by ETB receptor-coupled nitric oxide release in rat aorta. Am. J. Physiol. 1996;271:C923–C928. doi: 10.1152/ajpcell.1996.271.3.C923. [DOI] [PubMed] [Google Scholar]
- MCANULTY R.J., HERNANDEZ-RODRIGUEZ N.A., MUTSAERS S.E., COKER R.K., LAURENT G.J. Indomethacin suppresses the anti-proliferative effects of transforming growth factor-beta isoforms on fibroblast cell cultures. Biochem. J. 1997;321:639–643. doi: 10.1042/bj3210639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNAMARA C.A., SAREMBOCK I.J., GIMPLE L.W., FENTON J.W.D., COUGHLIN S.R., OWENS G.K. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J. Clin. Invest. 1993;91:94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOREL X., WALCH L., LABAT C., GASCARD J.P., DULMET E., BRINK C. Prostanoid receptors involved in the relaxation of human bronchial preparations. Br. J. Pharmacol. 1999;126:867–872. doi: 10.1038/sj.bjp.0702392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYSTEDT S., LARSSON A.K., ABERG H., SUNDELIN J. The mouse proteinase-activated receptor-2 cDNA and gene. Molecular cloning and functional expression. J. Biol. Chem. 1995;270:5950–5955. doi: 10.1074/jbc.270.11.5950. [DOI] [PubMed] [Google Scholar]
- PANG L., KNOX A.J. Effect of interleukin-1 beta, tumour necrosis factor-alpha and interferon-gamma on the induction of cyclo-oxygenase-2 in cultured human airway smooth muscle cells. Br. J. Pharmacol. 1997;121:579–587. doi: 10.1038/sj.bjp.0701152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAVORD I.D., TATTERSFIELD A.E. Bronchoprotective role for endogenous prostaglandin E2. Lancet. 1995;345:436–438. doi: 10.1016/s0140-6736(95)90409-3. [DOI] [PubMed] [Google Scholar]
- PAVORD I.D., WONG C.S., WILLIAMS J., TATTERSFIELD A.E. Effect of inhaled prostaglandin E2 on allergen-induced asthma. Am. Rev. Respir. Dis. 1993;148:87–90. doi: 10.1164/ajrccm/148.1.87. [DOI] [PubMed] [Google Scholar]
- PENE J., ROUSSET F., BRIERE F., CHRETIEN I., BONNEFOY J.Y., SPITS H., YOKOTA T., ARAI N., ARAI K., BANCHEREAU J., DE VRIES J.E. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc. Natl. Acad. Sci. U.S.A. 1988;85:6880–6884. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANGE S.P., PANG L., HOLLAND E., KNOX A.J. Selectivity of cyclo-oxygenase inhibitors in human pulmonary epithelial and smooth muscle cells. Eur. Respir. J. 2000;15:751–756. doi: 10.1034/j.1399-3003.2000.15d20.x. [DOI] [PubMed] [Google Scholar]
- RICCIARDOLO F.L.M., STEINHOFF M., AMADESI S., GUERRINI R., TOGNETTO M., TREVISANI M., CREMINON C., BERTRAND C., BUNNETT N.W., FABBRI L.M., SALVADORI S., GEPPETTI P. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am. J. Respir. Crit. Care. Med. 2000;161:1672–1680. doi: 10.1164/ajrccm.161.5.9907133. [DOI] [PubMed] [Google Scholar]
- SAMBRANO G.R., HUANG W., FARUQL T., MAHRUS S., CRAIK C., COUGHLIN S.R. Cathepsin G activates protease-activated receptor-4 in human platelets. J. Biol. Chem. 2000;275:6819–6823. doi: 10.1074/jbc.275.10.6819. [DOI] [PubMed] [Google Scholar]
- SARKER K.P., ABEYAMA K., NISHI J., NAKATA M., TOKIOKA T., NAKAJIMA T., KITAJIMA I., MARUYAMA I. Inhibition of thrombin-induced neuronal cell death by recombinant thrombomodulin and E5510, a synthetic thrombin receptor signaling inhibitor. Thromb. Haemost. 1999;82:1071–1077. [PubMed] [Google Scholar]
- SMITH W.G., THOMPSON J.M., KOWALSKI D.L., MCKEARN J.P. Inhaled misoprostol blocks guinea pig antigen-induced bronchoconstriction and airway inflammation. Am. J. Respir. Crit. Care. Med. 1996;154:295–299. doi: 10.1164/ajrccm.154.2.8756797. [DOI] [PubMed] [Google Scholar]
- STEINHOFF M., CORVERA C.U., THOMA M.S., KONG W., MCALPINE B.E., CAUGHEY G.H., ANSEL J.C., BUNNETT N.W. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp. Dermatol. 1999;8:282–294. doi: 10.1111/j.1600-0625.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- SUIDAN H.S., BOUVIER J., SCHAERER E., STONE S.R., MONARD D., TSCHOPP J. Granzyme A released upon stimulation of cytotoxic T lymphocytes activates the thrombin receptor on neuronal cells and astrocytes. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8112–8116. doi: 10.1073/pnas.91.17.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURGEON V.L., MILLIGAN C.E., HOUENOU L.J. Activation of the protease-activated thrombin receptor (PAR)-1 induces motoneuron degeneration in the developing avian embryo. J. Neuropathol. Exp. Neurol. 1999;58:499–504. doi: 10.1097/00005072-199905000-00009. [DOI] [PubMed] [Google Scholar]
- VANE J.R., BOTTING R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998;47:S78–S87. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]
- WATKINS D.N., PERONI D.J., LENZO J.C., KNIGHT D.A., GARLEPP M.J., THOMPSON P.J. Expression and localization of COX-2 in human airways and cultured airway epithelial cells. Eur. Respir. J. 2000;13:999–1007. doi: 10.1034/j.1399-3003.1999.13e12.x. [DOI] [PubMed] [Google Scholar]
- WOODWARD D.F., PEPPERL D.J., BURKEY T.H., REGAN J.W. 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH 6809), a human EP2 receptor antagonist. Biochem. Pharmacol. 1995;50:1731–1733. doi: 10.1016/0006-2952(95)02035-7. [DOI] [PubMed] [Google Scholar]


